Abstract
We investigated a series of coumarinyl-substituted aromatic sulfonamides as inhibitors of four carbonic anhydrase (CA, EC 4.2.1.1) isoforms with medical applications, the cytosolic hCA I, and II, and the transmembrane, tumor-associated hCA IX and XII. Compounds incorporating 7-methoxy-coumarin-4-yl-acetamide- tails and benzenesulfonamide and benzene-1,3-disulfonamide scaffolds showed medium potency inhibition of hCA I (KIs of 73 – 131 nM), effective hCA II inhibition (KIs of 9.1 – 36 nM) and less effective hCA IX and XII inhibition (KIs of 55-128 nM). Only one compound, the derivatized 4-amino-6-trifluoromethyl-benzene-1,3-disulfonamide with the coumarinyl tail, showed effective inhibition of the transmembrane isoforms, with KIs of 5.9 – 14.2 nM, although it was less effective as hCA I and II inhibitor (KIs of 36-120 nM). An X-ray crystal structure of hCA II in complex with 4-(7-methoxy-coumarin-4-yl-acetamido)-benzenesulfonamide (KI of 9.1 nM against hCA II) showed the intact inhibitor coordinated to the zinc ion from the enzyme active site by the sulfonamide moiety, and participating in a edge-to-face stacking with Phe131, in addition to other hydrophobic and hydrophilic interactions with water molecules and amino acid residues from the active site. Thus, sulfonamides incorporating coumarin rings have a distinct inhibition mechanism compared to the coumarins, and may lead to compounds with interesting inhibition profiles against various α-CAs found in mammals or parasites, such as Plasmodium falciparum.
1. Introduction
Coumarins were recently shown to constitute a novel class of inhibitors of the metalloenzyme carbonic anhydrase (CA, EC 4.2.1.1), whereas their mechanism of action is different from that of all other known inhibitors.1,2 Indeed most CA inhibitors (CAIs) investigated to date directly interact with the metal ion (which is Zn(II) in α-CAs) from the enzyme active site, directly coordinating to it (inorganic anions, sulfonamides and their isosteres, etc.)3,4 or anchoring to the zinc-bound water molecule/hydroxide ion through a network of hydrogen bonds (phenols,5 polyamines6) which stabilize the enzyme-inhibitor adduct. Whereas metal-complexing anions are weak CAIs, with affinities generally in the millimolar range,7 sulfonamides and their isosteres (sulfamates, sulfamides, etc) easily arrive to a low nanomolar inhibition potency.3,4 Phenols and polyamines have an intermediate potency between the two extremes mentioned above (micromolar – nanomolar range, depending on the isoform and the substitution pattern of the inhibitor scaffold).5,6 There are many X-ray crystal structures of adducts of all these classes of CAIs with several CA isoforms (of the 16 presently known in mammals),2,3 which undoubtedly prove these different binding modes of the inhibitor to the enzyme.7
However, sulfonamides remain the main chemotype of clinically used CAIs, with many such drugs available to date.3,4,8 Members of this class include aromatic, heterocyclic or aliphatic primary sulfonamides, but most drugs belong to the heterocyclic class.3,8 CAIs are clinically employed for the management of a variety of disorders connected to CA disbalances, such as glaucoma;3,8 in the treatment of edema due to congestive heart failure,3,9 or for drug-induced edema;3,9 as mountain sickness drugs,9 whereas other agents of this pharmacological class show applications as anticonvulsants,10,11 antiobesity12 or antitumor drugs/tumor diagnostic agents.3,13 As there are few isoform-selective inhibitors to date,3 new sulfonamides are continuously reported to find derivatives with better inhibition profiles as compared to the promiscuous, first generation inhibitors such as acetazolamide (5-acetamido-1,3,4-thiadiazole-2-sulfonamide).3 Recently, we have investigated some coumarinyl-substituted sulfonamides as inhibitors of the CA from the malaria producing protozoa Plasmodium falciparum.14 These compounds incorporate in their molecule both the coumarin ring, found in the new class of CAIs reported recently,1,2 as well as the classical benzenesulfonamide moiety normally associated with CA inhibition. As such coumarinyl-sulfonamides have not been investigated for the inhibition of thr mammalian CAs with medicinal chemistry applications, we report here such a study, investigating the inhibition of four human (h) hCA isoforms with a series of coumarinyl-substituted benzenesulfonamides. We were also interested to understand whether the presence of the substituted coumarin ring in these derivatives will lead to an inhibition mechanims typical of the coumarins (which are hydrolyzed to 2-hydroxy-cinnamic acids which subsequently occlude the entrance to the active site)1,2 or whether the binding to the enzyme occurs as that typical for sulfonamides, i.e., by coordination to the metal ion from the enzyme active site. To understand this, we also report a high resolution X-ray crystal structure of a coumarinyl-substituted benzenesulfonamide in adduct with the physiologically dominant isoform hCA II.
2. Results and discussion
2.1. Chemistry
The “tail approach” has been extensively used to develop sulfonamide CAIs possessing a range of desired physico-chemical properties, and consists in using aromatic/heterocyclic sulfonamide scaffolds to which tails that will induce water solubility (or other desired physico-chemical properties, e.g., enhanced liposolubility; membrane impermeability, etc.) are attached at the amino, hydroxy, imino or hydrazino moieties contained in such precursor scaffolds.15,16 Some coumarin-substituted sulfonamides of type 1-5 have also been reported by this approach,14 and they contain 7-methoxy-coumarin-4-yl-acetyl moieties, attached to the classical scaffolds of the aminobenzenesulfonamides (in para, meta and ortho positions, compounds 1-3) or to the 4-hydrazino-benzenesulfonamide (4) and 4-amino-6-trifluoromethyl-benzene-1,3-disulfonamide (5) scaffolds.
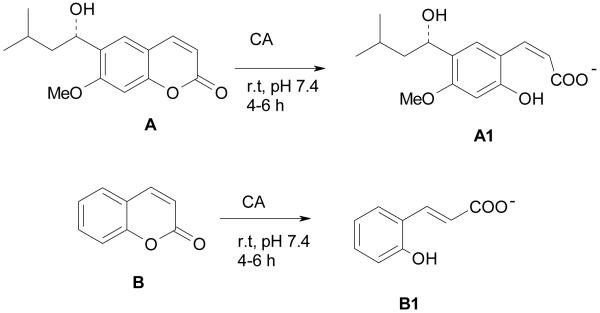
These compounds were prepared from the corresponding amino/hydrazino-aromatic sulfonamides and the 7-methoxy-coumarin-4-yl-acetyl chloride,14 by the classical tail approach,15,16 and were investigated for the inhibition of the α-CA from Plasmodium falciparum.14 Their activity against this enzyme was in the low micromolar range, but they showed no activity in vivo as antimalarials, in contrast to other types of such derivatives investigated by us.14 The substituted coumarinyl moieties were introduced in the molecules 1-5 in order to change their physico-chemical properties and obviously their interaction with the enzymes, but these compounds have not yet been tested as inhibitors of mammalian CAs. Interest in this class of derivatives reemerged recently after the report that coumarins act as a completely new class of CAIs.1,2 Indeed, the natural product coumarin A1 or the very simple non-substituted derivative B (and many of its congeners possessing various substitution patterns at the coumarin ring)2 act as effective CAIs against many of the mammalian isoforms CA I – CA XV. Furthermore, the real enzyme inhibitor is constituted by the hydrolyzed coumarins, such as compounds A1 and B1, formed from the original coumarins A and B, respectively (Scheme 1). They have been evidenced by X-ray crystallography of enzyme-inhibitor adducts and investigated in detail by kinetic methods.1,2 The 2-hydroxy-cinnamic acids thus formed (A1 and B1), bind in an unprecedented way to the enzyme,1,2 at the entrance of the active site cavity, plugging the entire entrance to it.
Scheme 1.
Formation of 2-hydroxy-cinnamic acids A1 and B1 by the CA-mediated hydrolysis of coumarins A and B.
Compounds 1-5 thus incorporate two chemotypes known to interact in very different ways with the CA active site: (i) the aromatic sulfonamide, classical CAI pharmacophore, and (ii) the substituted coumarin moieties. Both of them lead to low nanomolar and sometimes isoform-selective CAIs.1-4
2.2. CA inhibition
Inhibition of four physiologically relevant α-CA isoforms with compounds 1-5 and acetazolamide (5-acetamido-1,3,4-thiadiazole-2-sulfonamide, AZA, a clinically used drug) is presented in Table 1.17 hCA I and II (cytosolic, widespread enzymes) as well as hCA IX and XII (transmembrane, tumor-associated CAs) have been included in this study due to their relevance as targets/offtargets when developing CAIs. Indeed, CA II for example is the drug target for developing antiglaucoma CAIs,3,4 but it is an offtarget when considering CA IX/XII inhibition.13 In this latter case, only the transmembrane, tumor-associated isozymes (IX and XII) should be inhibited, as CA II may have the function of housekeeping enzyme, and its inhibition may lead to side effects. Inhibition of the malaria enzyme pfCA with compounds 1-5 are also shown in Table 1, for compariosn reasons, as these compounds were investigated earlier only for their interaction with this enzyme.14 Thus, we investigated in detail the inhibition of the coumarinyl-substituted sulfonamides 1-5 with these four human enzymes, i.e., hCA I, II, IX and XII.
Table 1.
Inhibition of human α-CAs and Plasmodium falciparum enzymes (hCA and pfCA) with sulfonamides 1-5, by a stopped-flow CO2 hydrase assay.17 Data for AZA are from ref.3 whereas data against pfCA from ref.14
The following structure-activity relationship (SAR) can be drawn from data of Table 1:
Compounds 1-5 were medium potency hCA I inhibtors, with inhibition constants in the range of 73-131 nM, being more effective than acetazolamide (KI of 250 nM). The best hCA I inhibitor was the sulfanilamide derivative 1, whereas its meta (2) and ortho (3) isomers were progressively less inhibitory. The hydrazino derivative 4 was slightly more active than 2 and 3, whereas the benzene-1,3-disulfonamide less active compared to 1. Thus, in this case the aromnatic sulfonamide head strongly influences activity for this small series of compounds, as hCA I inhibitors.
The physiologically dominant isoforms hCA II was highly inhibited by compounds 1-5 and acetazolamide. Compounds 1, 2, 4 and AZA were very effective CAIs, with KIs in the range of 9.1 – 15.8 nM whereas 3 and 5 were less effective (KIs of 36-79 nM). The best inhibitor was again 1, and SAR is rather similar to what obserbved above for the inhibition of hCA I. The least effective hCA II inhibitor was the orthanilamide derivative 3.
The tumor-associated isoforms hCA IX and XII were less prone to be inhibited by these sulfonamides, except compound 5 which behaved as an effective inhibitor of both isoforms, with inhibition constants of 5.9 – 14.2 nM (in the same range as the clinically used AZA, Table 1). The remaining derivatives 1-4 were medium potency hCA IX and hCA XII inhibitors, with KIs in the range of 59-113 nM against the first enzyme, and of 55-128 nM against the second one. It is obvious that SAR is different for the inhibition of the transmembrane versus the cytosolic isoforms with this small groups of CAIs. Indeed, the disulfonamide 5 was the best hCA IX and XII inhibitor, whereas the sulfanilamide 1 was the best hCA I and II inhibitor detected here.
As reported erlier,14 compounds 1-5 are less effective inhibitors of an α-CA from the malaria parasite P. falciparum, with inhibition constants in the range of 970 nM – 4.578 μM. AZA was one of the most effective pfCA inhibitors detected so far,14 with a KI of 315 nM. However, it has been proven that it is possible to inhibit the growth of the malaria parasite by inhibiting this enzyme with potent sulfonamide CAIs.18 Thus, it is possible to develop antimalarials based on the CAIs, although few nanomolar inhibitors of pfCA were reported to date.14,18
2.3. X-ray crystallography
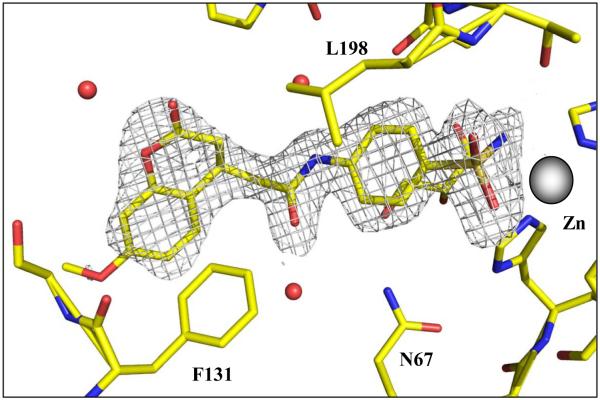
In order to understand why compound 1 is such a potent hCA II inhibitor, wheras it is less effective against other isoforms, we resolved its X-ray crystal structure in complex with this isozyme. From an initial refinement of the model of hCA II at 1.8 Å resolution, an |Fo – Fc| electron density omit map was calculated and revealed density consistent with the entire molecule compound 1 except for the methoxy moiety on the aromatic coumarin ring (Figure 1 and Table 2). Electron density consistent with a bound glycerol molecule was also evident, located adjacent to the aromatic ring of the benzene sulfonamide. The inhibitor is buried deep in the active site, with N1 of the sulfonamide bound to the zinc ion, at a distance of 2.2 Å.
Figure 1.
Stick representation compound 1 bound in the active site of hCA II. Atoms are coloured; zinc grey; sulfur, orange; oxygen, red; nitrogen, blue; and carbon, yellow. Solvent are depicted as red spheres. Amino acids are as labeled. The electron density is represented by a 2σ-weighted 2Fo - Fc Fourier map (grey mesh). Figure made using PyMOL.
Table 2.
Data and final model statistics of the hCA II – 1 adduct
| Data-collection statistics | |
| PDB Accession number | 3ML2 |
| Temperature (K) | 100 |
| Wavelength (Å) | 1.5418 |
| Space group | P 21 |
| Unit-cell parameters (Å,°) |
a = 42.14, b = 41.20, c =71.58, β =104.13 |
| Total number of measured reflections | 19896(1860)* |
| Resolution (Å) Rsym I/σ(I) Completeness (%) Redundancy |
23.1-1.8 (1.86-1.80) 6.5% (21.6%) 10.6 (3.2) 89.0 (89.0) 3.3 (3.5) |
| Final model statistics | |
|
aRcryst (%) bRfree(%) |
18.7 22.2 |
| Residue Nos. | 4-261 |
| No. of protein atoms (including alternate conformations) |
2060 |
| No. of drug atoms | 27 |
| No. of H2O molecules | 185 |
| R.m.s.d. for bond lengths (Å), angles (°) | 0.018, 1.7 |
| Ramachandran statistics (%) Most favored, additionally allowed and generously allowed regions |
88.0, 11.6, 0.5 |
| B factors (Å2) Average, main-, side-chain, inhibitor, solvent |
22.1, 27.2, 28.5, 32.1 |
Rsym = Σ |I - <I>|/ Σ <I>.
Rcryst = (Σ |Fo| - |Fc|/ Σ |Fobs| ) × 100.
Rfree is calculated in same manner as Rcryst, except that it uses 5% of the reflection data omitted from refinement.
Values in parenthesis represent highest resolution bin.
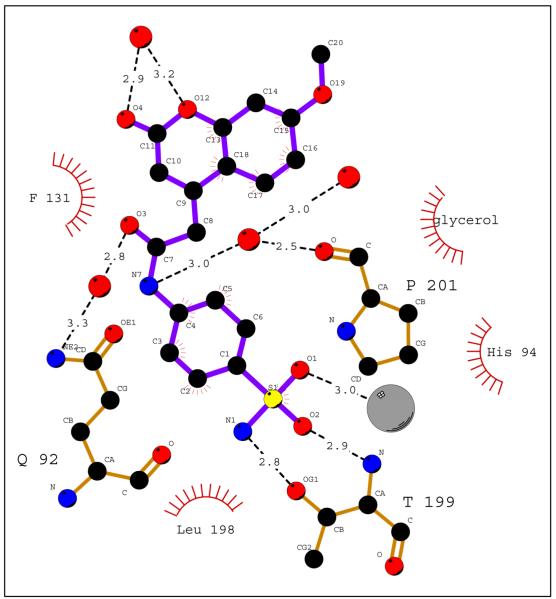
As with all sulfonamide compounds in complex with hCA II, for example AZA (PDB accession code 3hs4)7c, the nitrogen atom of the sulfonamide moiety is complexed with the active site zinc ion, at a distance of 2.2 Å. The O2 atom of the sulfonamide accepts a hydrogen bond from the main-chain nitrogen of Thr199, at a distance of 2.9 Å. No hydrogen bonds donate to O3 of the sulfonamide, but this atom is in Van der Waals contact with atoms near the active site zinc ion (Figures 2 and 3).
Figure 2.
Schematic of hCA II – compound 1 interactions. Hydrophobic contacts are indicated by red hash marks and H-bonds by black dashed lines. Atoms are coloured; zinc grey; sulfur, yellow; oxygen, red; nitrogen, blue; and carbon, black. Amino acids are as labeled. Figure made using Ligplot.
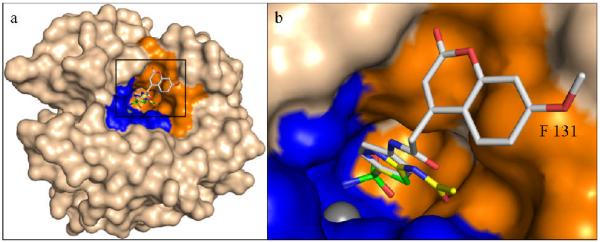
Figure 3.
Overall (a) and zoom-in (b) view of hCA II – sulfonamide 1 adduct, superposed onto acetazolamide (AZA) one (PDB accession code 3hs4)7c. Note the two compounds follow the same trajectory out of the active site with the extended coumarin heterocycle ring at an angle to the benzene ring with an edge-to-face interaction with the side chain of Phe131. hCA II is depicted as a surface representation (bulk solvent accessible area, light pink; hydrophilic and hydrophobic regions of the active site, blue and orange respectively). Compound 1 and AZA are represented as sticks. Compounds 1 (grey) and AZA (yellow) are represented as sticks. Non-carbon atoms of both compounds are colored as in Figure 1. Figure made using PyMOL (DeLano Scientific).
The benzene scaffold of compound 1 follows the same trajectory out of the active site, as the thiadiazole ring of acetazolamide AZA7c. The benzene core of the inhibitor is at van der Waals distance from the side chain of Leu98, and is sandwiched by the bound glycerol molecule, coming from the cryoprotectant solution. Hydrogen bonds from the three hydroxyl groups of the glycerol to the side chain nitrogen atoms of Gln92, Asn67, and Asn62 were also observed (Figures 1 and 2).
The extended coumarin heterocycle ring lies at an angle to the benzene ring, and is in an edge-to-face interaction with the side chain of Phe131. C10-C11 and O4 deviate slightly from the plane of the remaining nine atoms of the heterocyclic coumarin core, respectively. The torsion angles C9-C10-C11-O4 and C13-O12-C11-C10 are −9.1° and 7.4°, respectively. O4 interacts via a hydrogen bond with a solvent molecule and contacts the CG atom of Pro202 and the CD1 of Leu204. C10 has a hydrophobic interaction with the CD atom of Pro202, and C14 contacts the Cα atom of Gly132. In a packing contact with a symmetry-related molecule, the terminal methyl group C19 is 3.9 Å from the Cα and N atoms of Gly235.
Thus, its is obvious from the above data that the coumarin ring in compounds also incorporating a sulfonamide moiety, such as derivatives 1-5 investigated here, does not have the same fate as normal coumarins (without a zinc-binding group of the sulfonamide type in their molecule), which inhibit CAs after active-site mediated hydrolysis, as depicted in Scheme 1. On the contrary, similar to all sulfonamides investigated so far,3-7 the coumarinyl-substituted derivatives inhibit CAs by coordination of the sulfonamide moiety to the metal ion from the enzyme active site, and participation in hydrophobic and hydrophilic interactions of the organic scaffold of the inhibitor with amino acid residues known7 to be involved in the binding of CAIs, such as Thr199, Phe131, Pro201, etc. The X-ray data presented above also allow us to understand why compound 1 is a potent hCA II inhibitor and is less effective against hCA IX, one of the most important drug targets in this family of compounds. Indeed, as shown in Fig 2, a very important interaction between this inhibitor and hCA II is the stacking of the coumarin ring with Phe131, something evidenced earlier for other potent hCA II inhibitors.11d In hCA IX (PDB accession code 3iai), Phe131 from hCA II is “substituted” by a valine residue,13c and in hCA XII (PDB accession code 1jcz) Phe is an alanine residue,13d both of which are unable to participate in such an edge-to-face stacking interaction. This lack of interaction may thus explain the roughly 10- and 7-times weaker inhibition of hCA IX/hCA XII, respectively, over hCA II, with compound 1.
3. Conclusions
We investigated here a series of coumarinyl-substituted aromatic sulfonamides as inhibitors of four CA isoforms, the cytosolic hCA I, and II, and the transmembrane, tumor-associated isozymes hCA IX and XII. Compounds incorporating 7-methoxy-coumarin-4-yl-acetamide- tails and benzenesulfonamide and benzene-1,3-disulfonamide scaffolds showed medium potency inhibition of hCA I (KIs of 73 – 131 nM), effective hCA II inhibition (KIs of 9.1 – 36 nM) and less effective hCA IX and XII inhibition (KIs of 55-128 nM). Only one compound, the derivatized with the coumarinyl tail 4-amino-6-trifluoromethyl-benzene-1,3-disulfonamide, showed effective inhibition of the transmembrane isozymes, with KIs of 5.9 – 14.2 nM, although it was less effective as hCA I and II inhibitor (KIs of 36-120 nM). An X-ray crystal structure of hCA II in complex with 4-(7-methoxy-coumarin-4-yl-acetamido)-benzenesulfonamide (KI of 9.1 nM against this isozyme) showed the intact inhibitor coordinated to the zinc ion from the enzyme active site by the sulfonamide moiety, and participating in a edge-to-face stacking with Phe131, in addition to other hydrophobic and hydrophilic interactions with water molecules and amino acid residues from the active site. Thus, sulfonamides incorporating coumarin rings have a distinct inhibition mechanism compared to the coumarins, and may lead to compounds with interesting inhibition profiles against various enzymes of mammalian or parasitic (e.g., Plasmodium falciparum) origin.
4. Experimental
4.1. Chemistry
Sulfonamides 1-5 have been reported previously14 and were prepared by the tail approch from the corresponding amino-/hydrazino-sulfonamide and the acyl chloride.15,16 All reagents and buffers were the highest grade available, from Sigma-Aldrich, Milan, Italy.
4.2. CA inhibition
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity.17 Phenol red (at a concentration of 0.2 mM) was used as the indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining a constant ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10-100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants (5 different substyrate concentrations were used). For each inhibitor at least six traces of the initial 5-10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with distilled-deionized water. Experiments were done using 6 different inhibitor concentrations, varying from 100 μM to 0.1 nM. Inhibitor and enzyme solutions were preincubated together for 15 min - 24 h at room temperature (15 min) or 4 °C (all other incubation times) prior to assay, in order to allow for the formation of the E-I complex or for the eventual active site mediated hydrolysis of the inhibitor. Data reported in Table 1 show the inhibition after 15 incubation, as there were no diffeernces of inhibitory power when the enzyme and inhibitors were kept for longer periods in incubation.1 The inhibition constants were obtained by non-linear least-squares methods using PRISM 3, as reported earlier,1,2 and represent the mean from at least three different determinations.
4.3. X-ray crystallography
The plasmid encoding hCA II was transformed into E.coli BL21 cells through standard procedures and the transformed cells were expressed at 37 °C in LB medium containing 100 μg/ml ampicillin.19 hCA II production was induced by the addition of isopropyl thiogalactoside to a final concentration of 1mM at an O.D600 of 0.6 AU. The cells were harvested after 4hrs of post induction. The cell pellets were lysed and hCA II was purified through affinity chromatography using pAMBS resin as has been described elsewhere.20
Co-crystals of hCA II compound 1 complex were obtained using the hanging drop vapor diffusion method.21 10 μl drops (0.2 mM hCA II; 0.4 mM compound 1; 0.8 M sodium citrate; 50 mM Tris-Cl; pH 8.0) were equilibrated against 1ml of precipitant solution (1.6 M sodium citrate; 50mM Tris-Cl pH 8.0) at room temperature (~20 °C).7c Useful crystals were observed 4 days after the crystallization setup. A crystal was cryoprotected by quick immersion into 25% glycerol precipitant solution and flash-cooled by exposure to a gaseous stream of nitrogen at 100K. X-ray diffraction data were obtained using an R-AXIS IV++ image plate system with OsmicVarimax optics and a Rigaku RU-H3R Cu rotating anode operating at 50 kV and 22mA. The detector-crystal distance was set to 80 mm. The oscillation steps were 1° with a 6 min exposure per image. Indexing, integration, and scaling were performed using HKL2000. 22
The crystal structure of hCA II (PDB accession code: 2ili) 7c was used to obtain initial phases using PHENIX.23 The solvent molecules were removed and 5% of the unique reflections were selected randomly and excluded from the refinement data set for the purpose of Rfree calculations. Coordinates for the inhibitor were generated using the PRODRG2 website. 24 Goemetric restraints for inhibitor 1 were created using the eLBOW facility within the PHENIX software suite, and planar restraints for C10, C11, O3, and O4 were manually added. All manual rebuilding was done using Coot 25 and the geometry of the final refined model of the complex was checked using Procheck. 26
ACKNOWLEDGEMENTS
This research was financed in part by a grant of the 6th Framework Programme of the European Union (DeZnIT project) and by a 7th FP EU project (METOXIA) to AS and CTS, and in part by an NIH (GM 25154) and Maren grant to RM. RM would also like to thank the Centre of Structure Biology, University of Florida, for their continued support to help maintain the in-house X-ray facilities.
Footnotes
Coordinates and structure factors have been deposited in the Protein Data Bank as entry 3ml2.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.a) Maresca A, Temperini C, Vu H, Pham NB, Poulsen SA, Scozzafava A, Quinn RJ, Supuran CT. J. Am. Chem. Soc. 2009;131:3057. doi: 10.1021/ja809683v. [DOI] [PubMed] [Google Scholar]; b) Vu H, Pham NB, Quinn RJ. J. Biomol. Screen. 2008;13:265. doi: 10.1177/1087057108315739. [DOI] [PubMed] [Google Scholar]
- 2.a) Maresca A, Temperini C, Pochet L, Masereel B, Scozzafava A, Supuran CT. J. Med. Chem. 2010;53:335. doi: 10.1021/jm901287j. [DOI] [PubMed] [Google Scholar]; b Supuran CT. Bioorg. Med. Chem. Lett. 2010;20:3467. doi: 10.1016/j.bmcl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 3.a) Supuran CT. Nat. Rev. Drug Discov. 2008;7:168. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]; b) Supuran CT, Scozzafava A, Casini A. Development of sulfonamide carbonic anhydrase inhibitors (CAIs) In: Supuran CT, Scozzafava A, Conway J, editors. Carbonic anhydrase – Its Inhibitors and Activators. CRC Press; Boca Raton (FL): 2004. pp. 67–147. [Google Scholar]; c) Domsic JF, Avvaru BS, Kim CU, Gruner SM, Agbandje-McKenna M, Silverman DN, McKenna R. J. Biol. Chem. 2008;283:30766. doi: 10.1074/jbc.M805353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Supuran CT. Carbonic anhydrases as drug targets – general presentation. In: Supuran CT, Winum JY, editors. Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications. Wiley; Hoboken (NJ): 2009. pp. 15–38. [Google Scholar]; b) Winum JY, Rami M, Scozzafava A, Montero JL, Supuran C. Med. Res. Rev. 2008;28:445. doi: 10.1002/med.20112. [DOI] [PubMed] [Google Scholar]; c) Supuran CT, Scozzafava A, Casini A. Med. Res. Rev. 2003;23:146. doi: 10.1002/med.10025. [DOI] [PubMed] [Google Scholar]
- 5.a) Nair SK, Ludwig PA, Christianson DW. J. Am. Chem. Soc. 1994;116:3659. [Google Scholar]; b) Innocenti A, Vullo D, Scozzafava A, Supuran CT. Bioorg. Med. Chem. Lett. 2008;18:1583. doi: 10.1016/j.bmcl.2008.01.077. [DOI] [PubMed] [Google Scholar]; c) Innocenti A, Hilvo M, Scozzafava A, Parkkila S, Supuran CT. Bioorg. Med. Chem. Lett. 2008;18:3593–3596. doi: 10.1016/j.bmcl.2008.04.077. [DOI] [PubMed] [Google Scholar]; d) Innocenti A, Vullo D, Scozzafava A, Supuran CT. Bioorg. Med. Chem. 2008;16:7424. doi: 10.1016/j.bmc.2008.06.013. [DOI] [PubMed] [Google Scholar]; e) Barrese AA, 3rd, Genis C, Fisher SZ, Orwenyo JN, Kumara MT, Dutta SK, Phillips E, Kiddle JJ, Tu C, Silverman DN, Govindasamy L, Agbandje-McKenna M, McKenna R, Tripp BC. Biochemistry. 2008;47:3174. doi: 10.1021/bi702385k. [DOI] [PubMed] [Google Scholar]
- 6.Carta F, Temperini C, Innocenti A, Scozzafava A, Kaila K, Supuran CT. J. Med.Chem. 2010 doi: 10.1021/jm1003667. in press. [DOI] [PubMed] [Google Scholar]
- 7.a) Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G. X-Ray crystallography of CA inhibitors and its importance in drug design. In: Supuran CT, Winum JY, editors. Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications. Wiley; Hoboken: 2009. pp. 73–138. [Google Scholar]; b) Fisher SZ, Maupin CM, Budayova-Spano M, Govindasamy L, Tu CK, Agbandje-McKenna M, Silverman DN, Voth GA, McKenna R. Biochemistry. 2007;42:2930. doi: 10.1021/bi062066y. [DOI] [PubMed] [Google Scholar]; c) Sippel KH, Robbins AH, Domsic J, Genis C, Agbandje-McKenna M, McKenna R. Acta Cryst. 2009;D 65:992. doi: 10.1107/S1744309109036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Pastorekova S, Parkkila S, Pastorek J, Supuran CT. J. Enzyme Inhib. Med. Chem. 2004;19:199. doi: 10.1080/14756360410001689540. [DOI] [PubMed] [Google Scholar]; b) Supuran CT, Scozzafava A, Casini A. Development of sulfonamide carbonic anhydrase inhibitors. In: Supuran CT, Scozzafava A, Conway J, editors. Carbonic anhydrase – Its inhibitors and activators. CRC Press; Boca Raton: 2004. pp. 67–147. [Google Scholar]; c) Mincione F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors as ophthalomologic drugs. In: Supuran CT, Winum JY, editors. Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications. Wiley; Hoboken (NJ): 2009. pp. 139–154. [Google Scholar]
- 9.a) van Patot MC, Leadbetter G, 3rd., Keyes LE, Maakestad KM, Olson S, Hackett PH. High Alt. Med. Biol. 2008;9:289. doi: 10.1089/ham.2008.1029. [DOI] [PubMed] [Google Scholar]; b) Supuran CT. Curr. Pharm. Des. 2008;14:641. doi: 10.2174/138161208783877947. [DOI] [PubMed] [Google Scholar]
- 10.a) De Simone G, Vitale RM, Di Fiore A, Pedone C, Scozzafava A, Montero JL, Winum JY, Supuran CT. J. Med. Chem. 2006;49:5544. doi: 10.1021/jm060531j. [DOI] [PubMed] [Google Scholar]; b) De Simone G, Di Fiore A, Menchise V, Pedone C, Antel J, Casini A, Scozzafava A, Wurl M, Supuran CT. Bioorg. Med. Chem. Lett. 2005;15:2315. doi: 10.1016/j.bmcl.2005.03.032. [DOI] [PubMed] [Google Scholar]; c) Winum JY, Temperini C, El Cheikh K, Innocenti A, Vullo D, Ciattini S, Montero JL, Scozzafava A, Supuran CT. J. Med. Chem. 2006;49:7024. doi: 10.1021/jm060807n. [DOI] [PubMed] [Google Scholar]
- 11.a) Alterio V, Vitale RM, Monti SM, Pedone C, Scozzafava A, Cecchi A, De Simone G, Supuran CT. J. Am. Chem. Soc. 2006;128:8329. doi: 10.1021/ja061574s. [DOI] [PubMed] [Google Scholar]; b) Casini A, Antel J, Abbate F, Scozzafava A, David S, Waldeck H, Schafer S, Supuran CT. Bioorg. Med. Chem. Lett. 2003;13:841. doi: 10.1016/s0960-894x(03)00029-5. [DOI] [PubMed] [Google Scholar]; c) Weber A, Casini A, Heine A, Kuhn D, Supuran CT, Scozzafava A, Klebe G. J. Med. Chem. 2004;47:550. doi: 10.1021/jm030912m. [DOI] [PubMed] [Google Scholar]; d) Menchise V, De Simone G, Alterio V, Di Fiore A, Pedone C, Scozzafava A, Supuran CT. J. Med. Chem. 2005;48:5721. doi: 10.1021/jm050333c. [DOI] [PubMed] [Google Scholar]
- 12.a) Supuran CT, Di Fiore A, De Simone G. Expert Opin. Emerg. Drugs. 2008;13:383–392. doi: 10.1517/14728214.13.2.383. [DOI] [PubMed] [Google Scholar]; b) De Simone G, Di Fiore A, Supuran CT. Curr. Pharm. Des. 2008;14:655–660. doi: 10.2174/138161208783877820. [DOI] [PubMed] [Google Scholar]
- 13.a) Svastova E, Hulíkova A, Rafajova M, Zatovicova M, Gibadulinova A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, Pastorekova S. FEBS Lett. 2004;577:439. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]; b) Thiry A, Dogné JM, Masereel B, Supuran CT. Trends Pharmacol. Sci. 2006;27:566–573. doi: 10.1016/j.tips.2006.09.002. [DOI] [PubMed] [Google Scholar]; c) Alterio V, Hilvo M, Di Fiore A, Supuran CT, Pan P, Parkkila S, Scaloni A, Pastorek J, Pastorekova S, Pedone C, Scozzafava A, Monti SM, De Simone G. Proc. Natl. Acad. Sci. USA. 2009;106:16233. doi: 10.1073/pnas.0908301106. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Whittington DA, Waheed A, Ulmasov B, Shah GN, Grubb JH, Sly WS, Christianson DW. Proc. Natl. Acad. Sci. USA. 2001;98:9545. doi: 10.1073/pnas.161301298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krungkrai J, Krungkrai SR, Supuran CT. Bioorg. Med. Chem. Lett. 2008;18:5466. doi: 10.1016/j.bmcl.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 15.a) Borras J, Scozzafava A, Menabuoni L, Mincione F, Briganti F, Mincione G, Supuran CT. Bioorg. Med. Chem. 1999;7:2397. doi: 10.1016/s0968-0896(99)00190-x. [DOI] [PubMed] [Google Scholar]; b) Scozzafava A, Menabuoni L, Mincione F, Supuran CT. J. Med. Chem. 2002;45:1466. doi: 10.1021/jm0108202. [DOI] [PubMed] [Google Scholar]; c) Menabuoni L, Scozzafava A, Mincione F, Briganti F, Mincione G, Supuran CT. J. Enz. Inhib. 1999;14:457. doi: 10.3109/14756369909030336. [DOI] [PubMed] [Google Scholar]; d) Scozzafava A, Menabuoni L, Mincione F, Briganti F, Mincione G, Supuran CT. J. Med. Chem. 1999;42:2641. doi: 10.1021/jm9900523. [DOI] [PubMed] [Google Scholar]
- 16.a) Supuran CT, Scozzafava A. Bioorg. Med. Chem. 2007;15:4336. doi: 10.1016/j.bmc.2007.04.020. [DOI] [PubMed] [Google Scholar]; b) Steele RM, Batugo MR, Benedini F, Biondi S, Borghi V, Carzaniga L, Impagnietello F, Miglietta D, Chong WKM, Rajapakse R, Cecchi A, Temperini C, Supuran CT. Bioorg. Med. Chem. Lett. 2009;19:6565. doi: 10.1016/j.bmcl.2009.10.036. [DOI] [PubMed] [Google Scholar]; c) Vomasta D, Innocenti A, König B, Supuran CT. Bioorg. Med. Chem. Lett. 2009;19:1283. doi: 10.1016/j.bmcl.2009.01.079. [DOI] [PubMed] [Google Scholar]
- 17.Khalifah RG. J. Biol. Chem. 1971;246:2561. [PubMed] [Google Scholar]
- 18.a) Krungkrai J, Scozzafava A, Reungprapavut S, Krungkrai SR, Rattanajak R, Kamchonwongpaisan S, Supuran CT. Bioorg. Med. Chem. 2005;13:483. doi: 10.1016/j.bmc.2004.10.015. [DOI] [PubMed] [Google Scholar]; b) Krungkrai J, Krungkrai SR, Supuran CT. Curr. Top. Med. Chem. 2007;7:909. doi: 10.2174/156802607780636744. [DOI] [PubMed] [Google Scholar]; c) Krungkrai J, Supuran CT. Curr. Pharm. Des. 2008;14:631. doi: 10.2174/138161208783877901. [DOI] [PubMed] [Google Scholar]
- 19.Forsman CA, Behravan G, Osterman A, Jonsson BH. Acta. Chem. Scand. 1988;42:314. doi: 10.3891/acta.chem.scand.42b-0314. [DOI] [PubMed] [Google Scholar]
- 20.Khalifah RG, Strader DJ, Bryant SH, Gibson SM. Biochemistry. 1977;16:2241. doi: 10.1021/bi00629a031. [DOI] [PubMed] [Google Scholar]
- 21.McPherson A. Preparation and Analysis of Protein Crystals. 1st Ed. John Wiley & Sons; New York: 1982. [Google Scholar]
- 22.Pflugrath JW. Acta Cryst. 1999;D55:1718–1725. doi: 10.1107/s090744499900935x. [DOI] [PubMed] [Google Scholar]
- 23.Adams PD, Grosse-Kunstleve RW, Hung L-W, Ioerger TR, McCoy A, Moriarty J, Sauter NK, Terwilliger TC. Acta Cryst. 2002;D58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 24.Schuettelkopf AW, van Aalten DMF. Acta Cryst. 2004;D60:1355. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 25.Emsley P, Cowtan K. Acta Crystallogr. 2004;D60:2126. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 26.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. J. Appl. Cryst. 1993;26:283. [Google Scholar]