Abstract
T cell-directed therapies have become mainstays in the management of various autoimmune diseases and organ transplantation. The understanding of T cell biology has expanded greatly since the development of most agents currently in use. Here we discuss important recent discoveries pertaining to T helper cell differentiation, lineage commitment, and function. Within this context, we examine existing T cell-directed therapies, including new agents being evaluated in clinical and preclinical studies. We also use recent findings to speculate on novel targets.
Keywords: CD4+ T helper, autoimmune, immunosuppression, monoclonal antibody, therapy
Introduction
T cells have been implicated in the pathogenesis of a variety of immune and inflammatory disorders and recent gene-association studies have strengthened this link. Polymorphisms in T cell expressed genes, including signaling molecules and cytokine receptors, are now recognized to confer increased risk for various autoimmune diseases. These data in addition to abundant data from mouse models firmly support the notion that targeting T cells is a logical strategy for treating autoimmunity. Additionally, recent advances in our understanding of T cell biology have revealed surprising complexities in the heterogeneity and flexibility of T cells; these insights provide many new opportunities for intervention. In the present review, we will start by summarizing some of the important recent discoveries pertaining to T helper cell differentiation, lineage commitment, and function. We will then discuss existing T cell-directed therapies within this context, and then speculate on new opportunities.
T cell differentiation and function – the classic view
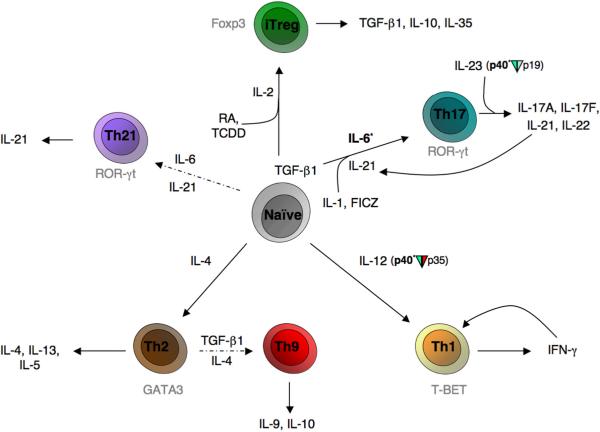
The diversity and complexity of microbial pathogens require equally diverse and sophisticated mechanisms for host defense. One mechanism by which immune responses are tailored to combat offending pathogens is through the differentiation of distinct subsets of CD4+ T helper cells. Through the selective production of cytokines, helper T cells coordinate the responses of other cellular components of the immune system. Classically, naïve CD4+ cells have been thought to differentiate into two possible lineages, T helper 1 (Th1) or T helper 2 (Th2) cells (Figure 1), which are defined by the cytokines these cells secrete when activated.1–5 Th1 cells produce interferon (IFN)-γ, which in turn activates phagocytic cells. This response confers protection against intracellular pathogens such as viruses, Mycobacteria, and protozoa. Th1 differentiation is promoted by a cytokine, interleukin (IL)-12, produced by antigen presenting cells (macrophages and dendritic cells), which activates the signal transducer and activator of transcription 4 (Stat4). Stat4-dependent signaling in conjunction with T cell receptor (TCR)-dependent signals, induces the expression of transcription factor T-box-expressed-in-T-cells (T-bet).5 Recently, in humans, it has been shown that STAT4 polymorphisms are associated with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and Sjogren's syndrome.6, 7 IFN-γ also activates Stat1 which further induces T-bet in an autocrine loop. This enforces IFN-γ production and Th1 differentiation and inhibits Th2 differentiation.
Fig 1. Naïve CD4+ T helper cell differentiation, expression of lineage defining transcription factors and effecter cytokines.
Pathways to established lineages are represented with solid arrows; more controversial lineage pathways are represented with dashed arrows. Transcription factors are labeled in gray. Factors currently being therapeutically targeted for T cells (phase III & above) appear in bold with an asterisk. `Plasticity' and established pathways of interconversion between lineages have not been diagrammed for simplicity. Select abbreviations: FICZ(6-formylindolo[3,2-b]carbazole/endogenous aryl hydrocarbon receptor ligand); RA(all-trans retinoic acid); TCDD(2,3,7,8-tetrachlorodibenzo-p-dioxin/synthetic aryl hydrocarbon receptor ligand)
On the other hand, Th2 cells selectively produce IL-4, IL-5, and IL-13. Th2 differentiation is promoted by IL-4 activation of Stat6, which up-regulates the expression of the transcription factor GATA-binding protein 3 (GATA-3).8 Sources of IL-4 that initiate Th2 differentiation include NK T cells, mast cells and basophils.9 Th2 cytokines are potent activators of B-cell IgE production, eosinophil recruitment, and mucosal expulsion mechanisms, and are essential for promoting host defense against helminths and other parasites. In addition, Th2 cells have been shown to mediate allergic diseases such as asthma, rhinitis, and atopic dermatitis. Th2 cytokines also inhibit Th1 differentiation.
The Th1/Th2 paradigm explained a great deal about the immune response to model pathogens. Moreover, understanding the rules that govern lineage commitment in differentiating T cells has provided many insights into the molecular basis of transcriptional control of lineage-specific T cell cytokines. However, this model also has a number of weaknesses, not the least of which is its ability to explain the pathogenesis of autoimmune disease.10
Th17 cells – the real bad boys
Classically, autoimmune diseases had been assumed to be associated with dysregulated Th1 responses. The demonstration that anti-IL-12p40 antibody was effective in the treatment of Crohn's disease (CD) and psoriasis was interpreted as supporting the notion that IL-12-dependent IFN-γ production and Th1 responses underlie the pathogenesis of autoimmunity.11, 12 However, it was subsequently shown in a number of models that IFN-γ deficiency exacerbated rather than ameliorated autoimmunity. The discovery of a new cytokine, IL-23, which comprises the IL-23p19 and the IL-12p40 subunits, began to clarify this paradox.13 Notably, IL-23p19-deficient mice or IL-12p40-deficient (IL-12/IL-23-deficient) mice were found to be resistant in models of autoimmune disease whereas IL-12p35-deficient mice had increased severity of disease.14 Next, it was recognized that IL-23 selectively induces the production of IL-17 in T cells and this in turn led to the notion that IL-17-producing T cells represent a new lineage of helper T cells, which are the major drivers of autoimmune disease and inflammation.15–17 Subsequently, polymorphisms in the IL-23 receptor gene have been linked to several autoimmune diseases.18–20
IL-17 is now recognized as the prototype of a family of proinflammatory cytokines including: IL-17 (IL-17A), IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25), and IL-17F.21 Th17 cells selectively produce IL-17A and IL-17F, which induce the production of various proinflammatory cytokines such as tumor necrosis factor α (TNF-α, IL-1β, IL-6, granulocyte-macrophage colony stimulating factor and various chemokines. As such, IL-17A and IL-17F are critical for inducing the recruitment of myeloid cells to sites of inflammation; though each appear to have selective importance in different tissues.22, 23. IL-17 is important in host defense against gram negative extracellular bacteria such as Klebsiella pneumonia and Bacteroides fragilis.24
It has subsequently become clear that the initial differentiation of Th17 cells requires more than just IL-23. Initially, it was puzzling that naïve CD4+ T cells did not express IL-23R. This led to the search for factors that initiate Th17 differentiation. We now know that the combination of IL-1, IL-6, and transforming growth factor-β1 (TGF-β1) in conjunction with TCR occupancy optimally induces Th17 differentiation in mice (Figure 1).25–27 As noted, in the classic Th1/Th2 paradigm, T cell subsets produce factors that promote their differentiation and constrain the differentiation to other fates. Interestingly, Th17 cells were found to selectively produce IL-21. Autocrine production of IL-21 by Th17 cells is now recognized to be an important factor that promotes Th17 differentiation and inhibits Th1 differentiation. Meanwhile, IL-23 function has been shown to be critical for expansion and pathogenicity of Th17 cells in vivo. In the absence of IL-23R, Th17 cells failed to generate large numbers of IL-17-producing progeny, accumulate in high numbers in tissue, or successfully initiate inflammation.28 The conditions required for human Th17 cell differentiation have been somewhat perplexing, but this may reflect in part differing dose response requirements for TGFβ-1 in mouse and man.29–32
Of note, IL-6, IL-21, and IL-23 all activate the transcription factor Stat3.33–35 Accordingly Th17 differentiation is abrogated in Stat3-deficient T cells and deletion of Stat3 in T cells abrogates models of autoimmunity.34–38 Humans with hyperimmunoglobulin E or Job's syndrome are for heterozygous mutations of Stat3, which produce a dominant negative protein that can block binding of the wild type Stat3 to candidate DNA binding sites; one consequence of this is the failure to generate Th17 cells.39–41 Stat3 regulation of Th17 differentiation appears to be remarkably direct – i.e Stat3 binds to the Il17 and Il21 loci, as detected by using chromatin immunoprecipitation assays. IL-6 and IL-23 promote IL-23R expression and this too appears to be Stat3-dependent.30, 42, 43
Like other T cell subsets, Th17 cells also have a lineage-specific transcription factor, namely the retinoic acid-related orphan receptor-γt (RORγt).31, 44 Mice lacking this transcription factor have reduced severity in models of autoimmune disease. A related retinoic acid related nuclear receptor, RORα also contributes to Th17 differentiation.45 As discussed below, Th17 cells also express another transcription factor in the steroid receptor superfamily – the aryl hydrocarbon receptor (AHR).46 This recent discovery is a fascinating insight as it provides a mechanism by which environmental stimuli and toxins can modulate T cell responses. Other transcription factors shown to play a positive role in Th17 differentiation include interferon regulatory factor (IRF)4 and Runx1.47, 48
Given their highly inflammatory nature, it should come as no surprise that there are many mechanisms in place to constrain the differentiation of Th17 cells. Remarkably, IL-2, IFN- γ17, IL-449, 50 and IL-27 all inhibit IL-17 production.51 In addition, retinoic acid, a Vitamin A metabolite and product of gut dendritic cells inhibits Th17 cells (discussed below).52–56
It should also be noted that recent work has suggested that CD4+ T cells, which preferentially produce IL-21, may represent a lineage distinct from Th17 cells. Specifically, it was reported that the conditions that drive optimal IL-21 and IL-17 production are different.57 Some reports label the selective IL-21 producers as follicular helper T (Tfh) cells, or T helper cells that regulate the step-wise development of antigen-specific B cell immunity in vivo.58 This fits with our increasing understanding of the importance of IL-21 in Tfh cell function. However, the designation of Tfh as a distinct lineage that aids B cell responses implies that only one lineage of Th cells has this capacity. It can be argued that multiple types of T cells with different capacities to selectively produce cytokines can take up residence in the follicular regions and regulate a spectrum of B cell immunity.59 In some respects, these notions get to the heart of what constitutes a T cell lineage (see below).
Regulatory T cells – the good guys and the bad boys are related!
CD4+ T cells have another critical fate, namely CD4+CD25+ regulatory T (Treg) cells, which we now know are critical for the maintenance of peripheral tolerance. CD4+CD25+ Treg cells express the transcription factor forkhead box protein 3 (Foxp3) and classic studies have amply demonstrated that the absence of Treg cells results in fatal autoimmunity in mouse and in man.60–64 Treg cells suppress proliferation of effector T cells and maintain self tolerance by down-regulation of immune responses. The mechanisms by which Tregs preserve peripheral tolerance are still controversial; however they preferentially express Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4), and the critical immunosuppressive cytokines TGFβ–1, IL-10 and IL-35.65
Naturally arising Treg (nTreg) cells are generated in the thymus, whereas naive T cells are converted in the periphery to become inducible Tregs (iTregs), especially in the gut. Common gamma chain cytokines are critical for nTreg development, and in vitro, IL-2 and TGF-β1 can convert CD4+CD25− naïve T cells into CD4+ CD25+ Foxp3+ Treg cells. IL-2 activates Stat5, which is essential for Treg cell development and in vitro conversion and appears to directly regulate the FOXP3 gene.66, 67 Of note, IL-2-, IL-2 receptor- and Stat5- deficient mice show enhanced T cell lympho-proliferation and severe autoimmunity, likely caused at least in part by a lack of Tregs.68, 69
The fact that TGF-β1 is important for the differentiation of both Treg and Th17 cells led to the idea that these lineages were related. Whereas, IL-2 in the context of TGFβ-1 promotes iTreg differentiation, this combination of cytokines inhibits Th17 differentiation.25–27, 37 Conversely, IL-6 and TGFβ-1 inhibit Foxp3 expression and enhance IL-17 production.25 Moreover, recent data also supports the idea that, depending on its local concentration, TGFβ–1 can promote either Th17 or Treg cell differentiation.70 As noted above, retinoic acid inhibits IL-17 but this factor enhances Foxp3 expression.52–56 More complex is the effect of arylhdrocarbon ligands. Remarkably, some ligands enhance Foxp3 expression; whereas other ligands promote IL-17 secretion. Equally surprising is that Foxp3 and RORγt appear to interact and can modulate each other's activity on target genes.70 Taken together, these data support the notion that Th17 and Treg cells are clearly related. This is a remarkable notion given the opposing functions of these cells.
Complexity and heterogeneity of T cells
Although some cytokines are clearly lineage restricted, other cytokines are made by multiple subsets of T cells. One example is IL-10, which was initially found in Th2 cells, but was subsequently linked with Treg cells. In fact, recent studies show that IL-10 can be made by virtually any CD4+ T cell subset including Th17 cells71.71 Interestingly, IL-27 not only downregulates IL-17, it also promotes IL-10 production.72 This might be viewed as a good example of the “lineage” vs. “flexible” view of T cell differentiation. What does one call a Th17 cell that has been exposed to IL-27? In contrast to the combination of TGF-β1 and IL-6, which both induces IL-17 and IL-10, IL-23 enhances IL-17 production alone in Th17 cells, promoting the pathogenicity of these cells.73
Another example of a cytokine that might not strictly fit with the “lineage” view of CD4+ helper cells is IL-22. IL-22 is a critical cytokine for host defense and has been proposed as a key mediator in psoriasis models, but also has important anti-inflammatory properties.74–78 Although IL-22 was also originally described as a Th1 cytokine, IL-22 has been reported to be preferentially produced by Th17 cells79; however, other data indicate that the cytokines that regulate optimal IL-22 production are distinct from those that generate IL-17.42 Yet, it is clear that IL-23 is a critical in vivo regulator of both IL-22 and IL-17.74, 77
Providing support for a more “flexible” view of Th cell differentiation is a recent study in which genome-wide epigenetic modifications were mapped in polarized Th1, Th2, Th17, & Treg cells and compared to unpolarized “naïve” CD4+ cells. Surprisingly, patterns of modification indicative of “poised” expression were noted in the loci of the “master regulators” Gata3 and T-bet, providing a potential mechanism for plasticity in Th17 and Treg cells.80 Supporting this notion were recent studies indicating that IL-17 producing cells have the propensity to become IFN-γ producers, but not the reverse.81, 82
Further blurring the classic Th1/Th2 dichotomy was a recent report of polarized Th2 cells being converted into stable IL-9 producers when cultured in the presence of the Th17/iTreg important cytokine TGF-β1.83 Clearly, there is still much to learn about T cell heterogeneity and plasticity within existing lineages.
Targeting T cells
Given their central role in modulating immune responses, it is logical to conclude that targeting T cells would be an effective means of accomplishing therapeutic immunosuppression. There are ample examples of existing therapies that support this contention. The ultimate goal is to refine therapies to improve efficacy and to minimize toxicity. In the next sections, we will consider present and potential strategies. Conceptually, we have divided these strategies into five different approaches, namely (1) therapies that deplete T cells, (2) therapies that alter T cell trafficking or adhesion, (3) therapies that block T cell receptor (TCR) function, (4) therapies that interfere with costimulatory signals and finally (5) therapies that interfere with cytokines that act on, or are produced by T cells.
Depletion of T cells using antibodies
An obvious approach for therapeutic immunsuppression, which has been in use for many years, is to simply deplete T cells. At first, polyclonal antibodies against thymocytes were used.84 Raised in either horses (ATGAM) or rabbits (Thymoglobulin), these polyclonal antibodies bind various T cell surface molecules and produce depletion of T cells through Fc receptor–mediated phagocytosis and complement-mediated lysis. Unfortunately, the polyclonal nature of such preparations affects many cell types and results in a nonspecific immunosuppression that leads to substantial infectious morbidity. Additionally, the repeated use of these agents can result in the formation of neutralizing antibodies to horse or rabbit immunoglobulin. Despite these problems, these agents are still used in certain high-risk transplant rejection cases and autoimmune diseases.
Next onto the scene, were the monoclonal antibodies (mAbs), which allowed for enhanced selectivity in depletion. The first of these to see substantial clinic use was OKT3, a mouse mAb to human CD3ε. It was the first Food and Drug Administration (FDA) approved monoclonal antibody for treatment in transplantation and remains one of the most potent immunosuppressive drugs available.85 Unfortunately, besides the usual risk of opportunistic infections and malignancy associated with immunosuppressants, OKT3 usage can also result in a sepsis-like syndrome referred to as “cytokine storm”. This severe complication occurs because anti-CD3 antibodies activate T cells, inducing rapid release of tumor necrosis factor (TNF) and other T cell-derived cytokines, which can lead to organ failure and even death.86 This risk has effectively limited OKT3's usage to the acute transplant rejection setting. In the treatment of less life-threatening autoimmune diseases, such as multiple sclerosis (MS), minimal efficacy was seen with considerable morbidity.87 To reduce this toxicity, modified anti-CD3 mabs have been engineered that produce an attenuated activating signal (Teplizumab, Otelixizumab, Visiluzimab).88 These agents produce milder T cell depletion. Additionally, they may induce apoptosis, promote tolerance, expand regulatory cells and produce anti-inflammatory cytokines like IL-10.89 These agents have shown promise in psoriatic arthritis,90 renal transplantation91 and recent onset type 1 diabetes.92–94 Oral administration of anti-CD3 antibodies has also been reported to induce regulatory T cells and ameliorate disease in autoimmune disease models.95, 96
CD52 is expressed on a variety of leukocytes and a mAb against CD52, alemtuzumab (Campath-1H), also very effectively depletes both B and T lymphocytes. This drug is efficacious in both solid-organ and hematological transplants, as well as refractory vasculitis, MS, and RA.97–101 Alemtuzumab's effects in RA appear to be transient, whereas its utility in early MS treatment are more durable; phase III clinical trials in the latter disease have been initiated.102 It has been argued that anti-CD52 could provide a costimulatory signal for CD4+ T cells. This phenomenon appears to lead to an induction of Tregs in vitro103 and CD4+ CD25high T cells are reportedly detected in relative abundance during the early reconstitution phase of alemtuxumab-treated patients. Curiously, a complication associated with alemtuzumab use is autoimmune thyroiditis.98
The paradoxical association of autoimmune disease induced by immunosupressives is not limited to alemtuxumab and is also seen in patients with inherited immunodeficiencies.104, 105 It should also be emphasized that CD52 is widely expressed, being found on lymphocytes, monocytes, macrophages, NK cells, certain granulocyte subpopulations, a proportion of bone marrow cells, and some CD34+ cells. Consequently, the depleting effect is not limited to T cells and pancytopenia is a common side effect.98
CD2 is a cell adhesion molecule expressed on mature T cells and most NK cells. Due to its ability to mediate interactions with antigen presenting cells, CD2 has mostly been a target for costimulation blockade (see below), however, several depleting anti-CD2 mAbs have also been developed.106, 107 Among these, BTI-322 has shown efficacy in acute kidney transplants108 and siplizumab (a humanized version of BTI-322) is being studied in nonmyeloablative stem cell transplantation, lymphoproliferative disorders, and psoriasis.109 In psoriasis, this drug resulted in a >75% reduction in severity in more than half of psoriasis patients. Adverse effects included flu-like symptoms and lymphopenia.110
In principle, depleting a specific subsets of T cells would be advantageous, especially if one could target surface molecules that did not trigger lymphocyte activation and production of cytokines. Disappointingly though, a variety of mAbs to at least three different epitopes of CD4 have failed to provide consistent efficacy in RA, despite substantial depletion of T cells.111, 112 This was initially thought to indicate a T cell-independent component of RA; however, we now know that targeting co-stimulatory molecules on T cells is efficacious (see below). One hypothesis is that the CD4 mAbs deplete beneficial regulatory subsets as well as pathogenic ones.113 Additionally, with the recognition of new T cell subsets, it is becoming increasingly obvious that further selective depletional strategies might be considered. Th1, Th2, Th17 and Treg cells have all been proposed to have distinctive surface receptors. Th1 cells express CCR5, CXCR3, and CXCR6,114–117 whereas Th2 cells express CCR4 and Spr44.114, 118–120 Th17 cells have been proposed to express CCR6, CCR4, and CCR2, but not CXCR3 or CCR5, although recent reports suggest increased heterogeneity within the Th17 lineage.121 Targeting of these chemokine receptors with mAbs is of great interest, but expression in multiple lineages, such as CCR4, CCR5, and more recently CCR6, significantly complicates matters.
Another alternative method to target autoreactive T cells is by promoting programmed cell death following restimulation.122, 123 CD95 (Fas) is responsible for TCR-induced apoptosis in CD4+ T cells and was an obvious early target for mAb therapy. Unfortunately, administration of the anti-Fas mAb Jo2 resulted in fulminant hepatitis, hemorrhage and death in mice due to Fas crosslinking on hepatocytes.124 Efforts are being made to eliminate Fc-mediated antibody crosslinking in the hopes of reducing this severe complication.125 Other strategies are being also being considered, such as, conjugating anti-Fas mAb to beads along with anti-HLA antibody molecules to generate “artificial killer antigen presenting cells” that could deplete antigen-specific T cells in a Fas/Fas ligand dependent fashion.126
Targeting lymphocyte trafficking and adhesion
A major means of regulating lymphocyte trafficking is through the action of chemokines, which have discrete effects on different lymphocyte populations. Chemokines signal through seven-transmembrane G protein-coupled receptors, which is a class of receptors that is highly “drugable”. As a result, interfering chemotaxis of immune cells seems like a reasonable therapeutic strategy. Theoretically, depending upon the chemokine receptor targeted, one could generate compounds that might affect a variety of leukocytes or have restricted effects on specific subsets.127 Work in this area is still very much ongoing, as investigators continue to elucidate the role of each chemokine in different subsets.
Thus far, the most well characterized immunosuppressant in this class is FTY720 (fingolimod), which interferes with the trafficking of all subsets of T cells. Sphingosine 1-phosphate (S1P) is abundant in plasma and lymph and promotes egress of lymphocytes from the thymus and lymphoid tissues via binding to the S1P type 1 receptor. FTY720 is a S1P analog and serves as a functional receptor antagonist by binding, and down-regulating S1P receptors, thus preventing migration of lymphocytes into blood and lymph.128 Human trials have shown that the drug reduces circulating lymphocyte counts by 85% and prevents acute rejection in transplant cases.129 It has also shown effectiveness in autoimmune disorders such as MS.130 Worth noting, is that signaling through the S1P type 1 receptor enhances IL-17 production.131, 132 FTY720 has also been reported to inhibit Th1 cells and enhance Treg activity.133 Though lymphocyte sequestration likely remains the primary mechanism by which FTY720 improves autoimmune pathology, inhibition of Th1 and Th17 cells also remains a possible contributory mechanism.
With FTY720 establishing the “proof of concept” in targeting of chemokine receptors, other agents are being developed and tested in the setting of autoimmune disease.127 Since Th1, Th2, Th17, and Treg cells may distinct chemokine receptor usage, selective chemokine receptor antagonists might be an effective way to target specific populations. A CCR5 antagonist, maraviroc, has been developed as an antiretroviral in HIV treatment and is safe and effective in the disease.134 In contrast, another CCR5 antagonists, vicriviroc and aplaviroc, have had unacceptable toxicities.135 In principle, CCR5 antagonists could also be useful based on their immunoregulatory properties and are effective in preclinical models.136 CCL25-CCR9 interaction are important in homing of T cells to the small intestine,137 and a small-molecule antagonist is being tested in CD.138 Likewise, CCL27-CCR10 and CCL17- or CCL22-CCR4 interactions play an important role in skin homing and therefore represent attractive targets for psoriasis and atopic dermatitis.139 Antagonists of CCR4 are also being tested,140 although expression of CCR4 expression on Tregs may be a complicating factor.141
Adhesion molecules are also essential for proper lymphocyte trafficking to areas of inflammation, and targeting such molecules has been another successful immunosuppressive strategy. Natalizumab, a mAb to α4 integrin, interferes with the adhesion of leukocytes to vascular endothelial cells through effects on CD49d (α4β1) and LPAM-1 (α4β7). Clinical trials in MS and CD have documented efficacy;142 however, in 2005, its use has been associated with progressive multifocal leukoencephalopathy (PML). This opportunistic viral infection of the brain, which is often fatal, was only noted when natalizumab therapy was accompanied with other immunosuppressive regimens. After initially withdrawing the drug, the FDA has allowed the drug to be used in MS with a “black box” warning, however further reports of PML have recently surfaced. Th1 and Th17 cells express high levels of α4β1, whereas Th17 cells alone expressed high levels of α4β7 in vivo.143 Specifically targeting α4β7 may be advantageous.144, 145
Another strategy is disrupting ICAM-1 (CD54)/LFA-1 (CD11a/CD18) interactions. LFA-1 is found on all T cells and is involved in recruitment to sites of inflammation. LFA-1 initially binds weakly to ICAM-1, but TCR signals change the conformation and increase binding affinity. Enlimomab, a murine mAb to human ICAM-1, showed limited efficacy in a small population of RA patients in phase I/II trials.146 However, subsequent trials in transplantion showed no efficacy.147 This drug was also associated with increased mortality rates among stroke patients on the drug. In contrast, efalizumab, a humanized mAb to human CD11a is efficacious for the treatment of psoriasis and severe atopic dermatitis.148–150. It is now FDA approved and appears to be effective in patients with severe atopic dermatitis as well.151 However, several recent reports of PML have arisen and the drug has been pulled from the market.
An anti-sense inhibitor of ICAM-1 synthesis, alicaforsen, may have some efficacy in CD152, but its utility is still being assessed153–157. Another important receptor/co-receptor pair that mediates adhesion is CD2 and LFA-3. Alefacept is an Fc-fusion protein comprising the extracellular portion of LFA-3, approved for the treatment of psoriasis and reported efficacious in atopic dermatitis and pyoderma gangrinosum.158–160 It is also being studied in graft versus host disease (GVHD) and renal transplantation.
Interfering with TCR-mediated signaling
Thanks to the efforts of many investigators, we know a great deal about the biochemistry of T cell activation.161–164 Activation of the transcription factor, nuclear factor of activated T cells (NFAT) is a key step in T cell activation and the production of cytokines. Interestingly, cyclosporin A and tacrolimus (FK506), highly successful immunosuppressive drugs that were generated empirically, were subsequently found to inhibit calcineurin-dependant dephosphorylation of NFAT proteins.165 Their usage has revolutionized solid organ transplant; in 2005 nearly 70% of kidney transplant recipients received tacrolimus as a component of their maintenance immunosuppression.166 Unfortunately, despite their clinical efficacy, use of calcineurin inhibitors is limited by toxicity due to the ubiquitous nature of their target. In addition to the usual enhanced risk of infection and malignancy, calcineurin inhibitors are associated with nephrotoxicity, neurotoxicity, hypertension, lipid abnormalities, glucose intolerance, hyperkalemia, hypomagnesemia and gastrointestinal disturbances.166 Such toxicities limit the application of these drugs in the treatment of autoimmune disease, despite their showing efficacy in atopic dermatitis, RA, cutaneous lupus erythematosus, MS, myasthenia gravis, and type I diabetes mellitus.
Despite these issues, the effectiveness of the calcineurin inhibitors strongly supports the idea that interference with TCR signaling is an effective immunosuppressive strategy. Conceptually, targeting molecules that have key functions only in lymphocytes should provide similar benefit with less toxicity. Early events in TCR signaling include the activation of the Src family kinases Lck and Zap-70, as well as Tec family protein tyrosine kinases. Three Tec family kinsases, Itk, Rlk, and Tec are all expressed in T cells. Itk knockout mice show defects in Th2 development and are resistant to asthma induction, whereas Rlk-deficient mice have a Th1 developmental abnormality.167 Of special interest in the Src family is Zap-70 which is only expressed in T and NK cells and whose deficiency results in severe combined immunodeficiency. Although a truly selective inhibitor has not yet been generated, it remains a very attractive target.168, 169 Also a possibility is targeting adaptor proteins (CARMA1, Bcl-10, and MALT1) that link TCR-induced signaling to NF-κB. Additionally, the novel PKC isoform, PKC theta, has become a target of increasing interest based on the phenotype of knock-out mice. Unlike the majority of PKCs, which are ubiquitous in distribution, PKC theta has restricted expression in skeletal muscle and T cells.170 Following TCR engagement, PKC theta is recruited into a membrane-proximal signaling complex and serves as an essential signaling intermediary in the pathway to IL-2 expression, affecting NF-κB, NFAT, and AP-1 signals.171, 172 PKC theta knock-out mice are resistant to mouse models of IBD and MS, possibly due to an impairment in Th17 differentiation.173; as such this enzyme appears to be an attractive target.
The inherent complication in general TCR signaling blockade, as well as in most of the other targeting strategies discussed in this review, is that both helpful and pathogenic responses are blocked, resulting in alleviation of symptoms at the cost of increased chance of infection and malignancy. The “Holy Grail” of treatment modalities would of course be to block pathogenic responses in an antigen-dependant manner. It has been long argued that TCR stimulation in the absence of costimulation, T cells results in anergy.174 An attractive targeting strategy is thus to provide T cells with their specific MHC-peptide complex to induce non-responsiveness. With this in mind, a single-chain two-domain (α1β1) MHC class II molecule capable of binding and forming stable complexes with antigenic peptide was generated. When loaded with cognate peptide, this recombinant T cell ligand (RTL), was able to selectively inhibit CD4+ T cells with this specificity.175 Subsequently, it has been shown to be effective in several animal models of MS and a phase I trial for MS has started with one of the RTL's, RTL1000.176, 177 One obvious problem with such acute specificity is that targeting of the wrong T cell set eliminates the efficacy of the treatment. For such a treatment strategy to be effective, it will be critical to define the specific autoreactive T cell population(s) in each autoimmune disease.
Targeting costimulatory and accessory molecules
An important co-stimulatory molecule on T cells is CD28, which binds the B7 family surface molecules CD80 and CD86 expressed on activated antigen presenting cells. CTLA-4 (CD152) is a CD28-related molecule upregulated in activated T cells, which competes with CD28 for CD80 and CD86 binding and effectively terminates costimulation. A fusion protein comprising the extracellular domain of CTLA-4 and the human IgG1 constant region (abatacept) is approved for use in RA and is currently being studied in many other autoimmune conditions, including inflammatory bowel disease (IBD), SLE, type I diabetes and MS.178 Curiously, abatacept is not an effective immunosuppressive drug for transplant rejection. However, mutagenesis was used to produce a molecule with tenfold higher affinity for B7 molecules, belatacept, which is currently being studied in the setting of allotransplantation. While this drug appears to be effective, post-transplant lymphoproliferative disease has been associated with its use.179 Galiximab is a mAb directed against CD80, which has shown efficacy in psoriasis but was associated with increased infections.180
Considering the relative success of abatacept in RA, direct targeting of CD28 with mAbs has also been attempted. Based on the logic that CD28 is important for the generation of Treg cells in mice, investigators used a superagonist anti-CD28 mAb TGN1412 in healthy human volunteers in hopes of selectively expanding this population of T cells. Unfortunately, the treatment resulted in a cytokine storm and multiorgan failure.181 Despite the abysmal failure of TGN1412, use of an antagonist antibody may still be a rationale strategy.
Additionally, attempts have been made to generate nondepleting antibodies against CD4. Zanolimumab has been studied in psoriasis, but had no significant effect on disease.182 Additionally, a phase II/III trial on patients with active RA who failed MTX and TNF blocking therapy has also been completed.183 It should be noted that zanolimumab does have some depleting activity and has been utilized in the treatment of T cell lymphomas.184
Blockade of costimulation through TNFR family members
A number of receptors in the TNF-receptor superfamily (TNFR) function as T cell costimulators in parallel with CD28.185 These include HVEM, GITR, 4-1BB, CD30, CD27, OX40, and DR3 and their TNF-family ligands. Although all of these receptors signal through TRAF adapter proteins to activate common signaling pathways such as NF-κB and MAP kinases, each receptor functions in vivo to boost specific T cell subsets at different phases of the T cell response. Like CD28, HVEM and CD27 function in T cell priming, whereas OX40, 4-1BB and DR3 share the property of specifically costimulating effector T cells, which predicts that blocking their action could be particularly important in reducing T-cell mediated immunopathology. OX40 sustains T cell effector responses through promoting the long-term survival of these cells, and functions more prominently in CD4+ responses, while 4-1BB has a similar role, but primarily in CD8+ T cell responses. DR3, another TNF receptor expressed specifically on T cells functions to enhance accumulation and function of CD4+ T cells at the site of inflammation in a variety of autoimmune disease models and has very little effect on primary T cell responses.186 This specificity predicts that blockade of individual TNF receptors may produce less generalized immunosuppression, allowing a targeted approach to alter T cell responses. In fact, agents blocking a number of TNF-TNFR family interactions critical in T cell biology have shown promise in pre-clinical studies and clinical trial settings. For instance, blocking anti-OX40-ligand antibodies are effective in treating EAE and an asthma model.187, 188 A humanized anti-CD70 monoclonal antibody has been engineered and may also be suitable for use in treatment trials of autoimmune disease.189
The TNF-family CD154 (CD40 ligand), is upregulated on activated T cells, and engages CD40 on antigen producing cells to produce various cytokines. Mutations of CD40 or CD40L underlie a primary immunodeficiency syndrome termed hyperimmunoglobulin M syndrome. As such, CD154 has also been suggested to be a potential therapeutic target. Preclinical transplantation model studies showed anti-CD154 to be very effective.190 However, the much anticipated trial of hu5C8, a humanized anti-CD154, in primary renal allografts was stopped prematurely due to the unexpected complication of thrombotic events. This has resulted in the cessation of the clinical development of anti-CD154 in both transplantation and autoimmunity.191 However, anti-CD40 has proven beneficial in mouse models of lupus192 and a new humanized anti-CD40 mAb, 4D11, is effective in kidney transplantation models.192, 193
Targeting cytokines and cytokine signaling
Both cytokines and their receptors are either expressed on the surface of cells or are secreted into the extracellular environment, making them prime targets for inhibition via mAbs and soluble receptor-Ig fusion proteins. However, there were several reasons why cytokine therapies might not be effective. Cytokines have redundant and complex actions and their effects include both proinflammatory and anti-inflammatory actions. Cytokines induce other cytokines and in many cases we do not fully understand the hierarchy of cytokine action in human diseases. However, despite these complexities, the success of TNF blocking therapies (etanercept, infliximab, adalimumab) are notable. These agents revolutionized the treatment of RA and other diseases including psoriatic arthritis, ankylosing spondylitis, psoriasis, and CD. Similarly, inhibition of IL-1 with recombinant IL-1 receptor antagonist, anakinra, is highly effective in diseases such as neonatal-onset multisystem inflammatory disease (NOMID) and adult-onset Still's disease, but is only modestly effective in RA.194, 195 Of interest, is that combined therapy with IL-1 and TNFα blockade in RA patients does not enhance efficacy and is associated with an increased incidence of serious infections, and the two treatments are not recommended for co-administration as a result.
Targeting T cell-derived cytokines or cytokines that act on T cells has already shown some measure of success. The mAbs daclizumab and basiliximab bind the IL-2 receptor α-subunit (CD25), blocking IL-2's autocrine growth signal. Both drugs seem to be well tolerated clinically and reduce the frequency of acute rejection in solid-organ transplantation.196, 197 Additionally, daclizumab has been shown to reduce disease severity in animal models of arthritis, and is being tested in uveitis, aplastic anemia and MS.198 However, IL-2's role in promoting differentiation of Treg cells and inhibiting differentiation of Th17 cells complicates matters. In both mice and humans, absence of one of the IL-2 receptor subunits results in autoimmune disease. Consequently, one might expect that interference with IL-2 signaling could precipitate autoimmunity. As of yet, this has not been seen with these drugs, but will need to be monitored in the future as a potential complication.
Also being targeted is the IL-2 receptor-β (CD122), which would ameliorate both IL-2 and IL-15 signaling. In a phase I trial for large granular lymphocyte leukemia, the murine monoclonal antibody for CD122, Mikβ1, did not improve clinical symptoms despite the effective inhibition of both IL-15 and IL-2 signaling in T cells.199 The treatment was not associated with autoimmunity, opening the door for further trials in autoimmune diseases. IL-15's proposed role in the maintenance of memory T cells and in the recruitment of T cells to sites of inflammation makes it a particularly attractive target and clinical trials in RA and psoriasis are underway.
Another important T cell-derived cytokine is IFN-γ. One approach for blunting Th1 mediated pathology has been the targeting of IFN-γ with the mAb fontolizumab. In a phase I–II trial, it was well tolerated and doubled the clinical response rate in patients with CD.200 However, a phase II trial in RA was recently terminated.183 Care is warranted in the blockade of IL-12/IFN-γ signaling however, as human mutations in this pathway escalate opportunistic infections.201 Also being evaluated as potential targets are the type I interferons; there is ample evidence that IFN-α plays a pivotal role in a number of autoimmune diseases, including SLE, thyroiditis, and diabetes.202 Type I IFNs are normally produced by plasmacytoid dendritic cells (pDC) in response to viral infections, but in certain autoimmune conditions such as SLE, these cells are also induced to synthesize IFN-α via Toll-like receptor (TLR) ligation by endogenous derived nucleic acids. Within this context, IFN-α leads to activation of autoreactive T cells.203 Neutralizing mAbs against anti-IFN-α have been developed and a recent phase I clinical trial using a single injection of MEDI-545 in patients with SLE reported a dose-dependent inhibition of type I IFN–inducible genes, as well a reduction in clinical disease activity. No safety problems appeared during this short-term study.204 Phase II studies in SLE are currently underway, as well as phase I studies in dermatomyositis and polymyositis.
As opposed to blocking discrete cytokines or their receptors, an alternative approach has been to target select functional components of their intracellular signaling pathways. Binding of interferons and many interleukins results in phosporylation of the Janus kinases (Jak1, Jak2, Jak3 and Tyk2) and the cytokine receptor subunits; this phosphorylation step is critical for the initiation of downstream signaling.205, 206 Of the Jaks, Jak3 seems to have the most discrete function, as it associates with only one cytokine receptor - the common gamma chain or γc. This is a shared receptor subunit that pairs with other ligand-specific subunits to form the receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21. Severe combined immunodeficiency, characterized by the absence of T cells and NK cells and impaired B cell function, can be caused by mutation of the gene encoding either γc or Jak3.207 Notably, no non-lymphoid tissue or organ is affected by the defect, suggesting that actions are restricted to hematopoietic cells. All of this very compelling data led to the development of an orally available, Jak3 antagonist, CP690,550, with nanomolar potency.208 The therapeutic effects of CP 690,550 have been evaluated in clinical trials in CD, psoriasis, RA and kidney transplantation.209 Overall, the results show that the compound appears to be an effective immunosuppressant. The toxicities that appear to be related to this compound include anemia and neutropenia, which might be the result of inhibiting other members of the Jak family.210
The discovery of a subset of myeloproliferative disorders (MPDs) associated with Jak2 mutations has stimulated the consideration of Jak2 as a reasonable therapeutic target. At present, there are at least fifteen clinical trials in MPDs using various inhibitors that target Jak2, only some of them selectively. One potent Jak1/2 inhibitor, INCB18424 (Incyte) has been studied in Phase I and II trials in patients with myeloproliferative disorders, but is also being tested in RA and psoriasis. Generation of a selective Tyk2 inhibitor is also of potential interest, given its importance in IL-12 and IL-23 signaling. In fact, mice with a mutation in Tyk2 have been shown to possess considerable resistance to the development of collagen-induced arthritis.211 One potential downfall of such a scheme is that Tyk2 is known to be important in IFN signaling and its blockade could lead to problems with viral infection, as observed in patients with Tyk2 deficiency.212 Just downstream of the Jaks, the STAT family of proteins might also serve as good potential targets and could theoretically be inhibited by phosphorylated peptidomimetics and “decoy” oligonucleotides.213 Additionally, the endogenous feedback inhibitors of the STATs, the SOCS family of proteins have been considered as a potential target.
mTOR (mammalian target of rapamycin) is a critical link in coupling cell growth stimuli with cell cycle progression downstream of cytokine receptors.214 Rapamycin, also known as sirolimus, is approved for use in allograft rejection, but in addition, there are currently three rapamycin derivatives undergoing clinical trials: temsirolimus, everolimus, and AP23573. Beyond benefits in transplant rejection, temsirolimus has shown effectiveness in RA and phase II MS trials.215 Some of these effects may be mediated by the recent finding that mTOR blockade promotes the differentiation of regulatory T cells while inhibiting the Th17 population.216 However, the ubiquitous nature of mTor means that targeting this molecule can result in a host of side-effects.
Tregs as a therapeutic modality
In both humans and mice, the importance of Foxp3-expressing Treg cells is well-established.61–64 Furthermore, transfer of isolated murine Treg cells routinely inhibits disease in models of autoimmune disease including: IBD, RA, MS, diabetes mellitus, SLE, and IPEX.217, 218 While administration of Tregs is straightforward in mice, there are a number of issues that must be addressed in human trials. The first is how to select pure populations of human Tregs. Tregs are defined as CD4+CD25+ cells; however, CD25 is also a marker of activated T cells, making it difficult to ascertain whether one is enriching Treg or effector cells. The second issue is that Foxp3 expression correlates less well with immunosuppressive activity in human cells than it does in the mouse, as activated human T cells also express Foxp3. Much effort at present is being devoted to finding other markers that reliably enrich Treg vs effector cells. One candidate is the IL-7 receptor (CD127) which has been proposed as a negative marker for Treg cells.219
In principle, one could also imagine inducing Treg cells from naïve precursors with the appropriate cytokine cocktail (e.g. TGFβ-1 and IL-2); however, this raises the issue of the stability of the Treg cell phenotype. Recent studies show that Treg cells have the capacity to differentiate into pro-inflammatory Th17 cells.220 Furthermore, their ability to produce TGFβ-1 can promote naïve cells to become either Treg or Th17 cells depending upon the cytokine milieu.27 The potential for flexibility and plasticity within the Treg and Th17 lineages is a fascinating issue, but certainly complicates the therapeutic use of Tregs. Such findings raise the disturbing possibility that administration of Tregs into a host with inflammation and high levels of IL-6 levels could backfire and enhance rather than alleviate pathology.
To avoid the issues of cell transfer, one might speculate that a logical approach to expand Tregs in vivo would be the administration of IL-2, given the role of IL-2 in Treg differentiation and the fact that IL-2 knockout mice develop severe autoimmunity221. However, it needs to be borne in mind that IL-2 is the prototypic T cell growth factor. It is a critical positive regulator of immune responses, having an essential role in T cell memory.222 It also is a potent inducer of proinflammatory cytokines. Based on this activity, IL-2 is an FDA approved drug for use in patients with renal cell carcinoma; however its use is limited by a sepsis-like, cytokine release syndrome, not exactly what one would expect to be beneficial for the treatment of autoimmune disease. Whether it will be possible to balance the pro- vs. anti-inflammatory actions of IL-2 in a therapeutically useful manner remains to be determined. Administration of TGFβ-1 has also been considered, but like IL-2, it too has pro-inflammatory actions, as well as profibrotic effects.223 As discussed below, retinoic acid and arylhydrocarbon receptor agonists can also regulate Foxp3 expression and affect the balance between Treg and Th17 differentiation.46, 55, 224, 225 As indicated, modified anti-CD3 antibodies may also exert their effect by promoting the production of Tregs and IL-10 from CD4+CD25− T cell precursors.226, 227 The suppressive action of these cells is thought to be TGF-β1 dependant and they express low levels of Foxp3.89
However, all of these therapies hinge on the assumption that an increase in the number of Treg cells would be beneficial for the treatment of autoimmune disease. The preclinical studies discussed above certainly suggest that Tregs can prevent autoimmunity, but few have shown that an enhancement of Treg numbers can be used in a therapeutic setting. In fact, Tregs have been shown to be present in high numbers at sites of local inflammation, suggesting that simply increasing their numbers may not prove therapeutic.228 Additionally, nonspecific immunosuppression and predisposition to infection or malignant disease is a huge concern with Treg therapies, especially if antigen specificity cannot be assured. This holds particularly true in autoimmunity due to the chronic nature of therapies for these diseases.
Given the complexities associated with administration or expansion of Tregs, it might be argued that it would be beneficial to simply administer Treg products. Recombinant human IL-10 was tried initially in patients with psoriasis, with some benefit.229 However, when used in patients with RA in a small study, it failed to show any clinical improvement.230 Studies in Wegener's granulomatosus patients (phase I) and psoriasis patients (phase II) have been completed, but the results have yet to be reported. Recently, it has been reported that IL-35, a heterodimeric cytokine comprising p35 and EBI3, is preferentially produced by Treg cells and has immunosuppressive actions.65 Interestingly, administration of IL-35 in an arthritis model expanded Tregs and decreased disease severity.231 However, because the suppressive effects of Tregs are thought to be multifactorial, involving both contact-dependant and –independent mechanisms, recapitulation of all of the effects of Treg suppression by administration of a single cytokine is likely to prove difficult.
Strategies for targeting Th17 cells
Because of the critical role of IL-17 in autoimmunity, much thought is presently being given to targeting Th17 cells. Ironically, before we even understood the differentiation of Th17 cells, an apparently effective therapy had been generated. Specifically, targeting the p40 subunit of IL-12 has been shown to be effective in the treatment of IBD and psoriasis.11, 12 However, we now know that p40 is a subunit of IL-12 and IL-23 and the salutary effects of this drug are more likely to be due to interference with the latter. Two recent phase III trials with an anti-p40 mAb, ustekinumab, showed significant improvement in psoriasis symptoms, with side-effects comparable to placebo treated groups.232, 233 Ustekinumab also provided significant improvement in a phase II psoriatic arthritis trial, but failed to demonstrate efficacy in an MS trial.234, 235 Clearly the utility of anti-p40 mAb in the treatment of a variety of other autoimmune diseases will be an intense area of investigation. In addition, it will be important to ascertain the relative benefit of targeting IL-23 selectively (anti-p19 mab) versus IL-12 and IL-23 jointly (anti-p40).
IL-6 is one of the most prominent cytokines found within inflamed joints. It has long been recognized as a critical proinflammatory cytokine and inducer of the acute phase response. More recently, we have learned that IL-6 is also a critical differentiation factor for Th17 cells. This new data has increased excitement over tocilizumab, a humanized mAb against IL-6 receptor α subunit. Very recent studies indicate that tocilizumab appears to be as efficacious as anti-TNF therapies in RA, both in terms of reducing symptoms and in inhibiting joint destruction.236–239 Additionally, this treatment has also been shown to be quite useful in the treatment of systemic-onset juvenile arthritis.240 This drug is approved for use in Japan and Europe in adult patients with moderate to severe RA who respond inadequately to other treatments.241. Because of the seemingly critical role of Th17 cells in murine models, MS would seem to be a very logical target for further study with this agent; however, the lack of efficacy of ustekinumab this setting gives one pause. It will also be interesting to see if tocilizumab has efficacy in SLE. Phase II trials of a subcutaneously administered mAb against IL-6 (CNTO 136) have also been initiated.183 With the demonstrated utility of tocilizumab in Th17 associated diseases, IL-21 might also be a good target, given its role as an autocrine positive regulator of Th17 differentiation.34, 42, 43, 242 In addition, IL-21 has critical functions in regulating B cells.243 Because Jak3 is essential for IL-21 signaling, capitalizing on this one aspect of Jak3 inhibitors might be the logical first choice for IL-21 signaling blockade.
More recently, retinoic acids have been shown to regulate Th17/Treg differentiation. Specifically, the production of endogenous retinoic acids by gut dendritic cells, or macrophages, promotes the expression of Foxp3 and inhibits IL-17 production.55, 224 All-trans retinoic acid (ATRA), commonly used topically for the treatment of acne vulgaris and keratosis pilaris, as well as systemically for the treatment of acute promyelocytic leukemia (APML), has effects both in vitro and in vivo – limiting IL-17 production and enhancing Foxp3 expression.54 However, it is a known teratogen and it is unknown whether ATRA has effects on fully polarized IL-17 producing memory cells or just undifferentiated effectors. At present, it is uncertain whether selective blockade of the lineage specific transcription factor, RORγt, or activators of retinoic acid receptors will be better clinical candidates for Th17 inhibition.
Another exciting development in the reciprocal regulation of Th17 and Treg cells has been the very recent finding that AHR ligands selectively influence the differentiation of both fates. Two separate studies showed that administration of 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous ligand for AHR, enhanced Th17 differentiation in vitro and enhanced the disease severity in mouse models of MS.46, 225 However, the administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), another AHR ligand derived from Agent Orange, instead induced Foxp3 and suppressed disease in the same models. It should be noted that these differences were certainly not “black and white” but more moderate in nature, with AHR knock-out mice still being susceptible to disease. Clinical translation of these findings could thus be quite challenging, especially considering that compounds like TCDD are extremely toxic. Blockade of the endogenous FICZ could be efficacious, but the effect is not likely to be dramatic and all of the downstream targets of FICZ are not known. Additionally, direct targeting of AHR itself is likely to be even less efficacious, as evidenced by the knock-out studies discussed above. Despite these potential therapeutic pitfalls, these findings are also of interest in that they provide a direct link between environmental toxins and immunoregulation.
Rather than inhibiting Th17 differentiation, another obvious strategy is the direct blockade of the Th17 signature cytokine, IL-17, through the use of a mAb. AIN457, an IL-17A mAb, has completed a phase I/II trial for psoriasis and phase I/II trials in RA and CD are underway.183 One complicating factor to this strategy is that two forms of IL-17 are secreted from T cells, e.g. IL-17A and IL-17F, and their respective functions have not yet been fully elucidated. Such complications can perhaps be avoided through the alternative targeting of the common receptor for both IL-17A and IL-17F, IL-17R. In fact, AMG 827, a fully human monoclonal antibody that binds to and blocks signaling via IL-17R, is currently undergoing phase I trials in psoriasis and phase I/II trials in RA are soon to recruit.183 However, all IL-17 signaling blocking strategies ignore the possibility that other Th17-associated cytokines may also be critical for autoimmunity, a hypothesis that is supported by the susceptibility of mice in certain disease models when IL-17 signaling is blocked.14, 244–247 IL-22 is one such Th17-associated cytokine, its blockade being shown to be curative in mouse models of psoriasis.75 While targeting IL-22 seems logical, this is another cytokine with complex and sometimes contradictory actions. In some settings, IL-22 has critical anti-inflammatory properties. Additionally, IL-22 plays a critical role in host-defense within the gut.74, 77
One final proposed target for the inhibition of Th17 cells is the chemokine receptor(s) selectively expressed by this subset. Chemokine receptor expression identifies subgroups of human memory CD4+ T cells: specifically, CCR6 and CCR4 positive cells are thought to be IL-17 producers.248 Joint targeting of these receptors has been given some consideration. Unfortunately, CCR6 is not a very selective marker, being expressed on approximately 50% of CD4+ memory PBLs.249 Additionally, CCR6 and CCR4 coexpression has been recently reported to also occur in Treg subsets.121 Interestingly, the ligand for CCR6, CCL20, has also been shown to be produced by Th17 cells and the CCR6/CCL20 signaling axis has been proposed as a paracrine signaling mechanism for recruitment of Th17 cells.31, 250 In support of this hypothesis, CCR6 deficiency or blockade protected against the development of arthritis and experimental autoimmune encephalomyelitis in mouse models.251 Especially in the CNS, CCR6+ T cells serve as “pioneers” that initiate inflammation, allowing other pathogenic T cells to follow.252 Taken together, the current data suggests that more work and characterization needs to be completed before definitive therapeutic targets can be developed.
Summary
The last few years have witnessed extraordinary advances in our understanding of the heterogeneity of T cells. In addition to Th1 and Th2 cells, we now know that T cells have other fates including Th17 and Treg; remarkably, these two new fates appear to be related with respect to the factors that control their differentiation. Furthermore, T cells also appear to have more flexibility in terms of cytokine production than initial models would have predicted. At present, there are many effective T cell-directed therapies both approved and in development. Moreover, recent advances reveal numerous opportunities for new therapeutic targets. The hope is that our improved understanding of the complexities of T cell differentiation and function will lead to the generation of effective new drugs with improved safety.
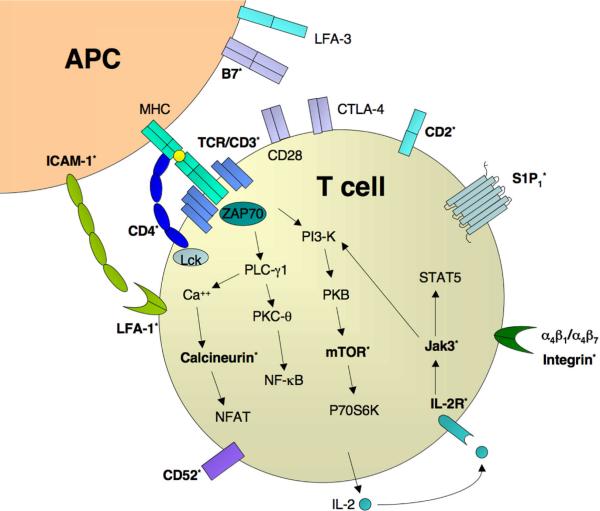
Fig 2. Therapeutic targets of T cells in autoimmunity and immunosuppresion.
A CD4+ T cell is activated by an APC through TCR binding of MHC bound antigen. CD28 and CTLA-4 compete for binding with B7 family members to result in costimulation or anergy, respectively. Downstream TCR signaling pathways, adhesion molecules, trafficking and cytokine receptors are also diagrammed. Factors currently being therapeutically targeted for T cells (phase III & above) appear in bold with an asterisk. Listing of relevant signaling pathways and factors is not exhaustive.
Table 1.
Drugs currently approved by the FDA or undergoing/completed Phase III trials, arranged according to the mechanism of action. Phase I–II trials are not listed.
| Name | Brand name | Mechanism of action | Target disease: FDA status |
|---|---|---|---|
| T cell depleting agents: | |||
| Lymphocyte Immune Globulin (horse), Anti-thymocyte Globulin (rabbit) | Atgam® (horse) Thymoglobulin® (rabbit) | Depleting horse/rabbit polyclonal anti-thymocyte antibody |
renal transplant: approved aplastic anemia: approved (Atgam) |
| Muronomab (OKT3) | Orthoclone OKT® 3 | Mouse anti-human CD3ε mAb | acute transplant rejection, GVHD: approved |
| Teplizumab (hOKT3γ1(Ala-Ala), MGA031)) | humanized OKT3 | T1DM: phase III | |
| Visilizumab | Nuvion® | humanized non-FcR-binding anti-CD3ε mAb | UC: phase III |
| Otelixizumab(TRX 4) | Tolerx® | humanized anti-CD3ε mAb | T1DM: phase III |
| Alemtuzumab | Campath® | humanized anti-CD52 mAb |
B cell CLL: approved MS: phase III |
| Targeting trafficking/adhesion: | |||
| Fingolimod (FTY720) | S1P receptor agonist | relapsing-remitting MS, renal transplant: phase III | |
| CCX282-B | Traficet-EN® | CCR9 inhibitor | CD: phase II/III |
| Natalizumab | Tysabri® | humanized anti-α4 integrin mAb |
relapsing-remitting MS: approved (TOUCH™ program) CD: phase III |
| Efalizumab | Raptiva® | humanized anti-CD11a mAb, blocks LFA-1 | chronic mod-severe plaque psoriasis: approved (pulled from market) |
| Alefacept | Amevive® | Fc fusion protein with extracellular portion of LFA-3, blocks CD2 |
chronic mod-severe plaque psoriasis: approved GVHD: phase III |
| Alicaforsen(ISIS 2302) | anti-sense ICAM-1 inhibitor | CD: phase III | |
| Targeting T cell receptor signaling: | |||
| Cyclosporine A | Gengraf®,Neoral®,Sandimmune® | calcineurin inhibitor | transplant, severe active RA, severe plaque psoriasis: approved |
| Tacrolimus (FK506) | Prograf® (systemic) Protopic® (topical) | calcineurin inhibitor |
transplant: approved mod-severe atopic dermatitis: approved (topical) UC, RA, myasthenia gravis, GVHD: phase III |
| Targeting costimulatory and accessory molecules: | |||
| Abatacept (BMS-188667) | Orencia® | Fc fusion protein with extracellular domain of CTLA-4, blocks CD28–CD80/86 interaction |
mod-severe RA: approved (2nd line agent) early RA, lupus nephritis, IBD, JIA: phase III |
| Belatacept (BMS-224818, LEA29Y) | same as Abatacept, higher affinity | transplant: phase II/III | |
| Zanolimumab (HuMax-CD4) | human anti-CD4 mAb, partially depleting | RA: phase II/III | |
| Targeting cytokines/cytokine signaling: | |||
| Daclizumab (HAT) | Zenapax® | humanized anti-Tac mAb: binds to the α-chain (CD25)of IL-2R |
renal transplant: approved GVHD, lung transplant: phase III |
| Basiliximab | Simulect® | chimeric mAb which blocks the α-chain of the IL-2R |
renal transplant: approved lung/liver transplant: phase III |
| CP690,550 | Jak3 inhibitor | RA: phase III | |
| Sirolimus | Rapamune® | mTOR inhibitor |
renal transplant: approved GVHD: phase III |
| Everolimus (RAD001) | Certican® | mTOR inhibitor | renal/heart transplant: approved in Europe and Canada, filed in the US |
| Ustekinumab (CNTO1275) | Stelara® | human anti-p40 mAb, blocks IL-12/IL-23 | psoriasis: approved in Europe and Canada, filed in the US |
| Tocilizumab (MRA) | Actemra® | humanized anti-IL-6 receptor mAb |
RA: approved in Europe and Japan, filed in US JIA: approved Japan, Phase III US |
Select abbreviations: CLL(chronic lymphocytic leukemia); CD (Crohn's disease); FcR(Fc receptor); GVHD(graft versus host disease); IBD(inflammatory bowel disease); JIA(juvenile idiopathic arthritis); mAb(monoclonal antibody); MS(multiple sclerosis); RA(rheumatoid arthritis); T1DM(type 1 diabetes mellitus); UC(ulcerative colitis)
Acknowledgements
S.M.S.T. is a National Institutes of Health-University of Oxford Biomedical Research Scholar.
Footnotes
Declaration of competing interests: The US National Institutes of Health and J.J.O. hold a patent related to Janus family kinases and identification of immune modulators, and have a Collaborative Research Agreement and Development Award with Pfizer. The US National Institutes of Health and R.M.S. hold a patent related to targeting the TL1A-DR3 interaction for immune modulation.
References
- 1.Dong C, Flavell RA. Th1 and Th2 cells. Curr Opin Hematol. 2001;8:47–51. doi: 10.1097/00062752-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic D, Sher A, Yap G. Th1/Th2 effector choice in parasitic infection: decision making by committee. Curr Opin Immunol. 2001;13:403–409. doi: 10.1016/s0952-7915(00)00234-x. [DOI] [PubMed] [Google Scholar]
- 3.Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunol Rev. 2004;202:203–222. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 4.Murphy KM. Fate vs choice: the immune system reloaded. Immunol Res. 2005;32:193–200. doi: 10.1385/IR:32:1-3:193. [DOI] [PubMed] [Google Scholar]
- 5.Szabo SJ, et al. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 6.Remmers EF, et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007;357:977–986. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korman BD, et al. Variant form of STAT4 is associated with primary Sjogren's syndrome. Genes Immun. 2008;9:267–270. doi: 10.1038/gene.2008.1. [DOI] [PubMed] [Google Scholar]
- 8.Lee GR, Fields PE, Flavell RA. Regulation of IL-4 gene expression by distal regulatory elements and GATA-3 at the chromatin level. Immunity. 2001;14:447–459. doi: 10.1016/s1074-7613(01)00125-x. [DOI] [PubMed] [Google Scholar]
- 9.Sokol CL, et al. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 11.Krueger GG, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 12.Mannon PJ, et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 13.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 14.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal S, et al. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 16.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton PR, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cargill M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Yang XO, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishigame H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 26.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 27.Veldhoen M, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 28.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acosta-Rodriguez EV, et al. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, et al. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 32.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei L, et al. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 36.Harris TJ, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 37.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Mathur AN, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 39.Holland SM, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 40.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minegishi Y, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 42.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 43.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 44.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 45.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber M, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci U S A. 2008;105:20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 50.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 52.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elias KM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2007 doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 55.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schambach F, et al. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 57.Suto A, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fazilleau N, et al. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakaguchi S. Regulatory T cells in the past and for the future. Eur J Immunol. 2008;38:901–937. doi: 10.1002/eji.200890012. [DOI] [PubMed] [Google Scholar]
- 62.Shevach EM, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 63.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 65.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 66.Burchill MA, et al. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 67.Yao Z, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007 doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sadlack B, et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 70.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008 doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vieira P, O'Garra A. Regula'ten' the gut. Nat Immunol. 2007;8:905–907. doi: 10.1038/ni0907-905. [DOI] [PubMed] [Google Scholar]
- 72.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 73.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 74.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma HL, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zenewicz LA, et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 78.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi G, et al. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veldhoen M, et al. Transforming growth factor-beta `reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 84.Starzl TE, et al. The clinical use of antilymphocyte globulin in renal homotransplantation. Transplantation. 1967;5(Suppl):1100–1105. doi: 10.1097/00007890-196707001-00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cosimi AB, et al. Use of monoclonal antibodies to T-cell subsets for immunologic monitoring and treatment in recipients of renal allografts. N Engl J Med. 1981;305:308–314. doi: 10.1056/NEJM198108063050603. [DOI] [PubMed] [Google Scholar]
- 86.Charpentier B, et al. Evidence that antihuman tumor necrosis factor monoclonal antibody prevents OKT3-induced acute syndrome. Transplantation. 1992;54:997–1002. doi: 10.1097/00007890-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 87.Weinshenker BG, et al. An open trial of OKT3 in patients with multiple sclerosis. Neurology. 1991;41:1047–1052. doi: 10.1212/wnl.41.7.1047. [DOI] [PubMed] [Google Scholar]
- 88.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 89.Belghith M, et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 90.Utset TO, et al. Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J Rheumatol. 2002;29:1907–1913. [PubMed] [Google Scholar]
- 91.Friend PJ, et al. Phase I study of an engineered aglycosylated humanized CD3 antibody in renal transplant rejection. Transplantation. 1999;68:1632–1637. doi: 10.1097/00007890-199912150-00005. [DOI] [PubMed] [Google Scholar]
- 92.Herold KC, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 93.Keymeulen B, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 94.Bresson D, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ochi H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 96.Ishikawa H, et al. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–2109. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 97.Hale G, et al. Removal of T cells from bone marrow for transplantation: a monoclonal antilymphocyte antibody that fixes human complement. Blood. 1983;62:873–882. [PubMed] [Google Scholar]
- 98.Coles AJ, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354:1691–1695. doi: 10.1016/S0140-6736(99)02429-0. [DOI] [PubMed] [Google Scholar]
- 99.Friend PJ, et al. Campath-1M--prophylactic use after kidney transplantation. A randomized controlled clinical trial. Transplantation. 1989;48:248–253. doi: 10.1097/00007890-198908000-00013. [DOI] [PubMed] [Google Scholar]
- 100.Hale G, Waldmann H. CAMPATH-1 monoclonal antibodies in bone marrow transplantation. J Hematother. 1994;3:15–31. doi: 10.1089/scd.1.1994.3.15. [DOI] [PubMed] [Google Scholar]
- 101.Isaacs JD, et al. CAMPATH-1H in rheumatoid arthritis--an intravenous dose-ranging study. Br J Rheumatol. 1996;35:231–240. doi: 10.1093/rheumatology/35.3.231. [DOI] [PubMed] [Google Scholar]
- 102.Buttmann M, Rieckmann P. Treating multiple sclerosis with monoclonal antibodies. Expert Rev Neurother. 2008;8:433–455. doi: 10.1586/14737175.8.3.433. [DOI] [PubMed] [Google Scholar]
- 103.Watanabe T, et al. CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. lin Immunol. 2006;120:247–259. doi: 10.1016/j.clim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 104.Villa A, et al. Genetically determined lymphopenia and autoimmune manifestations. Curr Opin Immunol. 2008;20:318–324. doi: 10.1016/j.coi.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 105.Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunol. 2008;122:1082–1086. doi: 10.1016/j.jaci.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 106.Branco L, et al. Selective deletion of antigen-specific, activated T cells by a humanized MAB to CD2 (MEDI-507) is mediated by NK cells. Transplantation. 1999;68:1588–1596. doi: 10.1097/00007890-199911270-00026. [DOI] [PubMed] [Google Scholar]
- 107.Xu Y, et al. The anti-CD2 monoclonal antibody BTI-322 generates unresponsiveness by activation-associated T cell depletion. Clin Exp Immunol. 2004;138:476–483. doi: 10.1111/j.1365-2249.2004.02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mourad M, et al. BTI-322 for acute rejection after renal transplantation. Transplant Proc. 1997;29:2353. doi: 10.1016/s0041-1345(97)00398-9. [DOI] [PubMed] [Google Scholar]
- 109.Spitzer TR, et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75:1748–1751. doi: 10.1097/01.TP.0000064211.23536.AD. [DOI] [PubMed] [Google Scholar]
- 110.Langley R, Roenigk H, McCall C. Phase I results of intravenous MEDI-507, an anti-T cell monoclonal antibody, for the treatment of psoriasis. J. Invest. Dermatol. 2001;117:817. [Google Scholar]
- 111.Choy EH, et al. Treatment of rheumatoid arthritis with single dose or weekly pulses of chimaeric anti-CD4 monoclonal antibody. Scand J Immunol. 1992;36:291–298. doi: 10.1111/j.1365-3083.1992.tb03102.x. [DOI] [PubMed] [Google Scholar]
- 112.Wendling D, et al. A randomized, double blind, placebo controlled multicenter trial of murine anti-CD4 monoclonal antibody therapy in rheumatoid arthritis. J Rheumatol. 1998;25:1457–1461. [PubMed] [Google Scholar]
- 113.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Kim CH, et al. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Loetscher P, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 116.Sallusto F, et al. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- 118.Bonecchi R, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D'Ambrosio D, et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 120.Imai T, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 121.Lim HW, et al. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 122.Critchfield JM, Lenardo MJ. Antigen-induced programmed T cell death as a new approach to immune therapy. Clin Immunol Immunopathol. 1995;75:13–19. doi: 10.1006/clin.1995.1046. [DOI] [PubMed] [Google Scholar]
- 123.Critchfield JM, et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 124.Ogasawara J, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 125.Saito M, et al. In SCID mice with transplanted joint tissues from rheumatism patients, a model mice of human rheumatoid arthritis, anti-human fas antibody (R-125224) distributes specifically to human synovium. Pharm Res. 2007;24:310–317. doi: 10.1007/s11095-006-9148-5. [DOI] [PubMed] [Google Scholar]
- 126.Schutz C, et al. Killer artificial antigen-presenting cells: a novel strategy to delete specific T cells. Blood. 2008;111:3546–3552. doi: 10.1182/blood-2007-09-113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 128.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 129.Brinkmann V, et al. FTY720: altered lymphocyte traffic results in allograft protection. Transplantation. 2001;72:764–769. doi: 10.1097/00007890-200109150-00002. [DOI] [PubMed] [Google Scholar]
- 130.Kappos L, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 131.Huang MC, et al. Th17 augmentation in OTII TCR plus T cell-selective type 1 sphingosine 1-phosphate receptor double transgenic mice. J Immunol. 2007;178:6806–6813. doi: 10.4049/jimmunol.178.11.6806. [DOI] [PubMed] [Google Scholar]
- 132.Liao JJ, Huang MC, Goetzl EJ. Cutting edge: Alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–5428. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- 133.Daniel C, et al. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178:2458–2468. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- 134.Waters L, et al. The impact of HIV tropism on decreases in CD4 cell count, clinical progression, and subsequent response to a first antiretroviral therapy regimen. Clin Infect Dis. 2008;46:1617–1623. doi: 10.1086/587660. [DOI] [PubMed] [Google Scholar]
- 135.Gulick RM, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196:304–312. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 136.Glass WG, et al. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol. 2004;172:4018–4025. doi: 10.4049/jimmunol.172.7.4018. [DOI] [PubMed] [Google Scholar]
- 137.Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Keshav S, Ungasher S, Zheng W. CCX282-B, an orally active inhibitor of chemokine receptor CCR9, shows anti-inflammatory & clinical activity in the treatment of Crohn's desease. Gastroenterology. 2007;132(Suppl):A–157. [Google Scholar]
- 139.Reiss Y, et al. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yokoyama K, et al. Discovery of potent CCR4 antagonists: Synthesis and structure-activity relationship study of 2,4-diaminoquinazolines. Bioorg Med Chem. 2008 doi: 10.1016/j.bmc.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 141.Bayry J, et al. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc Natl Acad Sci U S A. 2008;105:10221–10226. doi: 10.1073/pnas.0803453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Polman CH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 143.Cox CA, et al. Both Th1 and th17 are immunopathogenic but differ in other key biological activities. J Immunol. 2008;180:7414–7422. doi: 10.4049/jimmunol.180.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feagan BG, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 145.Behm BW, Bickston SJ. Humanized antibody to the alpha4beta7 integrin for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD007571. CD007571. [DOI] [PubMed] [Google Scholar]
- 146.Kavanaugh AF, et al. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis Rheum. 1994;37:992–999. doi: 10.1002/art.1780370703. [DOI] [PubMed] [Google Scholar]
- 147.Salmela K, et al. A randomized multicenter trial of the anti-ICAM-1 monoclonal antibody (enlimomab) for the prevention of acute rejection and delayed onset of graft function in cadaveric renal transplantation: a report of the European Anti-ICAM-1 Renal Transplant Study Group. Transplantation. 1999;67:729–736. doi: 10.1097/00007890-199903150-00015. [DOI] [PubMed] [Google Scholar]
- 148.Gordon KB, et al. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. Jama. 2003;290:3073–3080. doi: 10.1001/jama.290.23.3073. [DOI] [PubMed] [Google Scholar]
- 149.Leonardi CL, et al. Extended efalizumab therapy improves chronic plaque psoriasis: results from a randomized phase III trial. J Am Acad Dermatol. 2005;52:425–433. doi: 10.1016/j.jaad.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 150.Menter A, et al. Efficacy and safety observed during 24 weeks of efalizumab therapy in patients with moderate to severe plaque psoriasis. Arch Dermatol. 2005;141:31–38. doi: 10.1001/archderm.141.1.31. [DOI] [PubMed] [Google Scholar]
- 151.Takiguchi R, et al. Efalizumab for severe atopic dermatitis: a pilot study in adults. J Am Acad Dermatol. 2007;56:222–227. doi: 10.1016/j.jaad.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 152.Yacyshyn BR, et al. A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn's disease. Gastroenterology. 1998;114:1133–1142. doi: 10.1016/s0016-5085(98)70418-4. [DOI] [PubMed] [Google Scholar]
- 153.Miner PB, Jr., et al. Safety and efficacy of two dose formulations of alicaforsen enema compared with mesalazine enema for treatment of mild to moderate left-sided ulcerative colitis: a randomized, double-blind, active-controlled trial. Aliment Pharmacol Ther. 2006;23:1403–1413. doi: 10.1111/j.1365-2036.2006.02837.x. [DOI] [PubMed] [Google Scholar]
- 154.Schreiber S, et al. Absence of efficacy of subcutaneous antisense ICAM-1 treatment of chronic active Crohn's disease. Gastroenterology. 2001;120:1339–1346. doi: 10.1053/gast.2001.24015. [DOI] [PubMed] [Google Scholar]
- 155.van Deventer SJ, et al. A phase II dose ranging, double-blind, placebo-controlled study of alicaforsen enema in subjects with acute exacerbation of mild to moderate left-sided ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1415–1425. doi: 10.1111/j.1365-2036.2006.02910.x. [DOI] [PubMed] [Google Scholar]
- 156.Yacyshyn BR, et al. Double blind, placebo controlled trial of the remission inducing and steroid sparing properties of an ICAM-1 antisense oligodeoxynucleotide, alicaforsen (ISIS 2302), in active steroid dependent Crohn's disease. Gut. 2002;51:30–36. doi: 10.1136/gut.51.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yacyshyn BR, et al. Dose ranging pharmacokinetic trial of high-dose alicaforsen (intercellular adhesion molecule-1 antisense oligodeoxynucleotide) (ISIS 2302) in active Crohn's disease. Aliment Pharmacol Ther. 2002;16:1761–1770. doi: 10.1046/j.1365-2036.2002.01341.x. [DOI] [PubMed] [Google Scholar]
- 158.Foss CE, et al. An open-label pilot study of alefacept for the treatment of pyoderma gangrenosum. J Eur Acad Dermatol Venereol. 2008 doi: 10.1111/j.1468-3083.2008.02680.x. [DOI] [PubMed] [Google Scholar]
- 159.Moul DK, et al. Alefacept for moderate to severe atopic dermatitis: a pilot study in adults. J Am Acad Dermatol. 2008;58:984–989. doi: 10.1016/j.jaad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 160.Perlmutter A, et al. Alefacept revisited: Our 3-year clinical experience in 200 patients with chronic plaque psoriasis. J Am Acad Dermatol. 2008;58:116–124. doi: 10.1016/j.jaad.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 161.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 162.Matthews SA, Cantrell DA. The role of serine/threonine kinases in T-cell activation. Curr Opin Immunol. 2006;18:314–320. doi: 10.1016/j.coi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 163.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 164.Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nat Rev Immunol. 2006;6:56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- 165.Crabtree GR. Calcium, calcineurin, and the control of transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- 166.Bowman LJ, Brennan DC. The role of tacrolimus in renal transplantation. Expert Opin Pharmacother. 2008;9:635–643. doi: 10.1517/14656566.9.4.635. [DOI] [PubMed] [Google Scholar]
- 167.Schwartzberg PL, Finkelstein LD, Readinger JA. TEC-family kinases: regulators of T-helper-cell differentiation. Nat Rev Immunol. 2005;5:284–295. doi: 10.1038/nri1591. [DOI] [PubMed] [Google Scholar]
- 168.Hirabayashi A, et al. Structure-activity relationship studies of 5-benzylaminoimidazo[1,2-c]pyrimidine-8-carboxamide derivatives as potent, highly selective ZAP-70 kinase inhibitors. Bioorg Med Chem. 2009;17:284–294. doi: 10.1016/j.bmc.2008.10.070. [DOI] [PubMed] [Google Scholar]
- 169.Januchowski R, Jagodzinski PP. Trichostatin A down-regulates ZAP-70, LAT and SLP-76 content in Jurkat T cells. Int Immunopharmacol. 2007;7:198–204. doi: 10.1016/j.intimp.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 170.Baier G, et al. Molecular cloning and characterization of PKC theta, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J Biol Chem. 1993;268:4997–5004. [PubMed] [Google Scholar]
- 171.Manicassamy S, et al. Differential roles of PKC-theta in the regulation of intracellular calcium concentration in primary T cells. J Mol Biol. 2006;355:347–359. doi: 10.1016/j.jmb.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 172.Sun Z, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 173.Anderson K, et al. Mice deficient in PKC theta demonstrate impaired in vivo T cell activation and protection from T cell-mediated inflammatory diseases. Autoimmunity. 2006;39:469–478. doi: 10.1080/08916930600907954. [DOI] [PubMed] [Google Scholar]
- 174.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704–3712. [PubMed] [Google Scholar]
- 175.Burrows GG, et al. Two-domain MHC class II molecules form stable complexes with myelin basic protein 69–89 peptide that detect and inhibit rat encephalitogenic T cells and treat experimental autoimmune encephalomyelitis. J Immunol. 1998;161:5987–5996. [PubMed] [Google Scholar]
- 176.Link JM, et al. Monomeric DR2/MOG-35-55 recombinant TCR ligand treats relapses of experimental encephalomyelitis in DR2 transgenic mice. Clin Immunol. 2007;123:95–104. doi: 10.1016/j.clim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 177.Vandenbark AA. TCR peptide vaccination in multiple sclerosis: boosting a deficient natural regulatory network that may involve TCR-specific CD4+CD25+ Treg cells. Curr Drug Targets Inflamm Allergy. 2005;4:217–229. doi: 10.2174/1568010053586327. [DOI] [PubMed] [Google Scholar]
- 178.Reynolds J, Shojania K, Marra CA. Abatacept: a novel treatment for moderate-to-severe rheumatoid arthritis. Pharmacotherapy. 2007;27:1693–1701. doi: 10.1592/phco.27.12.1693. [DOI] [PubMed] [Google Scholar]
- 179.Vincenti F. Costimulation blockade in autoimmunity and transplantation. J Allergy Clin Immunol. 2008;121:299–306. doi: 10.1016/j.jaci.2008.01.002. quiz 307–298. [DOI] [PubMed] [Google Scholar]
- 180.Gottlieb AB, et al. Evaluation of safety and clinical activity of multiple doses of the anti-CD80 monoclonal antibody, galiximab, in patients with moderate to severe plaque psoriasis. Clin Immunol. 2004;111:28–37. doi: 10.1016/j.clim.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 181.Vitetta ES, Ghetie VF. Immunology. Considering therapeutic antibodies. Science. 2006;313:308–309. doi: 10.1126/science.1130482. [DOI] [PubMed] [Google Scholar]
- 182.Skov L, et al. HuMax-CD4: a fully human monoclonal anti-CD4 antibody for the treatment of psoriasis vulgaris. Arch Dermatol. 2003;139:1433–1439. doi: 10.1001/archderm.139.11.1433. [DOI] [PubMed] [Google Scholar]
- 183.clinicaltrials.gov [Accessed: March 2009]; http://clinicaltrials.gov/
- 184.Rider DA, et al. A human CD4 monoclonal antibody for the treatment of T-cell lymphoma combines inhibition of T-cell signaling by a dual mechanism with potent Fc-dependent effector activity. Cancer Res. 2007;67:9945–9953. doi: 10.1158/0008-5472.CAN-07-1148. [DOI] [PubMed] [Google Scholar]
- 185.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 186.Meylan F, et al. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29:79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nohara C, et al. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol. 2001;166:2108–2115. doi: 10.4049/jimmunol.166.3.2108. [DOI] [PubMed] [Google Scholar]
- 188.Seshasayee D, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McEarchern JA, et al. Engineered anti-CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood. 2007;109:1185–1192. doi: 10.1182/blood-2006-07-034017. [DOI] [PubMed] [Google Scholar]
- 190.Kirk AD, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 191.Kawai T, et al. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 192.Daikh DI, et al. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol. 1997;159:3104–3108. [PubMed] [Google Scholar]
- 193.Imai A, et al. A novel fully human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys. Transplantation. 2007;84:1020–1028. doi: 10.1097/01.tp.0000286058.79448.c7. [DOI] [PubMed] [Google Scholar]
- 194.Goldbach-Mansky R, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Scheinecker C, Redlich K, Smolen JS. Cytokines as therapeutic targets: advances and limitations. Immunity. 2008;28:440–444. doi: 10.1016/j.immuni.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 196.Nashan B, et al. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. Lancet. 1997;350:1193–1198. doi: 10.1016/s0140-6736(97)09278-7. [DOI] [PubMed] [Google Scholar]
- 197.Vincenti F, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med. 1998;338:161–165. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 198.Bielekova B, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl Acad Sci U S A. 2004;101:8705–8708. doi: 10.1073/pnas.0402653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Morris JC, et al. Preclinical and phase I clinical trial of blockade of IL-15 using Mikbeta1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proc Natl Acad Sci U S A. 2006;103:401–406. doi: 10.1073/pnas.0509575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hommes DW, et al. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease. Gut. 2006;55:1131–1137. doi: 10.1136/gut.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 202.Palucka AK, et al. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ronnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17:394–399. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wallac D, Petri M, al e. MEDI-545, an anti-interferon alpha monoclonal antibody, shows evidence of clinical activity in systemic lupus erythematosus. Arthritis Rheum. 2007;56:S526–S527. [Google Scholar]
- 205.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 206.O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Macchi P, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 208.Changelian PS, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 209.Ghoreschi K, Laurence A, O'Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be? Nat Immunol. 2009;10:356–360. doi: 10.1038/ni.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 211.Shaw MH, et al. A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity. Proc Natl Acad Sci U S A. 2003;100:11594–11599. doi: 10.1073/pnas.1930781100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Minegishi Y, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 213.O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 214.Yakupoglu YK, Kahan BD. Sirolimus: a current perspective. Exp Clin Transplant. 2003;1:8–18. [PubMed] [Google Scholar]
- 215.Kappos L, Barkhof F, al e. The effect of oral temsirolimus on new magnetic resonance imaging scan lesions, brain atrophy, and the number of relapses in multiple sclerosis: results from a randomised, controlled clinical trial. J Neurol. 2005;252(supplement 2):S46. [Google Scholar]
- 216.Kopf H, et al. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int Immunopharmacol. 2007;7:1819–1824. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 218.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 219.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xu L, et al. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 221.Sadlack B, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 222.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wahl SM. Transforming growth factor beta: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Denning TL, et al. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 225.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 226.You S, et al. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci U S A. 2007;104:6335–6340. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.You S, et al. Transforming growth factor-beta and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol Rev. 2006;212:185–202. doi: 10.1111/j.0105-2896.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 228.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Asadullah K, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–794. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Smeets TJ, et al. Analysis of serial synovial biopsies in patients with rheumatoid arthritis: description of a control group without clinical improvement after treatment with interleukin 10 or placebo. J Rheumatol. 1999;26:2089–2093. [PubMed] [Google Scholar]
- 231.Niedbala W, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 232.Leonardi CL, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 233.Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 234.Segal BM, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 235.Gottlieb A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633–640. doi: 10.1016/S0140-6736(09)60140-9. [DOI] [PubMed] [Google Scholar]
- 236.Emery P, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Genovese MC, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 238.Nishimoto N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–1167. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Smolen JS, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 240.Yokota S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 241.European Medicines Agency [Accessed: March 2009]; http://www.emea.europa.eu.
- 242.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 244.Izcue A, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 246.Ogawa A, et al. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 247.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 249.Singh SP, et al. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- 250.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 252.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of T(H)-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009 doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]