Abstract
BACKGROUND
Patients with amnestic mild cognitive impairment (MCI) demonstrate decline in everyday function. In this study, we investigated whether whole brain atrophy and apolipoprotein E (APOE) genotype are associated with the rate of functional decline in MCI.
METHODS
Participants were 164 healthy controls, 258 MCI patients, and 103 patients with mild Alzheimer’s disease (AD), enrolled in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). They underwent brain MRI scans, APOE genotyping, and completed up to 6 biannual Functional Activities Questionnaire (FAQ) assessments. Random effects regressions were used to examine trajectories of decline in FAQ across diagnostic groups, and to test the effects of ventricle-to-brain ratio (VBR) and APOE genotype on FAQ decline among MCI patients.
RESULTS
Rate of decline in FAQ among MCI patients was intermediate between that of controls and mild AD patients. Patients with MCI who converted to mild AD declined faster than those who remained stable. Among MCI patients, increased VBR and possession of any APOE ε4 allele were associated with faster rate of decline in FAQ. In addition, there was a significant VBR by APOE ε4 interaction such that patients who were APOE ε4 positive and had increased atrophy experienced the fastest decline in FAQ.
CONCLUSIONS
Functional decline occurs in MCI, particularly among patients who progress to mild AD. Brain atrophy and APOE ε4 positivity are associated with such declines, and patients who have elevated brain atrophy and are APOE ε4 positive are at greatest risk of functional degradation. These findings highlight the value of genetic and volumetric MRI information as predictors of functional decline, and thus disease progression, in MCI.
Keywords: MRI, Brain atrophy, APOE ε4, activities of daily living, MCI
1. INTRODUCTION
There is a rapid expansion in the proportion of older adults in most industrialized nations. In the United States, for example, the oldest-old (those aged 85 and older) is presently the fastest growing segment of the general population [1]. While many individuals remain independent in performance of activities of daily living into old age, aging is often accompanied by physiological changes that can impact everyday function [2, 3]. And, because preserved daily function is central to autonomy and quality of life in old age, identifying factors that predict functional decline among older adults, particularly those with putative neurodegenerative disorders, has become a clinical, scientific, and public health imperative [4–6].
Although they do not invariably incur a diagnosis of Alzheimer’s disease (AD) or other dementias when monitored longitudinally, persons with amnestic mild cognitive impairment (MCI) are widely believed to be in the transitional stage between normal aging and AD [7]. Accordingly, they have been the focus of several clinical [8] and epidemiological [9] studies aimed at understanding the dynamics of progression to AD. Interestingly, whereas diagnostic criteria for AD require both impairment in cognition and decline in everyday function [10, 11], a review of the literature reveals a notable disparity in the attention devoted to investigating the correlates of these indices of incident AD. Specifically, in contrast with advances in uncovering biomarkers of cognitive decline among patients with MCI [12, 13], relatively little is known about the biomarkers of functional decline in MCI. To our knowledge, only one study has examined neuroanatomic correlates of everyday function in MCI [14]. Moreover, the investigators used a pooled sample of participants ranging from cognitively healthy to moderately demented. Therefore, their findings are of questionable specificity to MCI. This situation represents a significant knowledge gap as impairments in everyday function impact the independence, psychological wellbeing, longevity, and economic viability of older adults and their families, and is a top reason for nursing home placement and eventual loss of personal autonomy [4, 15, 16]. Notably, functional restriction reliably predicts progression to AD among patients with MCI [17, 18].
In this study, we addressed this issue by examining the effects of whole brain atrophy and apolipoprotein E (APOE) genotype on rate of decline in everyday functional abilities among older adults with MCI. These biomarkers were chosen because of their established association with incident dementia in MCI [19, 20]. We hypothesized that greater whole brain atrophy will be associated with faster rate of functional decline and that possession of one or more copies of APOE ε4 allele (i.e., 2/4, 3/4, or 4/4) will be associated with faster rate of functional decline. Importantly, we expected to find a significant whole brain atrophy by APOE ε4 interaction, demonstrating that rate of functional decline is steepest among persons who have increased cerebral atrophy and are APOE ε4 positive.
As a precondition for examining the effects of these biomarkers on functional decline in MCI, we first established that patients with MCI experience measurable functional decline. Hence, we included cognitively-normal older adults and patients with mild Alzheimer’s disease (AD) for comparative purposes.
2. METHODS
2.1. Participants
Data for this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI; http://www.adni-info.org/). The ADNI was launched in 2003 by the National Institute on Aging and other entities (see Acknowledgments) as a 5-year public-private partnership, with the overall aim of identifying clinical and biomarker measures that provide the highest power for capturing longitudinal change and predicting transitions across the AD spectrum [21, 22]. Our analyses included all participants—164 controls, 258 MCI patients, and 103 mild AD patients—who had baseline data on the primary variables of interest (i.e., total ventricular volume, whole brain volume, APOE genotype, and a measure of everyday function) at the time of data download (November, 2008). Diagnosis of amnestic MCI was based on Petersen/Mayo criteria [7], operationalized as memory complaints, objective memory difficulties (established using education-specific cut scores on the delayed recall trial of Story A from the Logical Memory test), normal activities of daily living, global CDR score of 0.5, and MMSE scores ≥ 24. Diagnosis of mild AD required a global CDR of 0.5 or 1.0, MMSE scores between 20 and 26 (inclusive), and fulfillment of the NINCDS/ADRDA criteria for probable AD. Participants were evaluated at six-month intervals over 2 (mild AD) or 3 (controls and MCI) years. Informed consent was obtained from study participants and their families, and the study was approved by the local institutional review board at each participating site.
2.2. Functional assessment
Everyday function was assessed with the Pfeffer Functional Activities Questionnaire (FAQ) [23]. The FAQ is an informant-report inventory that inquires into an older adult’s ability to manage finances, complete forms, shop, perform games of skill or hobbies, prepare hot beverages, prepare a balanced meal, follow current events, attend to television programs, books or magazines, remember appointments, and travel out of the neighborhood. Ratings range from normal (0) to dependent (3), for a total of 30 points with higher scores indicating worse functional status.
2.3. MRI methods
Participants underwent high-resolution structural brain MRI scans using 1.5T scanners from General Electric or Siemens in accordance with standardized ADNI protocol [22]. Raw 3D T1-weighted MPRAGE images were downloaded from the ADNI site by Dr. Anders Dale and colleagues at the University of California, San Diego. Using an extensive set of methods operationalized in FreeSurfer—a semi-automated, 3D whole-brain segmentation/parcellation package (http://surfer.nmr.mgh.harvard.edu/)—they obtained volumetric quantifications of various neuroanatomical structures. The specific protocols involved have been described elsewhere [24, 25]. These protocols yield measurements comparable to those obtained via manual labeling and are sensitive to subtle cerebral changes across the dementia spectrum [26, 27]. The derived anatomical volumes were subsequently uploaded to the ADNI website for public access.
For the present analyses, the measures extracted from the ADNI website were baseline total ventricular and whole brain volumes. Total ventricular volume is a composite measure encompassing all ventricles. Whole brain volume is a summary measure of total brain parenchyma including the cerebrum, basal ganglia, diencephalon, and cerebellum. It does not include the ventricles or other cerebrospinal fluid spaces and is not equivalent to intracranial volume. Ventricle-to-brain ratio (VBR), our index of whole brain atrophy, was computed as [(total ventricular volume/whole brain volume)*100]. The VBR is a viable marker of disease progression in AD [28], reliably discriminates demented elders from their cognitively intact peers [29, 30], and is more sensitive to neuropsychological performance than either of its components [31]. The decision to use VBR as the index of cerebral atrophy was partly driven by the unavailability of total intracranial volume, with the result that appropriate corrections for inter-individual variations in head size could not be applied to either whole brain or total ventricular volumes. Of note, the analyses reported here were repeated with the uncorrected ventricular and whole brain volumes. Results were substantively unchanged, with the VBR having the most robust relationship with the FAQ.
2.4. APOE genotyping
EDTA blood samples were collected from participants during their screening visit and sent to the ADNI Biomarker Core at the University of Pennsylvania within 24 hours of collection where APOE genotyping was performed using TaqMan assays as described elsewhere [32].
2.5. Data analyses
Group differences on baseline demographic, clinical, APOE ε4, VBR, and FAQ variables were examined using one-way analysis of variance or chi square/Fisher exact tests. To establish that MCI patients experience functional decline, we fitted a random effects regression [33] that modeled change in FAQ scores as a function of baseline diagnostic status. In addition, because at baseline there were group differences in age, gender, education, and FAQ (see Table 1), we included all four variables and their respective interactions with time in the model. To further adjust for any influence that variations in baseline FAQ scores might have on rate of decline on the FAQ, analyses were begun at the six-month assessment such that, at baseline, all participants were assigned an FAQ score equal to the baseline FAQ grand mean [33].
Table 1.
Characteristics of study participants at baseline
| Variable | Controls, n = 164 | MCI, n = 258 | AD, n = 103 | p§ | post hoc |
|---|---|---|---|---|---|
| Age, mean (SD) | 76.03 (5.22) | 74.48 (7.25) | 75.11 (7.35) | .069 | C > M* |
| Female, % | 48.8 | 35.7 | 45.6 | .019 | C A > M** |
| Caucasian, % | 92.7 | 92.6 | 94.2 | .149 | ---- |
| Education, mean (SD) | 16.02 (2.88) | 15.67 (2.94) | 14.98 (3.29) | .022 | C M > A |
| On anti-dementia medication, % | 0.0 | 48.1 | 92.2 | .001 | A > M > C** |
| MMSE, mean (SD) | 29.16 (0.96) | 27.08 (1.79) | 23.54 (1.99) | .001 | C > M > A |
| CDR-global, % | |||||
| 0.0 | 100.0 | 0.0 | 0.0 | .001 | A > M > C† |
| 0.5 | 0.0 | 100.0 | 48.5 | ||
| 1.0 | 0.0 | 0.0 | 51.5 | ||
| APOE ε4, % | 26.8 | 56.2 | 66.0 | .001 | C < M A ** |
| ‡ VBR, mean (SD) | 3.65 (1.91) | 4.55 (1.91) | 5.15 (1.90) | .001 | A > M > C |
| ‡ FAQ, mean (SD) | 0.15 (4.32) | 3.95 (4.30) | 12.82 (4.28) | .001 | A > M > C |
MCI = mild cognitive impairment; AD = Alzheimer disease; MMSE = Mini-Mental State Examination; CDR = Clinical Dementia Rating scale; APOE = apolipoprotein E; VBR = ventricle-to-brain ratio; FAQ = Pfeffer Functional Activities Questionnaire.
p value for omnibus test of group differences.
Though the omnibus test for group differences in age was only marginally significant, we nonetheless conducted post hoc tests to further probe the omnibus finding because of the centrality of age in studies of dementia.
“Female,” “on anti-dementia medication,” and “APOE ε4” are dichotomous variables. Therefore, these are subsequent 2×2 chi square or Fisher’s exact tests, not pairwise comparisons.
“CDR–global” is a categorical variable. Therefore, these are subsequent 2×3 Fisher’s exact tests, not pairwise comparisons.
Adjusted for age, gender, and education.
C > M = control mean greater than MCI mean; C A > M = control and AD counts greater than MCI count; C M > A = control and MCI mean greater than AD mean; A > M > C = AD mean (count) greater than MCI and control means (counts), and MCI mean (count) greater than control mean (count); C < M A = control count less than MCI and AD counts; C > M > A = control mean greater than MCI and AD means, and MCI mean greater than AD mean.
To test the effects of VBR and APOE ε4 on rate of change in FAQ among patients with MCI, we fitted random effects regressions that separately modeled change in FAQ as a function of baseline VBR (Model A), APOE ε4 (Model B), and VBR and APOE ε4 (Model C). Age, baseline FAQ, and their respective interactions with time were entered as covariates to account for their potential impact on rate of change in FAQ. In addition, for the reason stated above, analyses were begun at the six-month assessment such that, at baseline, all MCI patients were assigned an FAQ of 3.95, which was their group mean at baseline (see Table 1).
In these within-MCI random effects models, the primary term of interest was the interaction between the substantive variables and time, specifically VBR*time (Model A), APOE ε4*time (Model B), and APOE ε4*VBR*time (Model C). A significant VBR*time in Model A would indicate that greater cerebral atrophy is associated with faster rate of decline on the FAQ; a significant APOE ε4*time in Model B would indicate that possession of one or more copies of the APOE ε4 allele is associated with steeper rate of decline on the FAQ; and finally a significant APOE ε4*VBR*time in Model C would demonstrate that VBR and APOE ε4 exert a synergistic effect on rate of functional decline such that MCI patients who have increased cerebral atrophy and are APOE ε4 positive decline the fastest. All analyses were performed using SPSS 16 (SPSS Inc., Chicago, IL). Only findings with a 2-tailed p value ≤ .05 were considered significant.
3. RESULTS
3.1. Baseline characteristics of study participants
At baseline MCI patients had worse MMSE and CDR-global scores compared to controls. Also, they were younger, had higher VBR and FAQ scores, and composed of proportionately less women, more persons on anti-dementia medications, and more persons positive for APOE ε4 than controls. Patients with mild AD had worse MMSE, CDR-global, VBR, and FAQ scores, fewer years of education, and proportionately more persons on anti-dementia medications compared to controls and MCI patients. In addition, they consisted of proportionately more women than MCI patients, and proportionately more APOE ε4 positive persons than controls (Table 1).
3.2. Longitudinal assessment and diagnostic outcome
On average, participants completed 4 study visits (range: 2 – 6). The variability in number of study visits completed is primarily because participants were recruited in biannual cohorts and on a rolling basis within each cohort. Random effects regression is uniquely capable of handling such imbalance, and reducing potential biases therefrom [33]. Over the course of the study, eighty-two MCI patients (31.8%) progressed to mild AD and 3 controls (1.8%) progressed to MCI.
3.3. Differential rate of change in FAQ across dementia spectrum
Whereas controls experienced a biannual increase (i.e., worsening) of 0.13 point (SE = 0.86, p = .879) in FAQ, patients with MCI experienced an increase of 1.35 points (SE = 0.16, p < .001), and mild AD patients experienced an increase of 2.12 points (SE = 0.26, p < .001). These gradations indicate that MCI patients experience functional decline that is intermediate between that experienced by controls and mild AD patients. An exploratory random effects analysis, controlling for age, baseline FAQ, and their interactions with time, was conducted to investigate potential differences in rate of change in FAQ between the 82 MCI patients who progressed to mild AD and the 176 patients who remained stable. This analysis revealed that the “converter MCI” group had a steeper rate of functional decline compared to the “stable MCI” group (Conversion*time estimate = 2.03, SE = 0.21, p < .001), consistent with the clinical view that functional degradation is a hallmark feature of AD.
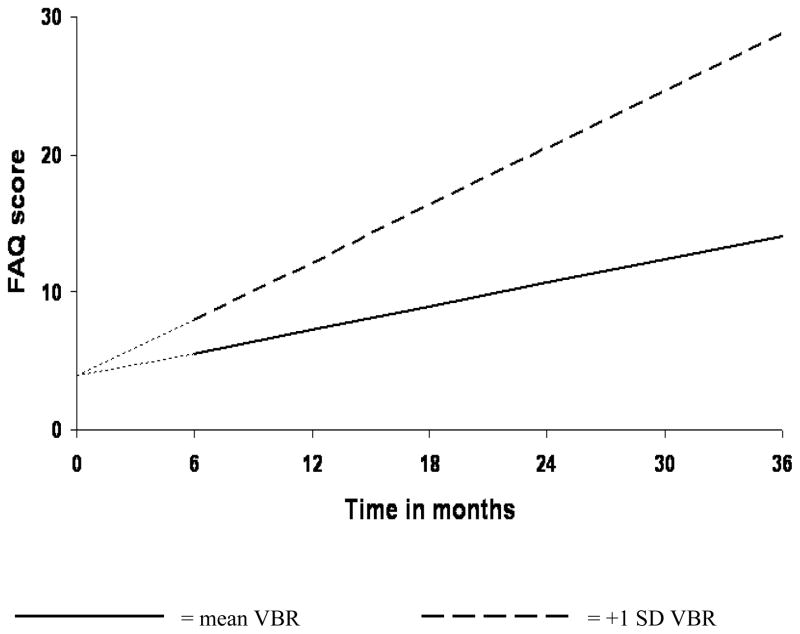
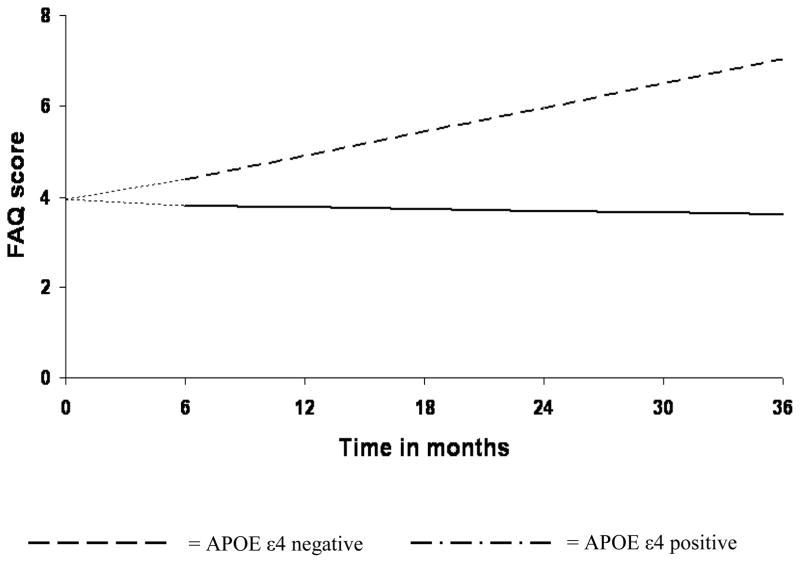
3.4. Effects of VBR and APOE ε4 genotype on rate of functional change in MCI
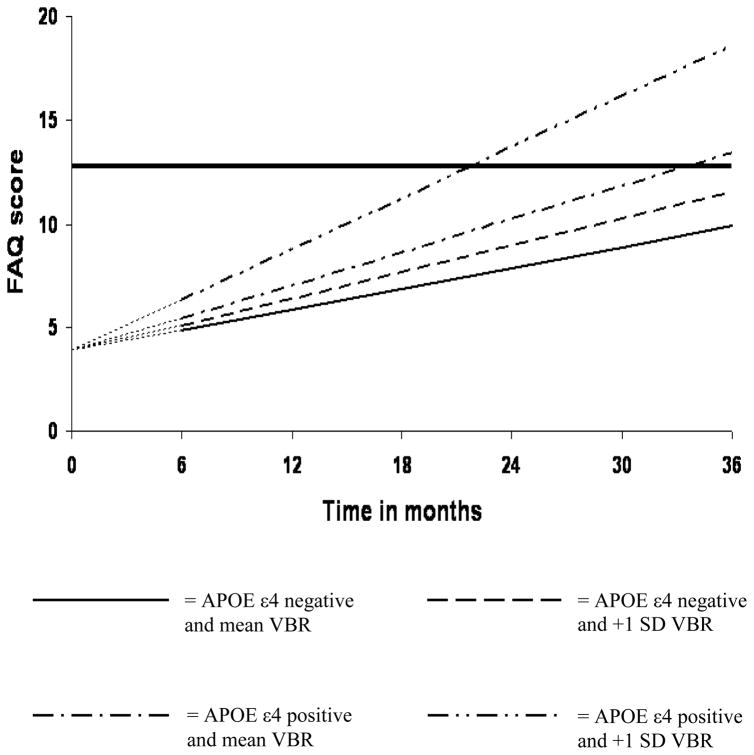
Table 2 details the results of the analyses that examined the independent and joint effects of VBR and APOE ε4 on rate of change in FAQ among patients with MCI. Model A revealed that for each percent increase in VBR, there was a significant 0.27-point biannual increase in FAQ. Figure 1 plots estimated FAQ scores across time for MCI patients whose VBR scores were at the mean (“mean VBR”) versus those whose VBR scores were one standard deviation above the mean (“+1SD VBR”). Model B revealed that APOE ε4 positive MCI patients experienced a significant 0.57-point biannual increase in FAQ compared to APOE ε4 negative patients. This is illustrated in Figure 2. Finally, VBR and APOE ε4 had a multiplicative effect on rate of change in FAQ as indicated by the APOE ε4*VBR*time term in Model C. Specifically, the effect of VBR on rate of change in FAQ was nonsignificant among APOE ε4 negative patients (see VBR*time term in Model C) whereas it was significant among APOE ε4 positive patients (estimate = 0.41, SE = 0.09, p < .001; not shown in Table 2). Figure 3 displays estimated FAQ scores across time for four mutually exclusive groups of MCI patients: (i) those who were APOE ε4 negative and had the mean VBR, (ii) those who were APOE ε4 negative and had VBR scores that were 1 SD above the mean, (iii) those who were APOE ε4 positive and had the mean VBR, and (iv) those who were APOE ε4 positive and had VBR scores that were 1 SD above the mean. Consistent with our hypothesis, these change trajectories revealed that functional decline was fastest for the last group, i.e., those patients who had increased VBR and possessed one or more copies of the APOE ε4 allele.
Table 2.
Independent and joint effects of VBR and APOE ε4 genotype on trajectories of functional change in MCI
| Effect | Estimate | Standard Error | p |
|---|---|---|---|
| A. VBR | |||
| Time a | 1.71 | 1.40 | .225 |
| VBR | −0.08 | 0.13 | .546 |
| VBR*time b | 0.27 | 0.07 | .001 |
| B. APOE ε4 | |||
| Time a | −0.04 | 1.13 | .973 |
| APOE ε4 | −0.02 | 0.45 | .961 |
| APOE ε4*time c | 0.57 | 0.22 | .009 |
| C. VBR and APOE ε4 | |||
| Time a | 1.01 | 1.38 | .467 |
| APOE ε4 | −0.39 | 0.48 | .425 |
| VBR | 0.21 | 0.17 | .215 |
| APOE ε4*VBR | −0.60 | 0.23 | .010 |
| VBR*time d | 0.13 | 0.08 | .122 |
| APOE ε4*time e | 0.59 | 0.24 | .014 |
| APOE ε4*VBR*time f | 0.28 | 0.11 | .016 |
Model adjusted for age, baseline FAQ, and their respective interactions with time. In addition, analyses were begun at month 6 to further correct for differences in FAQ at baseline. MCI = mild cognitive impairment; VBR = ventricle-to-brain ration; APOE = apolipoprotein E.
“Time” is the estimated biannual rate of change in FAQ for the reference group (Model A: MCI patients with mean VBR; Model B: MCI patients who are APOE ε4 negative; Model C: MCI patients who are APOE ε4 negative and have the mean VBR).
“VBR*time” in Model A is the estimated differential in biannual increase (i.e., worsening) in FAQ for each percent increase in VBR.
“APOE ε4*time” in Model B indicates the estimated differential in biannual increase (i.e., worsening) in FAQ for MCI patients who are APOE ε4 positive relative to those who are APOE ε4 negative.
“VBR*time” in Model C reflects the estimated differential in biannual increase (i.e., worsening) in FAQ for each percent increase in VBR among MCI patients who are APOE ε4 negative.
“APOE ε4*time” in Model C is the estimated differential in biannual increase (i.e., worsening) in FAQ due to being APOE ε4 positive among MCI patients who have the mean VBR.
The estimated differential in biannual increase (i.e., worsening) in FAQ for MCI patients who are APOE ε4 positive and have a percent increase in VBR relative to those who are APOE ε4 negative and have the mean VBR is given by the sum of the parameter estimates for “VBR*time,” “APOE ε4*time,” and “APOE ε4*VBR*time”
Figure 1. Increased VBR is associated with faster rate of functional decline in MCI.
Analysis was begun at month 6, with baseline FAQ treated as a covariate. Therefore, at baseline, all MCI patients were assigned an FAQ score of 3.95—the group mean at that assessment point. This is represented by the leading dashes in each line.
Figure 2. APOE ε4 positivity is associated with steeper rate of functional decline in MCI.
Analysis was begun at month 6, with baseline FAQ treated as a covariate. Therefore, at baseline, all MCI patients were assigned an FAQ score of 3.95—the group mean at that assessment point. This is represented by the leading dashes in each line.
Figure 3. Synergistic effect of VBR and APOE ε4 genotype on rate of functional decline in MCI.
Analysis was begun at month 6, with baseline FAQ treated as a covariate. Therefore, at baseline, all MCI patients were assigned an FAQ score of 3.95—the group mean at that assessment point. This is represented by the leading dashes in each line.
The heavy solid line represents an FAQ score of 12.8, the mean FAQ score of the AD patients at baseline.
4. DISCUSSION
Progressive decline in the ability to perform everyday activities is a core feature of AD and related dementias [34]. Consistent with its characterization as a prodrome for AD and other dementias [7], older adults with MCI exhibit detectable restrictions in daily function which, in turn, predict progression to AD [17, 18]. Yet, little is known about the AD-associated biomarkers that predispose patients with MCI to functional impairment. In this study, we examined two conceptually plausible candidates—whole brain atrophy and APOE ε4 genotype.
Our overarching aim was to delineate the functional significance of increased whole brain atrophy and APOE ε4 positivity in MCI. This is the first study to undertake such an investigation. Our analysis of differences in rates of decline in FAQ as a function of diagnostic status revealed that, relative to controls, patients with MCI experienced an additional 1.22-point biannual deterioration in function whereas patients with mild AD experienced an additional 2-point worsening. The observed trajectories are in accord with reports from prior investigations [4, 17, 35]. Furthermore, MCI patients who progressed to mild AD exhibited a faster rate of decline in FAQ compared to those who remained stable. Although the FAQ is a relatively coarse measure of daily function, the finding that it reliably distinguishes functional trajectories across the AD spectrum suggests that it is a valid measure of functional abilities in MCI, and is sensitive to change over time.
When examined independently, increased VBR and APOE ε4 positivity were each associated with a faster rate of decline in FAQ. When examined jointly, we found an interactive effect—the impact of VBR on rate of change in FAQ was three times (0.41/0.13) as large among APOE ε4 positive patients as among APOE ε4 negative patients. Overall, patients who had elevated VBR and were APOE ε4 positive experienced the most precipitous decline in function. This indicates that brain atrophy and APOE ε4 positivity have a synergistic relationship with regards to everyday functioning in MCI.
In addition to the characteristic accumulation of amyloid plaques and neurofibrillary tangles, disease progression in AD also results in the systematic and widespread loss of neurons and synapses [36]. Neuroimaging permits the surrogate visualization and quantification of this cell loss via indices of brain atrophy, such as VBR [37]. Since extent of brain atrophy arguably reflects severity of AD pathology, it is not surprising that those MCI patients who had increased VBR also experienced more rapid functional degradation. This conclusion is buttressed by our exploratory observation that MCI patients who progressed to mild AD exhibited a more rapid rate of decline in FAQ compared to those patients who remained stable. The finding that greater brain atrophy is associated with faster functional decline parallels prior reports of decline in cognitive function as a result of increased cerebral atrophy [12, 20]. Indeed, it is theoretically plausible that the effect of brain atrophy on functional decline is mediated by its effect on cognitive decline [14].
Possession of one or more copies of the APOE ε4 allele is a well-established risk factor for cognitive decline even among healthy older adults [38], and for the development of AD [19], though it has been shown to be neither necessary nor sufficient for the latter [39]. The precise mechanisms by which APOE ε4 impacts cognitive function or risk of AD is unclear. Possible explanations include its role in amyloid aggregation, fibrillization, and clearance [40], in neurofibrillary tangle formation [41], in regulation of the brain’s vasculature [42], in the metabolism of lipids [40], and in the modulation of other risk factors [43]. The association we found between APOE ε4 and rate of functional decline, as well as APOE ε4’s modulatory effect on the relationship between VBR and functional decline, is likely due to APOE ε4’s involvement in these biological processes. Even so, we note that prior attempts to link APOE ε4 to functional decline in the elderly have yielded inconsistent results, likely due to methodological variations [44].
The findings from this study have important clinical implications. With the ongoing race to develop disease-modifying drugs for AD, it has become critical to identify markers for monitoring disease progression and response to treatment [45]. Several studies have posited cerebral atrophy (whether of specific structures, of the whole brain, or simply as ventricular enlargement) as one such marker [28, 37, 46]. However, attention has been drawn to a need for these markers to be related not only to the underlying pathological process but, perhaps more importantly, to clinically meaningful outcomes [45]. The findings from this study suggest that VBR may be considered a bone fide marker because it is associated with functional abilities, in addition to cognitive performance [29–31]. Similarly, the ability to prevent, decelerate, or reverse limitations in complex daily activities could be a target outcome for MCI treatment studies [47].
This study demonstrated that cerebral atrophy and APOE ε4 status have prognostic value with respect to decline in everyday function; and that such functional degradation is more precipitous among patients who experience increased brain atrophy in the context of being APOE ε4 positive. Because progressive functional decline is a signature of AD, this study’s findings suggest that MCI patients who have increased brain atrophy and are APOE ε4 positive may be at elevated risk of progressing to AD. Indeed, if one considers an FAQ score of 12.8 (the mean FAQ score of the AD patients at baseline) the threshold for progression to AD, the trajectories displayed in Figure 3 suggests that APOE ε4 negative patients (whether with normal or elevated VBR) do not attain this threshold within the thirty six-month duration of the study; APOE ε4 positive patients with normal VBR attain this cutoff between 30 and 36 months; and APOE ε4 positive patients with elevated VBR reach this cutoff about one year earlier, between 18 and 24 months. This is potentially useful information for clinicians, patients, and their families with respect to long-term planning. In addition, identifying the class of MCI patients at increased risk for functional change may enable timely implementation of interventions to compress such changes, thereby improving quality of life for both patients and caregivers [4, 15, 16].
A possible limitation of this study is the use of an informant-report instrument in assessing everyday functioning. Although easy to obtain, report-based information is susceptible to biases such as those due to erroneous recall, social desirability, and the cognitive/psychological state of the reporter. In addition, FAQ’s relatively gross rating scale and generic nature may be considered limitations. Even so, as noted earlier, its ability to clearly distinguish the functional trajectories of control, MCI, and mild AD participants validates its use in MCI, especially in large-scale studies where the use of more elaborate functional measures may be logistically constrained. A common criticism of cerebral atrophy measures based on ventricular volume is that ventricular expansion could occur as a result of non-degenerative factors such as altered cerebrospinal fluid dynamics, chronic alcohol abuse, cardiovascular diseases, and treatment with diuretics [48]. These confounds were likely excluded via ADNI’s study entry criteria. Furthermore, though elevated VBR admittedly may be the result of ventricular expansion, brain tissue loss, or both, “in the ex vacuo state enlarged ventricular space” occurs only in proportion to dissolute brain parenchyma [31]. Thus, VBR veritably captures the extent of cerebral atrophy. Finally, the MCI patients in this study were of the amnestic variety. As such, it is not known whether our findings would replicate within non-amnestic MCI samples.
In summary, this study is the first to demonstrate that cerebral atrophy and APOE ε4 genotype have prognostic utility with regard to rate of functional decline among patients with MCI, and that their effects may be synergistic. Even so, it is important to highlight that a very recent study [49] found that the age of onset of sporadic AD was not necessarily a function of possession of ≥ 1 copies of the APOE ε4 allele. Rather, age of onset was influenced by possession of the longer forms of the polymorphic poly-T variant, rs10524523, in the translocase of the mitochondrial membrane 40 (TOMM40) homolog gene that is located in the same region of linkage disequilibrium as APOE. This finding was particularly robust among persons who were APOE ε3/4. It would be of great interest to determine whether TOMM rs10524523 poly-T length influences rate of prospective cognitive and functional decline in MCI to a greater extent than mere APOE carriership, especially among persons who are APOE ε3/4. Another avenue for future research is identifying VBR thresholds that have maximum power for predicting functional decline in MCI. Such thresholds may simultaneously serve in identifying MCI patients who are most likely to progress to AD, for enrollment in clinical trials. Finally, it would be informative to determine whether measures of regional atrophy (e.g., of medial temporal structures) are more sensitive to change in everyday function relative to the VBR and other measures of whole brain atrophy.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI; Principal Investigator: Michael Weiner; NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and through generous contributions from the following: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer’s Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the U.S. Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He W, Sengupta M, Velkoff VA, DeBarros KA. 65+ in the United States: 2005. Washington, DC: U.S. Government Printing Office; 2005. U.S. Census Bureau, Current Population Reports; pp. 23–209. [Google Scholar]
- 2.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 3.Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 4.Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, et al. The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology. 2008;22:531–44. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ. The cognitive correlates of functional status: A review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19:249–65. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 6.Moye J, Marson DC. Assessment of decision-making capacity in older adults: An emerging area of practice and research. J Gerontol B Psychol Sci Soc Sci. 2007;62:P3–P11. doi: 10.1093/geronb/62.1.p3. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Negash S. Mild cognitive impairment: An overview. CNS Spectrums. 2008;13:45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 9.Busse A, Angermeyer MC, Riedel-Heller SG. Progression of mild cognitive impairment to dementia: A challenge to current thinking. Br J Psychiatry. 2006;189:399–404. doi: 10.1192/bjp.bp.105.014779. [DOI] [PubMed] [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.APA. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: APA; 2000. text revision ed. [Google Scholar]
- 12.Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F, et al. Whole-brain atrophy rate and cognitive decline: Longitudinal MR study of memory clinic patients. Radiology. 2008;248:590–98. doi: 10.1148/radiol.2482070938. [DOI] [PubMed] [Google Scholar]
- 13.Buerger K, Ewers M, Andreasen N, Zinkowski R, Ishiguro K, Vanmechelen E, et al. Phosphorylated tau predicts rate of cognitive decline in MCI subjects: A comparative CSF study. Neurology. 2005;65:1502–03. doi: 10.1212/01.wnl.0000183284.92920.f2. [DOI] [PubMed] [Google Scholar]
- 14.Cahn-Weiner DA, Farias ST, Julian L, Harvey DJ, Kramer JH, Reed BR, et al. Cognitive and neuroimaging predictors of instrumental activities of daily living. J Int Neuropsychol Soc. 2007;13:747–57. doi: 10.1017/S1355617707070853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marson DC, Sawrie SM, Snyder S, McInturff B, Stalvey T, Boothe A, et al. Assessing financial capacity in patients with Alzheimer disease: A conceptual model and prototype instrument. Arch Neurol. 2000;27:877–84. doi: 10.1001/archneur.57.6.877. [DOI] [PubMed] [Google Scholar]
- 16.Desai AK, Grossberg GT, Sheth DN. Activities of daily living in patients with dementia: Clinical relevance, methods of assessment and effects of treatment. CNS Drugs. 2004;18:853–75. doi: 10.2165/00023210-200418130-00003. [DOI] [PubMed] [Google Scholar]
- 17.Peres K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: Impact on outcome. Neurology. 2006;67:461–66. doi: 10.1212/01.wnl.0000228228.70065.f1. [DOI] [PubMed] [Google Scholar]
- 18.Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, et al. Functional deficits in patients with mild cognitive impairment: Prediction of AD. Neurology. 2002;58:758–64. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Berry-Kravis E, Bennett DA. The apolipoprotein E epsilon4 allele and incident Alzheimer’s disease in persons with mild cognitive impairment. Neurocase. 2005;11:3–7. doi: 10.1080/13554790490903038. [DOI] [PubMed] [Google Scholar]
- 20.Spulber G, Niskanen E, Macdonald S, Smilovici O, Chen K, Reiman EM, et al. Whole brain atrophy rate predicts progression from MCI to Alzheimer’s disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.018. Epub. [DOI] [PubMed] [Google Scholar]
- 21.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jr, Jagust W, et al. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–29. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 24.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 25.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 26.Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, et al. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30:432–40. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 28.Bradley KM, Bydder GM, Budge MM, Hajnal JV, White SJ, Ripley BD, et al. Serial brain MRI at 3–6 month intervals as a surrogate marker for Alzheimer’s disease. Br J Radiol. 2002;75:506–13. doi: 10.1259/bjr.75.894.750506. [DOI] [PubMed] [Google Scholar]
- 29.Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Invest Radiol. 2001;36:539–46. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, et al. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007;21:14–24. doi: 10.1097/WAD.0b013e318032d2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bigler ED, Neeley ES, Miller MJ, Tate DF, Rice SA, Cleavinger H, et al. Cerebral volume loss, cognitive deficit and neuropsychological performance: Comparative measures of brain atrophy: I. Dementia J Int Neuropsychol Soc. 2004;10:442–52. doi: 10.1017/S1355617704103111. [DOI] [PubMed] [Google Scholar]
- 32.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer JD, Willett JB. Applied longitudinal data analysis. New York: Oxford University Press; 2003. [Google Scholar]
- 34.Knopman D, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practice parameter: Diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–53. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 35.Okonkwo OC, Griffith HR, Belue K, Lanza S, Zamrini E, Harrell LE, et al. Medical decision-making capacity in patients with mild cognitive impairment. Neurology. 2007;69:1528–35. doi: 10.1212/01.wnl.0000277639.90611.d9. [DOI] [PubMed] [Google Scholar]
- 36.Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer’s disease: What is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci. 1999;249 (Suppl 3):14–22. doi: 10.1007/pl00014168. [DOI] [PubMed] [Google Scholar]
- 37.Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, et al. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology. 2003;61:487–92. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- 38.Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, et al. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol. 2002;59:1154–60. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 39.Tanzi RE, Bertram L. New frontiers in Alzheimer’s disease genetics. Neuron. 2001;32:181–84. doi: 10.1016/s0896-6273(01)00476-7. [DOI] [PubMed] [Google Scholar]
- 40.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: The implications of systematic meta-analyses. Nature Reviews: Neuroscience. 2008;9:768–78. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 41.Nagy Z, Esiri MM, Jobst KA, Johnston C, Litchfield S, Sim E, et al. Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease. Neuroscience. 1995;69:757–61. doi: 10.1016/0306-4522(95)00331-c. [DOI] [PubMed] [Google Scholar]
- 42.Donahue JE, Johanson CE. Apolipoprotein E, amyloid-beta, and blood-brain barrier permeability in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:261–70. doi: 10.1097/NEN.0b013e31816a0dc8. [DOI] [PubMed] [Google Scholar]
- 43.Kivipelto M, Rovio S, Ngandu T, Kareholt I, Eskelinen M, Winblad B, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: A population-based study. Journal of Cellular and Molecular Medicine. 2008;12:2762–71. doi: 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melzer D, Dik MG, van Kamp GJ, Jonker C, Deeg DJ. The apolipoprotein E e4 polymorphism is strongly associated with poor mobility performance test results but not self-reported limitation in older people. J Gerontol A Biol Sci Med Sci. 2005;60:1319–23. doi: 10.1093/gerona/60.10.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaye JA. Methods for discerning disease-modifying effects in Alzheimer disease treatment trials. Arch Neurol. 2000;57:312–14. doi: 10.1001/archneur.57.3.312. [DOI] [PubMed] [Google Scholar]
- 46.Jack CR, Jr, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–31. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 (Suppl 2):S33–39. [PubMed] [Google Scholar]
- 48.Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC. Measuring atrophy in Alzheimer disease: A serial MRI study over 6 and 12 months. Neurology. 2005;65:119–24. doi: 10.1212/01.wnl.0000167542.89697.0f. [DOI] [PubMed] [Google Scholar]
- 49.Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics Journal. 2009 doi: 10.1038/tpj.2009.69. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]