Abstract
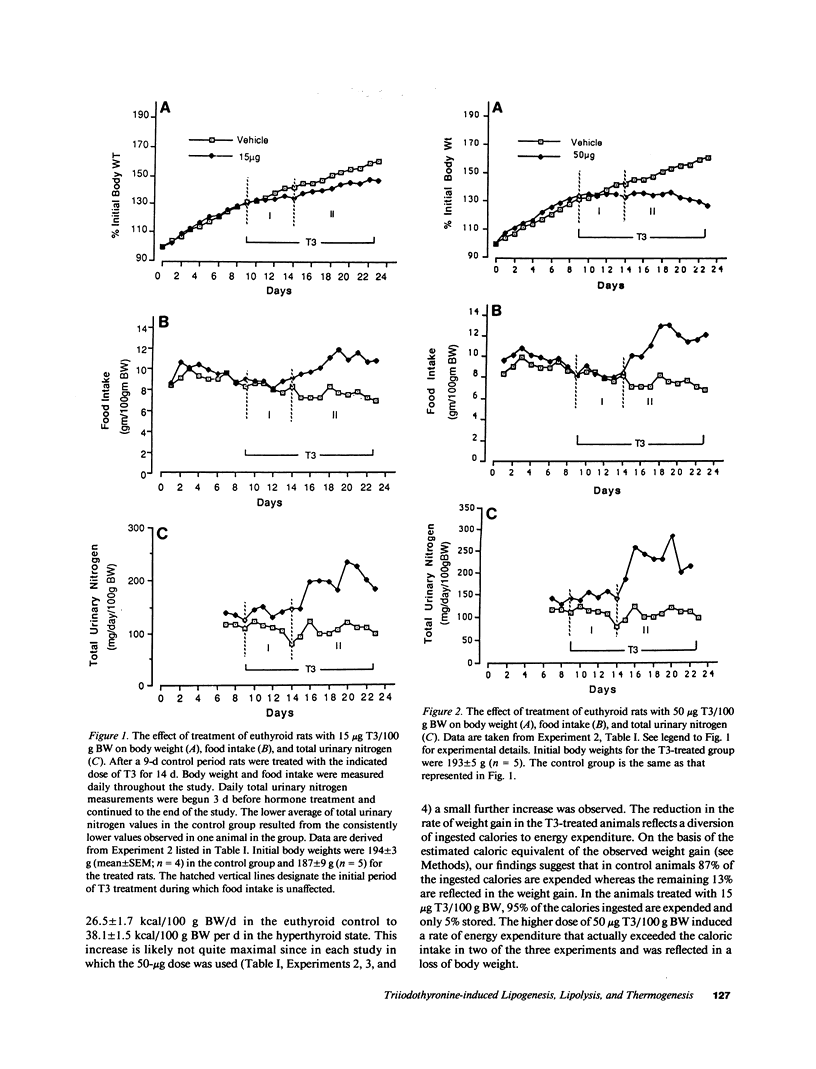
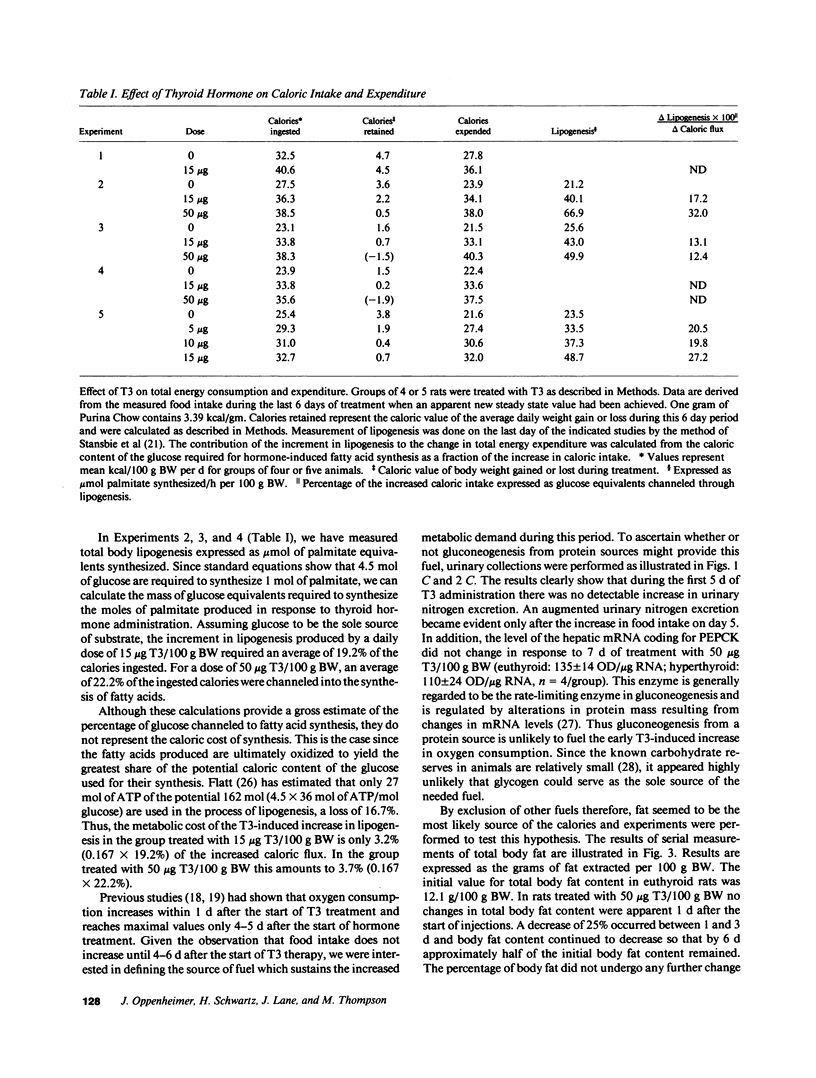
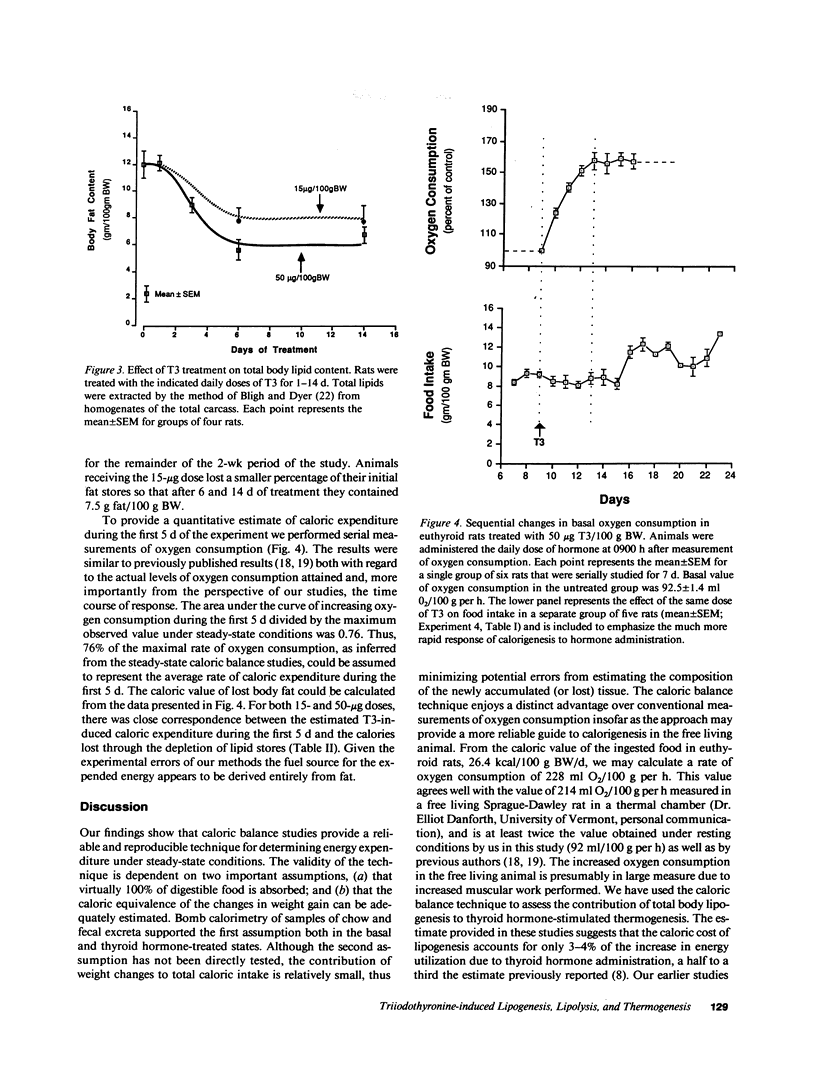
Metabolic balance studies were carried out to determine the interrelationships of thyroid hormone-induced lipogenesis, lipolysis, and energy balance in the free-living rat. Intraperitoneal doses of 15 micrograms triiodothyronine (T3)/100 g body wt per d caused an increase in caloric intake from 26.5 +/- 1.7 (mean +/- SEM) kcal/100 g per d to 38.1 +/- 1.5 kcal/100 g per d. Food intake, however, rose only after 4-6 d of treatment and was maximal by the 8th day. In contrast, total body basal oxygen consumption rose by 24 h and reached a maximum by 4 d. Since total urinary nitrogen excretion and hepatic phosphoenolpyruvate carboxykinase mRNA did not rise, gluconeogenesis from protein sources did not supply the needed substrate for the early increase in calorigenesis. Total body fat stores fell approximately 50% by the 6th day of treatment and could account for the entire increase in caloric expenditure during the initial period of T3 treatment. Total body lipogenesis increased within 1 d and reached a plateau 4-5 d after the start of T3 treatment. 15-19% of the increased caloric intake was channeled through lipogenesis, assuming glucose to be the sole substrate for lipogenesis. The metabolic cost of the increased lipogenesis, however, accounted for only 3-4% of the T3-induced increase in calorigenesis. These results suggest that fatty acids derived from adipose tissue are the primary source of substrate for thyroid hormone-induced calorigenesis and that the early increase in lipogenesis serves simply to maintain fat stores. Since the mRNAs coding for lipogenic enzymes rise many hours before oxygen consumption and lipolysis, these results suggest that T3 acts at least in part by an early coordinate induction of the genes responsible for these processes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABINEAU L. M., PAGE E. E. On body fat and body water in rats. Can J Biochem Physiol. 1955 Nov;33(6):970–979. [PubMed] [Google Scholar]
- BARKER S. B., KLITGAARD H. M. Metabolism of tissues excised from thyroxine-injected rats. Am J Physiol. 1952 Jul;170(1):81–86. doi: 10.1152/ajplegacy.1952.170.1.81. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bilezikian J. P., Loeb J. N. The influence of hyperthyroidism and hypothyroidism on alpha- and beta-adrenergic receptor systems and adrenergic responsiveness. Endocr Rev. 1983 Fall;4(4):378–388. doi: 10.1210/edrv-4-4-378. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Plasma noradrenaline and adrenaline in patients with thyrotoxicosis and myxoedema. Clin Sci Mol Med. 1973 Aug;45(2):163–171. doi: 10.1042/cs0450163. [DOI] [PubMed] [Google Scholar]
- Coulombe P., Dussault J. H., Walker P. Catecholamine metabolism in thyroid disease. II. Norepinephrine secretion rate in hyperthyroidism and hypothyroidism. J Clin Endocrinol Metab. 1977 Jun;44(6):1185–1189. doi: 10.1210/jcem-44-6-1185. [DOI] [PubMed] [Google Scholar]
- DEYKIN D., VAUGHAN M. RELEASE OF FREE FATTY ACIDS BY ADIPOSE TISSUE FROM RATS TREATED WITH TRIIODOTHYRONINE OR PROPYLTHIOURACIL. J Lipid Res. 1963 Apr;4:200–203. [PubMed] [Google Scholar]
- Dillmann W. H. Hormonal influences on cardiac myosin ATPase activity and myosin isoenzyme distribution. Mol Cell Endocrinol. 1984 Mar;34(3):169–181. doi: 10.1016/0303-7207(84)90173-4. [DOI] [PubMed] [Google Scholar]
- Edelman I. S., Ismail-Beigi F. Thyroid thermogenesis and active sodium transport. Recent Prog Horm Res. 1974;30(0):235–257. doi: 10.1016/b978-0-12-571130-2.50010-9. [DOI] [PubMed] [Google Scholar]
- Elks M. L., Manganiello V. C. Effects of thyroid hormone on regulation of lipolysis and adenosine 3',5'-monophosphate metabolism in 3T3-L1 adipocytes. Endocrinology. 1985 Sep;117(3):947–953. doi: 10.1210/endo-117-3-947. [DOI] [PubMed] [Google Scholar]
- Engfeldt P., Arner P., Bolinder J., Wennlund A., Ostman J. Phosphodiesterase activity in human subcutaneous adipose tissue in hyper- and hypothyroidism. J Clin Endocrinol Metab. 1982 Mar;54(3):625–629. doi: 10.1210/jcem-54-3-625. [DOI] [PubMed] [Google Scholar]
- Fisher J. N., Ball E. G. Studies on the metabolism of adipose tissue. XX. The effect of thyroid status upon oxygen consumption and lipolysis. Biochemistry. 1967 Mar;6(3):637–647. doi: 10.1021/bi00855a001. [DOI] [PubMed] [Google Scholar]
- Forciea M. A., Schwartz H. L., Towle H. C., Mariash C. N., Kaiser F. E., Oppenheimer J. H. Thyroid hormone-carbohydrate interaction in the rat: correlation between age-related reductions in the inducibility of hepatic malic enzyme by triiodo-L-thyronine and a high carbohydrate, fat-free diet. J Clin Invest. 1981 Jun;67(6):1739–1747. doi: 10.1172/JCI110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freake H. C., Schwartz H. L., Oppenheimer J. H. The regulation of lipogenesis by thyroid hormone and its contribution to thermogenesis. Endocrinology. 1989 Dec;125(6):2868–2874. doi: 10.1210/endo-125-6-2868. [DOI] [PubMed] [Google Scholar]
- Goodridge A. G. Regulation of malic enzyme synthesis by thyroid hormone and glucagon: inhibitor and kinetic experiments. Mol Cell Endocrinol. 1978 Jun;11(1):19–29. doi: 10.1016/0303-7207(78)90029-1. [DOI] [PubMed] [Google Scholar]
- Harris P. M. Changes in adipose tissue of the rat due to early undernutrition followed by rehabilitation. 1. Body composition and adipose tissue cellularity. Br J Nutr. 1980 Jan;43(1):15–26. doi: 10.1079/bjn19800060. [DOI] [PubMed] [Google Scholar]
- Jump D. B., Narayan P., Towle H., Oppenheimer J. H. Rapid effects of triiodothyronine on hepatic gene expression. Hybridization analysis of tissue-specific triiodothyronine regulation of mRNAS14. J Biol Chem. 1984 Mar 10;259(5):2789–2797. [PubMed] [Google Scholar]
- KENNEDY G. C. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953 Jan 15;140(901):578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Konstantinides F. N., Boehm K. A., Radmer W. J., Storm M. C., Adderly J. T., Weisdorf S. A., Cerra F. B. Pyrochemiluminescence: real-time, cost-effective method for determining total urinary nitrogen in clinical nitrogen-balance studies. Clin Chem. 1988 Dec;34(12):2518–2520. [PubMed] [Google Scholar]
- MAYER J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann N Y Acad Sci. 1955 Jul 15;63(1):15–43. doi: 10.1111/j.1749-6632.1955.tb36543.x. [DOI] [PubMed] [Google Scholar]
- Mariash C. N., Seelig S., Oppenheimer J. H. A rapid, inexpensive, quantitative technique for the analysis of two-dimensional electrophoretograms. Anal Biochem. 1982 Apr;121(2):388–394. doi: 10.1016/0003-2697(82)90498-5. [DOI] [PubMed] [Google Scholar]
- Miksicek R. J., Towle H. C. Changes in the rates of synthesis and messenger RNA levels of hepatic glucose-6-phosphate and 6-phosphogluconate dehydrogenases following induction by diet or thyroid hormone. J Biol Chem. 1982 Oct 10;257(19):11829–11835. [PubMed] [Google Scholar]
- Narayan P., Liaw C. W., Towle H. C. Rapid induction of a specific nuclear mRNA precursor by thyroid hormone. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4687–4691. doi: 10.1073/pnas.81.15.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Mariash C. N., Kinlaw W. B., Wong N. C., Freake H. C. Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev. 1987 Aug;8(3):288–308. doi: 10.1210/edrv-8-3-288. [DOI] [PubMed] [Google Scholar]
- ParízkoV'A J., Koutecký Z. The effect of age and different motor activity on fat content, lipoprotein-lipase activity and relative weight of internal organs, heart and skeletal muscle. Physiol Bohemoslov. 1968;17(2):179–189. [PubMed] [Google Scholar]
- Pilkis S. J., el-Maghrabi M. R., Claus T. H. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Biochem. 1988;57:755–783. doi: 10.1146/annurev.bi.57.070188.003543. [DOI] [PubMed] [Google Scholar]
- Pou M. A., Torresani J. Coordinated stimulation by triiodothyronine of fatty acid synthesis and isoproterenol-sensitive fatty acid release in two preadipocyte cell lines of lean or genetically obese mice. Horm Metab Res. 1989 Sep;21(9):468–472. doi: 10.1055/s-2007-1009265. [DOI] [PubMed] [Google Scholar]
- Rapiejko P. J., Watkins D. C., Ros M., Malbon C. C. Thyroid hormones regulate G-protein beta-subunit mRNA expression in vivo. J Biol Chem. 1989 Sep 25;264(27):16183–16189. [PubMed] [Google Scholar]
- Rohrer D., Dillmann W. H. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in the rat heart. J Biol Chem. 1988 May 25;263(15):6941–6944. [PubMed] [Google Scholar]
- Ros M., Northup J. K., Malbon C. C. Steady-state levels of G-proteins and beta-adrenergic receptors in rat fat cells. Permissive effects of thyroid hormones. J Biol Chem. 1988 Mar 25;263(9):4362–4368. [PubMed] [Google Scholar]
- Sestoft L. Metabolic aspects of the calorigenic effect of thyroid hormone in mammals. Clin Endocrinol (Oxf) 1980 Nov;13(5):489–506. doi: 10.1111/j.1365-2265.1980.tb03415.x. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait K. A., Kinlaw W. B., Mariash C. N., Oppenheimer J. H. Kinetics of induction by thyroid hormone of the two hepatic mRNAs coding for cytosolic malic enzyme in the hypothyroid and euthyroid states. Evidence against an obligatory role of S14 protein in malic enzyme gene expression. J Biol Chem. 1989 Nov 25;264(33):19784–19789. [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O., ARRHENIUS E., PEDERSEN S., HEDMAN R. The action of thyroid hormones at the cell level. Biochem J. 1963 Mar;86:408–428. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle H. C., Mariash C. N., Oppenheimer J. H. Changes in the hepatic levels of messenger ribonucleic acid for malic enzyme during induction by thyroid hormone or diet. Biochemistry. 1980 Feb 5;19(3):579–585. doi: 10.1021/bi00544a029. [DOI] [PubMed] [Google Scholar]
- Van Inwegen R. G., Robison G. A., Thompson W. J. Cyclic nucleotide phosphodiesterases and thyroid hormones. J Biol Chem. 1975 Apr 10;250(7):2452–2456. [PubMed] [Google Scholar]
- Wahrenberg H., Engfeldt P., Arner P., Wennlund A., Ostman J. Adrenergic regulation of lipolysis in human adipocytes: findings in hyper- and hypothyroidism. J Clin Endocrinol Metab. 1986 Sep;63(3):631–638. doi: 10.1210/jcem-63-3-631. [DOI] [PubMed] [Google Scholar]
- Weirich R. T., Schwartz H. L., Oppenheimer J. H. An analysis of the interrelationship of nuclear and plasma triiodothyronine in the sea lamprey, lake trout, and rat: evolutionary considerations. Endocrinology. 1987 Feb;120(2):664–677. doi: 10.1210/endo-120-2-664. [DOI] [PubMed] [Google Scholar]


