Abstract
The mammalian auditory system evolved to extract meaningful information from complex acoustic environments. Spectrotemporal selectivity of auditory neurons provides a potential mechanism to represent natural sounds. Experience-dependent plasticity mechanisms can remodel the spectrotemporal selectivity of neurons in primary auditory cortex (A1). Electrical stimulation of the cholinergic nucleus basalis (NB) enables plasticity in A1 that parallels natural learning and is specific to acoustic features associated with NB activity. In this study, we used NB stimulation to explore how cortical networks reorganize after experience with frequency-modulated (FM) sweeps, and how background stimuli contribute to spectrotemporal plasticity in rat auditory cortex. Pairing an 8–4 kHz FM sweep with NB stimulation 300 times per day for 20 days decreased tone thresholds, frequency selectivity, and response latency of A1 neurons in the region of the tonotopic map activated by the sound. In an attempt to modify neuronal response properties across all of A1 the same NB activation was paired in a second group of rats with five downward FM sweeps, each spanning a different octave. No changes in FM selectivity or receptive field (RF) structure were observed when the neural activation was distributed across the cortical surface. However, the addition of unpaired background sweeps of different rates or direction was sufficient to alter RF characteristics across the tonotopic map in a third group of rats. These results extend earlier observations that cortical neurons can develop stimulus specific plasticity and indicate that background conditions can strongly influence cortical plasticity
Keywords: Auditory plasticity, Rat, Nucleus basalis, Context dependent reorganization
Introduction
Sensory experience can alter the organization of adult somatosensory (Merzenich et al. 1990; Recanzone et al. 1992b), visual (Gilbert 1996), and auditory (Dimyan and Weinberger 1999; Kilgard et al. 2001b; Recanzone et al. 1993; Weinberger and Bakin 1998) cortex. The correlation between changes in neuronal responsiveness and behavioral performance (Ohl et al. 2001; Recanzone et al. 1992c; Super et al. 2001) suggests that these changes constitute the neural basis for perceptual learning (for a review see Das 1997). This reasoning is complemented by evidence that precise details of sensory experience differentially shape cortical plasticity. Tuning properties of cortical neurons change depending upon stimulus parameters, neuromodulatory influences, and contextual circumstances. Decreases as well as increases in neuronal responses to acoustic conditioned stimuli (CS+) have been documented, depending on various task parameters including task difficulty, type of training (i.e., fear conditioning or NB stimulation), or the presence of nonreinforced stimuli (CS−) (Bakin and Weinberger 1990; Dimyan and Weinberger 1999; Edeline and Weinberger 1993; Ohl and Scheich 1996).
Repeated pairing of a tone with foot shock results in a specific increase in response to the CS+ frequency, while presentation of the same tone and shock on an unpaired schedule leads to a general increase in response to all tones, called sensitization (Bakin and Weinberger 1990). When a tone is presented repeatedly without the shock, neurons in primary auditory cortex (A1) habituate and respond with fewer action potentials specifically to the repeated tone frequency (Condon and Weinberger 1991). The demonstration that RF plasticity is gated by arousal and is specific to input features indicates that sensory experience alters the neural representation of behaviorally relevant and irrelevant events (reviewed in Weinberger and Bakin 1998).
Neurons in the cholinergic nucleus basalis (NB) respond to stimuli that have been associated with either rewards or aversive stimuli (Sarter et al. 1999, 2001). Activation of the NB has been used as a substitute for behavioral arousal in gating plasticity and has been shown to create changes specific to features of sensory stimuli associated with increased NB activity. Cortical maps (Kilgard and Merzenich 1998a), RF size and structure (Dimyan and Weinberger 1999; Kilgard et al. 2001a; Metherate and Weinberger 1989; Weinberger and Bakin 1998), temporal response properties (Kilgard and Merzenich 1998b; Mercado et al. 2001), and combination sensitivity (Kilgard and Merzenich 2002) of A1 neurons can be altered by pairing different sounds with NB stimulation. In many cases the resulting plasticity parallels CS+ specific plasticity generated by behavioral training.
RF size can be increased or decreased depending on the spatial variability and modulation rate of sensory inputs associated with a behavioral task or NB stimulation. Modulated stimuli repeatedly delivered to one site on the receptor surface increase RF size and decrease response latency, while unmodulated stimuli delivered to different locations decrease RF size and increase response latency (Kilgard et al. 2001a; Recanzone et al. 1992a, 1993). Nonreinforced stimuli (CS−) also influence the expression of neural plasticity. Pairing foot-shock with a single tone frequency in the context of many others generates neural plasticity that is in the opposite direction compared to the plasticity induced by tone-shock pairing presented in a silent background (Bakin and Weinberger 1990; Ohl and Scheich 1996). The background sounds caused the best frequency of A1 neurons to move away from the tone associated with shock rather than toward it. Background tones also influence the expression of RF size. Pairing a single tone with NB stimulation results in a 20% increase in RF size. However, this RF expansion does not occur if the same tone-NB pairing is interleaved with flanking tones that are not associated with NB stimulation (Kilgard et al. 2001a). While these experiments and many others indicate that cortical plasticity is guided by sensory input patterns, the complex relationship between input patterns and plasticity remains poorly understood.
Frequency modulation is nearly ubiquitous in natural communication sounds. Frequency-modulated (FM) sweeps have been used to probe spectrotemporal coding by auditory neurons in several species (reviewed in Eggermont 2001). A1 neurons have been shown to be selective for FM parameters such as rate and direction (reviewed in Nelken 2002). Lesion studies indicate that FM direction discrimination depends on the integrity of primary auditory cortex (Kelly and Whitfield 1971; Wetzel et al. 1998). Here we report that experience with FM sounds influences plasticity in A1.
The aim of the current study was to determine how experience with FM stimuli in silence or in a background of contrasting sounds alters cortical responses to this important stimulus class. FM sweeps were paired with electrical activation of nucleus basalis to generate spectrotemporal plasticity in rat A1. In Experiment 1 a downward FM sweep covering a frequency range of one octave was repeatedly paired with NB stimulation. In Experiment 2 five different FM sweeps activating different regions of A1 were paired with NB stimulation. Experiment 3 was identical to Experiment 2 except that unpaired FMs of contrasting rates (faster or slower), or direction (upward sweeping) were interleaved with the five FM sweeps paired with NB stimulation. The results of this study were presented earlier in abstract form (Moucha et al. 2001a, 2001b).
Methods
Sixteen adult female Sprague-Dawley rats were used for this study. Nine rats were implanted with NB stimulation electrodes. The experimental data used in this study was derived from 398 A1 recording sites from these rats. Recordings from 259 A1 sites from seven rats served as control data (Table 1). All procedures were carried out in accordance with the guidelines laid down by the United States National Institute of Health and the University of Texas at Dallas Institutional Animal Care and Use Committee.
Table 1.
Experimental design
| Sounds paired with NB stimulation | Background sounds | No. of rats | No. of A1 sites | |
|---|---|---|---|---|
| Experiment 1: single FM sweep + NB | 8–4 kHz (160 ms) | None | 3 | 136 |
| Experiment 2: five FM sweeps + NB | 2–1, 4–2, 8–4, 16–8, and 32–16 kHz (160 ms) | None | 3 | 128 |
| Experiment 3: five FM sweeps + NB with background FMs interleaved | 2–1, 4–2, 8–4, 16–8, and 32–16 kHz (160 ms) | 1–2, 2–4, 4–8, 8–16, and 16–32 kHz (160 ms) 2–1, 4–2, 8–4, 16–8, and 32–16 kHz (40 or 640 ms) |
3 | 134 |
| Controls | None | None | 7 | 259 |
| Totals | – | – | 16 | 657 |
Chronic implantation and electrical stimulation
The NB stimulation used in this study was identical to our previous reports that include more detailed technical descriptions (Kilgard and Merzenich 1998a, 1998b, 1999, 2002; Kilgard et al. 2001a, 2001b). Briefly, experimental rats were anesthetized with pentobarbital and implanted with platinum bipolar stimulating electrodes into NB using sterile stereotaxic techniques (7.0 mm below cortical surface; 3.3 mm lateral and 2.3 mm posterior from bregma). Leads were attached to screws over cerebellum and cortex to allow recording of global electroencephalography during the subsequent sound-NB pairing phase of the study. After 2 weeks of recovery, each animal was placed in a sound shielded, calibrated test chamber and received approx. 300 pairings of an acoustic stimulus with NB stimulation per day for about 20 days under one of the three experimental conditions. Custom software was used to control the auditory stimuli (generated with a Tucker-Davis D/A converter, Alachua, Fla., USA) and trigger NB stimulation (train of 20 biphasic pulses, 100 Hz, 0.1 ms pulse width, current level 70–150 μA, beginning at the termination of each paired sweep).
Acoustic stimulation
In Experiment 1 the sound repeatedly paired with NB stimulation was a downward FM sweep (160 ms duration, 6.25 oct/s) spanning a single octave (8–4 kHz). In Experiments 2 and 3 five downward FM sweeps each spanning a different octave (2–1, 4–2, 8–4, 16–8, 32–16 kHz) were randomly interleaved and paired with NB stimulation (Fig. 1). Each sweep was presented at approx. 25 dB above rat hearing threshold based on behavioral and neural responses (Kelly and Masterton 1977; Kilgard and Merzenich 1999). Each of the five octaves was presented at 60, 55, 45, 40, and 50 dB SPL, respectively. Acoustic and electrical stimuli did not evoke any observable behavioral responses but did generate reliable electroencephalographic desynchronization for 1–2 s if stimulation occurred during slow wave sleep.
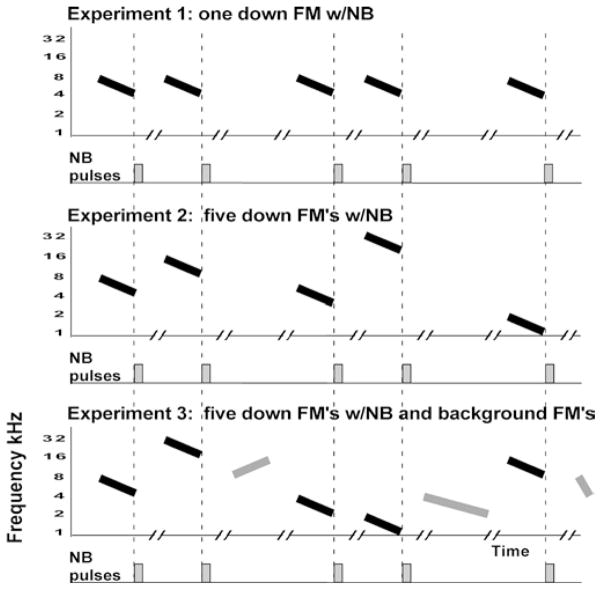
Fig. 1.
Schematic illustration of experimental design. Nucleus basalis (NB) stimulation was paired with downward one octave FM sweeps (160 ms duration) 300 times per day for 20 days. A In Experiment 1 an 8–4 kHz sweep was paired with NB stimulation (rectangle a train of 20 pulses). B In Experiment 2 five different one octave downward sweeps were paired with NB stimulation. C In Experiment 3 additional unpaired FM sweeps were randomly interleaved between the five sweeps paired NB stimulation. These background FM sweeps also spanned one octave, but were shorter (40 ms) or longer (640 ms) or swept in the opposite direction as the paired sounds. Stimuli or silent intervals were delivered every 10 s (line break 10-s gap). Total duration of daily exposure was 2–3 h
Neurophysiological recording
Twenty-four hours after the final NB stimulation session, responses of auditory cortex neurons to tones and FM sweeps were quantified with high-density microelectrode recordings. Animals were anesthetized with pentobarbital (50 mg/kg body weight) and maintained in a state of areflexia throughout the surgical procedure and during recordings. To ensure a proper level of anesthesia and stable physical condition, the animal’s electrocardiogram and blood oxygen concentration were also monitored. The skull was supported in a head holder leaving the ears unobstructed. The trachea was cannulated to ensure adequate ventilation. The cisternae magnum was drained of CSF to minimize cerebral edema. The auditory cortex was exposed via a wide craniotomy, the dura mater was resected, and a thin layer of viscous silicon oil was applied to prevent desiccation. Recording of action potentials were made in a double-walled sound chamber from two Parylene-coated tungsten microelectrodes (FHC, Bowdoinham, Me., USA) lowered orthogonally into right auditory cortex to a depth of 550 μm (layers IV/V). Multiunit data were collected from 30–65 sites in each animal. Neural signal was filtered (.3–8 kHz), amplified (×10,000), and resulting spike-waveforms crossing a fixed threshold were sampled. Tucker-Davis neurophysiology hardware and Brainware software were used for stimulus production, online spike sorting and data acquisition.
Frequency-intensity tuning curves were derived at each site, by presenting 81 frequencies spanning five octaves, at 16 intensities ranging between 0 and 75 dB (1296 total stimuli). Responses to FM sweeps of different direction, rates, and starting frequency were also recorded. Every FM sweep used in this study spanned a single octave. Six different sweep rates were presented: 20, 40, 80, 160, 320, and 640 ms (corresponding to 50, 25, 12.5, 6.25, 3.12, and 1.56 oct/s). FM sweeps always started at 1, 2, 4, 8, 16, or 32 kHz. At each site sweeps were presented that covered one octave above and one octave below the best frequency. The onset and offset of all sounds were linearly ramped over 3 ms. Responses to a total of 24 randomly interleaved FM stimuli (6 rates×2 octaves×2 directions) were recorded at each site. The sweeps had the same intensities as those used during NB stimulation (see above). Each sweep was repeated 30 times at 1-s intervals to minimize adaptation.
Data analysis
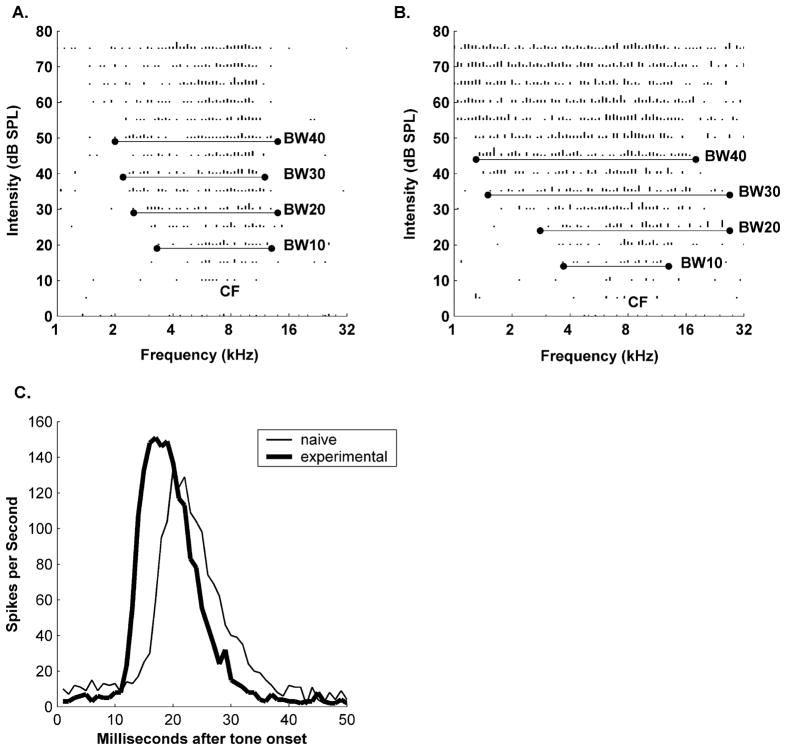
All analysis was conducted using custom MATLAB programs (MathWorks, Natick, Mass., USA). Tuning curve parameters were defined blind to experimental condition and recording location, using an interface specifically designed for this purpose. Thresholds, characteristic frequency (CF), response strength, minimum latency, time to end of response, and excitatory frequency tuning range or bandwidth (BW at 10, 20, 30, 40 dB over threshold) were determined for each site (Fig. 2a). Tone response strength was estimated as the average number of spikes in response to tones with intensities greater than threshold and frequencies within the maximum excitatory bandwidth for each site (as determined from the frequency-intensity tuning curves; Fig. 2a). Latency was derived from poststimulus time histogram (PSTH) created by summing responses to all the tones within each site’s tuning curve. The minimum latency was defined as the time from stimulus onset to the earliest consistent response. Peak latency was the time to the maximum instantaneous firing rate. The time to end of driven response was the time to return to spontaneous activity levels (Fig. 2c). A1 was defined on the basis of its short latency (8–20 ms) responses and continuous tonotopy. Boundaries were determined using nonresponsive and non-A1 sites.
Fig. 2.
Receptive field and latency plasticity after pairing NB stimulation with FM sweeps. Representative tuning curves from naive (A) and experimental (B) animals. Frequency intensity tuning curves were derived from responses to 81 frequencies spanning five octaves, at 16 intensities ranging between 0 and 75 dB. Length of line Proportional to the number of spikes. C Peristimulus time histograms (PSTH), for the recording sites in A, B. Minimum response latency is defined as the time from stimulus onset to the earliest consistent response. The time to the maximum response and end of response were also quantified. Decreased latencies and neuronal thresholds, and broadened bandwidths (BW at 10, 20, 30, and 40 dB above threshold) resulted from pairing NB stimulation with FM sweeps (Tables 1, 2)
Unpaired two tailed t tests were used to evaluate the effects of NB-FM pairing on frequency selectivity, response strength, latency, and direction selectivity.
FM direction selectivity (DS) was quantified using the following index: DS=(Rup−Rdown)/(Rup+Rdown), where R is the response (in number of spikes averaged from 30 repetitions) elicited by the upward or downward FM sweep. Spikes occurring from 8 ms after sweep onset until 40 ms after the end of each sweep were analyzed. The average spontaneous firing rate was estimated from the 8 ms before the onset of a driven response and subtracted. The responses of A1 neurons to pairs of sweeps that begin at a frequency within the excitatory receptive field and sweep one octave (up or down) are typically indistinguishable because the response is largely determined by the onset frequency which is identical for the two sweeps. Thus we compared sweeps that swept through the same octave in opposite directions (i.e., had reversed start and end frequencies, such as 4–8 vs. 8–4 kHz).
Results
In this study we compared responses to tones and FM sweeps from naive animals (n=259 sites from seven rats) with responses from animals who heard FM sweeps paired with NB stimulation (n=398 sites from nine rats). The three experimental groups differed in the number of FM sweeps paired with NB activation (1 or 5) and the presence or absence of background sweeps (0 or 15) interleaved between the paired sweeps (Table 1, Fig. 1).
Experiment 1
Animals in the first group heard a downward FM sweep (8–4 kHz, 160 ms duration) paired with NB activation 300 times per day over a period of 3 weeks. While repeated NB stimulation paired with an unmodulated tone nearly doubled the proportion of A1 neurons responding to the paired tone frequency (Kilgard and Merzenich 1998a), pairing the 8–4 kHz sweep with NB stimulation did not alter the A1 map of tone frequency. The proportions of cortex responding to 45 dB tones at 2, 4, 8, and 16 kHz in the experimental group were not significantly different from those in naive rats (36±7 vs. 38±4%, 37±6 vs. 44±3%, 43±3 vs. 45±6%, 41±4 vs. 39±3%, respectively).
Responses to upward and downward FM sweeps were recorded at each site to document changes in FM direction selectivity. Although A1 neurons in rats rarely exhibit a high degree of direction preference for one octave wide sweeps, some neurons do exhibit some preference. To measure direction selectivity we compared responses to FM pairs that swept through the same octave but in opposite directions (i.e., 8–4 kHz and 4–8 kHz). After pairing an 8–4 kHz sweep with NB stimulation no downward direction selectivity developed (DS=0.14±0.05 and 0.13±0.05 for control and experimental rats, respectively).
Although pairing a single FM sweep with NB stimulation did not alter direction preference or frequency topography, temporal and receptive field properties were altered in the region of auditory cortex activated by the paired sweep. NB activation paired with the 8–4 kHz sweep altered frequency selectivity, response threshold, latency, and strength of A1 neurons with best frequencies from 4 to 16 kHz (Table 2). In this experiment the FM sweep paired with NB activation was 8–4 kHz presented at approx. 25 dB above rat hearing and neuronal threshold (see “Methods”). Based on previous data from our laboratory at 20 dB over threshold the excitatory frequency bandwidth (BW20) of neurons in naive rats is approx. 2.0 octaves (see also Table 2). Thus an 8-kHz tone corresponding to the start frequency of the paired sweep would likely activate neurons with CF’s one octave below and above eight (4–16 kHz). The mean tone threshold in these neurons was 2 dB quieter than neurons in naive animals. Receptive field size (bandwidth at 20 dB above threshold) was increased by one-fifth of an octave. The minimum response latency was decreased by more than 2 ms (Fig. 2). Response threshold, frequency selectivity, and latency were not altered in the flanking regions of the A1 frequency map (Table 2). CF’s 1–4 kHz and 16–32 kHz are both towards the edges of the rat hearing range. It has been documented that neuronal thresholds are higher and receptive fields (BW) narrower for these neurons (Kelly and Masterton 1977; Kilgard and Merzenich 1999). The same trend is apparent in Table 2, thresholds were 26.45 dB, as compared to 15.0 dB for CF’s 4–16, and BW20 1.46 octaves as compared to 1.9 octaves. Because both CF groups (1–4 kHz and 16–32 kHz) showed no significant RF changes after FM-NB pairing their results were pooled. These results indicate that this paradigm generates plasticity that is specific to the region of the tonotopic map most strongly activated by the FM sweep. However, the average number of spikes evoked by tones within each site’s receptive field was increased across A1.
Table 2.
Sites with CFs 4 to 16 kHz vs. sites with CFs <4 and >16 kHz. Pairing an 8–4 FM sweep with NB stimulation generates plasticity in the region of the cortical map activated by the sweep. Values given are the mean and standard error of the mean. The distribution of CF values in control and experimental groups was not different. For each experimental group CF range (minimum and maximum value in kHz), mean (distance away of any CF from 1 kHz in octaves) and standard deviation (in octaves) were as follow: sites with CFs 4–16 kHz controls (4.23, 15.91, 3.14, 1.91), experimental (4.04, 15.85, 3.13, 1.97) and sites with CFs <4 and >16 controls (1.14, 31.92, 2.63, 4.80), experimental (1.31, 31.89, 3.07, 4.60). [N/A not applicable (FM 8–4 kHz or 4–8 kHz were not presented)]
| Sites with CFs 4 to 16 kHz |
Sites with CFs <4 and >16 kHz |
|||||
|---|---|---|---|---|---|---|
| 8 → 4 kHz sweep paired with NB (n=71) | Naive controls (n=130) | Change | 8 → 4 kHz sweep paired with NB (n=65) | Naive controls (n=129) | Change | |
| Threshold | 13.01±0.87 | 15.0±0.58 | −2.0 dB* | 24.71±1.01 | 26.45±0.82 | −1.73 dB |
| BW20 | 2.10±0.05 | 1.90±0.06 | 0.19 octaves* | 1.40±0.08 | 1.46±0.05 | −0.05 octaves |
| Minimum latency | 16.09±0.45 | 18.31±0.41 | −2.22 ms** | 16.83±1.10 | 17.50±0.63 | −0.66 ms |
| End of peak latency | 37.27±0.62 | 35.87±0.45 | 1.40 ms | 35.45±1.26 | 35.81±1.01 | −0.36 ms |
| Mean response to tones | 1.59±0.12 | 1.09±0.06 | 0.49 spikes** | 1.35±0.09 | 1.05±0.05 | 0.30 spikes** |
| Response to FM 8–4 kHz (160 ms) | 1.41±0.15 | 1.12±0.09 | 0.29 spikes | N/A | N/A | N/A |
| Response to FM 4–8 kHz (160 ms) | 1.79±0.20 | 1.46±0.14 | 0.33 spikes | N/A | N/A | N/A |
P<0.05,
P<0.01 vs. naive controls (two-tailed t tests)
Experiment 2
Earlier studies with spatially restricted stimuli also reported response plasticity in restricted regions of sensory maps (Bao et al. 2001; Irvine et al. 2001; Xerri et al. 1996). It is possible to generate plasticity that is not restricted by using inputs that are distributed across the receptor surface. For example, pairing NB stimulation with unmodulated tones of different frequencies (1.3, 2, 3, 4, 5, 7, 9, 11.2, and 14 kHz) narrowed receptive fields and increased the minimum latency of A1 neurons across the tonotopic map (Kilgard et al. 2001a). The aim of Experiment 2 was to determine whether distributing FM sweeps evenly across the cochlea could generate plasticity (in this case, broader RFs and shorter latencies as documented in Experiment 1) that generalized across the A1 frequency map. NB stimulation was paired with downward FM sweeps that spanned five different frequency ranges (2–1 kHz, 4–2 kHz, 8–4 kHz, 16–8 kHz, 32–16 kHz). In contrast to the earlier studies, no plasticity of any sort resulted from the repeated pairing (Table 3). A1 topography, receptive field properties, latency, and FM direction selectivity were all indistinguishable from naive rats. Response strength to downward FMs did not increase after the repeated pairing of down FM with NB stimulation; however, response strength to upward FM sweeps was somewhat decreased (Table 3).
Table 3.
Five downward FM sweeps paired with NB stimulation. Pairing multiple FM sweeps with NB stimulation does not lead to significant plasticity unless background (unpaired) sweeps are also presented. The distribution of CF values in control and experimental groups was not different. For each experimental group CF range (minimum and maximum value in kHz), mean (distance away of any CF from 1 kHz in octaves) and standard deviation (in octaves) were as follow: controls (1.14, 31.91, 2.87, 1.29), experiment 2 (1.20, 32.0, 3.05, 1.33), experiment 3 (1.18, 32.20, 2.88, 1.28)
| Naive controls (n=259) | Experiment 2 in silence (n=128) | Change | Experiment 3 with background (n=134) | Change | |
|---|---|---|---|---|---|
| Threshold | 20.85±0.62 | 19.20±0.72 | −1.65 dB | 15.96±0.84 | −4.89 dB** |
| BW20 | 1.67±0.04 | 1.72±0.06 | 0.05 octave | 1.85±0.09 | 0.18 octave* |
| Minimum latency | 17.75±0.30 | 18.39±0.52 | 0.64 ms | 15.89±0.38 | −1.86 ms** |
| End of peak latency | 35.57±0.39 | 36.68±0.71 | 1.11 ms | 33.05±0.46 | −2.51 ms** |
| Mean response to tones | 1.07±0.04 | 0.98±0.05 | −0.08 spikes | 1.07±0.06 | 0.00 spikes |
| Response to down 160 ms FM | 0.78±0.02 | 0.60±0.01 | −0.18 spikes | 0.42±0.02 | −0.36 spikes** |
| Response to up 160 ms FM | 0.98±0.01 | 0.77±0.01 | −0.21 spikes | 0.69±0.00 | −0.29 spikes** |
P<0.05,
P<0.01 vs. naive controls (two-tailed t tests)
Experiment 3
Previous evidence indicates that nonreinforced inputs can influence the expression of cortical plasticity in auditory, visual, and somatosensory modalities (Bakin and Weinberger 1990; Dimyan and Weinberger 1999; Edeline and Weinberger 1993; Kilgard et al. 2001a; Moore et al. 1999; Ohl and Scheich 1996). To test whether receptive field and FM response plasticity can be altered by background sounds we repeated Experiment 2 with additional unpaired FM sweeps randomly interleaved between the sweeps paired with NB activation (Fig. 1). The 15 background sweeps each spanned one octave but differed from the paired sweeps in that they were of opposite direction (i.e., upward sweeping FMs) or different frequency modulation rate (i.e., faster or slower), as described in “Methods” (Table 1). The additional sweeps were designed to provide greater contrast between paired and unpaired sounds that might influence the representation of the paired FM sweep.
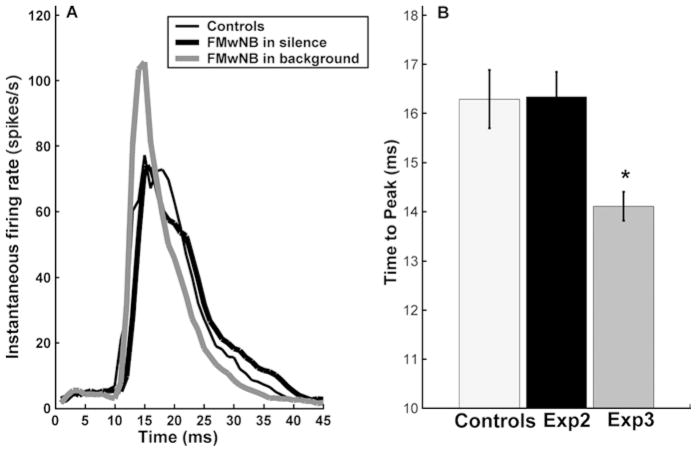
Frequency selectivity, response latency and threshold all significantly decreased as a result of adding background unpaired FMs. The changes in receptive field size and minimum latency were of the same magnitude as in Experiment 1 but generalized across the A1 frequency map (Table 3). The addition of contrasting FM sweeps decreased the minimum response threshold by nearly 5 dB compared to naive animals. The threshold decrease was significantly greater than the decrease observed in Experiment 1 (P<0.05, for neurons in the 4–16 kHz CF range). This experimental paradigm resulted in a decrease in average number of spikes elicited by all FM sweeps. However, the temporal characteristics of the response to FM sweeps was changed (Fig. 3). No preference for downward sweep direction or rate developed (data not shown).
Fig. 3.
Population PSTH. A Population PSTHs of responses to the paired FM sweeps in control and experimental groups. The addition of background FM sweeps interleaved with the sweeps paired with NB stimulation increased the response coherence of responses to the paired FM. B The addition of backgrounds sounds also decreased peak latency
In summary, NB stimulation paired with FM sweeps alters A1 responses as a function of both the number of paired sounds and the background in which the sounds were presented. Temporal sharpening and broader frequency tuning resulted from NB stimulation paired with FM sweeps that activated either a restricted region of the map or were presented in the context of contrasting FM sweeps. Since identical NB activation (strength, repetitions, time course) was associated with sound presentation in each experimental group, we conclude that the differential plasticity documented here was a result of the differential auditory experience of each group.
Discussion
The aim of this study was to explore aspects of experience-dependent plasticity in primary auditory cortex. We investigated how plasticity mechanisms activated by NB stimulation alter cortical response properties following experience with FM sounds. Our results show that experience with a single octave wide FM sweep alters receptive field properties (threshold and bandwidth), response strength, and minimum latency of auditory cortical neurons in a frequency specific manner. In contrast, experience with multiple FM sweeps spanning the rat hearing range does not alter receptive field properties or the temporal fidelity of A1 neurons. However, if the same auditory experience occurs in the context of background sounds, receptive fields and temporal response properties are changed across A1.
Receptive fields
Earlier studies documented that RF size can be altered by sensory input characteristics. Frequency discrimination training resulted in smaller RFs in auditory cortex (Recanzone et al. 1993). In contrast, a task requiring detection of amplitude modulation rate of a stimulus applied to a single digit resulted in RF broadening in somatosensory cortex (Recanzone et al. 1992b). Due to the large number of differences between these studies it was not possible to be sure whether task parameters or the pattern of sensory input resulted in the opposite effects on RF size. Such differential plasticity was also observed after pairing identical NB stimulation with different patterns of sensory inputs. Pairing NB stimulation with a single modulated tone broadened RFs, while pairing with two unmodulated tones more than an octave apart narrowed RFs. Sounds that were both modulated and distributed across the receptor surface resulted in intermediate RF plasticity. These results suggest that modulation rate and number of locations activated by the sensory input influence RF size.
The present findings may shed new light on a classical RF plasticity experiment conducted in monkeys. Several weeks of exposure to a spinning disk that brushed across several digits decreased RFs in somatosensory cortex (Jenkins et al. 1990). Because the original explanation of the spinning disk result was that moving stimuli generate narrow receptive fields (Merzenich et al. 1990), we had expected FM stimuli to decrease RF size in the present experiment. Our results (using the auditory analog of light brushes to the skin) indicate that the initial interpretation of the monkey experiment may be incomplete. It should be noted however that behavioral training likely engages other neuromodulatory systems than the NB stimulation used in the present study. Our observation that background stimuli can shape the expression of RF plasticity suggests many more studies are needed before we can reliably predict how untested sensory experiences influence cortical plasticity.
Response latency
Response latency of cortical neurons has also been shown to change as a function of sensory experience. Frequency discrimination training increased latencies of A1 neurons in owl monkeys (Recanzone et al. 1993), while temporal tasks decreased response latencies (Recanzone et al. 1992c). Results from NB stimulation experiments which attempted to mimic the patterns of sensory input in the primate studies closely parallel these findings (Kilgard et al. 2001a). Although some theoretical work has been carried out to understand the plasticity mechanisms that influence cortical response latency (Song et al. 2000), little is known about the effects of complex input patterns. At present the most parsimonious explanation of the latency results following behavioral training or NB stimulation is that modulated inputs tend to decrease response latency while nonmodulated stimuli tend to increase latency. A complex sound sequence (tone-tone-noise) paired with NB activation caused a 30% decrease in cortical processing time (Kilgard and Merzenich 2002). In the present study, experience with an 8–4 kHz FM sweep caused minimum response latency to decrease within the region of the map activated by the input. When multiple FM sweeps were paired with NB activation such that all regions of the map were equally activated neuronal latencies were not affected. However, when unpaired background sounds were interleaved with the paired sounds, latencies decreased across the A1 frequency map. These results suggest that the spectral and temporal features of both behaviorally relevant and irrelevant sounds have the potential to influence the response latencies of cortical neurons.
Response strength
The number of spikes evoked by a tone can also be modified by experience (Engineer et al. 2001). Pairing modulated tones (i.e., 15-Hz train of 9-kHz tones) with NB stimulation increased spikes per tone by 40%(Kilgard et al. 2001a), while pairing unmodulated tones of the same frequency had no effect on neural excitability. Our observation of increased response strength after pairing NB stimulation with FM stimuli supports an earlier report by Mercado and colleagues (2001). It is not yet clear why response strength was not increased after pairing five different FM sweeps with NB stimulation. Distributing the inputs across five octaves resulted in fewer paired inputs to each cortical sector. Although this could explain the lack of response strength plasticity, it would not explain why RF and latency plasticity were equally strong in Experiments 1 and 3. In Experiment 3 the addition of background sounds resulted in more sounds activating each region, with the distinction that not all were paired with NB stimulation.
Background stimuli
Studies of associative learning have shown that neuronal tuning can change both towards the conditioned frequency, when presented in silence (Bakin and Weinberger 1990; Dimyan and Weinberger 1999), or away from the paired frequency, when unpaired stimuli are presented (Ohl and Scheich 1996). Our current results indicate that background unpaired FM sweeps have a significant influence on RF and latency plasticity in A1. Stimuli or silent intervals were delivered every 10 s (Fig. 1) therefore this influence cannot be attributed to the effect of acetylcholine at the time the background sounds are presented. Introducing only 1-s separation between the sensory input and NB activation is sufficient to block NB-induced plasticity (Metherate and Ashe 1991, 1993). Another consideration in interpreting the effects attributed to the background sounds is the difference in stimulation rate in our experimental conditions. Because we kept NB stimulation identical in each experimental group, pairing of five different FM sweeps (approx. 300 times/day for about 20 days) resulted in fewer pairings of each sweep (i.e., frequency interval) in the group in which a single FM sweep was paired (approx. 300 times/day for about 20 days). However, the interpretation that this might lead to less plasticity is partly incorrect when we take into consideration the different effects observed in our third group which received identical pairing in the presence of unpaired background sounds. We favor the interpretation that the differential plasticity in Experiments 2 and 3 is due to the difference in background conditions between these two experiments, since stimulation rate was the same. Such dependence of plastic effects on contextual circumstances may improve learning in noisy environments.
Responses to FM sweeps
Neurons in several species have been shown to exhibit precise spectrotemporal selectivity to behaviorally relevant complex stimuli (Wang et al. 1995; for a review see Suga 1989). Our experiments were designed to explore the neural mechanisms that may give rise to this selectivity. Plasticity induced by cholinergic modulation creates changes in cortical responses specific to the experienced stimulus, thus by pairing NB activation with FM sweeps we investigated whether neurons can become selective for this class of sounds. Earlier studies of FM exposure during development have suggested that direction selectivity of auditory neurons can be altered by sensory experience (Clopton and Winfield 1976; Poon et al. 1990). We observed no change in direction selectivity toward the direction of the paired sweep. This seems to be in accord with a recent study by Mercado and colleagues (2001) using FM-NB stimulation pairing; however, we did not see a general increase in response strength to FM sounds as they reported. We show that neurons had a slight preference for upward direction which confirms findings by other investigators studying this species (Gaese and Ostwald 1995; Ricketts et al. 1998). Direct comparison, however, with previous studies is made difficult due to differences in FM parameters used (both frequency range and rate). Some discrepancies could be explained by the difference in the way that FM sweeps are generated. For example, direction selectivity maps differ between linear and logarithmic FMs (Nelken and Versnel 2000), and between continuous (Shamma et al. 1993) and separate sweeps (Heil et al. 1992; Mendelson et al. 1993). Thus it appears that FM direction selectivity depends strongly on the paradigm used to measure it.
Comparison of plasticity generated by rapidly modulated and unmodulated tones
The present results are part of a series of experiments that use identical NB stimulation paired with different sounds to determine how sensory features influence cortical response plasticity. However, most of the experiments completed to date have focused on relatively simple tonal stimuli. The long-term goal is to understand how complex input patterns direct neural plasticity. Despite the limited set of sounds tested so far, a number of important generalizations are apparent. The decreased threshold observed in the current study appears to be specific to FM stimuli as it was not observed when NB stimulation was paired with amplitude modulated or unmodulated pure tones (Table 4). Increased response strength was observed only after amplitude- or frequency-modulated tones that activated a restricted region of A1 were paired with NB stimulation. Shorter minimum latency and broader receptive fields resulted from pairing NB stimulation with narrow band FM sounds or unmodulated tones that activate a restricted receptor region. Unmodulated inputs distributed across the cochlea was the only combination that lengthened latency and narrowed receptive fields. Although FM sweeps and pure tones generate very similar patterns of local activation, pairing a single FM sweep with NB stimulation does not generate the map plasticity that results from pairing a simple tone.
Table 4.
Sounds paired with NB stimulation. Different forms of plasticity result from pairing single or multiple tones that are unmodulated, amplitude-modulated (15 Hz), or frequency-modulated (1 octave decrease in 160 ms) paired with identical NB stimulation (left plasticity resulting from pairing a single FM sweep or a tone with NB stimulation were similar, right plasticity resulting from pairing multiple FM sweeps or tones with NB stimulation were dissimilar)
| Unmodulated single tone | Unmodulated single tone + background | Single tone AM | Single FM | Multiple tones | Multiple tones AM | Multiple FMs | Multiple FMs + background | |
|---|---|---|---|---|---|---|---|---|
| Threshold | – | – | – | Decrease | – | – | – | Decrease |
| Mean response to tones | – | – | Increase | Increase | – | – | – | – |
| BW20 | Increase | – | Increase | Increase | Decrease | Increase | – | Increase |
| Minimum latency | Decrease | Decrease | Decrease | Decrease | Increase | – | – | Decrease |
| Map plasticity | Increase | Increase | Increase | – | – | – | – | – |
The observation that increases in bandwidth often cooccur with decreases in latency suggests the two effects could be related. For example, stronger afferent inputs could explain these effects. However, the processes giving rise to the observed changes are likely to be complex. Receptive field expansion or contraction and latency shortening or lengthening could result from (a) lowering or raising of spike thresholds, (b) increased or decreased synaptic strength, (c) added or reduced number of connections, (d) reduced or increased inhibition, and/or (e) shifts in the balance of inputs toward thalamocortical or corticocortical synapses, respectively.
Conclusions
In summary, we show (a) that differential changes in receptive field properties of cortical neurons can be induced in adult animals by altering sensory experience, and that (b) background sounds play an important role in shaping cortical plasticity. Temporal sharpening and broader tuning curves resulted from pairing one octave FM sweeps with NB stimulation only when restricted to one region of the map or presented in the context of background FM sweeps. These findings add to a growing body of evidence that input statistics play an important role in guiding representational plasticity that contributes to perceptual learning.
Effective rehabilitation following peripheral or central nervous system damage appears to rely on plasticity mechanisms that are likely to be guided by sensory experience. A more complete understanding of the influence-specific forms of experience have on the cerebral cortex will be useful in designing more effective strategies for improving functional recovery (Grimby et al. 2003). The current results indicate the sensory context within which rehabilitation is conducted may be as important as the tasks themselves in stimulating plasticity.
Acknowledgments
We thank Amanda Puckett and Wei Wei Dai for assistance with animal training and colony management and we are grateful to Jessica Vasquez for help with neurophysiological recordings. Two anonymous reviewers provided insightful comments. This study was funded in part by RO3-DC04534 (to M.P.K.).
References
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Clopton BM, Winfield JA. Effect of early exposure to patterned sound on unit activity in rat inferior colliculus. J Neurophysiol. 1976;39:1081–1089. doi: 10.1152/jn.1976.39.5.1081. [DOI] [PubMed] [Google Scholar]
- Condon CD, Weinberger NM. Habituation produces frequency-specific plasticity of receptive fields in the auditory cortex. Behav Neurosci. 1991;105:416–430. doi: 10.1037//0735-7044.105.3.416. [DOI] [PubMed] [Google Scholar]
- Das A. Plasticity in adult sensory cortex: a review. Network Comput Neural Syst. 1997;8:R33–R76. [Google Scholar]
- Dimyan MA, Weinberger NM. Basal forebrain stimulation induces discriminative receptive field plasticity in the auditory cortex. Behav Neurosci. 1999;113:691–702. doi: 10.1037//0735-7044.113.4.691. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Receptive field plasticity in the auditory cortex during frequency discrimination training: selective retuning independent of task difficulty. Behav Neurosci. 1993;107:82–103. doi: 10.1037//0735-7044.107.1.82. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Between sound and perception: reviewing the search for a neural code. Hear Res. 2001;157:1–42. doi: 10.1016/s0378-5955(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Pandya PK, Vazquez JL, Rathbun DL, Moucha R, Kilgard MP. Society for neuroscience. Vol. 27. San Diego: 2001. Auditory experience improves response characteristics in rat primary auditory cortex neurons. [Google Scholar]
- Gaese BH, Ostwald J. Temporal coding of amplitude and frequency modulation in the rat auditory cortex. Eur J Neurosci. 1995;7:438–450. doi: 10.1111/j.1460-9568.1995.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Gilbert CD. Learning and receptive field plasticity. Proc Natl Acad Sci U S A. 1996;93:10546–10547. doi: 10.1073/pnas.93.20.10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby G, Eriksson P, Nilsson M, Sjolund B. Neurobiology provides a scientific foundation for rehabilitation. Report from an international symposium. Lakartidningen. 2003;100:2052–2055. [PubMed] [Google Scholar]
- Heil P, Rajan R, Irvine DR. Sensitivity of neurons in cat primary auditory cortex to tones and frequency-modulated stimuli. II. Organization of response properties along the ‘iso-frequency’ dimension. Hear Res. 1992;63:135–156. doi: 10.1016/0378-5955(92)90081-w. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Rajan R, Brown M. Injury- and use-related plasticity in adult auditory cortex. Audiol Neurootol. 2001;6:192–195. doi: 10.1159/000046831. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Masterton B. Auditory sensitivity of the albino rat. J Comp Physiol Psychol. 1977;91:930–936. doi: 10.1037/h0077356. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Whitfield IC. Effects of auditory cortical lesions on discriminations of rising and falling frequency-modulated tones. J Neurophysiol. 1971;34:802–816. doi: 10.1152/jn.1971.34.5.802. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998b;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Distributed representation of spectral and temporal information in rat primary auditory cortex. Hear Res. 1999;134:16–28. doi: 10.1016/s0378-5955(99)00061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Order-sensitive plasticity in adult primary auditory cortex. Proc Natl Acad Sci U S A. 2002;99:3205–3209. doi: 10.1073/pnas.261705198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol. 2001a;86:326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez JL, Rathbun DL, Engineer ND, Moucha R. Spectral features control temporal plasticity in auditory cortex. Audiol Neurootol. 2001b;6:196–202. doi: 10.1159/000046832. [DOI] [PubMed] [Google Scholar]
- Mendelson JR, Schreiner CE, Sutter ML, Grasse KL. Functional topography of cat primary auditory cortex: responses to frequency-modulated sweeps. Exp Brain Res. 1993;94:65–87. doi: 10.1007/BF00230471. [DOI] [PubMed] [Google Scholar]
- Mercado E, Bao S, Orduna I, Gluck MA, Merzenich MM. Basal forebrain stimulation changes cortical sensitivities to complex sound. Neuroreport. 2001;12:2283–2287. doi: 10.1097/00001756-200107200-00047. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Recanzone GH, Jenkins WM, Grajski KA. Adaptive mechanisms in cortical networks underlying cortical contributions to learning and nondeclarative memory. Cold Spring Harb Symp Quant Biol. 1990;55:873–887. doi: 10.1101/sqb.1990.055.01.082. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Res. 1991;559:163–167. doi: 10.1016/0006-8993(91)90301-b. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Acetylcholine produces stimulus-specific receptive field alterations in cat auditory cortex. Brain Res. 1989;480:372–377. doi: 10.1016/0006-8993(89)90210-2. [DOI] [PubMed] [Google Scholar]
- Moore CI, Nelson SB, Sur M. Dynamics of neuronal processing in rat somatosensory cortex. Trends Neurosci. 1999;22:513–520. doi: 10.1016/s0166-2236(99)01452-6. [DOI] [PubMed] [Google Scholar]
- Moucha R, Pandya PK, Vazquez J, Engineer N, Rathbun D, Kilgard MP. Association for research in otolaryngology. St. Petersburg Beach: 2001a. Plasticity of spectrotemporal coding in primary auditory cortex enabled by cholinergic modulation. [Google Scholar]
- Moucha R, Pandya PK, Vazquez JL, Engineer ND, Rathbun DL, Kilgard MP. Society for neuroscience. Vol. 27. San Diego: 2001b. Background stimuli contribute to cortical plasticity in rat primary auditory cortex. [Google Scholar]
- Nelken I. Feature detection by the auditory cortex. In: Popper RRF, editor. Integrative functions in the mammalian auditory pathway. Vol. 1. Springer; Berlin Heidelberg New York: 2002. pp. 358–416. [Google Scholar]
- Nelken I, Versnel H. Responses to linear and logarithmic frequency-modulated sweeps in ferret primary auditory cortex. Eur J Neurosci. 2000;12:549–562. doi: 10.1046/j.1460-9568.2000.00935.x. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Scheich H. Differential frequency conditioning enhances spectral contrast sensitivity of units in auditory cortex (field Al) of the alert Mongolian gerbil. Eur J Neurosci. 1996;8:1001–1017. doi: 10.1111/j.1460-9568.1996.tb01587.x. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Scheich H, Freeman WJ. Change in pattern of ongoing cortical activity with auditory category learning. Nature. 2001;412:733–736. doi: 10.1038/35089076. [DOI] [PubMed] [Google Scholar]
- Poon PW, Chen XY, Hwang JC. Altered sensitivities of auditory neurons in the rat midbrain following early postnatal exposure to patterned sounds. Brain Res. 1990;524:327–330. doi: 10.1016/0006-8993(90)90710-s. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM. Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response zone in cortical area 3a. J Neurophysiol. 1992a;67:1057–1070. doi: 10.1152/jn.1992.67.5.1057. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992b;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Schreiner CE. Changes in the distributed temporal response properties of SI cortical neurons reflect improvements in performance on a temporally based tactile discrimination task. J Neurophysiol. 1992c;67:1071–1091. doi: 10.1152/jn.1992.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts C, Mendelson JR, Anand B, English R. Responses to time-varying stimuli in rat auditory cortex. Hear Res. 1998;123:27–30. doi: 10.1016/s0378-5955(98)00086-0. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Turchi J. Basal forebrain afferent projections modulating cortical acetylcholine, attention, and implications for neuropsychiatric disorders. Ann N Y Acad Sci. 1999;877:368–382. doi: 10.1111/j.1749-6632.1999.tb09277.x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Shamma SA, Fleshman JW, Wiser PR, Versnel H. Organization of response areas in ferret primary auditory cortex. J Neurophysiol. 1993;69:367–383. doi: 10.1152/jn.1993.69.2.367. [DOI] [PubMed] [Google Scholar]
- Song S, Miller KD, Abbott LF. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat Neurosci. 2000;3:919–926. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- Suga N. Principles of auditory information-processing derived from neuroethology. J Exp Biol. 1989;146:277–286. doi: 10.1242/jeb.146.1.277. [DOI] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VA. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) Nat Neurosci. 2001;4:304–310. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Beitel R, Schreiner CE. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J Neurophysiol. 1995;74:2685–2706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Bakin JS. Learning-induced physiological memory in adult primary auditory cortex: receptive fields plasticity, model, and mechanisms. Audiol Neurootol. 1998;3:145–167. doi: 10.1159/000013787. [DOI] [PubMed] [Google Scholar]
- Wetzel W, Ohl FW, Wagner T, Scheich H. Right auditory cortex lesion in Mongolian gerbils impairs discrimination of rising and falling frequency-modulated tones. Neurosci Lett. 1998;252:115–118. doi: 10.1016/s0304-3940(98)00561-8. [DOI] [PubMed] [Google Scholar]
- Xerri C, Coq JO, Merzenich MM, Jenkins WM. Experience-induced plasticity of cutaneous maps in the primary somatosensory cortex of adult monkeys and rats. J Physiol Paris. 1996;90:277–287. doi: 10.1016/s0928-4257(97)81438-6. [DOI] [PubMed] [Google Scholar]