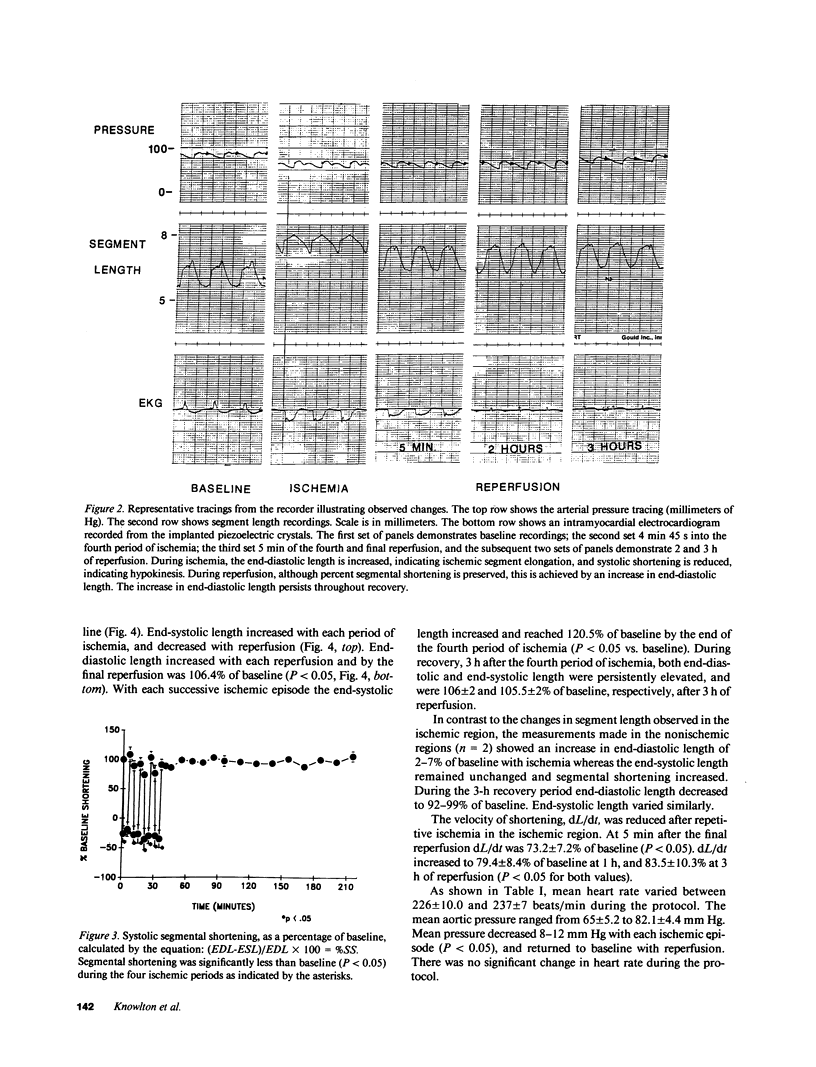
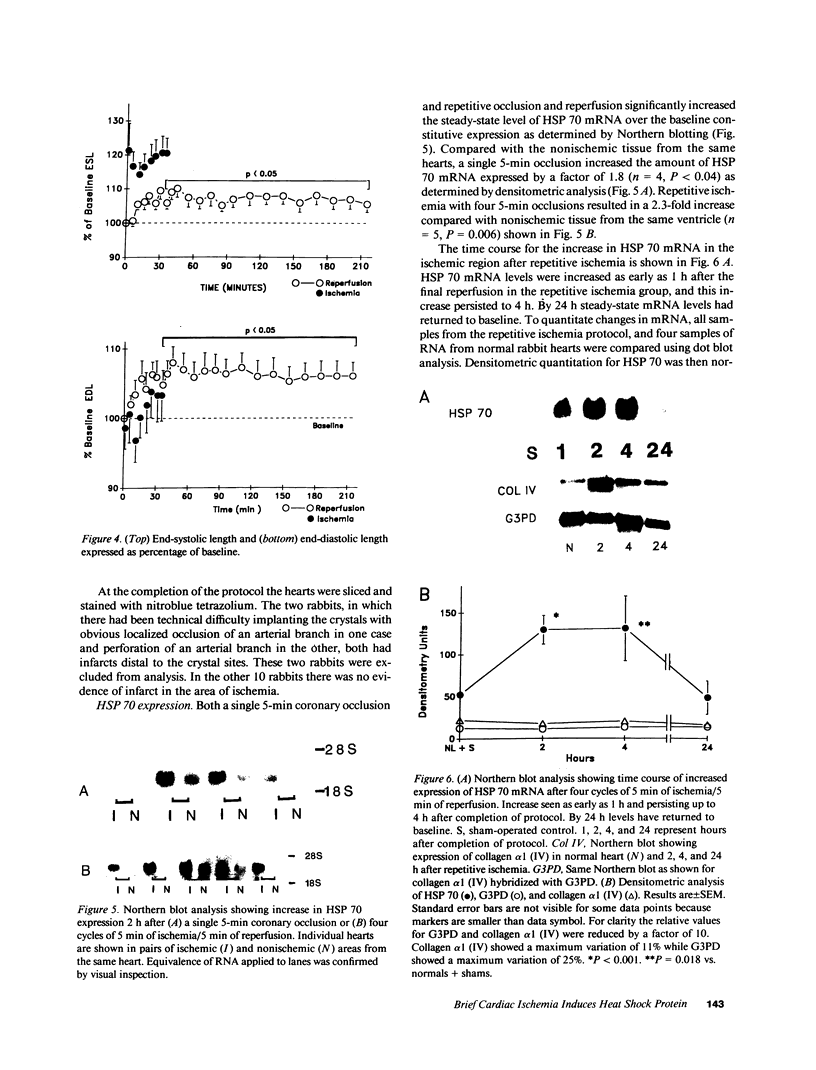
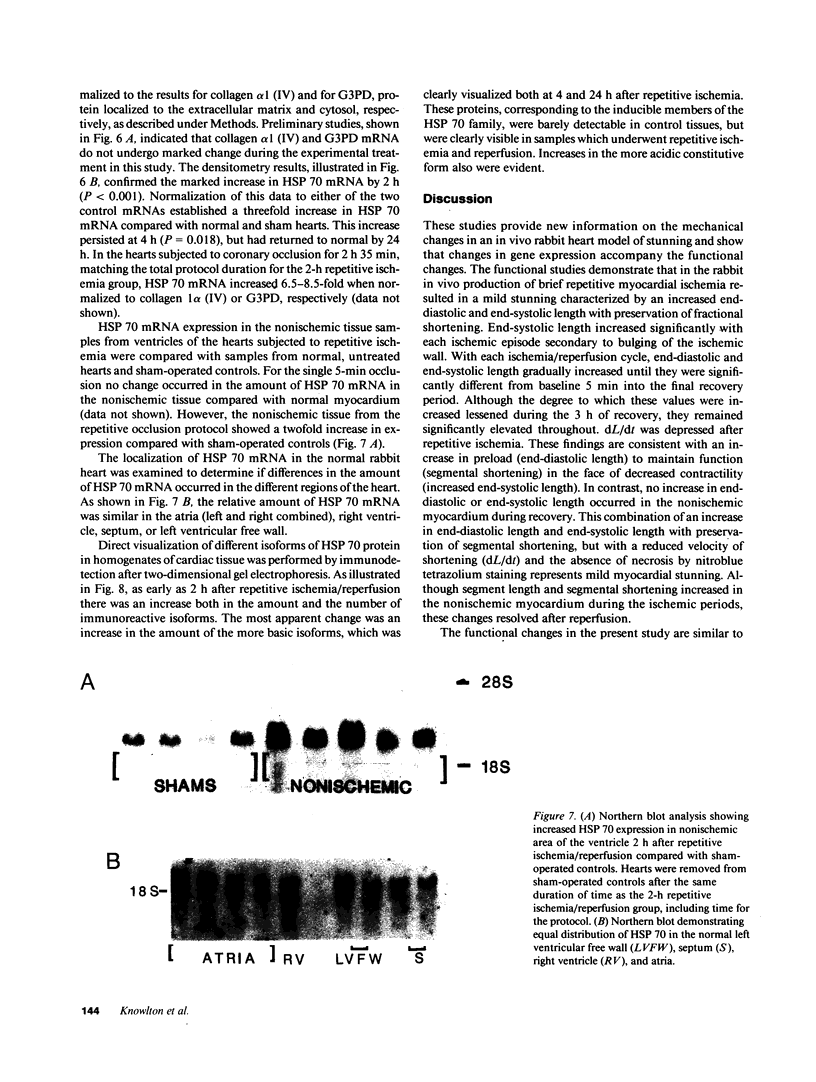
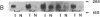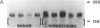Abstract
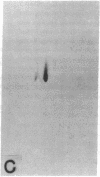
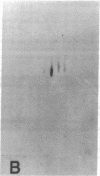
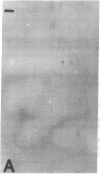
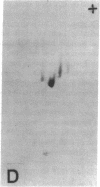
The effect of brief myocardial ischemia on the expression of heat shock protein (HSP 70) was examined in an in vivo rabbit model of myocardial ischemia using Northern blotting. Functional studies were carried out in the open-chested anesthetized rabbit. The large marginal branch of the left circumflex was occluded four times for 5 min. Using piezoelectric crystals implanted midwall in the ischemic zone, end-diastolic length, end-systolic length, and percent segmental shortening were assessed. Expression of HSP 70 was measured by Northern blotting. A single 5-min coronary occlusion doubled the expression of HSP 70 whereas four cycles of 5 min of ischemia/5 min of reperfusion resulted in a threefold increase in HSP 70 mRNA (P less than 0.001). Measurements with the piezoelectric crystals showed mild myocardial dysfunction concomitant with the increase in HSP 70. This increase in HSP 70 mRNA after repetitive brief ischemia was transient, occurring as early as 1 h and returning to baseline by 24 h after ischemia. Western blot analysis with a monoclonal antibody to HSP 70 was used to compare sham and postischemic myocardial HSP 70 levels. Changes in the amount of HSP 70 were evident as early as 2 h and were even more striking at 24 h.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolli R., Patel B. S., Hartley C. J., Thornby J. I., Jeroudi M. O., Roberts R. Nonuniform transmural recovery of contractile function in stunned myocardium. Am J Physiol. 1989 Aug;257(2 Pt 2):H375–H385. doi: 10.1152/ajpheart.1989.257.2.H375. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982 Dec;66(6):1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- Burdon R. H. Heat shock and the heat shock proteins. Biochem J. 1986 Dec 1;240(2):313–324. doi: 10.1042/bj2400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush L. R., Buja L. M., Samowitz W., Rude R. E., Wathen M., Tilton G. D., Willerson J. T. Recovery of left ventricular segmental function after long-term reperfusion following temporary coronary occlusion in conscious dogs. Comparison of 2- and 4-hour occlusions. Circ Res. 1983 Aug;53(2):248–263. doi: 10.1161/01.res.53.2.248. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Connelly C. M., Leppo J. A., Weitzman P. W., Vogel W. M., Apstein C. S. Effect of coronary occlusion and reperfusion on myocardial blood flow during infarct healing. Am J Physiol. 1989 Aug;257(2 Pt 2):H365–H374. doi: 10.1152/ajpheart.1989.257.2.H365. [DOI] [PubMed] [Google Scholar]
- Connelly C., Vogel W. M., Hernandez Y. M., Apstein C. S. Movement of necrotic wavefront after coronary artery occlusion in rabbit. Am J Physiol. 1982 Nov;243(5):H682–H690. doi: 10.1152/ajpheart.1982.243.5.H682. [DOI] [PubMed] [Google Scholar]
- Currie R. W. Effects of ischemia and perfusion temperature on the synthesis of stress-induced (heat shock) proteins in isolated and perfused rat hearts. J Mol Cell Cardiol. 1987 Aug;19(8):795–808. doi: 10.1016/s0022-2828(87)80390-5. [DOI] [PubMed] [Google Scholar]
- Currie R. W., Karmazyn M., Kloc M., Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988 Sep;63(3):543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- DeBoer L. W., Ingwall J. S., Kloner R. A., Braunwald E. Prolonged derangements of canine myocardial purine metabolism after a brief coronary artery occlusion not associated with anatomic evidence of necrosis. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5471–5475. doi: 10.1073/pnas.77.9.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcayre C., Samuel J. L., Marotte F., Best-Belpomme M., Mercadier J. J., Rappaport L. Synthesis of stress proteins in rat cardiac myocytes 2-4 days after imposition of hemodynamic overload. J Clin Invest. 1988 Aug;82(2):460–468. doi: 10.1172/JCI113619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann W. H., Mehta H. B., Barrieux A., Guth B. D., Neeley W. E., Ross J., Jr Ischemia of the dog heart induces the appearance of a cardiac mRNA coding for a protein with migration characteristics similar to heat-shock/stress protein 71. Circ Res. 1986 Jul;59(1):110–114. doi: 10.1161/01.res.59.1.110. [DOI] [PubMed] [Google Scholar]
- Edington B. V., Whelan S. A., Hightower L. E. Inhibition of heat shock (stress) protein induction by deuterium oxide and glycerol: additional support for the abnormal protein hypothesis of induction. J Cell Physiol. 1989 May;139(2):219–228. doi: 10.1002/jcp.1041390202. [DOI] [PubMed] [Google Scholar]
- Ellis S. G., Wynne J., Braunwald E., Henschke C. I., Sandor T., Kloner R. A. Response of reperfusion-salvaged, stunned myocardium to inotropic stimulation. Am Heart J. 1984 Jan;107(1):13–19. doi: 10.1016/0002-8703(84)90126-1. [DOI] [PubMed] [Google Scholar]
- Foster E., DeJong D., Connelly C., Apstein C. S. Failure of nifedipine and reperfusion to reduce infarct size relative to region at risk as measured by NADH fluorophotography. Circulation. 1984 Sep;70(3):506–512. doi: 10.1161/01.cir.70.3.506. [DOI] [PubMed] [Google Scholar]
- Gallagher K. P., Stirling M. C., Choy M., Szpunar C. A., Gerren R. A., Botham M. J., Lemmer J. H. Dissociation between epicardial and transmural function during acute myocardial ischemia. Circulation. 1985 Jun;71(6):1279–1291. doi: 10.1161/01.cir.71.6.1279. [DOI] [PubMed] [Google Scholar]
- Heyndrickx G. R., Baig H., Nellens P., Leusen I., Fishbein M. C., Vatner S. F. Depression of regional blood flow and wall thickening after brief coronary occlusions. Am J Physiol. 1978 Jun;234(6):H653–H659. doi: 10.1152/ajpheart.1978.234.6.H653. [DOI] [PubMed] [Google Scholar]
- Heyndrickx G. R., Millard R. W., McRitchie R. J., Maroko P. R., Vatner S. F. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975 Oct;56(4):978–985. doi: 10.1172/JCI108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A. A., Burrier R. E., Brecher P. Rabbit heart fatty acid-binding protein. Isolation, characterization, and application of a monoclonal antibody. Circ Res. 1989 Oct;65(4):981–988. doi: 10.1161/01.res.65.4.981. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Condon M. R., Blumberg B., Barlow D. P., Quinones S., Saus J., Pihlajaniemi T. Extensive homology between the carboxyl-terminal peptides of mouse alpha 1(IV) and alpha 2(IV) collagen. J Biol Chem. 1987 Jun 25;262(18):8496–8499. [PubMed] [Google Scholar]
- Kusuoka H., Porterfield J. K., Weisman H. F., Weisfeldt M. L., Marban E. Pathophysiology and pathogenesis of stunned myocardium. Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest. 1987 Mar;79(3):950–961. doi: 10.1172/JCI112906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R., Ware J., Kloner R. A. Absence of a cumulative deterioration of regional function during three repeated 5 or 15 minute coronary occlusions. Circulation. 1984 Feb;69(2):400–408. doi: 10.1161/01.cir.69.2.400. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Translational efficiency of heat-induced messages in Drosophila melanogaster cells. J Mol Biol. 1980 Feb 25;137(2):151–158. doi: 10.1016/0022-2836(80)90322-8. [DOI] [PubMed] [Google Scholar]
- Mehta H. B., Popovich B. K., Dillmann W. H. Ischemia induces changes in the level of mRNAs coding for stress protein 71 and creatine kinase M. Circ Res. 1988 Sep;63(3):512–517. doi: 10.1161/01.res.63.3.512. [DOI] [PubMed] [Google Scholar]
- Milarski K. L., Welch W. J., Morimoto R. I. Cell cycle-dependent association of HSP70 with specific cellular proteins. J Cell Biol. 1989 Feb;108(2):413–423. doi: 10.1083/jcb.108.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C. E., Jennings R. B., Reimer K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986 Nov;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Hill M. L., Jennings R. B. Prolonged depletion of ATP and of the adenine nucleotide pool due to delayed resynthesis of adenine nucleotides following reversible myocardial ischemic injury in dogs. J Mol Cell Cardiol. 1981 Feb;13(2):229–239. doi: 10.1016/0022-2828(81)90219-4. [DOI] [PubMed] [Google Scholar]
- Sabbah H. N., Marzilli M., Stein P. D. The relative role of subendocardium and subepicardium in left ventricular mechanics. Am J Physiol. 1981 Jun;240(6):H920–H926. doi: 10.1152/ajpheart.1981.240.6.H920. [DOI] [PubMed] [Google Scholar]
- Sarzani R., Claffey K. P., Chobanian A. V., Brecher P. Hypertension induces tissue-specific gene suppression of a fatty acid binding protein in rat aorta. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7777–7781. doi: 10.1073/pnas.85.20.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder E., Kieso R. A., Laughlin D., Schröder M., Meng R., Kerber R. E. Altered response of reperfused myocardium to repeated coronary occlusion in dogs. J Am Coll Cardiol. 1987 Oct;10(4):898–905. doi: 10.1016/s0735-1097(87)80286-3. [DOI] [PubMed] [Google Scholar]
- Schulz R., Miyazaki S., Miller M., Thaulow E., Heusch G., Ross J., Jr, Guth B. D. Consequences of regional inotropic stimulation of ischemic myocardium on regional myocardial blood flow and function in anesthetized swine. Circ Res. 1989 Jun;64(6):1116–1126. doi: 10.1161/01.res.64.6.1116. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H., Roberts A. F., Raine A. E. Energy metabolism in reperfused heart muscle: metabolic correlates to return of function. J Am Coll Cardiol. 1985 Oct;6(4):864–870. doi: 10.1016/s0735-1097(85)80496-4. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. M., Astein C. S., Arthur J. H., Pirzada F. A., Hood W. B., Jr Persistence of myocardial injury following brief periods of coronary occlusion. Cardiovasc Res. 1976 Nov;10(6):678–686. doi: 10.1093/cvr/10.6.678. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984 Apr 10;259(7):4501–4513. [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J. The mammalian heat shock (or stress) response: a cellular defense mechanism. Adv Exp Med Biol. 1987;225:287–304. doi: 10.1007/978-1-4684-5442-0_26. [DOI] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]