Abstract
Sendai virus (SeV), a natural mouse pathogen, shows considerable promise as a candidate vaccine for human parainfluenza virus-type 1 (hPIV-1), and also as a vaccine vector for other serious pathogens of infants including respiratory syncytial virus (RSV). In an effort to define correlates of immunity, we examined the virus-specific serum antibody of cotton rats inoculated intranasally (I.N.) with SeV. Virus-specific antibody forming cells (AFCs) were also measured in the bone marrow, because these are considered responsible for durable serum antibody levels in other viral systems. Results showed that a single SeV inoculation was sufficient to induce virus-specific serum antibodies and bone marrow-resident AFCs that persisted for as many as 8 months post-vaccination. Given that the predominant SeV-specific serum antibody isotype was IgG, an isotype that traffics poorly to the upper respiratory tract (URT), we asked if local nasal and lung-associated antibodies and AFCs were also present. Studies showed that: (i) SeV-specific antibodies appeared in the URT and lower respiratory tract (LRT) within seven days after immunization, (ii) corresponding AFCs were present in the diffuse-NALT (d-NALT) and lung, (iii) AFCs in the d-NALT and lung peaked at approximately six weeks and persisted for the lifetime of the animal, reaching a level exceeding that of the bone marrow by an order of magnitude, (iv) IgA was the dominant isotype among AFCs in the d-NALT and lung at 4-weeks post-vaccination and thereafter, and (v), antibody and AFC responses associated with the prevention of lung infection when animals were challenged with hPIV-1 just one week after vaccination.
Keywords: Parainfluenza virus, nasal-associated lymphoid tissue, upper respiratory tract, antibody forming cells, hPIV-1 protection
1. INTRODUCTION
The human parainfluenza virus-type 1 (hPIV-1) is responsible for serious human respiratory infections in the pediatric population. Recent surveys suggest that hPIV-1 may cause as many as 48,000 hospitalizations in the United States per year, often due to severe laryngotracheobronchitis (croup). In addition to the significant morbidity caused by hPIV-1 among children, millions of dollars are spent each year on hospital care, home care, and absence from work by parents and guardians (www.who.int/vaccine_research [1–4]).
Sendai virus (SeV), the mouse parainifluenza virus-type 1, was first discovered in Sendai Japan during an epidemic of fatal pneumonitis in human newborns [5]. The virus was isolated from mice which had been inoculated with patient samples in an attempt to amplify the human pathogen. Whereas SeV was originally thought to be the etiologic agent of human disease [6;7]. researchers have since determined that it is a pathogen of mice and not of humans [8]. During laboratory studies of SeV, the profound sequence and antigenic similarities between SeV and hPIV-1 were recognized [9–12], as was the potential for SeV to serve as a Jennerian (xenotropic) vaccine for protection against hPIV-1. SeV has been shown to grow transiently in the upper and lower respiratory tract of non-human primates, conferring complete protection against hPIV-1 challenge with no evidence of adverse events [13;14]. Its attenuation in chimpanzees was greater than that of bovine parainfluenza virus-type 3, a vaccine candidate that was well tolerated in pediatric clinical trials [14–16]. With the advent of reverse genetics, recombinant SeVs were also produced and proven protective in cotton rat studies against respiratory syncytial virus (RSV) and human parainfluenza virus-type 3 (hPIV-3) [17–21]. The results from animal models are encouraging, but cannot fully predict immunogenicity and risk-benefit in humans. FDA-approved clinical trials have therefore been initiated with unmodified SeV. Thus far, the vaccine has been well tolerated in both adults and children ([22] and unpublished results).
The attraction of Sev as a vaccine vehicle is based in part on its ability to elicit lifelong immunity against respiratory pathogens in small animal models following a single I.N. inoculation [23]. The current report describes an effort to define the immune correlates of SeV-mediated protection using the cotton rat model system. Results showed that shortly after a single I.N. inoculation with SeV, virus-specific IgG appeared in the serum and both IgG and IgA antibodies appeared in the upper and lower respiratory tract (URT/LRT). In addition, an extraordinary number of IgA and IgG-producing Sev-specific AFCs could be found in the d-NALT and lung. These SeV-specific AFCs persisted for as long as 8 months post-vaccination without a requirement for a booster immunization. The AFC in the d-NALT and lung achieved numbers far superior to those in the bone marrow, spleen, cervical lymph nodes (CLN) and lower airways (bronchoalveolar lavage, BAL), and associated with complete protection of the LRT from hPIV-1 challenge just seven days after vaccination.
2. MATERIALS AND METHODS
2.1 Animals and inoculations
Cotton rats (Sigmodon hispidus, ≥ 6 weeks of age) were purchased from Ace Animals (Boyertown, PA). Groups of 2–5 animals were vaccinated with 2×106 EID50 SeV by the I.N. route for subsequent evaluation. Challenges were conducted seven days after vaccination, by I.N. administration of 3×106 PFU C35 hPIV-1 (ATCC, Rockville, MD). All tests were conducted in replicate.
2.2 Preparation of samples for ELISA and ELISPOT assays
To harvest d-NALT [24;25] cotton rats were sacrificed and skin, lower jaws, soft palates (including the attached o-NALT), muscles, cheek bones and teeth were removed from the heads. Remaining snouts were cut into small pieces, after which cells were released by digestion with 4mg/ml collagenase in PBS at 37°C for 30 min. Cells were first washed with PBS and then suspended in PBS and layered onto a 40/75% discontinuous sucrose gradient. After centrifugation at 600 × g for 30 min, cells were collected from the gradient interface for assay. The cells were washed 2× in PBS and suspended in RPMI1640 plus 10% heat inactivated FBS (R10). Lungs were suspended and similarly processed by collagenase digestion and purification on percoll gradients. Bone marrow, CLN, spleen and blood cells were collected, suspended and washed prior to assay. BAL was collected by three washes with 1.0 ml PBS. Nasal washes were collected after clipping the trachea, by flushing the upper airway with 0.5 ml PBS. All experiments were conducted in replicate.
2.3 ELISA
For the detection of SeV-specific antibodies, sucrose-gradient purified SeV was lysed in disruption buffer (0.5% TritonX-100, 0.6M KCl, 0.05M Tris pH7.8), and diluted with PBS to yield 10 µg/ml for the coating of 96-well ELISA plates. After overnight incubation, plates were washed with PBS and blocked with PBS containing 1% bovine serum albumin (BSA, Sigma, St Louis, MO). Serum, nasal wash or BAL samples from vaccinated and control animals were diluted in PBS and 1% BSA and incubated on plates for 1h at 37°C Plates were then washed 6× with PBS-Tween 20 (.05%) and incubated with alkaline phosphatase- conjugated goat anti mouse IgG (gamma specific) or goat anti mouse IgA (Southern Biotechnologies Assoc.) diluted 1:1000 in PBS and 1% BSA, for 1 hr at 37°C. Plates were washed 6× with PBS-Tween and developed by addition of p-nitrophenyl phosphate substrate (1mg/ml) in diethanolamine buffer (pH 9.8). Assays were read at OD 405 nm after 30 min. All experiments were conducted in replicate.
2.4 ELISPOT
The ELISPOT plate was coated with disrupted SeV overnight. The membrane was blocked for one hour with RPMI plus 10% serum (R10). Media were aspirated and 100ul fresh R10 media added back to the wells. Next, 105 cells in 100 µl R10 were added to each well. The plates were incubated at 37°C, 5% CO2 for 3 hours. After washing 3× with PBS and 3× with PBS-Tween 20, 100ul of alkaline phosphatase-conjugated goat anti IgG or IgA antibodies (specified above) in 1% BSA were added to each well. After overnight incubation, the antibody was removed and plates were developed with 1 mg/ml BCIP/NBT (Sigma Aldrich). The plate was incubated at room temperature and monitored for spot appearance. The exposure was stopped by rinsing plates with water. Plates were dried and spots were counted using a Nikon dissecting scope. All experiments were conducted in replicate.
2.5 Virus measurement
Three days after I.N. viral challenge with hPIV-1, cotton rats were sacrificed and lungs were harvested for measurement of virus titer. Lungs were homogenized on ice in 2 ml PBS with a mechanical Dounce homogenizer (PowerGen125 PCR Tissue Homogenizing kit; Fisher Scientific). Homogenates were centrifuged to remove debris and supernatants were collected. Serially diluted lung supernatants in DMEM (with supplements of 0.1% BSA, 0.5 mg/ml gentamicin, 2mM L-glutamine and 5 µg/ml acetylated trypsin) were added to wells of LLC-MK2 cell monolayers. After a 5 day incubation at 37°C, 50 µl supernatants from wells were used for hemagglutination (HA) assays with an equal volume of chicken red blood cells for 30–60 min. TCID50 were calculated using the Reed-Muench formula. All experiments were conducted in replicate.
3. RESULTS
3.1 Serum antibody is long-lasting in SeV-primed cotton rats
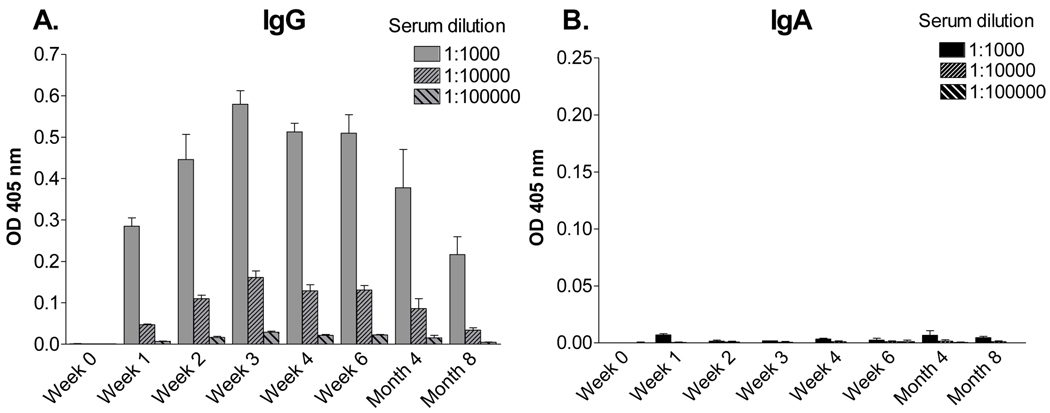
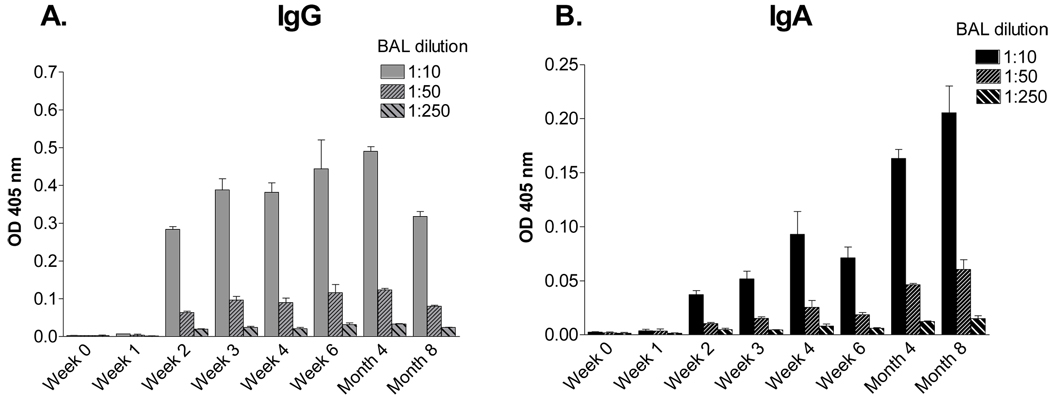
Previous studies in our laboratory have shown that SeV, a mouse parainfluenza virus-type 1 (PIV-1), can elicit protective immune responses in cotton rats that last for the lifetime of the animal [23]. In the current study, to identify potential correlates of protection, we measured IgG and IgA responses over an 8 month time course after SeV exposure. Plates were coated with purified, disrupted SeV as a PIV-1 target; previous studies have shown that SeV-specific antibodies are highly cross-reactive with hPIV-1 [11]. As shown by representative results in Figure 1, there was a serum antibody response toward SeV with a titer exceeding 104 by day seven post-inoculation. IgG was predominant in the sera, while levels of SeV-specific IgA were negligible. Antibodies persisted throughout the 8 month time course. These results showed that unlike the situation for many subunit vaccines [26;27], SeV elicited long-term serum antibodies without a requirement for a booster inoculation.
Figure 1. Long-sustained virus-specific IgG, but not IgA in the serum of SeV-primed cotton rats.
Cotton rats were inoculated I.N. with 2 × 106 EID50 SeV. At various time points during an eight month period, groups of animals were tested for SeV-specific serum antibodies by ELISA. Values represent the mean and standard error for replicates from 2 animals per group.
3.2 AFCs in the bone marrow correlate with durable serum antibody levels
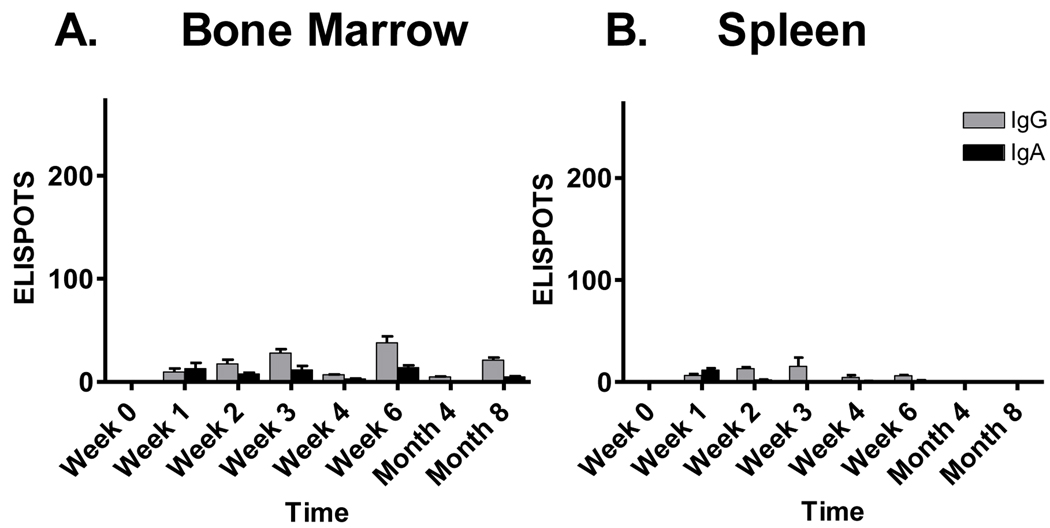
Previous studies in other model systems have demonstrated that long-term bone marrow-resident AFCs can associate with durable serum antibody responses [28;29]. We therefore asked if virus-specific AFCs could be found in the bone marrow of SeV-primed cotton rats as a potential source of serum antibodies. As shown in Figure 2, this was the case, although numbers were low. AFCs with SeV specificity could be recognized by day seven and lasted throughout the 8 month time course. Except for the earliest sampling date, IgG was the dominant isotype produced by these cells. AFCs were also examined in peripheral blood lymphocytes, but these levels were extremely low (data not shown). When spleens were examined (Figure 2B), AFCs could be measured up until week 6 post-vaccination. Studies suggested that while an inductive phase of immune activity occurred in the spleen, the more durable virus-specific AFCs were bone marrow-resident.
Figure 2. Sustained virus-specific AFCs in the bone marrow but not spleen of SeV-primed cotton rats.
Cotton rats were inoculated I.N. with 2 × 106 EID50 SeV and monitored for eight months thereafter for SeV-specific ELISPOTS in the bone marrow and spleen. The Y axis shows the ELISPOT number per 105 plated lymphocytes. Values represent the mean and standard error for replicates from 2 animals per group.
3.3 Virus specific antibody responses in the nasal wash exhibit an IgA-biased isotype profile
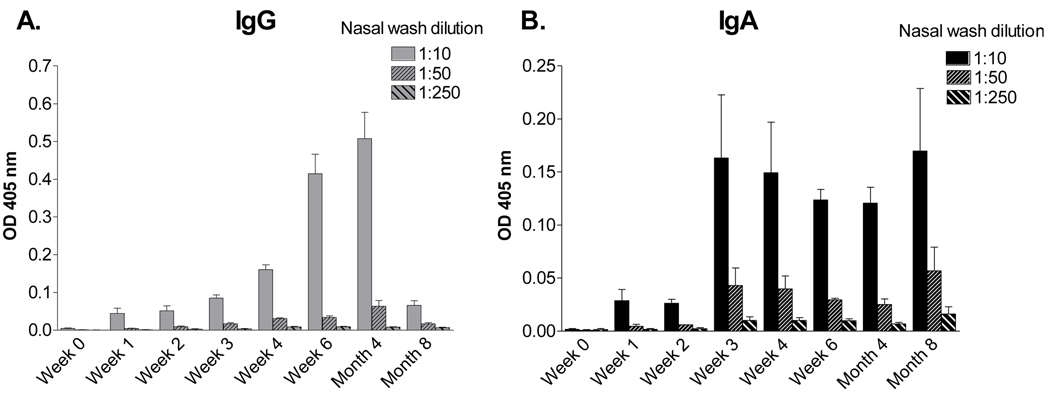
The induction of high serum titers is an attractive feature of the SeV vaccine, but antibody localized to the nasal passage is better positioned to counter virus at its portal of entry. We therefore collected nasal wash and conducted ELISAs with serially diluted samples to measure SeV-specific antibodies. The levels of antibodies were low in the nasal wash compared to levels in the serum, possibly because antibodies are tethered to secretory component on cells lining the respiratory tract and are not easily released from the lumen wall ([30], discussed further below). Similar to the situation for serum antibodies, we found that antibodies appeared rapidly. In this case, the IgA isotype was prevalent and sustained, whereas IgG peaked and waned by the eight month time point (Figure 3).
Figure 3. IgA and IgG in the nasal wash of SeV-primed animals.
Cotton rats were inoculated I.N. with 2 × 106 EID50 SeV and monitored for eight months thereafter for SeV-specific antibodies in the nasal wash by ELISA. Values represent the mean and standard error for replicates from 4 (IgG) and 2 (IgA) animals per group.
3.4 High-frequency and durable SeV-specific AFCs appear in the NALT
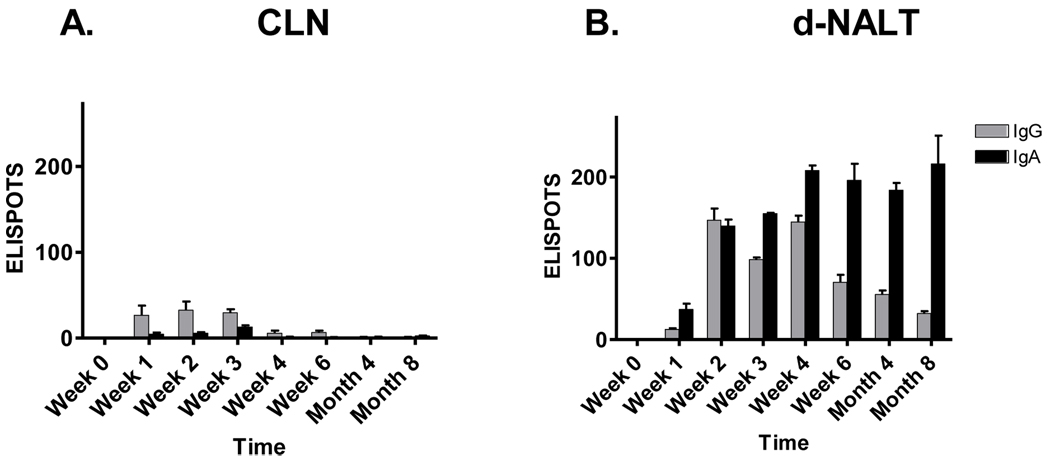
We next questioned whether AFCs might also be found in tissues lining the airways of the URT. Nasal-associated lymphoid tissue (NALT) is generally categorized as either organized (o-NALT) or diffuse (d-NALT). The former exists as two parallel strips of lymphoid tissue positioned along the nasal septum, attached to the upper palate [25]. Like other organized lymph (e.g. cervical lymph nodes (CLN) of the URT or peyers patches of the gastro-intestinal tract) the o-NALT is considered to be an inductive site for the initiation of immune responses. The d-NALT refers to interstitial cells surrounding the nasal passage. Given that the cellularity of o-NALT is very low, we chose to harvest CLN and d-NALT as respective inductive and effector sites in the URT. As shown by representative results in Figure 4, a small number of SeV-specific AFCs appeared in the CLN at early time points, but waned thereafter. In contrast, there were abundant AFCs in the d-NALT throughout the 8 month time course. In fact, AFC numbers in the d-NALT exceeded those in the bone marrow by approximately 10-fold. The isotype profile showed some similarity to that of nasal wash antibodies; there was a rise and fall of IgG-producing cells, but IgA-producing cells were sustained at high levels for as long as 8 months after immunization. The unpaired student T test was used to show that at 8 months, IgA ELISPOTs were significantly higher in d-NALT compared to CLN, bone marrow, or spleen (p<.05 in each case). A photograph of IgA ELISPOT wells developed at the 8 month time point is shown (Figure 5).
Figure 4. Sustained IgA and IgG AFC in the d-NALT.
Cotton rats were inoculated I.N. with 2 × 106 EID50 SeV and monitored for eight months to measure AFC responses in the CLN and d-NALT. The Y axis shows the ELISPOT number per 105 plated lymphocytes. Values represent the mean and standard error for replicates of 2 animals per group.
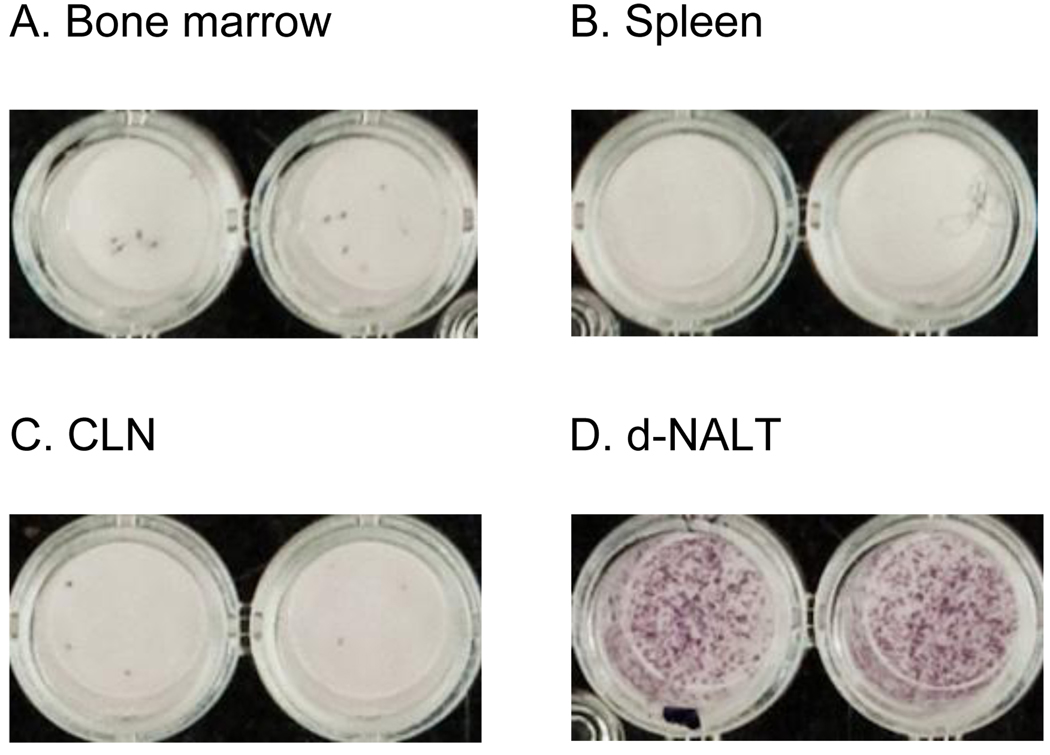
Figure 5. AFCs in d-NALT following SeV inoculation.
Eight months following the inoculation of cotton rats with 2 × 106 EID50 SeV, AFCs were measured in respiratory and systemic tissues. Shown are representative results for (A) Bone marrow, (B) spleen, (C) CLN and (D) d-NALT.
3.5 Virus-specific antibodies and AFC in the LRT
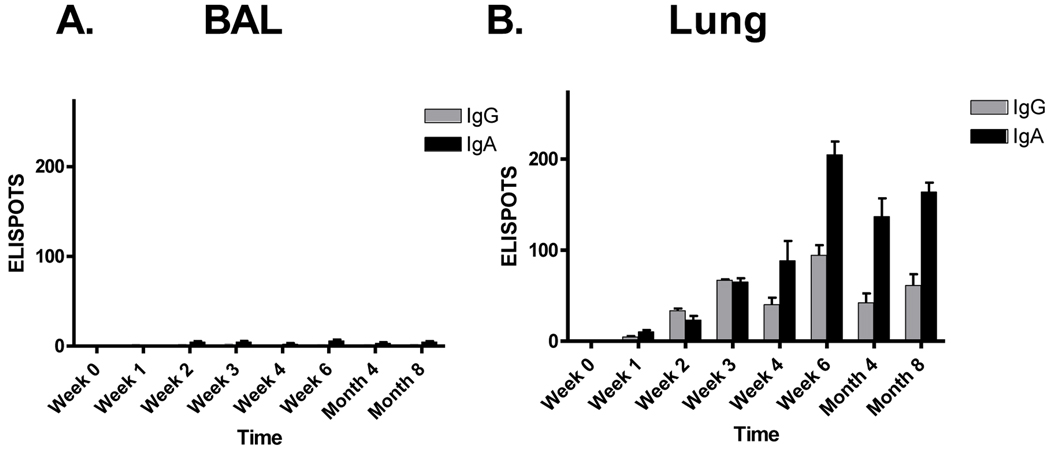
The full time-course of IgG and IgA isotypes in the BAL was also evaluated. As shown in Figure 6, there was significant antibody in the BAL for 8 months. As shown in Figure 7, the numbers of AFCs in the BAL were negligible. In lung tissues, both IgG and IgA AFC were recognized early and were maintained throughout the 8 month period. IgA and IgG patterns were somewhat similar to those of free antibody in the BAL (Figure 6), consistent with the suggestion that AFCs in the lung parenchyma secrete antibodies into the lumen of the LRT.
Figure 6. IgA and IgG in the LRT.
Cotton rats were inoculated I.N. with 2 × 106 EID50 SeV and monitored for eight months thereafter for SeV-specific antibodies in the BAL. Values represent the mean and standard error for replicates from 2 animals per set.
Figure 7. AFCs in the lung reflect virus-specific antibody in the BAL.
Groups of cotton rats were inoculated I.N. with 2 × 106 EID50 SeV and monitored for eight months to measure AFC responses in the BAL and lung. The Y axis shows the ELISPOT number per 105 plated lymphocytes. Values represent the mean and standard error for replicates of 2 animals per set.
3.6 AFC appearance correlates with rapid protection against human parainfluenza virus type 1 challenge
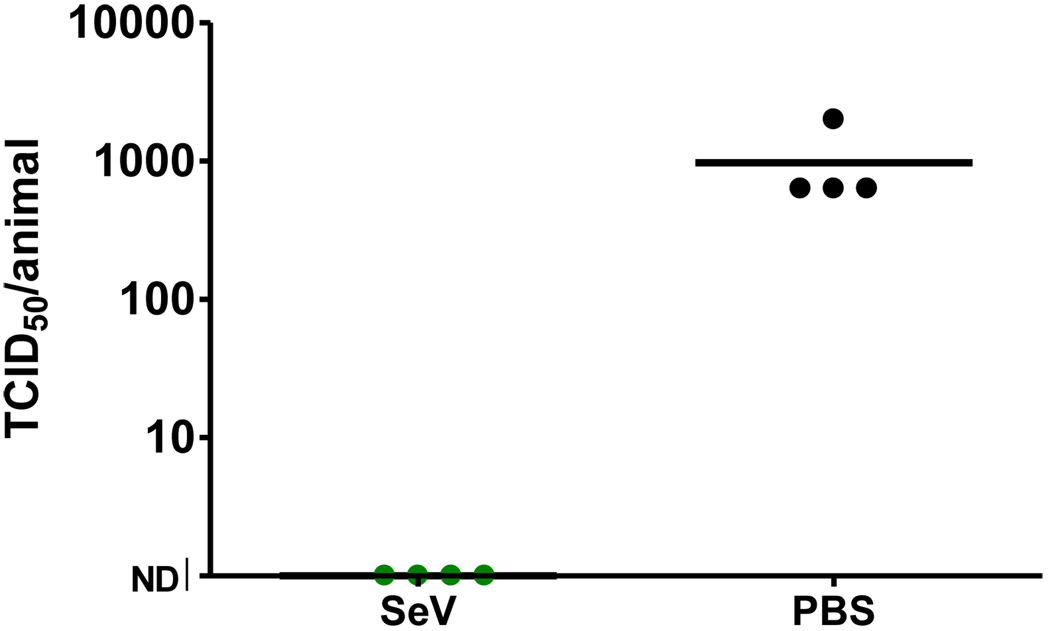
Previous studies with SeV-based vaccines have demonstrated that protective immune responses are generated within one month of immunization and persist for the lifetime of an animal [23]. If the appearance of antibody and AFCs serves as an immune correlate of protection, we hypothesized that protection might also be apparent as early as one week after vaccination. To test this hypothesis, animals were immunized I.N. with SeV and then challenged seven days later I.N. with 3 × 106 PFU hPIV-1. As shown by a representative experiment in Figure 8, challenge virus was detected in four of four control animals, but in none of the four vaccinated cotton rats. A Fishers exact test showed that results were significantly different between test and control animals (p<.05). Data demonstrated that protective immunity was induced within one week of a single inoculation with SeV.
Figure 8. Protection against challenge with hPIV-1 within seven days of vaccination.
Cotton rat groups were inoculated I.N. with 3 × 106 EID50 SeV. After seven days, animals were challenged I.N. with hPIV-1 (C35). On day three, animals were sacrificed for lung titer. Each point represents the viral load in an individual animal with 4 animals per group. As shown, there was no lung infection in vaccinated animals, but all four PBS control animals were infected.
4. DISCUSSION
4.1 Antibodies and antibody forming cells in SeV primed cotton rats
The study described in this report was initiated to identify immune correlates for the protection induced by SeV vaccines. We demonstrated that serum IgG antibody responses appeared within one week and were sustained for the lifetime of the animals after a single I.N. inoculation with SeV. Bone marrow-resident AFCs appeared with similar kinetics, suggesting that these were the effectors responsible for sustained serum antibody levels, as has been described in other viral systems (see below,[28;29;31]). However, we considered that bone marrow-resident cells and serum antibodies could not fully explain the protective immunity conferred by SeV against respiratory pathogens, because: (i) unlike IgA, IgG traffics poorly to the URT (illustrated by studies with intravenously administered monoclonal and polyclonal antibodies [32–38]) and (ii) the SeV-specific serum antibody responses were deficient in IgA, a common isotype of the respiratory tract. We therefore examined URT and LRT tissues for SeV-specific antibodies and AFCs. We discovered an extraordinary number of SeV-specific IgA producing cells in the d-NALT and lung, sustained for at least eight months after one SeV inoculation. Results suggest that the cells localized in d-NALT perpetually secrete antibodies to survey the upper airway. These antibodies would be a first line of defense against invading pathogens. IgA is uniquely structured for poly-Ig mediated transcytosis across epithelial cells and retention on the lining of the airway by secretory component. The antibodies are best positioned for capture and destruction of invading pathogens and the prevention of virus-mediated disease.
4.2 Variable IgA/IgG ratios in the upper and lower respiratory tract
A comparison of IgA and IgG antibodies in the URT and LRT revealed some subtle differences. In each case IgA/IgG ratios improved with time. In nasal wash, the predominance of IgA was most pronounced at the eight month time point. The IgA/IgG levels in the LRT were slightly lower, perhaps because there is a greater capacity for serum IgG to penetrate the LRT (and a greater capacity of polymeric IgA to penetrate the URT [34;37;39]). However, the subtle IgA/IgG differences were also apparent among URT and LRT-resident AFCs in that the IgA/IgG ratio was most pronounced in the d-NALT at the latest stage of analysis. IgA expression by an AFC requires a gene switch rearrangement which juxtaposes V-D-J immunoglobulin sequences upstream of the C-alpha gene. This switch rearrangement can in some cases occur very early after a viral infection, and in the absence of cognate T cell help [40–42]. Propensity for switch rearrangements to C-alpha is enhanced in the presence of factors such as TGF-beta, IL-5, IL-6, IL-10, retinoic acid and IgA-inducing protein [40–48]. Apparently, the signals received by SeV-specific B cells that are destined for residence in the URT versus LRT are not identical. B cell contacts with antigen presenting cells, T-helper cells, and soluble factors are each likely to influence isotype display in the upper and lower respiratory tract.
As might be expected, the isotype profiles of d-NALT AFCs were fairly similar to those of free antibodies in the nasal wash, and profiles of lung AFCs were fairly similar to those of free antibodies in the BAL. Possibly, these similarities reflect a direct relationship between effector cells and secreted products, yet one might have predicted that the large numbers of AFCs lining the airways would have yielded higher titers of free antibodies in the airways. It is perhaps the case that the relatively low antibody titers (compared to serum titers) were a reflection of antibody tethering to the airway lining, either via secretory component or Fc receptors. We expect that future immunohistochemical studies with labeled SeV antigens may reveal that the majority of SeV-specific antibodies in the airway are stably bound to the lumen wall and are not easily released by a nasal wash.
4.3 What is the derivation of d-NALT AFCs?
Surprisingly, even though d-NALT-resident AFCs may be critical to the control of respiratory virus infections, their biology and trafficking patterns are not well understood. The priming of B cells associated with mucosal tissues usually initiates when antigen enters organized lymph nodes or tissues (e.g. o-NALT and CLN of the URT or peyers patches of the gastro-intestinal tract). This uptake can be supported by specialized antigen-sampling cells [49]. Following B cell activation and proliferation in germinal centers of organized tissue, B cells traffic through efferent vessels to the thoracic duct and into the bloodstream. They may then return to mucosal tissues to exert their effector function. The term ‘common mucosal immune system’ denotes a degree of interplay between immune responses that are generated in the mucosa. In other words, when an antigen is administered at one mucosal site, immune responses can be recognized at another (e.g. the administration of recombinant adenovirus I.N. route can induce a secretory immune response in the genital tract [50]). Nonetheless, cells are often ‘imprinted’ at their site of first antigen exposure, after which membrane integrins can direct their preferential return to adjacent tissues (e.g. cells primed in the peyers patches of the gut will often traffic back to the gut lamina propria [51]). In this regard, the digestive tract has been more extensively studied than the respiratory tract [52–55]. Elements that may influence the trafficking of URT-primed B Cells include the integrins CCR10 and α4β1, and corresponding ligands CCL28 and VCAM1 [56;57], but the detail of URT/LRT homing remains to be determined. How precisely do integrins affect the trafficking of SeV-specific AFCs to the d-NALT versus lung? Do SeV-specific d-NALT AFCs most often derive from the o-NALT or CLN? When o-NALT and CLN are removed (a situation shown to render variable effects in the context of other respiratory pathogens [58–60]) do SeV specific d-NALT cells preferentially derive from mediastinal lymph node cells or cells from more distal tissues? These questions remain unanswered and are each worthy of further investigation.
4.4 The durability of antibodies and AFCs after SeV infection
The long-sustained nature of SeV-specific AFCs is reminiscent of other respiratory virus infections emphasizing the value of the replication competent vaccine strategy [61;62]. In other laboratories, researchers have shown that mice can be primed with influenza virus or LCMV to yield highly durable systemic and local immune responses [28;31;41;42;61;63]. Furthermore, the adoptive transfer of bone marrow-resident AFC from virus-primed mice establishes persistent virus-specific serum antibody levels in naïve mouse recipients [31]. Durable immune responses are also recognized in humans following administration of virus-based vaccines. For example, humans who receive a single inoculation with the smallpox vaccine (vaccinia virus, VV) as children can maintain smallpox-specific antibody and T cell responses for many decades thereafter [64]. A single immunization with a recombinant VV vaccine can also generate durable antibody activities in humans toward passenger gene products [65]. The live virus vaccines thus differ dramatically from subunit vaccines, the latter of which tend to induce transient responses that can fall below detection unless booster vaccinations are implemented [26].
The mechanisms responsible for the extraordinary virus-specific AFC persistence observed in this study, often associated with replication competent virus infections, are not fully understood [66]. One argument has been that the persistence of memory and/or effector cells for long periods after a virus infection is dependent on the maintenance of antigen for constitutive lymphocyte activation [31]. Indeed, antigen can be detected for weeks or months after a live virus infection has been cleared [67]. The sensitivity of the polymerase chain reaction further allows detection of viral nucleic acid for extremely long periods after a virus exposure in animal models and in humans [68].. On the other hand, there is compelling evidence that the persistence of immune cells does not always require antigen. For example [69] using mice that were transgenic for human CD20 and an immunoglobulin heavy chain allele that conferred antibody specificity to the 4-hydroxy-3-nitrophenyl acetyl (NP) hapten, researchers found that they could prime cells with NP and then deplete memory B cells without affecting the NP-specific AFC population, demonstrating that antigen-driven memory cells were not required for AFC persistence [69]. In a separate experiment, researchers used a cre-lox system in mice to prime B cells and then destroy the capacity of activated cells to produce antibodies against the priming antigen [70]. They found that the cells were nonetheless maintained. Taken together, these experiments show that both antigen-dependent and -independent factors influence the impressive durability of antibodies and AFCs after a single viral inoculation.
4.5 Immune correlates of SeV-mediated protection
The results of the current work correlate an early (day seven) appearance of AFCs in the respiratory tract with protective immunity against hPIV-1 Although a direct cause-effect relationship can be considered, other factors may lead to SeV-mediated protection. By day seven post-vaccination we measured antibodies and AFCs in URT and LRT, but we also scored serum antibodies and AFCs (albeit of smaller numbers) in bone marrow and spleen. Moreover our preliminary studies have since identified CD8+ T cells in the d-NALT soon after SeV inoculations (data not shown). These virus-specific CD8+ T cells may assist antibodies by destroying virus-infected cells [71]. In addition, CD4+ T cells have been shown to clear virus, even when B cell and CD8+ T cell effectors are absent [72]. It is therefore likely that protection against challenge is mediated not by a single lymphocyte population, but by a composite of B cell, T cell and innate immune activities.
Acknowledgements
This work was supported in part by NIH NIAID grants P01 AI054955 and R01 AI088729, NIH NCI grant P30-CA21765 and the American-Lebanese Syrian Associated Charities (ALSAC). We thank Tom Fabrizio and Amy Bogard for excellent assistance with ELISPOT assays. We thank Dr. Mark Sangster for useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Henderson FW. Pulmonary infections with respiratory syncytial virus and the parainfluenza viruses. Semin Respir Infect. 1987 Jun;2(2):112–121. [PubMed] [Google Scholar]
- 2.Henrickson KJ, Kuhn SM, Savatski LL. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin Inf Dis. 1994;18:770–779. doi: 10.1093/clinids/18.5.770. [DOI] [PubMed] [Google Scholar]
- 3.Henrickson KJ, Hoover S, Kehl KS, Hua W. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr Infect DIs J. 2004 Jan;23(1 Suppl):S11–S18. doi: 10.1097/01.inf.0000108188.37237.48. [DOI] [PubMed] [Google Scholar]
- 4.Heilman CA. From the National Institute of Allergy and Infectious Diseases and the World Health Organization. Respiratory syncytial and parainfluenza viruses. J Infect Dis. 1990 Mar;161(3):402–406. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- 5.Ishida N, Homma M. Sendai virus. Adv Virus Res. 1978;23:349–383. doi: 10.1016/s0065-3527(08)60103-7. [DOI] [PubMed] [Google Scholar]
- 6.Tai F-H, Chiu S, Ma C-C. Seroepidemiologic studies of Sendai virus infection in Taiwan. Taiwan I Hsueh Hui Tsa Chih. 1967;66:312–318. [PubMed] [Google Scholar]
- 7.Stark JE, Heath RB. The development of antibodies against Sendai virus in childhood. Arch Gesamte Virusforsch. 1967;20:438–444. doi: 10.1007/BF01275224. [DOI] [PubMed] [Google Scholar]
- 8.Chanock RM, Murphy BR, Collins PL. Parainfluenza viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4th ed. Philadelphia PA: Lippincott Williams and Wilkins; 2001. pp. 1341–1379. [Google Scholar]
- 9.Lyn D, Gill DS, Scroggs RA, Portner A. The nucleoproteins of human parainfluenza virus type 1 and Sendai virus share amino acid sequences and antigenic and structural determinants. J Gen Vir. 1991;72:983–987. doi: 10.1099/0022-1317-72-4-983. [DOI] [PubMed] [Google Scholar]
- 10.Gorman WL, Gill DS, Scroggs RA, Portner A. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and sendai virus have high structurefunction similarity with limited antigenic cross-reactivity. Virology. 1990;175:211–223. doi: 10.1016/0042-6822(90)90201-2. [DOI] [PubMed] [Google Scholar]
- 11.Smith FS, Portner A, Leggiadro RJ, Turner EV, Hurwitz JL. Age-related development of human memory T-helper and B-cell responses toward parainfluenza virus type-1. Virology. 1994 Dec;205(2):453–461. doi: 10.1006/viro.1994.1665. [DOI] [PubMed] [Google Scholar]
- 12.Dave VP, Allan JE, Slobod KS. Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology. 1994;199:376–383. doi: 10.1006/viro.1994.1135. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz JL, Soike KF, Sangster MY, et al. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine. 1997;15:533–540. doi: 10.1016/s0264-410x(97)00217-x. [DOI] [PubMed] [Google Scholar]
- 14.Skiadopoulos MH, Surman SR, Riggs JM, et al. Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology. 2002 May 25;297(1):153–160. doi: 10.1006/viro.2002.1416. [DOI] [PubMed] [Google Scholar]
- 15.Wyke Coelingh KL, Winter CC, Tierney EL, London WT, Murphy BR. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J Infect Dis. 1988 Apr;157(4):655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg DP, Walker RE, Lee MS, et al. A bovine parainfluenza virus type 3 vaccine is safe and immunogenic in early infancy. J Infect Dis. 2005 Apr 1;191(7):1116–1122. doi: 10.1086/428092. [DOI] [PubMed] [Google Scholar]
- 17.Takimoto T, Hurwitz JL, Coleclough C, et al. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol. 2004 Jun;78(11):6043–6047. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takimoto T, Hurwitz JL, Zhan X, et al. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunol. 2005;18(2):255–266. doi: 10.1089/vim.2005.18.255. [DOI] [PubMed] [Google Scholar]
- 19.Zhan X, Hurwitz JL, Krishnamurthy S, et al. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007 Dec 17;25(52):8782–8793. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan X, Slobod KS, Krishnamurthy S, et al. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine. 2008 Jun 25;26(27–28):3480–3488. doi: 10.1016/j.vaccine.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voges B, Vallbracht S, Zimmer G, et al. Recombinant Sendai virus induces T cell immunity against respiratory syncytial virus that is protective in the absence of antibodies. Cell Immunol. 2007 Jun;247(2):85–94. doi: 10.1016/j.cellimm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Slobod KS, Shenep JL, Lujan-Zilbermann J, et al. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 2004 Aug 13;22(23–24):3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 23.Jones B, Zhan X, Mishin V, et al. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine. 2009 Mar 13;27(12):1848–1857. doi: 10.1016/j.vaccine.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis SS. Nasal vaccines. Adv Drug Deliv Rev. 2001 Sep 23;51(1–3):21–42. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 25.Asanuma H, Thompson AH, Iwasaki T, et al. Isolation and characterization of mouse nasal-associated lymphoid tissue. J Immunol Methods. 1997 Mar 28;202(2):123–131. doi: 10.1016/s0022-1759(96)00243-8. [DOI] [PubMed] [Google Scholar]
- 26.Koff RS. Immunogenicity of hepatitis B vaccines: implications of immune memory. Vaccine. 2002 Nov 1;20(31–32):3695–3701. doi: 10.1016/s0264-410x(02)00405-x. [DOI] [PubMed] [Google Scholar]
- 27.Zuckerman JN. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol. 2006 Feb;78(2):169–177. doi: 10.1002/jmv.20524. [DOI] [PubMed] [Google Scholar]
- 28.Hyland L, Sangster M, Sealy R, Coleclough C. Respiratory virus infection of mice provokes a permanent humoral immune response. J Virol. 1994;68:6083–6086. doi: 10.1128/jvi.68.9.6083-6086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995 Mar;69(3):1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy K, Travers P, Walport M. Janeway's Immunobiology. 7 ed. New York, NY: Garland Science; 2008. [Google Scholar]
- 31.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998 Mar;8(3):363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 32.Siber GR, Leombruno D, Leszczynski J, et al. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis. 1994 Jun;169(6):1368–1373. doi: 10.1093/infdis/169.6.1368. [DOI] [PubMed] [Google Scholar]
- 33.Sami IR, Piazza FM, Johnson SA, et al. Systemic immunoprophylaxis of nasal respiratory syncytial virus infection in cotton rats. J Infect Dis. 1995 Feb;171(2):440–443. doi: 10.1093/infdis/171.2.440. [DOI] [PubMed] [Google Scholar]
- 34.Renegar KB, Small PA., Jr. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991 Mar 15;146(6):1972–1978. [PubMed] [Google Scholar]
- 35.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008 Mar;82(5):2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prince GA, Hemming VG, Horswood RL, Chanock RM. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985;3:193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 37.Prince GA, Horswood RL, Chanock RM. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985 Sep;55(3):517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeVincenzo JP. Factors predicting childhood respiratory syncytial virus severity: what they indicate about pathogenesis. Pediatr Infect DIs J. 2005 Nov;24(11 Suppl):S177–S183. doi: 10.1097/01.inf.0000187274.48387.42. discussion. [DOI] [PubMed] [Google Scholar]
- 39.Johnson S, Oliver C, Prince GA. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997 Nov;176(5):1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 40.Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, Doherty PC. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J Exp Med. 2003 Oct 6;198(7):1011–1021. doi: 10.1084/jem.20021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sealy R, Surman S, Hurwitz JL, Coleclough C. Antibody response to influenza infection of mice: different patterns for glycoprotein and nucleocapsid antigens. Immunology. 2003 Apr;108(4):431–439. doi: 10.1046/j.1365-2567.2003.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coleclough C, Sealy R, Surman S, Marshall DR, Hurwitz JL. Respiratory vaccination of mice against influenza virus: dissection of T- and B-cell priming functions. Scand J Immunol. 2005 Jul;62(Suppl 1):73–83. doi: 10.1111/j.1365-3083.2005.01613.x. [DOI] [PubMed] [Google Scholar]
- 43.Mora JR, Von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008 Mar;1(2):96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- 44.Mora JR, Von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009 Feb;21(1):28–35. doi: 10.1016/j.smim.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006 Nov 17;314(5802):1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 46.Endsley MA, Njongmeta LM, Shell E, et al. Human IgA-inducing protein from dendritic cells induces IgA production by naive IgD+ B cells. J Immunol. 2009 Feb 15;182(4):1854–1859. doi: 10.4049/jimmunol.0801973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawanishi H, Saltzman LE, Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer's patches that switch sIgM B cells to sIgA B cells in vitro. J Exp Med. 1983;157:433. doi: 10.1084/jem.157.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehrhardt RO, Strober W, Harriman GR. Effect of transforming growth factor (TGF)-beta 1 on IgA isotype expression. TGF-beta 1 induces a small increase in sIgA+ B cells regardless of the method of B cell activation. J Immunol. 1992 Jun 15;148(12):3830–3836. [PubMed] [Google Scholar]
- 49.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 50.Gallichan WS, Rosenthal KL. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995 Nov;13(16):1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 51.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003 Jul 3;424(6944):88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 52.Kiyono H, Fukuyama S. NALT versus Peyer's patch-mediated mucosal immunity. Nat Rev Immunol. 2004 Sep;4(9):699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009 Dec;70(6):505–515. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 54.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007 Jul 26;25(30):5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Hiroi T, Yanagita M, Iijima H, et al. Deficiency of IL-5 receptor alpha-chain selectively influences the development of the common mucosal immune system independent IgA-producing B-1 cell in mucosa-associated tissues. J Immunol. 1999 Jan 15;162(2):821–828. [PubMed] [Google Scholar]
- 56.Kunkel EJ, Kim CH, Lazarus NH, et al. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. 2003 Apr;111(7):1001–1010. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003 Apr 1;170(7):3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 58.Sabirov A, Metzger DW. Intranasal vaccination of infant mice induces protective immunity in the absence of nasal-associated lymphoid tissue. Vaccine. 2008 Mar 17;26(12):1566–1576. doi: 10.1016/j.vaccine.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiley JA, Tighe MP, Harmsen AG. Upper respiratory tract resistance to influenza infection is not prevented by the absence of either nasal-associated lymphoid tissue or cervical lymph nodes. J Immunol. 2005 Sep 1;175(5):3186–3196. doi: 10.4049/jimmunol.175.5.3186. [DOI] [PubMed] [Google Scholar]
- 60.Ying X, Chan K, Shenoy P, Hill M, Ruddle NH. Lymphotoxin plays a crucial role in the development and function of nasal-associated lymphoid tissue through regulation of chemokines and peripheral node addressin. Am J Pathol. 2005 Jan;166(1):135–146. doi: 10.1016/S0002-9440(10)62239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang B, Hyland L, Hou S. Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J Virol. 2001 Jun;75(11):5416–5420. doi: 10.1128/JVI.75.11.5416-5420.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singleton R, Etchart N, Hou S, Hyland L. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J Virol. 2003 Nov;77(21):11303–11311. doi: 10.1128/JVI.77.21.11303-11311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joo HM, He Y, Sangster MY. Broad dispersion and lung localization of virus-specific memory B cells induced by influenza pneumonia. Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3485–3490. doi: 10.1073/pnas.0800003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003 Nov 15;171(10):4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 65.Slobod KS, Lockey TD, Howlett N, et al. Subcutaneous administration of a recombinant vaccinia virus vaccine expressing multiple envelopes of HIV-1. Eur J Clin Microbiol Infect Dis. 2004 Feb;23(2):106–110. doi: 10.1007/s10096-003-1075-3. [DOI] [PubMed] [Google Scholar]
- 66.Slifka MK, Ahmed R. Long-term humoral immunity against viruses: revisiting the issue of plasma cell longevity. Trends Microbiol. 1996 Oct;4(10):394–400. doi: 10.1016/0966-842X(96)10059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gray D. Immunological memory: a function of antigen persistence. Trends Microbiol. 1993 May;1(2):39–41. doi: 10.1016/0966-842x(93)90026-n. [DOI] [PubMed] [Google Scholar]
- 68.Sikkel MB, Quint JK, Mallia P, Wedzicha JA, Johnston SL. Respiratory syncytial virus persistence in chronic obstructive pulmonary disease. Pediatr Infect DIs J. 2008 Oct;27(10 Suppl):S63–S70. doi: 10.1097/INF.0b013e3181684d67. [DOI] [PubMed] [Google Scholar]
- 69.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008 Mar 25;105(12):4802–4807. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000 Oct 5;407(6804):636–642. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- 71.Kast WM, Roux L, Curren J, et al. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown SA, Hurwitz JL, Zirkel A, et al. A recombinant Sendai virus is controlled by CD4+ effector T cells responding to a secreted human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 2007 Nov;81(22):12535–12542. doi: 10.1128/JVI.00197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]