Abstract
Just over a century ago Paul Ehrlich received the Nobel Prize for his studies of immunity. This review describes one of his legacies, the histochemical description of the mast cell, and the research that has ensued since then. After a long period of largely descriptive studies, which revealed little about the biological role of the mast cell, the field was galvanized in the 1950s by the recognition that the mast cell was the main repository of histamine and a key participant in anaphylactic reactions. Although the mast cell was long-viewed in these terms, recent research has now shown that the mast cell also plays a key role in innate and adaptive immune responses, autoimmune disease, and possibly tissue homeostasis by virtue of its expression of a diverse array of receptors and biologically active products. In addition, the responsiveness of mast cells to immunological and pathological stimulants is highly modulated by the tissue cytokine environment and by synergistic, or inhibitory, interactions among the various mast cell receptor systems. This once enigmatic cell of Paul Ehrlich has proved to be both adaptable and multifunctional.
Keywords: Adaptive immunity, Autoimmune diseases, Innate immunity, Mast cell, Paul Ehrlich
Introduction
The past year was the 130th anniversary of Paul Ehrlich's presentation of his doctoral thesis at the Medical Faculty of Leipzig University [1] and the 100th anniversary of his Nobel Prize in “physiology or medicine” which he shared with Elie Metchnikoff. The work and achievements of Elie Mitchnikoff were described in the December issue of the European Journal of Immunology [2, 3]. This review is dedicated to Paul Ehrlich. Among Ehrlich's accomplishments were his descriptions of the tissue mast cell and, sometime later, the blood basophil. Subsequent work by his students and others verified the soundness of Ehrlich's studies but the biological function of the mast cell remained an enigma throughout a period when advances were made in immunity, to which Ehrlich contributed much, and physiological transmitters such as histamine. These advances set the context for studies in the 1950s that culminated in the discoveries that the mast cell was the major repository for histamine and participated in inflammatory allergic diseases.
Nevertheless, enigmas remained. Histamine release failed to account for all the symptoms of mast cell activation. Also, the perceived role of mast cells in allergic conditions such as hay fever, asthma, and anaphylactic shock begged the question as to why evolution had endowed mammalian species with a lethal abundance of mast cells in tissues without obvious benefits to the host. These enigmas have been addressed in part by the realization that mast cells play a significant role in both innate and adaptive immunities. The downside is that the mast cell is also responsible, perhaps aberrantly so, for certain autoimmune diseases as well as for allergic phenomena. This review discusses these and other aspects of mast cell function. These include the phenotypic adaptability of the mast cell to its anatomical and pathological environment and the fact that the mast cell is provisioned with multiple types of receptors as well as mediators. Both characteristics now provide a reasonable explanation for the multifunctional role of this cell.
Paul Ehrlich's description of the mast cell and basophil
In his thesis, Ehrlich discussed the chemical and histological properties of the basic aniline dyes and then devoted one chapter to the histological application of these dyes in human tissues. He described aniline-positive cells in connective tissues, which he named “Mastzellen” in the belief that they had a nutritional function because of their granules. The German word “mast” denotes a “fattening” or “suckling” function. He also noted that, although mast cells were localized around blood vessels in connective tissues, they were not part of the perivascular system and could be distinguished from a heterogenous group of cells referred to as “Plasmazellen” by Waldemeyer. In particular, the granules of mast cells contained an undefined substance that reacted metachromatically with aniline dyes. He emphasized that identification should be based on the metachromatic reactivity of mast cells and not solely on their morphological appearance, a novel concept at that time. His first published hand drawings of mast cells [4] are illustrated in Fig. 1.
Figure 1.
Paul Ehrlich and his sketch of the distribution of mast cells in various tissues in Fig. 1 in [4]. This publication is available on the Paul-Ehrlich-Institut website for Paul Ehrlich.
Ehrlich applied his staining techniques to blood cells and laid the foundations of hematology by describing the affinity of various leukocytes for specific dyes [5, 6]. He recognized cells with basophilic granules that stained metachromatically and surmised that these blood “mast cells”, like their blood counterparts the eosinophil and neutrophils, were derived from precursor cells in the BM. In contrast, the tissue mast cell was derived from and resided in connective tissue. As will be noted later, these conclusions were partially, but not totally, correct. Apart from his observations that mast cells are abundant in states of chronic inflammation and in tumors, which he ascribed to the nutritional requirements of these tissues, the function of mast cells eluded him as it did others who followed him. An excellent account of Ehrlich's early studies on mast cells can be found in [7].
Subsequent studies by other workers were largely histological and reaffirmed the soundness of Ehrlich's work. In the meantime, seemingly separate lines of research on the allergic response and histamine were being pursued, whose connections to the mast cell (and basophil) became evident 60 years later.
The allergic response and the identification of IgE as the reaginic factor
Although the symptoms of allergic reactions were recognized long before, the scientific era began in 1869 when Charles Blakely in England performed the first skin test on himself to test the idea that his hay fever was caused by pollen. He noted that pollen did indeed cause an urticarial reaction. Portier and Richet introduced the term anaphylaxis in 1902 to describe the symptoms of anaphylactic shock produced by administration of extracts of Physalia tentacles to dogs that had survived an initial injection several weeks earlier [8]. Prince Albert of Monaco had suggested that Richet examine the toxic principles of Physalia while both were cruising on the Prince's yacht in 1901 (Fig. 2). For this and his subsequent studies, Richet received the Nobel Prize five years after Ehrlich. Pirquet in Vienna had, independently, coined the term “allergy” in 1906 after noting that some patients had more severe reactions to a second dose of horse serum antitoxin or smallpox vaccine, which he attributed to the formation of a pathogen-interacting “antibody” [9].
Figure 2.
The postage stamp issued by the Principality of Monaco commemorating the discovery of anaphylaxis. The stamp illustrates the circumstances that resulted in the research of Richet and Portier on anaphylaxis for which Richet received the Nobel prize. Details are described in the text (photograph of a stamp in the author's possession).
Concepts were further refined in 1921 when Prausnitz passively sensitized himself by intracutaneous injection of serum from his allergic patient, Küstner, and referred to the transferrable factor as reagin or reaginic antibody [10]. Later, Landsteiner defined the roles of antibodies (agglutinins) and antigen (haptens) and demonstrated that polyvalent but not monovalent hapten could elicit allergic reactions [11]. Ishizaka and Campbell re-examined this phenomenon much later and concluded that aggregation of two or more antibodies in a single antigen–antibody complex on the cell surface was sufficient to initiate passive cutaneous anaphylaxis [12]. It was not until 1967 that the reaginic antibody was finally identified by Ishizaka and Ishizaka as γE-antibodies (IgE), a minor component (0.005%) of the Ig pool [13, 14].
H substance and histamine
In 1910, Dale and colleagues began a series of studies on histamine that extended over a period of 20 years [15]. They noted that the pharmacological actions of histamine were reminiscent of the effects of tissue extracts and resembled the symptoms of anaphylactic shock. By 1927 they had successfully purified and identified histamine from several organs. Meanwhile, Lewis and his colleagues demonstrated that a substance, cautiously called “H substance”, with properties identical to histamine was released from skin during injury and antigen challenge (see [16] for specific citations). The isolation of histamine from skin by Harris [17] in amounts that could account for the wheal-and-flare reaction left little doubt that “H substance” was histamine or that histamine was involved in reactions to injury or anaphylactic phenomena. When Dale summarized the current state of knowledge in 1929 [15] he stated that “We have no justification for assuming that histamine is the only constituent which injury liberates...”, a prophesy fulfilled with the later discoveries of the eicosanoids and cytokines.
Research paths coalesce and link mast cells to histamine and anaphylaxis
The prelude was the discovery that heparin, initially isolated from dog liver, which contains an abundance of mast cells, was the metachromatic factor in mast cells. In 1937, almost 60 years after Ehrlich's description of the mast cell, Holmgren and Wilander reported that tissues with large numbers of “Ehrlichschen Mastzellen” were particularly rich in heparin [18]. Ten years later, it was shown that both histamine and heparin were released from dog liver during anaphylactic shock [19].
The presence of histamine in mast cells was rigorously established in a notable series of studies by Riley and West in the period 1952–1956 (for a complete account see [20]). They first examined pathological lesions known to be rich in mast cells such as urticaria pigmentosa and noted a correlation between histamine content and mast cell count [20, 21]. Solid mastocytomas in dog were found to be as enriched in histamine as they were in heparin [22, 23]. Similar correlations were observed in normal tissues with widely different mast cell populations [21, 24]. These results indicated that the mast cell was the predominant source of histamine in many tissues.
The connection to anaphylaxis was established following reports that certain organic bases (“histamine liberators”) elicited wheal-and-flare reactions when administered locally or anaphylactic shock when administered systemically. Stilbamidine, in particular, was fluorescent and Riley took advantage of this property to identify its site of deposition as the mast cell [25]. A polymeric compound known as compound 48/80 induced degranulation of mast cells [26] and became the mainstay for subsequent studies. Fawcett [27] and Riley and West [28], for example, found good correlations between serum hista-mine and disruption of mast cells with graded doses of compound 48/80 in vivo. Shortly thereafter, Mota and Vugman reported similar correlations in a guinea pig model of anaphylaxis [29].
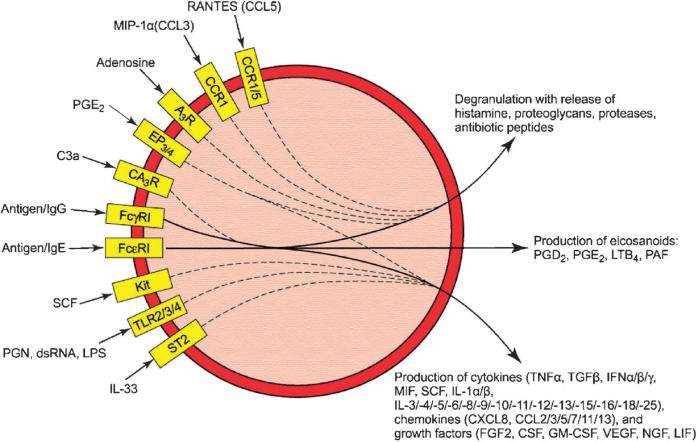
The recognition that anaphylaxis was linked, at least in part, to histamine release from mast cells raised new questions. As Dale had noted, it seemed likely that other inflammatory factors participated in anaphylaxis, one being a mysterious “slow-reacting substance of anaphylaxis” (SRS-A). In fact, mast cells produce a surprisingly large array of biologically active products (Fig. 3). Some, such as histamine and various neutral proteases, are stored in granules. Others, such as the eicosanoids and cytokines, are generated de novo after stimulation. The diversity of these products allows the mast cells to participate in a wide variety of biological functions (Fig. 4).
Figure 3.
Examples of synergistic interactions among mast cell receptors. The figure depicts well-documented examples of receptor ligands that augment responses to antigen either through FcεRI or, in the case of C3a, through FcγRI. Some of these ligands enhance primarily degranulation while others enhance primarily cytokine production. Yellow boxes denote receptor subtypes involved, solid lines denote the responses to antigen via FcεRI or FcγRI, and dashed lines indicate the “feed in” to these responses. The effects on eicosanoid production have not been systematically examined. See text for details and abbreviations.
Figure 4.
Mast cell-mediated pathways that lead to innate and adaptive immune responses to pathogenic stimulants. The stimuli could be IgE- or IgG-directed antigens, complement components, TLR ligands, alone or in combination. The innate immune responses include inflammatory responses to histamine and eicosanoids and antimicrobial effects through release of proteases and antibiotic peptides from granules as well as recruitment of leukocytes and macrophages by eicosanoids and chemokines. Adaptive immune responses include cytokine/chemokine-mediated recruitment of naïve T cells and APC to regional lymph nodes for antigen presentation or direct activation of T cells by TNF-α and a costimulatory ligand called OX40L.
Other mast cell products: the eicosanoids, mast cell proteases, proteoglycans, cytokines, chemokines, and antibiotic peptides
The eicosanoids
As in other types of cells, stimulated mast cells rapidly synthesize inflammatory lipids (collectively known as eicosanoids) from arachidonic acid that is produced from membrane phospholipids after activation of phospholipase A2. These include the leukotrienes (LT), the prostaglandins (PG), and platelet-activating factor. All of these lipids increase microvascular permeability and produce long-lasting wheal-and-flare responses in skin and bronchoconstriction in lung [30].
Research on the eicosanoids began with the discovery of a spasmogenic slow-reacting substance (i.e. SRS-A), distinct from histamine, by Feldberg and Kellaway in 1938 [31]. SRS-A was subsequently shown by Brocklehurst in 1960 to be generated in lung tissue upon anaphylactic stimulation [32] and later by Austen and colleagues in lung mast cells in an IgE-dependent manner[33, 34]. The exact nature of SRS-A remained elusive until 1979 when Samuelsson and coworkers elucidated the structure of LTC4 as a glutathionyl adduct of arachidonic acid [35]. It then became apparent that SRS-A was generated from arachidonic acid through the 5′-lipoxygenase pathway and consisted of three related cysteinyl-LT, LTC4, LTD4, and LTB4 (reviewed in [36]). Contrary to the situation in vivo, primary cultures of human mast cells (HMC) produce little cysteinyl-LT in response to antigen unless first primed with IL-4 or the combination of SCF and IL-3 or IL-5 (reviewed in [36]). That mast cell responses can be so modified is a theme that we will return to frequently.
The production of PG in mast cells was first reported by Austen and colleagues in 1979 when they noted production of high amounts of PGD2, along with smaller amounts of other PG, in ionophore-stimulated rat mast cells [37] and later in anti-IgE-stimulated rat and HMC [38]. Subsequent studies in many laboratories have confirmed that mast cells can produce substantial amounts of PGD2, which is detectable within minutes of stimulation.
Mast cell proteases and proteoglycans
By 1960 mast cells were known to contain an enzymatically detectable chymotrypsin-like [39] or a trypsin-like esterase activity [40]. These enzymes were purified in the 1980s and renamed tryptase [41] and chymase [42]. A metalloexopeptidase, carboxypeptidase A, was also purified from lung and skin mast cells [43]. Since 1989, all three enzymes as well as numerous variants of tryptase and chymase have been cloned from various sources to reveal different profiles of expression of these enzymes among mast cells from different tissues and species [44].
The heterogeneous expression of the mast cell proteases (designated as MCP) is best exemplified in mouse. The major mast cell proteases in mouse are the chymases (mMCP-1, -2, -4, -5, and -9), tryptases (mMCP-6, -7, -11, and mTMT, a transmembrane tryptase), and carboxypeptidase (mMC-CPA). Mast cells in mouse airway and intestinal mucosa express mMCP-1 and mMCP-2, whereas those in skin and peritoneum express mMCP-4, -5, and -6 as well as mMCCPA. These two subsets of mast cells are referred to as mucosal and serosal (or connective tissue) mast cells, respectively. The mast cell proteases in humans show evolutionary divergence from their homologs in mouse and other rodents [44]. As in mouse, the human proteases are differentially expressed in mast cell subtypes. Human tryptase is present in mast cells in mucosal tissues, whereas mast cells in skin and intestinal submucosa contain tryptase, chymase, and carboxypeptidase. These subtypes are designated MCT and MCTC accordingly.
The mast cell proteases are the major protein constituent in granules and much is now known about their structure, genetic relationships, and enzymatic properties [30, 45–48]. Although the biologically relevant substrates are not always obvious, mast cells confer innate protection against snake and insect venoms [49], parasite infestation [50, 51], and bacterial peritonitis [52] through proteolytic degradation of exogenous or endogenous toxic peptides (Fig. 4). The proteases also contribute to the inflammation, matrix destruction, and tissue remodeling in allergic diseases [46].
The proteases form inactive complexes with proteoglycans within mast cell granules. Heparin was the first proteoglycan to be rigorously identified as a highly sulfated glycosaminoglycan (reviewed in [45]). However, the different staining characteristics of skin mast cells compared with mucosal mast cells or cultured BM-derived mast cells (BMMC) [53, 54] suggested that the latter cells contained a less sulfated glycosaminoglycan that was subsequently identified as chondroitin sulfate E [55]. It transpired later that both types of glycosaminoglycans contain a peptide core (hence the term proteoglycan), called serglycin, with a segment consisting of alternating serine and glycine residues (see [45] for details). Seven to ten heparin or chondroitin chains are attached to serine residues of each serglycin core to form macromolecules that regulate expression and packaging of distinct sets of proteases [45]. This macro-molecular arrangement leaves the mast cell proteoglycans uniquely resistant to extracellular proteases, which may help stabilize the proteoglycan/protease complex once it is secreted from mast cells [56].
Cytokines, chemokines, and growth factors
In 1989 Plaut [57] and Wodnar-Filipowicz [58], along with their coworkers, reported that stimulated mast cells produced and released IL-3, IL-4, IL-5, IL-6, and GM-CSF. Shortly thereafter, Gordon and Galli in 1990 reported that mast cells were a biologically relevant source of both preformed and antigen-inducible TNF-α [59]. The list of antigen-inducible factors in rodent mast cell lines has much expanded since then to include inflammatory and immunomodulatory cytokines, chemokines, and growth factors (as listed in Fig. 3). The same is true for HMC [60], which also contain preformed SCF that, upon release, may then act locally as a mast cell growth factor [61]. The biological relevance of many of these factors is unclear but it is apparent that the pattern of cytokine gene expression varies according to the type of stimulus and conditioning of the mast cell by external cytokines [30].
Antimicrobial peptides
Mast cell granules in fish [62], mouse, and humans [63] also contain antimicrobial peptides that accumulate at sites of epithelial injury and infection [64]. The antimicrobial peptides in fish (piscidin) and mammals (cathelicidin) are small membrane-interacting amphipathic a-helical peptides. Their microbial lytic activity spans a broad spectrum of pathogens including bacteria, enveloped viruses, fungi, and protozoa. Cathelicidin can also act as a chemoattractant for neutrophils, another source of cathelicidin. Reconstitution studies with mast-cell-deficient mice and mast cells derived from these and cathelicidin-deficient mice indeed suggest that mast cells protect against skin infection, directly or indirectly through recruitment of neutrophils [64].
Mast cell lineage and heterogeneity
Mast cells and basophils are derived from pluripotent hematopoietic stem cells but their developmental pathways appear to be different [65] although some issues remain unresolved [66]. It is believed that HMC arise from CD34+ stem cells in BM, circulate in the blood as progenitors, and then acquire their mature phenotype within tissues, processes regulated by SCF, IL-3, IL-4, IL-9, nerve growth factor, and probably other factors [60]. SCF, a mast cell growth factor, is produced mainly by stromal cells where it expressed on the cell surface or released in soluble form [67]. Kit (CD117), the receptor for SCF, is expressed on hematopoietic stem cells and is retained on mast cells throughout their development and differentiation but is down-regulated during differentiation of other BM-derived cells including basophils. In contrast to mast cells, basophils reach their mature state in BM before release into blood.
The observations of Enerbäck in 1966 of two histochemically distinct populations of mast cells [53, 68, 69] and in 1974 that one of these subsets, the mucosal mast cell, was resistant to compound 48/80 [70] led to the concept of mast cell “heterogeneity”. This concept that was reinforced by the differences in expression of proteoglycans and proteases in serosal and mucosal mast cells [71, 72]. Nevertheless, these two subsets exhibit “plasticity”. When BMMC from normal mice were transferred to mast-cell-deficient mice, the cells retained their mucosal cell phenotype at tissue sites that normally bear this phenotype while adopting a serosal mast cell phenotype at sites that contain such cells [73]. A similar change in phenotype was noted on co-culture of BMMC with fibroblasts [74]. IL-3 and SCF were found to be two factors driving mast cell differentiation to, respectively, either the mucosal (i.e. chondroitin sulfate E positive) [55] or serosal (i.e. heparin positive) [75] phenotypes.
Subsequent studies have validated the idea that mast cells are conditioned by their cytokine environment under normal or pathological situations [76]. The “heterogeneity” and “plasticity” of mast cells undoubtedly contribute to their multifunctional capability in immunological and pathological processes.
IgE, its high affinity receptor (FcεRI), and studies of FcεRI-mediated responses in cell cultures
IgE is produced following presentation of antigen by APC to Th2 cells at local lymph nodes. Release of IL-4 and IL-13 from Th2 cells induces B cells to switch production of IgM to antigen-specific IgE by a complex process that involves additional stringent levels of regulation [77]. IgE may sensitize mast cells to antigen locally [78], thus minimizing systemic reactions, and promote survival of the mast cells [79]. As noted earlier, circulating levels of IgE are normally very low and increases are diagnostic of severe allergic disease.
The identification of the putative receptors for IgE was aided by the discovery of IgE-producing immunocytomas from rats [80] and the successful culture of a rat basophilic leukemia (RBL) tumor mast cell line in 1974 [81]. The subsequent development of monoclonal antibody technology allowed the preparation of mouse IgE of defined antigen specificity [82]. The original RBL cell line bound IgE but was unresponsive to antigen. Under curious circumstances, secreting RBL cell lines arose spontaneously [83] including the now widely used RBL-2H3 cell line [84]. With these reagents at hand, Metzger and his colleagues began characterizing the IgE receptor. The cardinal features of this receptor (reviewed in [85]) were that it was expressed in abundant numbers (3–6 × 106 receptors/cell) in both normal and RBL-2H3 mast cells and bound IgE with high affinity (KD 10−11) and specificity. Aggregation of just two or three receptors with chemically cross-linked dimers and higher oligomers of IgE triggered degranulation. The purified receptor consisted of four subunits, the IgE-binding subunit α, β, and two disulfide-linked γ-subunits. These studies culminated in the cloning of the individual FcεRI subunits and expression of the full receptor in 1989 [86]. Studies in several laboratories have since revealed that rodent FcεRI is invariably expressed as an αβγ2 tetrameric structure, whereas human FcεRI is expressed as either the tetramer (αβγ2) or a trimer (αγ2) [87]. Studies with transgenic mice suggest that the human β-subunit functions as an amplifier of FcεRI surface expression and signaling and may thus enhance allergic reactions in vivo (reviewed in [88]). In contrast, an alternatively spliced truncated β-isoform (βT) inhibits FcεRI expression [89]. For this reason, it is suggested that FcεRIβ gene could serve as a regulator of allergic responses. Also, polymorphisms within the FcεRIβ gene are associated with various atopic diseases in humans although none have been directly linked to the allergic phenotype [90].
The α-subunit is unique to FcεRI. However, the β and γ subunits are shared with other Fc receptors; the β-subunit with the low-affinity IgG receptor, FcγRIII, and the γ-subunit with both FcγRIII and the high-affinity IgG receptor, FcγRI [87]. Although FcεRI is expressed predominantly in mast cells, it is also expressed, albeit at substantially lower numbers, in blood leukocytes, dendritic cells, and Langerhans cells.
FcεRI-mediated signaling processes have been examined in most detail in rodent mast cell lines. RBL-2H3 cells were widely used for this line of research once it was established that antigen-induced degranulation of these cells was associated with increases in cytosolic calcium, phosphoinositide hydrolysis, and activation of protein kinase C [91–93]. However, RBL-2H3 cells express constitutively active Kit, which limits their usefulness because background Kit-mediated signals enhance mast cell signaling and function (see the following section). The development of protocols for generation of primary cultures of mast cells from mouse BM (BMMC) and other tissue sources [94, 95] circumvented this and other problems associated with transformed cells. Research on HMC was handicapped by the lack of reactive human cell lines until the advent of human LAD tumor mast cell lines [96] and protocols for generation of mature HMC from CD34+ peripheral blood cells [97]. Much has been learnt about signaling processes and mediator release with these cell lines and, regardless of the phenotypic and species differences, all appear to engage the same basic signaling components when activated via FcεRI (reviewed in [98, 99]). Nevertheless, such studies reveal signaling processes that pertain to FcεRI alone. FcεRI-mediated signals and mediator release are substantially influenced by the tissue environment in which the mast cell resides. The same is true for other mast cell receptors and responses. Therefore, there are inherent problems in extrapolating data from cell culture to the situation in vivo, which may vary from one tissue or species to another and during pathological changes in tissues.
Kit (CD117) and use of Kit/Kit ligand-defective mice for studies of mast cell function in vivo
Kit, a tyrosine kinase receptor, is essential not only for homing and differentiation of mast cells in tissues but also for optimal FcεRI-mediated signaling and physiologic responses [99] and, in this respect, is a potential target for therapy [100, 101]. The importance of Kit is most apparent from the absence of mast cells in mice with a deletion or inactivating mutation of Kit (W/Wv and Wsh/Wsh) or a Kit ligand mutation (Sl/Sld). These mouse strains have proved valuable in unmasking the biological roles of mast cells in vivo, with certain caveats [66], as will become evident later in this review. Activating mutations of Kit are associated with adult onset mastocytosis in humans and mastocytomas in dog [101, 102]. One such mutation (D816V) is found in the corresponding codon of c-kit in the RBL-2H3, P815, and HMC-1 neoplastic mast cell lines.
TLR and innate immunity
Until the mid 1990s, mast cells were viewed primarily as mediators of allergic reactions and as combatants of parasite infestation [103]. In 1996, mast cells were shown to protect mice from acute bacterial infection [104, 105]. The discovery that the mammalian homologs of Drosophilia TLR are pathogen-recognizing receptors and are expressed on mast cells indicated that mast cells could directly participate in host defense. The primary response to TLR ligands is the production of inflammatory cytokines rather than degranulation. The initial reports from 2001 to 2003 focused on TLR2 and TLR4 [106–111] but since then other functional TLR (1, 3, 6, 7, and 9) have been discovered on mast cells (reviewed in [112]). Each of these receptors recognizes a distinct category of microbial products such as peptidoglycans (PGN) by TLR2, dsRNA by TLR3, LPS by TLR4, and bacterial DNA and CpG-containing DNA by TLR9. Some TLR rely on co-receptors, which by themselves have no signaling capabilities. A well-studied example is the interaction of TLR4 with CD14, which is essential for binding of LPS to a TLR4/CD14 complex. A recent example is dectin-1, which acts as a co-receptor for TLR2 in mast cells for the detection of fungal zymosan [113].
Expression of TLR may vary among different subsets of mast cells [112] and conditions although this topic warrants more systematic investigation. For example, mast cells derived from human cord blood lack TLR4 under certain culture conditions but thenexpress functional TLR4 on exposure to IFN-γ or IL-4 [110, 111].
Other mast cell receptors
Receptors for complement components
The presence of complement receptors on mast cells provides another mechanism for responding to pathogens either directly or indirectly [114]. Complement peptides elicit histamine release from mast cells and a wheal-and-flare reaction in skin [115]. The relevant receptors include not only those for C3a (C3aR) and C5a (C5aR/CD88) but also those for C3b (CR3/CD11b-CD18) and C4b (CR4/CD11c-CD18). Together, these receptors would permit responses to cleaved complement components such as C3a and C5a as well as bacterial/complement complexes that contain C3b and C4b (reviewed in [116]). C3a and C5a are potent chemoattractants for mast cells [117]. In addition, C3a, but not C5a, stimulates degranulation and production of the chemokines MCP-1 (CCL2) and RANTES (CCL5) [118, 119]. A possible scenario is that C3a and C5a recruit mast cells to sites of complement activation and, at this location, C3a-stimulated production of chemokines by mast cells promotes recruitment of APC and T cells. PGN-induced recruitment of Langerhans cells to lymph nodes, for example, is not observed in mast cell- or complement 3-deficient mice [120].
C3a contains a C-terminal sequence that is essential for its anaphylatoxic and chemotactic activity and an upstream sequence that is reported to interact with FcεRI and thus negatively regulates FcεRI-mediated signaling events, degranulation, and TNF-α production ([121] and citations therein). Of potential therapeutic benefit, peptide derivatives of this inhibitory motif exhibit similar inhibitory activity even in mast cell lines that do not appear to express C3a receptors.
Fcγ receptors
Mast cells can be positively or negatively regulated through multimeric IgG receptors (FcγR) [122]. Of these, FcγRI and FcγRIII are activating receptors and are inducible. Expression of FcγRI is induced by low concentrations of IFN-γ [96, 123]. Cells so treated can be stimulated through either FcεRI or FcγRI to release a similar array of mediators. Expression of FcγRIII is also inducible and is up-regulated by co-culture of mouse BMMC with fibroblasts. Under this circumstance, the cells can be activated by cross-linking of FcγRIII [124] or aggregated IgG [125]. FcγRIIB is an inhibitory receptor as described below.
Regulation of FcεRI-mediated responses through other receptors
The responses to antigen can be substantially augmented by the presence of other stimulatory ligands (Fig. 3). Antigen-induced cytokine production is markedly enhanced by co-stimulation with PGE2, SCF, TLR ligands, and IL-33, whereas degranulation is enhanced by ligands of G protein-coupled receptors such as adenosine, PGE2, macrophage inflammatory protein 1α (MIP-1α), and RANTES. Additive responses are observed on co-stimulation through receptors for C3a and FcγRI (reviewed in [99, 126]). The synergistic interactions can be quite remarkable. In the case of TLR ligands, robust production of inflammatory cytokines is observed with concentrations of antigen and TLR ligands that by themselves minimally stimulate cytokine production [127].
The inhibitory receptors are receiving increasing attention because they may provide ideal therapeutic targets for mast cell-related disorders [128]. They include FcγRIIB [129, 130], various members of the sialic acid-binding Ig-like lectin (Siglec) family of receptors [131], mast cell function-associated antigen [132], and leukocyte Ig-like receptors among others [133]. These receptors are monomeric transmembrane proteins with either multiple Ig-like domains (FcγRIIB, Siglecs, leukocyte Ig-like receptors) or a C-type lectin domain (mast cell function-associated antigen) in their extracellular segments. All have one or more ITIM in their cytosolic domains, which, when phosphorylated, recruit phosphatases such as SHIP and thereby reverse activating phosphorylation events [133]. In regard to function, FcγRIIB [130] and Siglec 8 [131] negatively regulate FcεRI-mediated signaling and Kit-dependent mast cell proliferation. FcγRIIB also appears to ameliorate the severity of experimental allergic encephalomyelitis (EAE) in mice [134]. The biological relevance of other inhibitory receptors in mast cells is unknown.
Induction of acquired immunity
In addition to acting as first responders to microbial agents [114], mast cells coordinate the recruitment of immune cells within lymph nodes to initiate adaptive immune responses [135]. Early evidence that mast cells set the stage for an adaptive immune response was the finding by Wang and coworkers in 1998 that induction of contact dermatitis in a mouse model is accompanied by migration of mast cells to regional lymph nodes [136] and in such a location mast cells produce MIP-1β to promote recruitment of T cells [136, 137]. Later studies extended these findings and provided additional evidence that mast cells play a critical role in lymph node hypertrophy and the engagement of T cells with APC upon bacterial infection [138], Leishmania major parasite infection [139], contact dermatitis [140, 141], and IgE-dependent cutaneous anaphylaxis [142]. The mast cell mediators involved varied according to the experimental model and stimulant used. TNF-α seemed to play a predominant role after bacterial infection [138], contact dermatitis [141], and IgE crosslinking [120]; only a partial role in the responses to the bacterial component, PGN [120]; and no role in mast cell-dependent lymph node hypertrophy following mosquito bites [143]. Other mast cell mediators such as MIP-1β [136, 137], IL-1 [144], and histamine [142] have also been implicated in cell recruitment. Mast cells also express ligands that activate co-stimulatory molecules on T cells. One such ligand, OX40L, has been identified as enhancing T-cell activation following antigen stimulation [145].
The studies to date suggest that activated mast cells promote migration of peripheral lymphocytes [138], Langerhans cells [142], and dendritic cells [146] to regional lymph nodes and thus facilitate the interaction of APC with Th cells as a prelude to T-cell/B-cell collaboration [147] or cytotoxic T-cell activation [148] (Fig. 4). The link between mast cells and a cytotoxic T-cell response was demonstrated in mice by topical application of a TLR7 ligand (imiquimod) as adjuvant along with an MHC-class-1-restricted cytotoxic T-lymphocyte peptide [144]. In this model, mast-cell-derived IL-1 and TNF-α mediated different phases of the response, namely, inflammation, migration of Langerhans cells, and lymph node hypertrophy. The reaction was severely impaired in mast-cell-deficient mice but this impediment could be bypassed by immunization of defective mice with peptide-loaded dendritic cells.
Autoimmune diseases and hypersensitivity reactions
In addition to FcεRI-mediated allergic reactions, the detrimental effects of mast cell activation extend to various autoimmune diseases [149]. Early reports noted high concentrations of IgE, degranulated mast cells, and mast-cell-derived chemoattractants in the subepidermal skin blisters of patients with bullous pemphigoid [150, 151]. Others have since reported that these lesions contain high levels of histamine, eosinophils, and IgE autoantibodies [152], which elicit lesions reminiscent of bullous phemphigoid when injected into skin of athymic mice [153]. This disease results from formation of autoantibodies against two proteins, BP180 and BP230, located in the dermal-epidermal adhesion plaques (hemidesmosomes) of skin. The disease can be reproduced in normal but not mast-cell-deficient neonatal mice by intradermal injection of IgG antibodies against murine BP180 or BP230 [154] except for one feature. The skin lesions are characterized by neutrophil infiltration in the mouse model and eosinophil infiltration in humans [152].
Rheumatoid arthritis (a Type III hypersensitivity resulting from IgG–immune complexes) also has the hallmarks of a mast cell-dependent disease [155]. Mast cells accumulate in arthritic synovial fluid and their degranulation and cytokine production correlates with severity of disease. Mast-cell-deficient mice fail to develop the disease when injected with serum from arthritic K/BxN mice [156, 157]. K/BxN mice spontaneously develop IgG antibodies against glucose-6-phosphatase isomerase. The resulting immune complex initiates synovial inflammation that involves the C5a complement cascade, neutrophils, cytokines such as TNF-α and IL-1β, and Fc receptors [158]. The progression of disease is significantly retarded in recipient FcγRIII-deficient mice but can be reconstituted by supply of FcR-positive BM cells [157]. Mast cells have also been implicated in a collagen-induced arthritis in mice [149, 158] in which activation of mast cells by IL-33 through the IL-33 receptor (ST2) plays a critical role in the disease process [159]. IL-33 is expressed in synovial membranes from patients with rheumatoid arthritis and expression of IL-33 is markedly enhanced by TNF-α and IL-1β in cultures of the patients’ synovial fibroblasts [159]. It is proposed that IL-33, along with IgG, stimulates synovial mast cells to generate additional cytokines and thus amplify the inflammatory response. Mast cells have a well-documented role in the Arthus reaction, which, like rheumatoid arthritis, is dependent on the formation of antigen–antibody complexes. The ensuing inflammatory response is not observed in mast-cell-deficient mice [160] and is dependent on the activation of mast cells through FcγRIII [161].
Mast cells appear to participate in Type IV hypersensitivity reactions including multiple sclerosis and its experimental counterpart, EAE in mice (reviewed in [149]). There are numerous reports of correlations between progression of disease and localization of mast cells since the first such observation by Neuman [162] shortly after the description of the mast cell by Ehrlich. Degranulation of these cells [163] and the presence of mast cell proteases in cerebrospinal fluid [164] have also been reported. EAE is induced by immunization of genetically susceptible mice with myelin peptides although the causative agent in humans is unknown. The most compelling evidence for the involvement of mast cells in EAE is its delay in onset and diminished severity in mast-cell-deficient mice and restoration of susceptibility by administration of BMMC [165] apparently by mechanism that requires expression of Fcg receptors on mast cells [166].
Other autoimmune disorders thought to be influenced by mast cells, but less well studied in this regard, are type 1 diabetes, Guillain–Barré syndrome, scleroderma, ulcerative colitis, Crohn's disease, Sjögren's syndrome, chronic idiopathic urticaria, and thyroid eye disease. As reviewed in detail by others [149, 158], the evidence to date is circumstantial and is based on the presence of mast cells or activated mast cells in the affected tissues.
Non-inflammatory functions of mast cells: immune tolerance, angiogenesis, and wound healing
The participation of mast cells in adaptive, innate, and autoimmunity [148, 167] is now well recognized but there is also increasing awareness that mast cells play a positive role in noninflammatory processes such as immune tolerance, angiogenesis, and tissue remodeling during wound healing [168]. However, many aspects of the contributions of mast cells to these processes remain unclear.
The possible participation of mast cells in immune suppression is best illustrated in the Treg-dependent allograft tolerance model in mice. In contrast to CD4+ Th1 and Th2 cells that enable rejection of allogeneic tissue grafts, Treg cells can suppresses rejection. The first hint of an interaction between Treg cells and mast cells was the report in 2001 by Zelenika and colleagues of increased expression of specific markers of both cell types in syngeneic skin grafts or tolerated allogeneic grafts [169]. Here, production of IL-9 by Treg cells was thought to promote recruitment and activity of mast cells in tolerant tissue. As reported recently, mast-cell-deficient mice failed to develop tolerance to allogeneic grafts but did so after inoculation with BMMC from normal mice in an IL-9-dependent manner [170]. Although the donors and recipients were not fully syngeneic, and therefore subject to some background rejection, the results are enticing and could have therapeutic implications once the mast cell factors that favor tolerance are identified. There is also evidence for direct interaction between Treg cells and mast cells through the OX40–OX40L connection [171]. This interaction is reported to suppress FcεRI-mediated mast cell activation in vitro and dampen the allergic response in vivo.
An angiogenic role for mast cells has long been suspected because of their intimate proximity to blood and lymphatic vessels, their tendency to accumulate in hemangiomas, polyps, tumors, and other tissues associated with angiogenesis, and their production of FGF-2, vascular endothelial growth factor, IL-8, TGF-β, TNF-α, histamine, heparin, and tryptase, all of which have demonstrated angiogenic activity [172]. Much of the supporting evidence is correlative and based on the use of pharmacologic blocking agents such as the mast cell stabilizing compounds cromolyn and salbutamol. Recent examples include the possible participation of mast cells in angiogenesis in human basal cell carcinoma through production of vascular endothelial growth factor and IL-8 [173] and in rat cervix during pregnancy [174]. Studies based on reconstitution of mast cells in W/Wv mice and use of cromolyn provide alternative evidence for mast-cell-dependent angiogenesis in arthritic lesions in the K/BxN mouse model [175] and Myc-driven cancer of pancreatic β cells in mice [176]. The daunting challenge will be to dissect out the individual roles of mast cells and other participating cells, as well as their chemical mediators, in the various phases of angiogenesis.
Another process of long-standing interest is tissue regeneration and wound healing. Kahlson proposed such a role for histamine almost 50 years ago [177]. Since then it has been claimed that mast cells control all phases of healing from the initial inflammatory response to re-epthelialization and tissue remodeling [178]. The circumstantial evidence is that mast cells accumulate in scar tissue, keloids, hypertrophic scars, and the edges of healing wounds and can produce fibroblast as well as keratinocyte growth factors in addition to angiogenic and matrix remodeling factors [178, 179]. Wound healing models in both animal and human skin have been widely used to study recruitment and potential roles of mast cells but two recent examples illustrate the uncertainty in the field at this time. Mast cell reconstitution studies in W/Wv mice suggested that wound closure was dependent on histamine release from mast cells [180]. In this study, wound closure was delayed in W/Wv mice, whereas this was not the case in another study [181]. Instead, collagen remodeling appeared to be impaired during the final phase of wound healing [181]. As with angiogenesis, many issues need clarification before adequate assessment can be made of the role of mast cells in wound healing.
Tumor growth
Among the specific observations of Ehrlich that still have relevance today was the abundance of mast cells in tumors, particularly carcinomas, where, as his student Westphal noted, mast cells accumulate at the periphery rather than the interior of the tumor nodules [182]. Subsequent studies provided ample evidence for increased populations of mast cells in such tumors as mammary adenocarcinomas, basal cell carcinomas, melanomas, neurofibromatosis, and Hodgkin's lymphoma [183]. Moreover, the extent of mast cell infiltration correlated with tumor histology and prognosis in patients with multiple myeloma and Hodgkin's lymphoma [184]. Nevertheless, there are examples of negative correlations with improved survival of patients with certain tumors and there are theoretical reasons as to why this might be so [184].
One focus of interest is the possible involvement of mast cells in the inflammation associated with malignancy. Depending on the type of tumor, inflammation may exist before malignant changes or be induced with the onset of malignancy but in either case inflammation may be conducive to tumor growth [185]. As noted earlier, mast cells produce growth-promoting and angiogenic factors that may further enhance progression of tumors [173, 176, 184, 186]. However, the connections are still unclear because tumor-associated macrophages and fibroblasts also produce growth and angiogenic factors.
Much of the evidence linking tumor growth, or regression, to specific mast cell factors is circumstantial and controversial. Even in studies with W/Wv mast-cell-deficient mice, some groups report increased susceptibility to tumors while others found diminished tumor growth. These discordant findings have been attributed to variable contributions of mast cells to different phases of tumor growth and the types of experimental models used [187]. As is apparent from recent reviews [183, 184, 188], research is handicapped by the correlative nature of clinical research, the diversity of experimental models, the array of mediators produced by mast cells, and not least the complexity of tumor biology. Nevertheless, the evidence in total makes a plausible case that the mast cell can regulate tumor growth and could be a potential target for adjuvant therapy [183] in some but not all types of cancers [184].
Therapeutic implications
While suppression of mast cell activation is an obvious therapeutic strategy for the treatment of allergic and autoimmune diseases, recent findings suggest that enhancement of mast cell activity may have therapeutic benefit in other conditions. The positive role of mast cells in adaptive immune responses could be exploited in vaccine adjuvant therapy [146]. The feasibility of this approach was demonstrated by subcutaneous or nasal administration of mast cell secretagogues (such as compound 48/80) along with vaccine antigens, which together prevented the lethal effects of anthrax toxin and vaccinia virus infection in mice. Imiquimod, a TLR7 agonist, has been used successfully as a mast-cell-activating adjuvant for activation of cytotoxic T cells in mice [144]. The potential advantage of these approaches would be the strong cellular immune response, which is not observed with alum, the only clinically approved adjuvant. With respect to suppression of mast cell activation, clarification of the biology of inhibitory receptors might provide new therapeutic opportunities, particularly if any one subset of receptors is largely restricted to mast cells.
Concluding remarks
Just as the mast cell adapts to its tissue environment, it may have been equally adept in evolution. Mast-cell-like cells are present in lowly urochordates. These granulated cells contain heparin/tryptase complexes, degranulate in response to compound 48/80, and possibly serve as a primitive innate immune system [189]. Mast cells in fish have acquired antimicrobial peptides [62], but not histamine [190], and in higher vertebrates, a panoply of immune receptors as the immune system has evolved. Among vertebrate species, the mast cell has acquired different subsets of proteases [45] and a tendency to accumulate in specific tissues [20] such as the liver of dog and peritoneal cavity of rat, which may reflect the immunological challenges faced by a given species. There is insufficient data to know whether evolutionary pressures have produced distinct differences among mammalian species in expression of mast cell receptors and cytokines. Nevertheless, it is clear from these considerations that one suit does not fit all subpopulations of mast cells and caution should be exercised in extrapolating data from cell culture to the whole animal or from animals to humans.
Ehrlich, having speculated on the nutritional function of mast cells, left it to others to elucidate the actual role of these cells by wistfully hoping that one day mast cells would be found to have an interesting function [191]. Only in the last few decades have Ehrlich's hopes been largely fulfilled. Nevertheless, contemporary reviews such as this one are probably interim accounts of the mast cells. Although the beneficial roles of the mast cell in innate and adaptive immune responses are increasingly clear, the purpose of the IgE pathway, other than its detriment to host, is still not obvious. Is it primarily a memory and amplification mechanism for past microbial infections that were initially handled through the TLR and complement systems? The normally parsimonious regulation of IgE production, the specifi-city of IgE for mast cells and basophils, and the remarkable synergy between antigen and TLR ligands would be consistent with such a mechanism.
Acknowledgements
Geoffrey B. West who, being my external examiner in 1959, sparked my interest in the mast cell; James C. Metcalfe and John P. Moore for directing my attention toward calcium and the phosphoinositides; Henry Metzger who enlightened me on various aspects of FcεRI aggregation; Alasdair M. Gilfillan and many colleagues for invigorating discussions and, not least, the intramural program of National Heart, Lung, and Blood Institute at the National Institutes of Health for supporting my research over the past 40 years.
Abbreviations
- BMMC
BM-derived mast cells
- LT
leukotrienes
- PG
prostaglandins
- PGN
peptidoglycans
- RBL
rat basophilic leukemia
- SRS-A
slow-reacting substance of anaphylaxis
Footnotes
Conflict of interest: The author declares no financial or commercial conflict of interest.
References
- 1.Ehrlich P. Thesis. Leipzig University; Beiträge zur Theorie und Praxis der histologischen Färbung. 6-17-1878. [Google Scholar]
- 2.Gordon S. Elie Metchnikoff: Father of natural immunity. Eur. J. Immunol. 2008;38:3257–3264. doi: 10.1002/eji.200838855. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A. From phagocyte diversity and activation to probiotics: Back to Metchnikoff. Eur. J. Immunol. 2008;38:3269–3273. doi: 10.1002/eji.200838918. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich P. Beiträge zur Kenntnis der Anilinfärbungen und ihrer Verwendung in der mikroskopischen Technik. Arch. mikr. Anat. 1877;13:263–277. [Google Scholar]
- 5.Ehrlich P. Farbenanalytische Untersuchungen zur Histologie und Klinik des Blutes. Hirschwald; Berlin: 1891. [Google Scholar]
- 6.Ehrlich P, Lazarus A. Die Anämie, 1. Normale und pathologische Histologie des Blutes, Holder, Wien (revised edition published in 1909) 1898.
- 7.Crivellato E, Beltrami C, Mallardi F, Ribatti D. Paul Ehrlich's doctoral thesis: a milestone in the study of mast cells. Br. J. Haematol. 2003;123:19–21. doi: 10.1046/j.1365-2141.2003.04573.x. [DOI] [PubMed] [Google Scholar]
- 8.Portier P, Richet C. De l'action anaphylactique de certains venins. C. R. Soc. Biol. (Paris) 1902;54:170–172. [Google Scholar]
- 9.Silverstein AM. Clemens Freiherr von Pirquet: explaining immune complex disease in 1906. Nat. Immunol. 2000;1:453–455. doi: 10.1038/82691. [DOI] [PubMed] [Google Scholar]
- 10.Prausnitz C, Küstner H. Studien über die Überempfindlichkeit. Zentralbl. Bakteriol. Parasitenk. Infektionskr. Abt. 1, Orig. 1921;86:160–169. [Google Scholar]
- 11.Landsteiner K. Experiments on anaphylaxis to azoproteins. J. Exp. Med. 1924;39:631–637. doi: 10.1084/jem.39.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishizaka K, Campbell DH. Biologic activity of soluble antigen–antibody complexes. IV. The inhibition of the skin reactivity of soluble complexes and the PCA reaction by heterologous complexes. J. Immunol. 1959;83:116–126. [PubMed] [Google Scholar]
- 13.Ishizaka K, Ishizaka T. Identification of γE-antibodies as a carrier of reaginic activity. J. Immunol. 1967;99:1187–1198. [PubMed] [Google Scholar]
- 14.Ishizaka K. Human reaginic antibodies. Annu. Rev. Med. 1970;21:187–200. doi: 10.1146/annurev.me.21.020170.001155. [DOI] [PubMed] [Google Scholar]
- 15.Dale HH. Croonian Lectures on some chemical factors in the control of the circulation. Lancet. 1929;213:1179–1183. 1233–1237, 1285–1290. [Google Scholar]
- 16.Beaven MA. Histamine: Its role in physiological and pathological processes. Monogr. Allergy. 1978;13:1–113. [PubMed] [Google Scholar]
- 17.Harris KE. Observations upon a histamine-like substance in skin extracts. Heart. 1927;14:161–176. [Google Scholar]
- 18.Holmgren H, Wilander O. Beitrag zur Kenntnis der Chemie und Funktion der Ehrlichschen Mastzellen. Z. Mikrosk. Anat. Forsch. 1937;42:242–278. [Google Scholar]
- 19.Rocha e Silva M, Scroggie AE, Fidlar E, Jacques LB. Liberation of histamine and heparin by peptone from isolated dog's liver. Proc. Soc. Exp. Biol. Med. 1947;64:141–146. doi: 10.3181/00379727-64-15727. [DOI] [PubMed] [Google Scholar]
- 20.Riley JF. The Mast Cells, E.&S. Livingstone; Edinburgh and London: 1959. [Google Scholar]
- 21.Riley JF, West GB. Histamine in tissue mast cells. J. Physiol. 1952;117:72P–73P. [PubMed] [Google Scholar]
- 22.Cass R, Riley JF, West GB, Head KW, Stroud SW. Heparin and histamine in mast-cell tumours from dogs. Nature. 1954;174:318–319. doi: 10.1038/174318b0. [DOI] [PubMed] [Google Scholar]
- 23.Riley JF, West GB. The occurence of histamine in mast cells. In: Rocha e Silva M, editor. Handbook of Experimental Pharmacology. Histamine and Anti-Histaminics. Springer Verlag; New York: 1966. pp. 116–135. [Google Scholar]
- 24.Riley JF, West GB. The presence of histamine in tissue mast cells. J. Physiol. 1953;120:528–537. doi: 10.1113/jphysiol.1953.sp004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley JF. The effects of histamine-liberators on the mast cells of the rat. J. Pathol. Bacteriol. 1953;65:471–479. doi: 10.1002/path.1700650219. [DOI] [PubMed] [Google Scholar]
- 26.Paton WDM. Compound 48/80: a potent histamine-liberator. Brit. J. Pharmacol. 1951;6:499–508. doi: 10.1111/j.1476-5381.1951.tb00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fawcett DW. An experimental study of mast cell degranulation and regeneration. Anat. Rec. 1955;121:29–51. doi: 10.1002/ar.1091210104. [DOI] [PubMed] [Google Scholar]
- 28.Riley JF, West GB. Tissue mast cells: studies with a histamine-liberator of low toxicity (compound 48/80). J. Pathol. Bacteriol. 1955;69:269–282. doi: 10.1002/path.1700690135. [DOI] [PubMed] [Google Scholar]
- 29.Mota I, Vugman I. Effects of anaphylactic shock and compound 48/80 on the mast cells of the guinea pig lung. Nature. 1956;177:427–429. doi: 10.1038/177427a0. [DOI] [PubMed] [Google Scholar]
- 30.Castells M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol. Allergy Clin. North Am. 2006;26:465–485. doi: 10.1016/j.iac.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Feldberg W, Kellaway DH. Liberation of histamine and formation of lyscithin-like substances by cobra venom. J. Physiol. 1938;94:187–226. doi: 10.1113/jphysiol.1938.sp003674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brocklehurst WE. The release of histamine and the formation of slow reacting substance (SRS-A) during anaphylactic shock. J. Physiol. 1960;151:416–435. doi: 10.1113/jphysiol.1960.sp006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orange RP, Austen WG, Austen KF. Immunological release of histamine and slow-reacting substance of anaphylaxis from human lung. I. Modulation by agents influencing cellular levels of cyclic 3′,5′-adenosine monophosphate. J. Exp. Med. 1971;134:136s–148s. [PubMed] [Google Scholar]
- 34.Ishizaka T, Ishizaka K, Orange RP, Austen KF. The capacity of human immunoglobulin E to mediate the release of histamine and slow reacting substance of anaphylaxis (SRS-A) from monkey lung. J. Immunol. 1970;104:335–343. [PubMed] [Google Scholar]
- 35.Murphy RC, Hammarström S, Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc. Natl. Acad. Sci. USA. 1979;76:4275–4279. [PubMed] [Google Scholar]
- 36.Austen K,F. The Mast Cell and the Cysteinyl Leukotrienes. Mast Cells and Basophils: Development, Activation and Roles in Allergic/Autoimmune Disease (Novartis Foundation Symposium 271) Wiley; Chichester: 2005. pp. 166–178. [DOI] [PubMed] [Google Scholar]
- 37.Roberts LJ, Lewis RA, Oates JA, Austen KF. Prostaglandin thromboxane, and 12-hydroxy-5,8,10,14-eicosatetraenoic acid production by ionophore-stimulated rat serosal mast cells. Biochim. Biophys. Acta. 1979;575:185–192. doi: 10.1016/0005-2760(79)90020-1. [DOI] [PubMed] [Google Scholar]
- 38.Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ. Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J. Immunol. 1982;129:1627–1631. [PubMed] [Google Scholar]
- 39.Benditt EP, Rase M. An enzyme in mast cells with properties like chymotrypsin. J. Exp. Med. 1959;110:451–460. doi: 10.1084/jem.110.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glenner GG, Cohen LA. Histochemical demonstration of a species-specific trypsin-like enzyme in mast cells. Nature. 1960;185:846–847. doi: 10.1038/185846a0. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells. Purification and characterization. J. Biol. Chem. 1981;256:11939–11943. [PubMed] [Google Scholar]
- 42.Schechter NM, Choi JK, Slavin DA, Deresienski DT, Sayama S, Dong G, Lavker RM, et al. Identification of a chymotrypsin-like proteinase in human mast cells. J. Immunol. 1986;137:962–970. [PubMed] [Google Scholar]
- 43.Goldstein SM, Kaempfer CE, Proud D, Schwartz LB, Irani AM, Wintroub BU. Detection and partial characterization of a human mast cell carboxypeptidase. J. Immunol. 1987;139:2724–2729. [PubMed] [Google Scholar]
- 44.Caughey GH. New developments in the genetics and activation of mast cell proteases. Mol. Immunol. 2002;38:1353–1357. doi: 10.1016/s0161-5890(02)00087-1. [DOI] [PubMed] [Google Scholar]
- 45.Stevens RL, Adachi R. Protease–proteoglycan complexes of mouse and human mast cells and importance of their β-tryptase–heparin complexes in inflammation and innate immunity. Immunol. Rev. 2007;217:155–167. doi: 10.1111/j.1600-065X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 46.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007;217:141–154. doi: 10.1111/j.1600-065X.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol. Allergy Clin. North Am. 2006;26:451–463. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv. Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 49.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 50.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl. Acad. Sci. USA. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, Clouthier DE, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 53.Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol. Microbiol. Scand. 1966;66:303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- 54.Razin E, Cordon-Cardo C, Good RA. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc. Natl. Acad. Sci. USA. 1981;78:2559–2561. doi: 10.1073/pnas.78.4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Razin E, Ihle JN, Seldin D, Mencia-Huerta JM, Katz HR, LeBlanc PA, Hein A, et al. Interleukin 3: a differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J. Immunol. 1984;132:1479–1486. [PubMed] [Google Scholar]
- 56.Ghildyal N, Friend DS, Stevens RL, Austen KF, Huang C, Penrose JF, Sali A, Gurish MF. Fate of two mast cell tryptases in V3 mastocytosis and normal BALB/c mice undergoing passive systemic anaphylaxis: prolonged retention of exocytosed mMCP-6 in connective tissues, and rapid accumulation of enzymatically active mMCP-7 in the blood. J. Exp. Med. 1996;184:1061–1073. doi: 10.1084/jem.184.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of FcεRI or to calcium ionophores. Nature. 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 58.Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989;339:150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- 59.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 60.Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin. Exp. Allergy. 2008;38:4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- 61.de Paulis A, Minopoli G, Arbustini E, de Crescenzo G, Dal Piaz F, Pucci P, Russo T, Marone G. Stem cell factor is localized in, released from, and cleaved by human mast cells. J. Immunol. 1999;163:2799–2808. [PubMed] [Google Scholar]
- 62.Silphaduang U, Noga EJ. Peptide antibiotics in mast cells of fish. Nature. 2001;414:268–269. doi: 10.1038/35104690. [DOI] [PubMed] [Google Scholar]
- 63.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: Mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 64.Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL. Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J. Immunol. 2008;180:7565–7573. doi: 10.4049/jimmunol.180.11.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitamura Y, Kasugai T, Arizono N, Matsuda H. Development of mast cells and basophils: processes and regulation mechanisms. Am. J. Med. Sci. 1993;306:185–191. doi: 10.1097/00000441-199309000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol. Rev. 2007;217:304–328. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 67.Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, March CJ, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 68.Enerbäck L. The gut mucosal mast cell. Monograph. Allergy. 1981;17:222–232. [Google Scholar]
- 69.Enerbäck L. Berberine sulphate binding to mast cell polyanions: a cytofluorometric method for the quantitation of heparin. Histochemistry. 1974;42:301–313. doi: 10.1007/BF00492678. [DOI] [PubMed] [Google Scholar]
- 70.Enerbäck L, Lundin PM. Ultrastructure of mucosal mast cells in normal and compound 48-80-treated rats. Cell Tissue Res. 1974;150:95–105. doi: 10.1007/BF00220383. [DOI] [PubMed] [Google Scholar]
- 71.Bienenstock J, Befus AD, Denburg J, Goodacre R, Pearce F, Shanahan F. Mast cell heterogeneity. Monogr. Allergy. 1983;18:124–128. [PubMed] [Google Scholar]
- 72.Enerbäck L. The differentiation and maturation of inflammatory cells involved in the allergic response: mast cells and basophils. Allergy. 1997;52:4–10. doi: 10.1111/j.1398-9995.1997.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 73.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, Asai H, et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J. Exp. Med. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levi-Schaffer F, Austen KF, Gravallese PM, Stevens RL. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc. Natl. Acad. Sci. USA. 1986;83:6485–6488. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc. Natl. Acad. Sci. USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol. Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hellman L. Regulation of IgE homeostasis, and the identification of potential targets for therapeutic intervention. Biomed. Pharmacother. 2007;61:34–49. doi: 10.1016/j.biopha.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IgE and the basis of allergic disease. Annu. Rev. Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 79.Kawakami T, Kitaura J. Mast cell survival and activation by IgE in the absence of antigen: a consideration of the biologic mechanisms and relevance. J. Immunol. 2005;175:4167–4173. doi: 10.4049/jimmunol.175.7.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bazin H, Querinjean P, Beckers A, Heremans JF, Dessy F. Transplantable immunoglobulin-secreting tumours in rats. IV. Sixty-three IgE-secreting immunocytoma tumours. Immunology. 1974;26:713–723. [PMC free article] [PubMed] [Google Scholar]
- 81.Kulczycki A, Isersky C, Metzger H. The interaction of IgE with rat basophilic leukemia cells. I. Evidence for specific binding of IgE. J. Exp. Med. 1974;139:600–616. doi: 10.1084/jem.139.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, Klinman NR, Katz DH. Monoclonal dinitrophenyl-specific murine IgE antibody: Preparation, isolation, and characterization. J. Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- 83.Siraganian RP, Metzger H. Evidence that the “mouse mastocytoma” cell line (MCT-1) is of rat origin. J. Immunol. 1978;121:2584–2585. [PubMed] [Google Scholar]
- 84.Barsumian EL, Isersky C, Petrino MG, Siraganian RP. IgE-induced histamine release from rat basophilic leukemia cell lines: Isolation of releasing and nonreleasing clones. Eur. J. Immunol. 1981;11:317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- 85.Metzger H, Alcaraz G, Hohman R, Kinet JP, Pribluda V, Quarto R. The receptor with high affinity for immunoglobulin E. Annu. Rev. Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- 86.Blank U, Ra C, Miller L, White K, Metzger H, Kinet JP. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 87.Kinet JP. The high affinity IgE receptor (FcεRI): From physiology to pathology. Annu. Rev. Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 88.Kraft S, Rana S, Jouvin MH, Kinet JP. The role of the FcεRI β-chain in allergic diseases. Int. Arch. Allergy Immunol. 2004;135:62–72. doi: 10.1159/000080231. [DOI] [PubMed] [Google Scholar]
- 89.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat. Rev. Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 90.Morar N, Willis-Owen SA, Moffatt MF, Cookson WO. The genetics of atopic dermatitis. J. Allergy Clin. Immunol. 2006;118:24–34. doi: 10.1016/j.jaci.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 91.Beaven MA, Rogers J, Moore JP, Hesketh TR, Smith GA, Metcalfe JC. The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J. Biol. Chem. 1984;259:7129–7136. [PubMed] [Google Scholar]
- 92.Beaven MA, Moore JP, Smith GA, Hesketh TR, Metcalfe JC. The calcium signal and phosphatidylinositol breakdown in 2H3 cells. J. Biol. Chem. 1984;259:7137–7142. [PubMed] [Google Scholar]
- 93.Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells: Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J. Biol. Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 94.Rossi GL, Di Comite V, Olivieri D. Mast cell cultures: bench to bedside. Clin. Exp. Allergy. 1998;28:1182–1190. doi: 10.1046/j.1365-2222.1998.00376.x. [DOI] [PubMed] [Google Scholar]
- 95.Jensen B,M, Swindle EJ, Iwaki S, Gilfillan AM. Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr. Protoc. Immunol. 2006:3.23.1–3.23.13. [Google Scholar]
- 96.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metclafe DD. Characterization of a novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI and FcγRI. Leukemia Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 97.Kirshenbaum AS, Metcalfe DD. Growth of human mast cells from bone marrow and peripheral blood-derived CD34+ pluripotent progenitor cells. Methods Mol. Biol. 2006;315:105–112. doi: 10.1385/1-59259-967-2:105. [DOI] [PubMed] [Google Scholar]
- 98.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 99.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 100.Jensen BM, Metcalfe DD, Gilfillan AM. Targeting Kit activation: a potential therapeutic approach in the treatment of allergic inflammation. Inflamm. Allergy Drug Targets. 2007;6:57–62. doi: 10.2174/187152807780077255. [DOI] [PubMed] [Google Scholar]
- 101.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 102.Beaven MA, Hundley TR. Mast cell-related diseases: Genetics, signaling pathways, and novel therapies. In: Finkel TH, Gutkind JS, editors. Signal Transduction and Human Disease. Wiley; Hoboken, NJ: 2003. pp. 307–355. [Google Scholar]
- 103.Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr. Opin. Immunol. 1999;11:53–59. doi: 10.1016/s0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 104.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 105.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 106.Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J. Immunol. 2001;167:2250–2256. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]
- 107.McCurdy JD, Lin TJ, Marshall JS. Toll-like receptor 4-mediated activation of murine mast cells. J. Leukoc. Biol. 2001;70:977–984. [PubMed] [Google Scholar]
- 108.Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, Ogawa H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: Distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J. Immunol. 2003;170:1625–1629. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 110.Varadaradjalou S, Feger F, Thieblemont N, Hamouda NB, Pleau JM, Dy M, Arock M. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur. J. Immunol. 2003;33:899–906. doi: 10.1002/eji.200323830. [DOI] [PubMed] [Google Scholar]
- 111.Okumura S, Kashiwakura J, Tomita H, Matsumoto K, Nakajima T, Saito H, Okayama Y. Identification of specific gene expression profiles in human mast cells mediated by Toll-like receptor 4 and FcεRI. Blood. 2003;102:2547–2554. doi: 10.1182/blood-2002-12-3929. [DOI] [PubMed] [Google Scholar]
- 112.Marshall JS. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 113.Olynych TJ, Jakeman DL, Marshall JS. Fungal zymosan induces leukotriene production by human mast cells through a dectin-1-dependent mechanism. J. Allergy Clin. Immunol. 2006;118:837–843. doi: 10.1016/j.jaci.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 114.Metz M, Siebenhaar F, Maurer M. Mast cell functions in the innate skin immune system. Immunobiology. 2008;213:251–260. doi: 10.1016/j.imbio.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 115.Johnson AR, Hugli TE, Müller-Eberhard HJ. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975;28:1067–1080. [PMC free article] [PubMed] [Google Scholar]
- 116.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 117.Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J. Immunol. 1996;157:1693–1698. [PubMed] [Google Scholar]
- 118.Venkatesha RT, Berla TE, Zaidi AK, Ali H. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol. Immunol. 2005;42:581–587. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 119.Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through FcγRI: additive effects of C3a. Clin. Immunol. 2004;110:172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 120.Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J. Immunol. 2006;177:1755–1762. doi: 10.4049/jimmunol.177.3.1755. [DOI] [PubMed] [Google Scholar]
- 121.Andrasfalvy M, Peterfy H, Toth G, Matko J, Abramson J, Kerekes K, Vamosi G, et al. The β subunit of the type I Fcε receptor is a target for peptides inhibiting IgE-mediated secretory response of mast cells. J. Immunol. 2005;175:2801–2806. doi: 10.4049/jimmunol.175.5.2801. [DOI] [PubMed] [Google Scholar]
- 122.Tkaczyk C, Okayama Y, Metcalfe DD, Gilfillan AM. Fcγ receptors on mast cells: activatory and inhibitory regulation of mediator release. Int. Arch. Allergy Immunol. 2004;133:305–315. doi: 10.1159/000077213. [DOI] [PubMed] [Google Scholar]
- 123.Woolhiser MR, Okayama Y, Gilfillan AM, Metcalfe DD. IgG-dependent activation of human mast cells following up-regulation of FcγRI by IFN-γ. Eur. J. Immunol. 2001;31:3298–3307. doi: 10.1002/1521-4141(200111)31:11<3298::aid-immu3298>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 124.Katz HR, Raizman MB, Gartner CS, Scott HC, Benson AC, Austen KF. Secretory granule mediator release and generation of oxidative metabolites of arachidonic acid via FcγR bridging in mouse mast cells. J. Immunol. 1992;148:868–871. [PubMed] [Google Scholar]
- 125.Katz HR, Arm JP, Benson AC, Austen KF. Maturation-related changes in the expression of FcγRII and FcγRIII on mouse mast cells derived in vitro and in vivo. J. Immunol. 1990;145:3412–3417. [PubMed] [Google Scholar]
- 126.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcεRI-mediated mast cell activation. Immunol. Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcεRI and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ott VL, Cambier JC. Activating and inhibitory signaling in mast cells: new opportunities for therapeutic intervention? J. Allergy Clin. Immunol. 2000;106:429–440. doi: 10.1067/mai.2000.109428. [DOI] [PubMed] [Google Scholar]
- 129.Daëron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J. Clin. Invest. 1995;95:577–585. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Malbec O, Daëron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol. Rev. 2007;217:206–221. doi: 10.1111/j.1600-065X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 131.Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, Ryu SD, et al. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J. Allergy Clin. Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 132.Guthmann MD, Tal M, Pecht I. A secretion inhibitory signal transduction molecule on mast cells is another C-type lectin. Proc. Natl. Acad. Sci. USA. 1995;92:9397–9401. doi: 10.1073/pnas.92.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li L, Yao Z. Mast cell and immune inhibitory receptors. Cell Mol. Immunol. 2004;1:408–415. [PubMed] [Google Scholar]
- 134.Robbie-Ryan M, Tanzola MB, Secor VH, Brown MA. Cutting edge: Both activating and inhibitory Fc receptors expressed on mast cells regulate experimental allergic encephalomyelitis disease severity. J. Immunol. 2003;170:1630–1634. doi: 10.4049/jimmunol.170.4.1630. [DOI] [PubMed] [Google Scholar]
- 135.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 136.Wang HW, Tedla N, Lloyd AR, Wakefield D, McNeil PH. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J. Clin. Invest. 1998;102:1617–1626. doi: 10.1172/JCI3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tedla N, Wang HW, McNeil HP, Di GN, Hampartzoumian T, Wakefield D, Lloyd A. Regulation of T lymphocyte trafficking into lymph nodes during an immune response by the chemokines macrophage inflammatory protein (MIP)-1 α and MIP-1 β. J. Immunol. 1998;161:5663–5672. [PubMed] [Google Scholar]
- 138.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat. Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 139.Maurer M, Lopez Kostka S, Siebenhaar F, Moelle K, Metz M, Knop J, von Stebut E. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006;20:2460–2467. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- 140.Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 141.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J. Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 142.Jawdat DM, Albert EJ, Rowden G, Haidl ID, Marshall JS. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J. Immunol. 2004;173:5275–5282. doi: 10.4049/jimmunol.173.8.5275. [DOI] [PubMed] [Google Scholar]
- 143.Demeure CE, Brahimi K, Hacini F, Marchand F, Peronet R, Huerre M, St-Mezard P, et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J. Immunol. 2005;174:3932–3940. doi: 10.4049/jimmunol.174.7.3932. [DOI] [PubMed] [Google Scholar]
- 144.Heib V, Becker M, Warger T, Rechtsteiner G, Tertilt C, Klein M, Bopp T, et al. Mast cells are crucial for early inflammation, migration of Langerhans cells, and CTL responses following topical application of TLR7 ligand in mice. Blood. 2007;110:946–953. doi: 10.1182/blood-2006-07-036889. [DOI] [PubMed] [Google Scholar]
- 145.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 146.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, Abraham SN. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat. Med. 2008;14:536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 147.Pulendran B, Ono SJ. A shot in the arm for mast cells. Nat. Med. 2008;14:489–490. doi: 10.1038/nm0508-489. [DOI] [PubMed] [Google Scholar]
- 148.Heib V, Becker M, Taube C, Stassen M. Advances in the understanding of mast cell function. Br. J. Haematol. 2008;142:683–694. doi: 10.1111/j.1365-2141.2008.07244.x. [DOI] [PubMed] [Google Scholar]
- 149.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu. Rev. Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 150.Baba T, Sonozaki H, Seki K, Uchiyama M, Ikesawa Y, Torisu M. An eosinophil chemotactic factor present in blister fluids of bullous pemphigoid patients. J. Immunol. 1976;116:112–116. [PubMed] [Google Scholar]
- 151.Wintroub BU, Mihm MC, Jr., Goetzl EJ, Soter NA, Austen KF. Morphologic and functional evidence for release of mast-cell products in bullous pemphigoid. N. Engl. J. Med. 1978;298:417–421. doi: 10.1056/NEJM197802232980803. [DOI] [PubMed] [Google Scholar]
- 152.Dimson OG, Giudice GJ, Fu CL, Van den Bergh F, Warren SJ, Janson MM, Fairley JA. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J. Invest. Dermatol. 2003;120:784–788. doi: 10.1046/j.1523-1747.2003.12146.x. [DOI] [PubMed] [Google Scholar]
- 153.Fairley JA, Burnett CT, Fu CL, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J. Invest. Dermatol. 2007;127:2605–2611. doi: 10.1038/sj.jid.5700958. [DOI] [PubMed] [Google Scholar]
- 154.Leighty L, Li N, Diaz LA, Liu Z. Experimental models for the autoimmune and inflammatory blistering disease, bullous pemphigoid. Arch. Dermatol. Res. 2007;299:417–422. doi: 10.1007/s00403-007-0790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol. Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 156.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 157.Corr M, Crain B. The role of FcγR signaling in the K/B x N serum transfer model of arthritis. J. Immunol. 2002;169:6604–6609. doi: 10.4049/jimmunol.169.11.6604. [DOI] [PubMed] [Google Scholar]
- 158.Benoist C, Mathis D. Mast cells in autoimmune disease. Nature. 2002;420:875–878. doi: 10.1038/nature01324. [DOI] [PubMed] [Google Scholar]
- 159.Xu D, Jiang H-R, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Nat. Acad. Sci. USA. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang Y, Ramos BF, Jakschik BA. Augmentation of reverse Arthus reaction by mast cells in mice. J. Clin. Invest. 1991;88:841–846. doi: 10.1172/JCI115385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sylvestre DL, Ravetch JV. A dominant role for mast cell Fc receptors in the Arthus reaction. Immunity. 1996;5:387–390. doi: 10.1016/s1074-7613(00)80264-2. [DOI] [PubMed] [Google Scholar]
- 162.Neuman J. Über das Vorkommen der sogneannten “Mastzellen” bei pathologischen Veränderungen des Gehirns. Arch. Pathol. Anat. Physiol. Virchows. 1890;122:378–381. [Google Scholar]
- 163.Brenner T, Soffer D, Shalit M, Levi-Schaffer F. Mast cells in experimental allergic encephalomyelitis: characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J. Neurol. Sci. 1994;122:210–213. doi: 10.1016/0022-510x(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 164.Rozniecki JJ, Hauser SL, Stein M, Lincoln R, Theoharides TC. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann. Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 165.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J. Exp. Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown MA, Tanzola MB, Robbie-Ryan M. Mechanisms underlying mast cell influence on EAE disease course. Mol. Immunol. 2002;38:1373–1378. doi: 10.1016/s0161-5890(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 167.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Henz BM. Exploring the mast cell enigma: a personal reflection of what remains to be done. Exp. Dermatol. 2008;17:91–99. doi: 10.1111/j.1600-0625.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 169.Zelenika D, Adams E, Humm S, Lin CY, Waldmann H, Cobbold SP. The role of CD4+ T-cell subsets in determining transplantation rejection or tolerance. Immunol. Rev. 2001;182:164–179. doi: 10.1034/j.1600-065x.2001.1820113.x. [DOI] [PubMed] [Google Scholar]
- 170.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 171.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40–OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Crivellato E, Beltrami CA, Mallardi F, Ribatti D. The mast cell: an active participant or an innocent bystander? Histol. Histopathol. 2004;19:259–270. doi: 10.14670/HH-19.259. [DOI] [PubMed] [Google Scholar]
- 173.Aoki M, Pawankar R, Niimi Y, Kawana S. Mast cells in basal cell carcinoma express VEGF, IL-8 and RANTES. Int. Arch. Allergy Immunol. 2003;130:216–223. doi: 10.1159/000069515. [DOI] [PubMed] [Google Scholar]
- 174.Bosquiazzo VL, Ramos JG, Varayoud J, Munoz-de-Toro M, Luque EH. Mast cell degranulation in rat uterine cervix during pregnancy correlates with expression of vascular endothelial growth factor mRNA and angiogenesis. Reproduction. 2007;133:1045–1055. doi: 10.1530/REP-06-0168. [DOI] [PubMed] [Google Scholar]
- 175.Kneilling M, Hultner L, Pichler BJ, Mailhammer R, Morawietz L, Solomon S, Eichner M, et al. Targeted mast cell silencing protects against joint destruction and angiogenesis in experimental arthritis in mice. Arthritis Rheum. 2007;56:1806–1816. doi: 10.1002/art.22602. [DOI] [PubMed] [Google Scholar]
- 176.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat. Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 177.Kahlson G. A place for histamine in normal physiology. Lancet. 1960;1:67–71. doi: 10.1016/s0140-6736(60)92896-8. [DOI] [PubMed] [Google Scholar]
- 178.Noli C, Miolo A. The mast cell in wound healing. Vet. Dermatol. 2001;12:303–313. doi: 10.1046/j.0959-4493.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 179.Artuc M, Steckelings UM, Henz BM. Mast cell-fibroblast interactions: human mast cells as source and inducers of fibroblast and epithelial growth factors. J. Invest. Dermatol. 2002;118:391–395. doi: 10.1046/j.0022-202x.2001.01705.x. [DOI] [PubMed] [Google Scholar]
- 180.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20:2366–2368. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- 181.Iba Y, Shibata A, Kato M, Masukawa T. Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int. Immunopharmacol. 2004;4:1873–1880. doi: 10.1016/j.intimp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 182.Westphal E. Über Mastzellen. In: Ehrlich P, editor. Farbenanalytische Untersuchungen. Hirschwald; Berlin: 1891. pp. 17–41. [Google Scholar]
- 183.Theoharides TC, Conti P. Mast cells: the JEKYL and HYDE of tumor growth. Trends Immunol. 2004;25:235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 184.Galinsky DS, Nechushtan H. Mast cells and cancer-No longer just basic science. Crit. Rev. Oncol. Hematol. 2008;68:115–130. doi: 10.1016/j.critrevonc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 185.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 186.Dimitriadou V, Koutsilieris M. Mast cell-tumor cell interactions: for or against tumour growth and metastasis? Anticancer Res. 1997;17:1541–1549. [PubMed] [Google Scholar]
- 187.Wedemeyer J, Galli SJ. Decreased susceptibility of mast cell-deficient KitW/KitW-V mice to the development of 1, 2-dimethylhydrazine-induced intestinal tumors. Lab. Invest. 2005;85:388–396. doi: 10.1038/labinvest.3700232. [DOI] [PubMed] [Google Scholar]
- 188.Conti P, Castellani ML, Kempuraj D, Salini V, Vecchiet J, Tete S, Mastrangelo F, et al. Role of mast cells in tumor growth. Ann. Clin. Lab. Sci. 2007;37:315–322. [PubMed] [Google Scholar]
- 189.Cavalcante MC, Allodi S, Valente AP, Straus AH, Takahashi HK, Mourão PA, Pavão MS. Occurrence of heparin in the invertebrate Styela plicata (Tunicata) is restricted to cell layers facing the outside environment. An ancient role in defense? J. Biol. Chem. 2000;275:36189–36196. doi: 10.1074/jbc.M005830200. [DOI] [PubMed] [Google Scholar]
- 190.Reite OB. Comparative physiology of histamine. Physiol. Rev. 1972;52:778–819. doi: 10.1152/physrev.1972.52.3.778. [DOI] [PubMed] [Google Scholar]
- 191.Ehrlich P. Beiträge zur Kenntnis der granulierten Bindegewebszellen und der eosinophilen Leukocyten. Arch. Anat. Physiol. (Leipzig) 1879;3:166–169. [Google Scholar]