Case Report
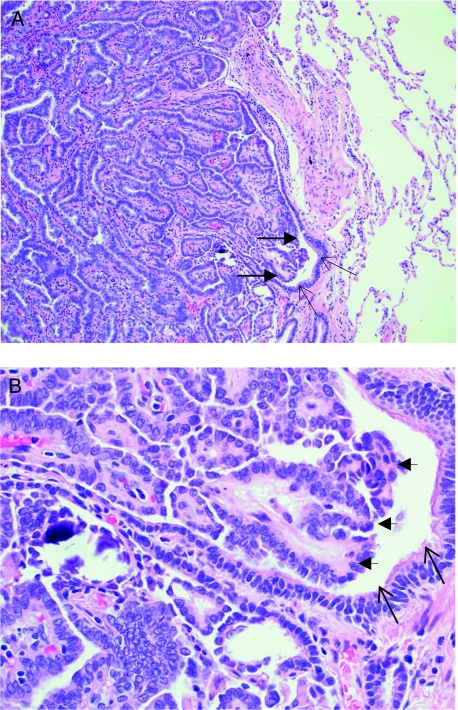
A man aged 76 years with known metastatic papillary thyroid carcinoma presented with progressive cough and dyspnea on exertion over a 4-month period. At the time of diagnosis in 2001, he had a total thyroidectomy with a limited neck dissection and received 151 mCi sodium iodide 131 (131I). In 2002, he had a follow-up chest radiograph that showed multiple pulmonary nodules. He subsequently underwent a video-assisted thoracoscopic surgery lung biopsy in 2004 that confirmed the presence of metastatic papillary thyroid carcinoma. Histologic sections of the left upper lobe and lingula showed multiple foci, up to 0.5 cm in greatest dimension, of metastatic papillary thyroid carcinoma, which involved approximately 30% of the bronchioles. The structures of some of the involved bronchioles were completely destroyed, and some bronchiolar lumens were almost completely occluded (Figs 1A, 1B). Lymphatic involvement by tumor was identified, but there was no vascular invasion. Pulmonary function tests prior to the lung biopsy procedure were normal (Fig 2, Table 1). The patient received a second treatment with 119 mCi 131I in January 2005.
Figure 1.
Metastatic papillary thyroid carcinoma involves the bronchiole with luminal occlusion. Hematoxylin and eosin stain of lung biopsy, original magnification, × 100 (A) and × 400 (B). Thin arrows indicate bronchiolar epithelium; large arrows demonstrate tumor invading and occluding the lumen.
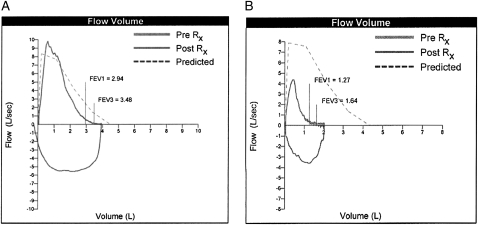
Figure 2.
Flow-volume loops from spirometry performed in December 2004 and September 2008 show the development of severe obstructive lung disease. Rx = bronchodilator treatment.
Table 1.
—Spirometry Values Over the Course of Treatment With 131I
| Parameter | Initial, December 2004 | Pre-131I, September 2008 | Post-131I, December 2008 | Progression of Disease | Post-Sorfenib, August 2009 | |
| April 2009 | April 2009 PB | |||||
| FEV1, L | 2.94 | 1.03 | 1.31 | 0.85 | 0.90 | 1.11 |
| FVC, L | 3.93 | 1.73 | 2.15 | 1.54 | 1.66 | 1.90 |
| FEV1/FVC, % | 75 | 60 | 62 | 55 | 54 | 58 |
Both FEV1 and FVC increase post-131I treatment, indicating improvement in airflow obstruction. As the disease progressed, the patient’s obstruction became worse. Two months after treatment with sorafenib, the degree of obstruction improved. 131I = sodium iodide 131; PB = postbronchodilator.
The patient was seen by his primary physician 1 month prior to pulmonary consultation in 2008 for worsening shortness of breath. He was a life-long nonsmoker who had no personal or family history of asthma or reactive airways disease. He denied secondary smoke or occupational exposures. He had no orthopnea, paroxysmal nocturnal dyspnea, chest pain, or palpitations. His medications at the time of consultation included levothyroxine; vitamins A, C, and E; and occasional guaifenesin and pseudoephedrine for cough. Physical examination revealed cyanosis and an oxygen saturation of 80% on room air. The patient did not have stridor. He had mild supraclavicular adenopathy. He had inspiratory and expiratory squeaks and scattered expiratory wheezes. The remainder of the examination was unremarkable.
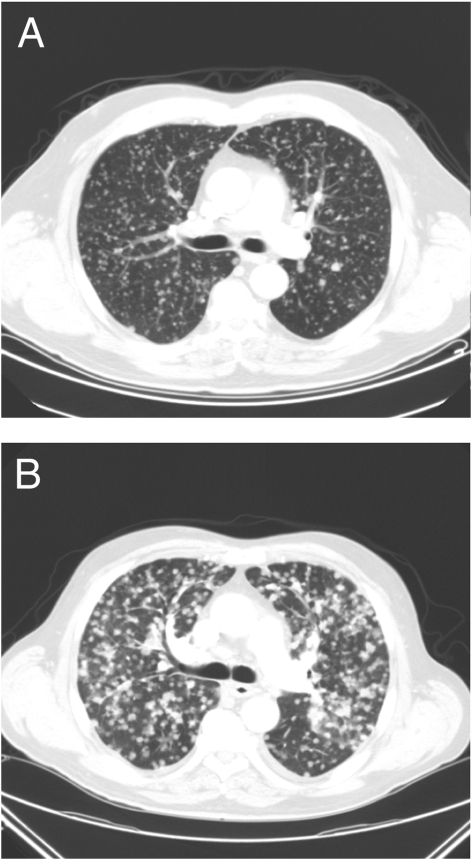
A chest CT scan was performed to evaluate progression of the patient’s disease and exclude pulmonary embolism (Fig 3A). This scan was compared with an earlier CT scan of his chest from 2006 (Fig 3B). The comparison demonstrated a significant increase in the number of pulmonary nodules and increased adenopathy. There were no pulmonary emboli or endobronchial lesions or evidence of pneumonitis noted.
Figure 3.
Chest CT scan showing innumerable tiny bilateral pulmonary nodules consistent with metastatic papillary thyroid carcinoma (A). Chest CT scan 2 years later showing progression of metastatic disease (B).
The patient had pulmonary function testing (Fig 2) that demonstrated severe obstructive lung disease with an FEV1 of 1.03 L (33% predicted), an FVC of 1.73 L (40% predicted), and an FEV1/FVC ratio of 0.60. A bronchodilator challenge failed to reveal reversible airway obstruction. The flow volume loops did not suggest an extrathoracic airway obstruction. These spirometric values were significantly reduced from those obtained in 2004 before his video-assisted thoracoscopic surgery biopsy. A diffusing capacity corrected for level of hemoglobin was moderately reduced at 48% predicted. Resting and ambulatory oximetry confirmed the presence of hypoxemia that was correctable with 2- to 4-L/min oxygen supplementation through nasal cannula. A total lung capacity measured through helium dilution was moderately reduced at 68% predicted. Both maximal inspiratory and expiratory pressures were > 100% predicted.
The patient subsequently underwent repeat treatment with 176 mCi 131I. His spirometry and dyspnea improved. Two months after treatment with 131I, his FEV1 increased by 280 mL, his FVC increased by 420 mL, and his FEV1/FVC ratio increased to 61%. The patient did not receive further 131I because of the good initial response and concerns that he would develop pulmonary toxicity from additional doses. Six months after 131I treatment, he developed worsening dyspnea and more severe airflow obstruction (FEV1, 0.85 L [27% predicted]; FVC, 1.54 L [35% predicted]). Repeat bronchodilator challenge again failed to reveal a positive response. He ultimately was referred for treatment with sorafenib, which is ongoing. This treatment resulted in improvement in his dyspnea and degree of hypoxemia, and repeat pulmonary function tests performed 2 months after treatment showed improvement in the degree of airflow obstruction (Fig 2, Table 1).
What is the diagnosis?
Diagnosis: Airway obstruction caused by widespread tumor metastases
Discussion
Clinical Discussion
Thyroid carcinoma is relatively uncommon, accounting for only 1% of all new malignancies. Approximately 94% of thyroid carcinomas originate from follicular epithelia and are divided into papillary (the most common) and follicular subtypes.1 Ten percent to 15% of patients with papillary thyroid carcinomas will develop metastases,2 80% of which occur within the thoracic cavity. Two distinct types of metastasis occur with papillary thyroid carcinoma. Miliary metastases or multiple nodules may occur within the lung parenchyma similar to those seen in our patient, but a more localized pulmonary infiltration with widespread lymphadenopathy and pleural effusions also is possible.3 Lung metastases often are associated with respiratory symptoms, particularly dyspnea. They have been reported to be the most common fatal complication, listed as the cause of death in up to 46% of patients with thyroid carcinoma.4 Widespread tumor infiltration of the pulmonary parenchyma is well known to precipitate dyspnea and hypoxia, and there are many cases of reported airflow obstruction related to direct tumor invasion of central airways, such as the trachea and major bronchi.5,6 However, we have identified no other reports of small airway obstruction precipitated by tumor metastases. Our patient had no evidence of upper airway or central airway obstruction on flow-volume loops, but he did have characteristic airflow obstruction seen with airways diseases, such as bronchiolitis, asthma, or emphysema. He had no history of asthma, no reversibility on bronchodilator challenge, and no history of tobacco use, therefore suggesting that his airflow obstruction was secondary to the metastatic tumors. This finding was corroborated by histopathology showing significant tumor invasion of the bronchioles, lack of vascular invasion, and improvement in airflow obstruction following therapy for carcinoma. We hypothesize that the reduction in the diffusing capacity of the lung for carbon monoxide seen in our patient was multifactorial but most likely related to direct obliteration of the alveolar capillary interface by the tumor. The reduction in total lung capacity also was likely secondary to the extensive tumor infiltration in his chest, thus limiting the extent of lung expansion. There was no evidence for neuromuscular weakness, as his maximal inspiratory and expiratory pressures both were above predicted values.
Pathologic Discussion
The mainstays of treatment of papillary thyroid carcinoma include surgical resection, neck dissection, and 131I treatment, depending on the extent of disease.7 The treatment of metastatic lung disease depends on the size of the pulmonary lesions, avidity of the tumor for radioiodine, and stability of the metastatic lesions. 131I is used universally for micronodular metastases, and depending on the avidity of 131I, also can be used for macronodular disease.7 Pulmonary complications of 131I, including pneumonitis and fibrosis, are rare and tend to be dose related.8,9 The American Thyroid Association Guideline Taskforce recommends pulmonary function testing only when there is suspicion of pneumonitis or fibrosis secondary to radioiodine.7 Our patient had no conclusive evidence for radioiodine toxicity. Although it could be argued that the increase in oxygen requirements after 6 months may have been related to pneumonitis, there was no radiographic evidence of pneumonitis on chest CT scan, and his symptoms again improved after treatment with sorafenib, further corroborating the impact of reducing tumor burden to alleviate airflow obstruction. Our patient’s severe hypoxia was likely multifactorial in etiology partly due to extensive tumor obliteration of the alveolar capillary interface and partly due to the degree of airflow obstruction. Our patient’s restrictive lung disease is explained by the extensive tumor burden in his chest that was likely decreasing compliance and limiting lung expansion. There was no evidence for neuromuscular weakness. The presence of restrictive lung disease does not diminish the finding of partially reversible airflow obstruction seen after treatment of the metastatic papillary thyroid carcinoma.
Radiologic Discussion
Response to therapy typically is monitored by radiographic methods, such as radioiodine full-body scanning, fluorodeoxyglucose PET scan, CT scan, and MRI. These methods rely on visual determination of disease burden and often can be difficult to quantify, particularly when disease is extensive. Inspiratory and expiratory chest CT scan classically have been used to detect the presence of focal air trapping in other bronchiolar diseases such as noxious or toxic inhalation, including tobacco use, bronchiolitis obliterans (BOOP), diffuse pan bronchiolitis, asthma, and chronic granulomatous disease.10-14 It has been postulated that for BOOP, asthma, and smoking-related lung disease, particularly for very early or localized airway disease, chest CT imaging indicating air trapping may be the most sensitive method for detection.10,15,16 Nevertheless, there has been debate on how to accurately score air trapping on chest CT scan because the exact CT scan protocols used in the studies examining these diseases often differ, and there is variable interobserver variability. Editorials on this subject note that pulmonary function tests are more likely to miss focal areas of air trapping because they reflect a more global function of the lung. When the lung disease is more diffuse, such as in BOOP, there is a significant correlation between degree of air trapping and FEV1.13 Even though our patient had diffuse disease, he did not undergo inspiratory and expiratory chest CT imaging, but rather, his disease regression and progression were effectively monitored using pulmonary function tests. Initial spirometric values indicating airflow obstruction correlated with chest CT images, showing innumerable enlarging pulmonary nodules and the gross pathology of small airway infiltration by metastatic tumors. The airflow obstruction and degree of hypoxemia determined by serial measurements of ambulatory oximetry were significantly improved after treatment with 131I and sorafenib, suggesting that the initial hypoxia was not related to radioiodine toxicity. When the disease progressed, the degree of airflow obstruction and hypoxemia worsened. Pulmonary function tests were used as a noninvasive method of disease monitoring, and enabled early recognition of disease progression in this patient. He ultimately was referred for sorafenib treatment, which currently is ongoing. Repeat pulmonary function tests performed 2 months after treatment already have shown improvement in the degree of airflow obstruction. We do not suggest that patients be routinely monitored with pulmonary function testing, but spirometry was useful in the case presented. Chest CT imaging instead remains the standard method for monitoring metastatic disease progression and response to therapy. In this context, CT imaging may identify metastatic nodules, effusions, and endobronchial or peribronchial lesions. Chest imaging also may be necessary to evaluate pulmonary emboli, unrecognized emphysema, and infiltrates that may suggest infection or pneumonitis.
Conclusion
Metastatic papillary thyroid carcinoma is an uncommon condition that generally has a relatively good prognosis. However, in cases of severe tumor burden and widespread metastases, the prognosis may be poor. We highlight pulmonary physiology in a patient with metastatic papillary thyroid carcinoma, namely airway obstruction due to infiltrating tumor metastases. To our knowledge, this condition has not been described previously. We demonstrate that airflow obstruction partially improved after treatment with 131I. The decrease in airflow obstruction correlated well with other clinical indices of improvement, such as dyspnea and degree of hypoxia. We were able to noninvasively evaluate disease progression in this particular patient by demonstrating worsening airflow obstruction, and the patient was subsequently referred for experimental therapy. However, radiographic imaging remains the mainstay of monitoring for metastatic disease progression and response to therapy.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Dr Paul Ladenson of Johns Hopkins University for his assistance in the management of this patient, specifically for the sorafenib treatment, and Dr Mark Utell for his critical appraisal of this manuscript.
Footnotes
Funding/Support: This work is supported by the following grants from the National Institutes of Health: T32 HL66988 and NHLBI-R01 HL75432.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Sherman SI. Thyroid carcinoma. Lancet. 2003;361(9356):501–511. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 2.Schlumberger MJ, Torlantano M. Papillary and follicular thyroid carcinoma. Best Pract Res Clin Endocrinol Metab. 2000;14(4):601–613. doi: 10.1053/beem.2000.0105. [DOI] [PubMed] [Google Scholar]
- 3.Hoie J, Stenwig AE, Kullmann G, Lindegaard M. Distant metastases in papillary thyroid cancer. A review of 91 patients. Cancer. 1988;61(1):1–6. doi: 10.1002/1097-0142(19880101)61:1<1::aid-cncr2820610102>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura Y, Shimizu K, Nagahama M, et al. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999;84(11):4043–4049. doi: 10.1210/jcem.84.11.6115. [DOI] [PubMed] [Google Scholar]
- 5.Patel PC, Millman B, Pellitteri PK, Woods EL. Papillary thyroid carcinoma presenting with massive angioinvasion of the great vessels of the neck and chest. Otolaryngol Head Neck Surg. 1997;117(6):S117–S120. doi: 10.1016/s0194-5998(97)70076-7. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly MJ, Considine N, McShane DP. Upper airway invasion by well-differentiated thyroid carcinoma. J Laryngol Otol. 1993;107(8):752–754. doi: 10.1017/s002221510012434x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association Guidelines Taskforce Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16(2):109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Sogutlu G, Leard L, et al. Lung transplantation for pulmonary metastases and radiation-induced pulmonary fibrosis after radioactive iodine ablation of extensive lung metastases from papillary thyroid carcinoma. Thyroid. 2007;17(4):367–369. doi: 10.1089/thy.2006.0234. [DOI] [PubMed] [Google Scholar]
- 9.Dorn R, Kopp J, Vogt H, Heidenreich P, Carroll RG, Gulec SA. Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. J Nucl Med. 2003;44(3):451–456. [PubMed] [Google Scholar]
- 10.Mastora I, Remy-Jardin M, Sobaszek A, Boulenguez C, Remy J, Edme JL. Thin-section CT finding in 250 volunteers: assessment of the relationship of CT findings with smoking history and pulmonary function test results. Radiology. 2001;218(3):695–702. doi: 10.1148/radiology.218.3.r01mr08695. [DOI] [PubMed] [Google Scholar]
- 11.Mendelson DS, Roggeveen M, Levin SM, Herbert R, de la Hoz RE. Air trapping detected on end-expiratory high-resolution computed tomography in symptomatic World Trade Center rescue and recovery workers. J Occup Environ Med. 2007;49(8):840–845. doi: 10.1097/JOM.0b013e3180d09e87. [DOI] [PubMed] [Google Scholar]
- 12.Hartman TE, Primack SL, Lee KS, Swensen SJ, Müller NL. CT of bronchial and bronchiolar diseases. Radiographics. 1994;14(5):991–1003. doi: 10.1148/radiographics.14.5.7991828. [DOI] [PubMed] [Google Scholar]
- 13.de Jong PA, Dodd JD, Coxson HO, et al. Bronchiolitis obliterans following lung transplantation: early detection using computed tomographic scanning. Thorax. 2006;61(9):799–804. doi: 10.1136/thx.2005.053249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godoy MC, Vos PM, Cooperberg PL, Lydell CP, Phillips P, Müller NL. Chest radiographic and CT manifestations of chronic granulomatous disease in adults. AJR Am J Roentgenol. 2008;191(5):1570–1575. doi: 10.2214/AJR.07.3482. [DOI] [PubMed] [Google Scholar]
- 15.Park CS, Müller NL, Worthy SA, Kim JS, Awadh N, Fitzgerald M. Airway obstruction in asthmatic and healthy individuals: inspiratory and expiratory thin-section CT findings. Radiology. 1997;203(2):361–367. doi: 10.1148/radiology.203.2.9114089. [DOI] [PubMed] [Google Scholar]
- 16.Lee KW, Chung SY, Yang I, Lee Y, Ko EY, Park MJ. Correlation of aging and smoking with air trapping at thin-section CT of the lung in asymptomatic subjects. Radiology. 2000;214(3):831–836. doi: 10.1148/radiology.214.3.r00mr05831. [DOI] [PubMed] [Google Scholar]