Abstract
Each year, a larger proportion of the Earth's surface is urbanized, and a larger proportion of the people on Earth lives in those urban areas. The everyday nature, however, that humans encounter in cities remains poorly understood. Here, we consider perhaps the most urban green habitat, street medians. We sampled ants from forty-four medians along three boulevards in New York City and examined how median properties affect the abundance and species richness of native and introduced ants found on them. Ant species richness varied among streets and increased with area but was independent of the other median attributes measured. Ant assemblages were highly nested, with three numerically dominant species present at all medians and additional species present at a subset of medians. The most common ant species were the introduced Pavement ant (Tetramorium caespitum) and the native Thief ant (Solenopsis molesta) and Cornfield ant (Lasius neoniger). The common introduced species on the medians responded differently to natural and disturbed elements of medians. Tetramorium caespitum was most abundant in small medians, with the greatest edge/area ratio, particularly if those medians had few trees, whereas Nylanderia flavipes was most abundant in the largest medians, particularly if they had more trees. Many of the species encountered in Manhattan were similar to those found in other large North American cities, such that a relatively small subset of ant species probably represent most of the encounters humans have with ants in North America.
Introduction
Urban and suburban areas are increasing in number and population density globally [1], [2]. Worldwide, 2003 was the first year that more people lived in urban areas than in rural ones [3]. With these shifts, the recognition of the role of urban ecological health and conservation is increasing [3], [4], [5]. Barring apocalyptic catastrophes [e.g., 6], urban and suburban habitats will be among the most common habitats on Earth for many generations to come and the most common setting for human interactions with wild species [5]. However, urban environments vary (and have long varied) in their diversity and ecological health from complex urban gardens to habitats superficially devoid of life such as the glass walls of skyscrapers [7]. Similarly, the species found in these habitats range from pests and pathogens, to species beneficial to conservation, ecosystem health, human health or human appreciation of and interaction with nature [8], [9]. A central question then for urban ecology is to understand which species persist in urban environments and what governs their diversity and composition.
Reviews emphasize that urban ecology has become an increasing area of research [e.g. 10], [11], [12]. However, we still better understand more “natural” landscape elements within urban habitats than we do constructed elements such as buildings, sidewalks, roads, and medians which constitute the majority of urban areas [4]. Studies tend to focus on urban habitats that mimic more natural habitats, such as domestic gardens [13], brownfield sites [14], parks [15], [16] and forest patches [17]. Understanding the ecology of more highly developed habitats – whether the tops of buildings, sidewalks or street medians – is more difficult. In these places, natural processes interact with active management in ways that may or may not reconcile well with ecological models developed for oceanic islands and forest fragments in “seas” of agriculture [18].
It is likely that aside from observations of rats and roaches in buildings, the little patches of grass and trees in street medians are where urbanites are most likely to see “nature,” whether in the form of a nesting bird or foraging ants. A connection to nature has been hypothesized to serve important functions for psychological and emotional well-being of people in general, and urbanites in particular [e.g. 19], [20], [21]. However, little to nothing is known about the ecology of medians as green patches, areas that if not quite islands are at least visually isolated. If medians are able to conserve a diversity of native species and thereby connect urbanites to nature, that effect will have broad implications [22], [23], [24]. Conversely, if these communities are depauperate wastelands, a relatively barren nature will be the kind experienced by most urbanites. If we can understand what facilitates survival of native species in the most impacted and frequently experienced of green habitats, we can understand how to maintain diversity in other, more ‘natural’ urban habitats.
Among the taxa best able to persist in highly managed urban environments are a subset of arthropods, particularly common insects such as ants. Ants are found in nearly all urban habitats (from woodlands to kitchens and picnics), can be diverse in urban settings [25], [26], [27], are conspicuous, and can have strong ecological [28], [29] and economic impacts [30]. In natural habitats, ant species can function as predators, prey, detritivores, seed dispersers, and herbivores [29], [31]. In urban habitats, ants are likely to also play an important role in scavenging and cleaning cities of human detritus and waste [32]. Where ants are rare, it seems possible that carrion and feces may even be slower to decompose and more likely to accumulate. Ants have been successfully used as indicators of the ecosystem health of different agricultural practices, as well as of the environmental change that such practices inadvertently bring [33], [34], [35]. Ants might also provide a measure of the richness and types of ecological interactions to which urban humans are exposed.
Here we had two objectives which we achieved by sampling 44 medians along Broadway, Park Avenue, and the West Side Highway on Manhattan Island of New York City. First, we sought to characterize the ant community of these “greenways” of one of the most urbanized cities in North America. The New York City metropolitan area is home to around 21 million people. The city alone houses more than 8 million people [3]. Second, within this highly managed environment, we sought to understand how different characteristics of street medians influence the species richness and composition of ants. We test each of a series of potential correlates of both ant species richness and the abundance of two key introduced ant species, Tetramorium caespitum and Nylanderia flavipes, both of which are known to be present in New York City.
Theory developed in natural settings predicts that larger medians should have more species [36], [37], [38], [39] as should medians with greater vegetative complexity [40], [41], [42]. On the other hand, in urban environment, unique aspects of the managed environment might prove more important. For example, the presence of garbage bins, disturbance, and availability of potential nesting sites (concrete structures and dead wood) might disproportionately influence ant communities [32], [43]. Both of these possibilities assume that ants respond to medians as “islands” of forest-like habitat in a sea of inhospitable city. Medians may be islands for species that forage only in green spaces. On the other hand, some species may forage and live in both medians and the surrounding cement and/or patches of green life even smaller than the medians, such as plants in sidewalk cracks. If many species forage both in medians and between them, ant species richness may be independent of attributes of medians and instead be invariant across replicates. The two focal introduced ant species might respond either to natural or anthropogenic features of medians. Studies of invasive ants tend to emphasize the extent to which they are facilitated by disturbance [44], but whether this is true within cities is ambiguous [45].
As an additional measure of the composition of the median ant faunas, we tested whether depauperate medians tended to have a nested subset of species found at more diverse medians.
Methods
Study locations
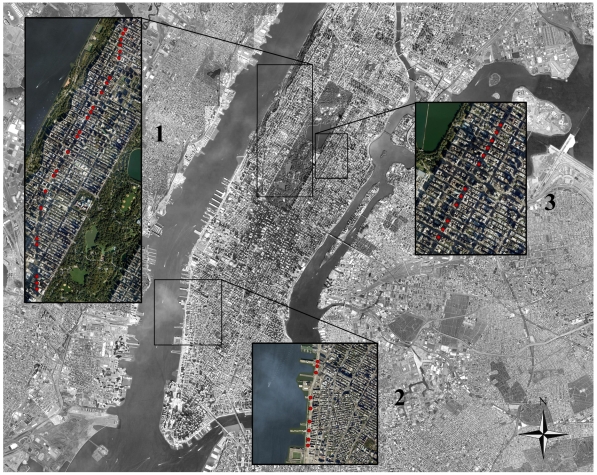
The 44 medians included in this study were located on three major avenues in New York City (Figure 1). These medians were Broadway (23 medians), Park Avenue (12 medians), and West Side Highway (nine medians, WSH in further text). Each of these avenues includes medians that are surrounded by roads on all four sides. The avenues are sufficiently far apart as to preclude frequent dispersal of ants between them, and the medians are sufficiently separated from each other so as to sample independent colonies (i.e. colonies are not foraging from one median to another).
Figure 1. Locations of the 44 street medians included in this study.
1 – Broadway, 2 – West Side Highway, 3 – Park Avenue.
Ant sampling
Sampling was carried out between 12 June and 28 July 2006. Five unbaited pitfall traps were placed inside each median, three of which were randomly chosen for inclusion in the study and subsequent sorting; this design accommodated occasional trap disturbances by natural or human means. Pitfall traps were plastic drinking cups (7×9 cm) filled with propylene glycol car antifreeze as a killing agent [46]. Pitfall traps sample a subset of all ant species, but are a useful measure of the ants foraging on the ground-surface [46], which are those that human New Yorkers are also likely to see. Since all of the medians included in this study were rectangular, the traps were placed in a manner that ensured that each third of the median contained at least one trap. Traps were emptied after four days, and all the specimens were preserved in 75% ethyl alcohol. After setting the traps, free-roaming ants were also hand sampled using an aspirator for three minutes on each of three locations inside each median to collect any that were in sight, for a total of nine minutes of active hand collecting in each median. All ants collected were preserved in 75% ethyl alcohol. A total of 65 medians were initially sampled, 21 of which had to be excluded from the study because more than two of the traps were removed from them by cleaning crews, stepped on or filled with mowed grass. During laboratory analysis of the traps collected from the 44 remaining medians, ants were separated from non-ant arthropods, identified to species and counted. Ant specimens were deposited in the collection of R. R. Dunn in the Department of Biology at North Carolina State University.
Environmental variables
Variables measured across each median included both natural and anthropogenic variables likely to influence invertebrate communities. Natural environmental variables included: the number of trees higher than 2 m, percent of canopy cover above 2 m, percent of canopy cover below 2 m, presence of dead wood, non-ant arthropod abundance, and the number of plant species. Anthropogenic variables were the percent of ground covered by ground-covering plants (in horticulture, low-lying plants that are used to cover the ground, and that typically propagate by running rhizomes and/or stolons), number of subway vents (each of which was approximately 6 m by 4 m), number of garbage bins, percent of ground covered by mulch, and number of concrete structures (including man-holes, fire hydrants, and electrical fuse boxes). Each of these variables was used either because previous studies have found them to have an effect on arthropod community species richness [13], [27], [47], [48], [49], or because they were found to be prominent and potentially important features of the medians during the sampling period (as with the number of subway vents and garbage bins).
When counting the number of plant species, only perennials were included because most “seasonal” plants were actively replaced on a monthly basis (Pecarevic personal communication with maintenance crews). The richness of both plants and ants was measured as species density, in other words the total number of species sampled in a given median. We used Chao2 and ICE (Incidence-based coverage estimator) as species richness estimators to estimate the total number of species that would have been sampled had sampling gone to completion both overall and for each of the sets of the three sets of medians considered [76]. Dead wood such as logs and tree stumps were recorded as either present or absent from a median.
Percent tree cover was estimated as the proportion of a median that included trees when seen from one side. All variables measured as percentages (mulch cover, ground covering plants, and canopy cover below 2 m) were estimated in the same manner.
Data analysis
Species richness models
Ordinary least squares regression was used to consider the correlates of ant species richness among medians. In all models, street (Broadway, Park Avenue, or WSH) was included as a variable, to account for differences among medians due to the circumstances associated with each street, their management regime, or other unmeasured factors. A priori, the spatial effects due to within median differences seemed the most likely to influence patterns in ant richness and composition. However, to test for additional spatial effects, such as might be associated with spatial autocorrelation in unmeasured independent variables, we tested for spatial autocorrelation in the residuals from the best model within each median. No significant autocorrelation was detected within any street and so we do not consider these spatial effects further. Prior to analysis, all continuous variables were log-transformed to better conform to the normality assumptions of parametric tests, with the exception of those expressed as percentages which were arcsine square root transformed, again for the purpose of normalizing the data [50]. All statistical analyses were done using JMP [51].
We began with a model including all of the variables of interest (number of trees higher than 2 m, percent of canopy cover above 2 m, percent of canopy cover below 2 m, presence of dead wood, the number of plant species, percent of ground covered by ground-covering plants, number of subway vents, number of garbage bins, percent of ground covered by mulch, and number of concrete structures). We then simplified the model by removing non-significant variables (α = 0.05) in a stepwise fashion [52], to explore the relative contributions of the various terms included in the full models. Although stepwise simplification approaches have been criticized [53], there is no perfect approach to choosing among multiple predictor variables in the absence of prior models [54]. We interpret our “best model” as being the best model in terms of accounting statistically for variation in ant species richness, though we acknowledge that causal mechanisms may be more complicated. As such, we present the correlation matrixes for pairwise comparisons among all possible variables in the supplement (Figure S1).
Nestedness
In habitats that include disturbance, low richness sites often include a nested subset of those species in high richness sites. At the opposite extreme, low richness sites might contain a unique subset of species not found in more diverse sites. Our species by site data were tested for nestedness using the Nestedness Calculator [55] available at a website from the Field Museum in Chicago (http://www.fieldmuseum.org/research_collections/zoology/nested.htm). This program measures the extent of the order present in nested presence-absence matrices. A perfectly ordered or nested system, absent of all randomness, is characterized as maximally “cold” (0°), whereas a system absent of all order may be labeled as maximally “hot” (100°) [56]. A Monte Carlo simulation was used to test whether the data were significantly different from a random sample. The matrix was randomized 500 times. The measurements of temperature from the observed data were then compared to the distribution of values from the randomized matrices.
Results
Ant community composition
A total of 6,619 individual ants of 13 species and 11 genera was recorded from the street medians of Manhattan (Table 1). Species richness estimators indicate that were sampling to have gone to completion 12 species would have been detected on Broadway, 8 on Park Avenue, 12 on the West Side Highway, and 14 overall. The three numerically most dominant species (Table 1) accounted for most of the individual ants sampled (94%). These were the Pavement ant (Tetramorium caespitum), the Thief ant (Solenopsis molesta), and the Cornfield ant (Lasius neoniger). These were also the three species found at the most sites. All three of these species are frequently encountered in urban and suburban environments across a broad geographic area [48], [57], [58]. In addition to these three species, Nylanderia flavipes [59] was also very frequently sampled, although not numerically dominant. Of the four most common species, two (L. neoniger and S. molesta) are native, and two (T. caespitum and N. flavipes) are introduced (Table 1). Both native and introduced ant species coexist in Manhattan in relatively large numbers. Although the most diverse and least diverse medians sampled (eight and two species, respectively) represented a large range, most medians were similar in their ant species richness. In fact, 31 of the 44 medians had between three and five ant species.
Table 1. Ant species found in New York City medians.
| Species | No. of medians collected | % of medians collected | Number of individuals | % of total number of individuals | Status |
| Tetramorium caespitum | 41 | 93.2% | 3484 | 52.6% | Introduced |
| Lasius neoniger | 39 | 88.6% | 1695 | 25.6% | Native |
| Solenopsis molesta | 28 | 63.6% | 1022 | 15.4% | Native |
| Nylanderia flavipes | 23 | 52.3% | 195 | 3.0% | Introduced |
| Camponotus pennsylvanicus | 7 | 15.9% | 17 | 0.3% | Native |
| Lasius alienus | 5 | 11.4% | 40 | 0.6% | Native |
| Brachymyrmex depilis | 4 | 9.1% | 10 | 0.2% | Native |
| Formica subsericea | 4 | 9.1% | 57 | 0.9% | Native |
| Monomorium minimum | 4 | 9.1% | 38 | 0.6% | Native |
| Pheidole tysoni | 4 | 9.1% | 40 | 0.6% | Native |
| Aphaenogaster rudis | 2 | 4.6% | 4 | 0.1% | Native |
| Formica sp. II | 2 | 4.6% | 3 | 0.1% | Native |
| Pachycondyla chinensis | 1 | 2.3% | 4 | 0.1% | Introduced |
Correlates of ant abundance and species richness
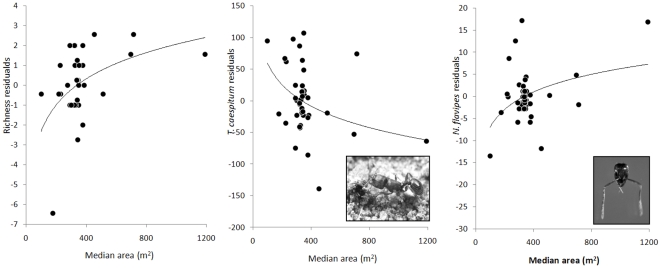
Given the similarity among medians in their ant species composition and richness, relatively little variation was left to be explained by possible biotic and abiotic correlates. In stepwise regression, the only significant factor in the models was the street on which the medians were located. Once “street,” was accounted for, the only other variable that improved the model was the area of the median, with larger medians having more species (Table 2, Figure 2). As our approach here is correlational, it begs for a second, more experimental step to disentangle the mechanistic basis of the correlation of richness with area.
Table 2. Correlates of ant species richness, where each site (N = 44) is an individual street median.
| SSE | DF | MSE | R2 | |
| Full model | 87.4 | 41 | 2.1 | 0.47 |
Figure 2. The relationship between ant species richness, T. caespitum abundance and N. flavipes abundance and area after we accounted for other variables significant in the final models.
Species richness and N. flavipes abundance increase with area, whereas the abundance of T. caespitum declines.
Correlates of introduced species abundance
The abundance of the introduced ant species T. caespitum was negatively correlated with tree density. Medians that were less forest-like had more T. caespitum (Table 3). The other abundant introduced ant species N. flavipes, on the other hand, was most abundant at the medians that were more forest-like, increasing when a larger proportion of the median was tree covered (Table 4).
Table 3. Correlates of the abundance of the pavement ant, Tetramorium caespitum.
| SSE | DF | MSE | R2 | |
| Full model | 93772.9 | 39 | 2404.4 | 0.59 |
Table 4. Correlates of the abundance of the introduced ant, Nylanderia flavipes.
| SSE | DF | MSE | R2 | |
| Full model | 1099 | 39 | 28.2 | 0.50 |
Nestedness
Using the Nestedness Calculator [55] the temperature of the ant fauna of Manhattan medians was found to be 11.97° out of a possible 100°, in other words strongly nested. After randomizing the matrix 500 times, average temperature of the system was found to be 55.21° (S.D. = 5.12°), using the Monte Carlo test. The probability that temperature below 11.97° will be drawn was P = 1.8×10−17. It is therefore safe to conclude that the species inhabiting the medians are very nested. Species poor sites contain a subset of those species found at species rich sites.
Discussion
In perhaps the most often viewed green space in the world, the medians of New York City streets, we found thirteen different species of ants and evidence that even more species are likely to occur. The species collected included subterranean species that tend aphids, much in the way that humans tend cattle (Lasius neoniger), a human commensal species (Tetramorium caespitum) that appears to nest preferentially under cement, and a keystone, seed-dispersing forest species (Aphaenogaster rudis). The most abundant species (Tetramorium caespitum) is introduced, but the second most abundant species (Lasius neoniger) and the majority of species overall were native. On Broadway, species native to North America coexist alongside those native to other regions of the world. Manhattan is, if not quiet a melting pot of ant species, at least a mixing bowl.
In contrast to the case for many natural ecosystems [60], [61], [62], few of the environmental variables we considered were significantly correlated with the species richness of ants in medians. Why some medians had more ant species than did others remains enigmatic. What is clear is that the species assemblages were strongly nested, i.e. the primary differences in species richness from one median to the next was due to the presence of rare species in the most diverse medians. While it is unclear from our analyses what allows rarer species we sampled to persist in medians, it is clear they do. Some of these species may be more common than is apparent given our sampling method. Future studies that employ additional forms of sampling that catch arboreal, soil and litter dwelling ants, are likely to yield more species than we were able to detect. Even those species we collected deserve additional study in a natural history, ecological or evolutionary context. For example, a median on West Side Highway close to the Hudson River has Pheidole tysoni living on it. This species can be found within a walking distance of several million people and is geographically widespread, but like most insect species, even its most basic biology is poorly known.
Introduced ant species are often described as being favored by disturbance [15]. In New York City, we found patterns in introduced ant species abundance to be more complex. The four ubiquitous species (T. caespitum, L. neoniger, S. molesta, and N. flavipes) were found to coexist on almost all of the medians observed. In contrast, the pavement ant, T. caespitum, was most abundant in small medians with the greatest edge to area ratio, and particularly if those medians had few trees. This is consistent with a study of urban ants carried out in San Francisco [73], where this species was found only in parks where the dominant vegetation type was herbaceous. Nylanderia flavipes, on the other hand, was most abundant in the largest medians, particularly if they had more trees. These results reconcile well with observations of the natural history of these species, which are often thought of as pavement (T. caespitum [48], [63]) and forest (N. flavipes [25], [64]) specialists. Nylanderia flavipes is Asian, potentially Japanese, in origin and has spread to the United States via imported plant material [64], [65]. This species nests in leaf-litter, rotten wood, and in the soil of grasslands and forests [15], a natural history that reconciles well with our observation that tree cover was a predictor of the abundance of this species in medians. T. caespitum is considered a human commensal because of its close association with humans [66], [67]. It often nests under bricks and in sidewalk crevices [48]. T. caespitum is highly territorial, omnivorous, and has a foul taste (at least to myrmecologists) which makes it unpalatable to predators [66], [68], [69], all of which may predispose it to life in cities, though why it does better where more cement is present, both in New York and elsewhere, remains unknown.
Four individuals of the Asian Needle Ant (Pachycondyla chinensis) were discovered during this survey. This ant has recently been recognized as a public health threat in the southeastern United States (South Carolina, Georgia, North Carolina, and Virginia) [70], [77], but it had not been identified in the state of New York until the sampling done during this study [71]. In some parts of North Carolina, this species is now the most common ant (70 – 80% of all individuals encountered) where it is present [72]. The great potential abundance of this species, combined with the high incidence with which its stings cause anaphylactic shock, suggests the appearance of this species in New York State and particularly in densely populated Manhattan should be taken seriously. In the late spring of 2007, an additional survey of the leaf-litter in the median in which P. chinensis was collected was carried out, but no additional individuals were captured. Additional studies in Manhattan, and New York more generally, are necessary to understand if the individuals we sampled were a nascent and unsuccessful propagule from the south or part of the expanding range of this species. It is interesting to note that in a study carried out by Yamaguchi [15], P. chinensis was found inhabiting the same area as Nylanderia flavipes in their native Japan, as is also apparently the case in New York City.
While the species richness within the medians studied here was relatively high compared to less human dominated habitats, it is notable that many of the species we encountered were similar (though importantly not identical) to those found in other North American cities, such that a relatively small subset of ant species probably represent most of the encounters humans have with ants in much of the United States and Canada. In the seven studies of urban habitats in North American cities of which we are aware [16], [17], [28], [48], [57], [73], all but one of the studies [28] shared some species with those found in our study. Even in a study of San Diego, California, where the surrounding natural habitat is semi-desert rather than temperate forest (Table 5), many species were shared. Additionally, two of the most abundant species in New York were also among the most abundant species in two other cities. Whatever effects these species are having on urban ecosystems, they are carrying them out across a very wide geographic range.
Table 5. Comparison of the species most frequently sampled in this study, with those in other urban ant studies.
| Reference | Region | Distance from Manhattan, NY (km) | Total Species | Most abundant species | Shared species (with this study) | Notes |
| This study | Manhattan, NY, USA | 0 | 14 | T. caespitum , L. neoniger , S. molesta , N. flavipes | 13/13 | |
| Danoff-Burg & Melnick (2004) | Manhattan, New York City, USA | 0 | 24 | N. flavipes , Prenolepis imparis, Ponera pennsylvanica, L. neoniger , L. alienus, Aphaenogaster rudis | 7/13 | Unpublished data |
| Nuhn & Wright (1979) | Raleigh, NC, USA | 650 | 59 | Pheidole vinlandica, Forelius pruinosus, Monomorium minimum, T. caespitum , L. neoniger , Nylanderia sp. parvula complex, Aphaenogaster spp. | 10/13 | Habitat surveyed was the North Carolina State University Campus in Raleigh |
| Lessard & Buddle (2005) | Quebec, Canada | 715 | 24 | Aphaenogaster sp., Camponotus pennsylvanicus, Formica glacialis, L. alienus, L. neoniger , T. caespitum | 6/13 | Only the urban aspect of this study |
| Thompson & McLachlan (2007) | Manitoba, Canada | 2000 | 10 | C. pennsylvanicus, F. glacialis, Lasius pallitarsis | 1/13 | Only the urban aspect of this study |
| Sanford et al (2009) | Lake Tahoe basin, USA | 3800 | 42 | Formica sybilla, Formica lassioides, Myrmica tahoensis, Camponotus modoc, Formica accreta, Camponotus vicinus, T. sessile | 0/13 | Only the most urbanized of the sites characterized by this study. |
| Suarez et al (1998) | Coastal southernCalifornia | 4000 | 46 | L. humile, Dorymyrmex insanus, Prenolepis imparis, Solenopsis molesta, Leptothorax andrei | 2/13 | |
| Clarke et al (2008) | San Francisco | 4600 | 15 | Linipithema humile, Temnothorax andrei, Monomorium ergatogyna, T. sessile | 2/13 |
The number of shared species is in some cases an estimate as in some studies certain ant types were only identified to the genus level. For studies that had such separation of results, only the urban aspect was considered. Shared species indicates the number of species found in this study that were also found in each of the other studies, where the maximum would be 13/13.
The rare species in Manhattan, as in other cities need to be studied in order to understand how they persist and how we might favor their continued persistence. Yet, the more successful and common species may be the ones from which we have the most to learn. Recently, the origins [74] and life history of urban populations of Tapinoma sessile were considered. Menke et al. [74] together with Buczkowski [75] found that many or even most urban colonies of T. sessile have life history traits that are absent in colonies living in natural habitats (multiple queens and large colonies in particular). These life history traits have arisen independently in different lineages of the species in association with the transition to city life [74]. Whether other urban species have also made these convergent shifts is unknown. While there are many ways to be a New Yorker, it may be that there are fewer ways to be an urban ant.
Supporting Information
The correlation and scatter plot matrix for pairwise comparisons among all possible variables. (% high canopy = percent of canopy cover above 2 m, % mulch = percent of ground covered by mulch, % high canopy = percent of canopy cover below 2 m, percent of ground covered by ground-covering plants, trees>2 m = number of trees higher than 2 m, plant sp. = number of plant species)
(1.73 MB TIF)
Acknowledgments
We would like to thank the New York City Parks Department for allowing us to proceed with our work. Thanks also to Benoit Guenard, Andrea Lucky, Sean Menke and two anonymous reviewers for reading earlier drafts and offering their helpful comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: RRD was supported by a United States Department of Energy (DOE) National Institute for Climatic Change Research (DOE-NICCR) grant, DOE Program for Ecosystem Research (DOE-PER) grant (DE-FG02-08ER64510), a National Science Foundation (NSF) Career grant and a National Aeronautics and Space Administration (NASA) Biodiversity grant (NNX09AK22G). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pauchard A, Mauricio A, Eduardo P, Roberto U. Multiple effects of urbanization on the biodiversity of developing countries: The case of a fast-growing metropolitan area (Concepción, Chile). Biological conservation. 2006;127:272–281. [Google Scholar]
- 2.UN. World Urbanization Prospects: The 2007 Revision. 2008. In:Secretariat DoEaSAotUN, editor. New York.
- 3.Cohn JP. Urban wildlife. BioScience. 2005;55:201–205. [Google Scholar]
- 4.Miller JR, Hobbs RJ. Conservation where people live and work. Conservation Biology. 2002;16:330–337. [Google Scholar]
- 5.Dunn RR, Gavin MC, Sanchez MS, Solomon JN. The Pigeon Paradox: Dependence of Global Conservation on Urban Nature. Conservation Biology. 2006;20:1814–1816. doi: 10.1111/j.1523-1739.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- 6.Weisman A. New York: Picador; 2008. The World Without Us.432 [Google Scholar]
- 7.Rebele F. Urban Ecology and Special Features of Urban Ecosystems. Global Ecology and Biogeography Letters. 1994;4:173–187. [Google Scholar]
- 8.Herbert RA, Herbert KGS. Behavior of Peregrine Falcons in the New York City Region. The Auk. 1965;82:64–94. [Google Scholar]
- 9.Mehtälä J, Vuorisalo T. Changing values of urban biodiversity: a reply to Miller. Trends in Ecology & Evolution. 2006;21:116–117. doi: 10.1016/j.tree.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre NE, Knowles-Yanez K, Hope D. Urban ecology as an interdisciplinary field: differences in the use of “urban” between the social and natural sciences. Urban Ecosystems. 2000;4:5–24. [Google Scholar]
- 11.Pickett STA, Cadenasso ML, Grove JM, Nilon CH, Pouyat RV, et al. Urban ecological systems: Linking Terrestrial Ecological, Physical, and Socioeconomic Components of Metropolitan Areas. Urban Ecosystems. 2001;32:127–157. [Google Scholar]
- 12.Savard JPL, Clergeau P, Mennechez G. Biodiversity concepts and urban ecosystems. Landscape and Urban Planning. 2000;48:131–142. [Google Scholar]
- 13.Smith RM, Warren PH, Thompson K, Gaston KJ. Urban domestic gardens (VI): environmental correlates of invertebrate species richness. Biodiversity and Conservation. 2006;V15:2415–2438. [Google Scholar]
- 14.Small EC, Sadler JP, Telfer MG. Carabid beetle assemblages on urban derelict sites in Birmingham, UK. Journal of Insect Conservation. 2003;6:233–246. [Google Scholar]
- 15.Yamaguchi T. Influence of urbanization on ant distribution in parks of Tokyo and Chiba City, Japan II. Analysis of species. Entomological Science. 2005;8:17–25. [Google Scholar]
- 16.Suarez AV, Bolger DT, Case TJ. Effects of fragmentation and invasion on native ant communities in coastal southern California. Ecology. 1998;79:2041–2056. [Google Scholar]
- 17.Thompson B, McLachlan S. The effects of urbanization on ant communities and myrmecochory in Manitoba, Canada. Urban Ecosystems. 2007;10:43–52. [Google Scholar]
- 18.Niemelä J. Ecology and urban planning. Biodiversity and Conservation. 1999;8:119–131. [Google Scholar]
- 19.Chawla L. Life paths into effective environmental action. The Journal of Environmental Education. 1999;31:15–26. [Google Scholar]
- 20.Louv R. Chapel Hill, NC: Algonquin Books; 2008. Last Child in the Woods: Saving Our Children from Nature-Deficit Disorder.390 [Google Scholar]
- 21.Chawla L. Significant life experiences revisited: a review of research on sources of environmental sensitivity. The Journal of Environmental Education. 1998;29:11–29. [Google Scholar]
- 22.Angold PG, Sadler JP, Hill MO, Pullin A, Rushton S, et al. Biodiversity in urban habitat patches. Science of the Total Environment. 2005;360:196–204. doi: 10.1016/j.scitotenv.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer V. Droscher T, Fraser DA, editors. Green Links and Urban Biodiversity-an Experiment in Connectivity. Vancouver, British Columbia. 2003.
- 24.Beier P, Noss RF. Do habitat corridors provide connectivity? Conservation Biology. 1998;12:1241–1252. [Google Scholar]
- 25.Kondoh M, Kitazawa Y. Ant Communities on the Campus of UOEH and in an Adjacent Natural Forest. Journal of UOEH. 1984;6:221–234. [Google Scholar]
- 26.Na JPS, Lee CY. Identification key to common urban pest ants in Malaysia. Tropical Biomedicine. 2001;18:1–17. [Google Scholar]
- 27.Klotz JH, Mangold JR, Vail KM, Davis LR, Patterson RS. A Survey of the Urban Pest Ants (Hymenoptera: Formicidae) of Peninsular Florida. The Florida Entomologist. 1995;78:109–118. [Google Scholar]
- 28.Sanford MP, Manley PN, Murphy DD. Effects of urban development on ant communities: Implications for ecosystem services and management. Conservation Biology. 2008;23:131–141. doi: 10.1111/j.1523-1739.2008.01040.x. [DOI] [PubMed] [Google Scholar]
- 29.Folgarait PJ. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodiversity and Conservation. 1998;7:1221–1244. [Google Scholar]
- 30.Klotz JH, Hansen L, Pospischil R, Rust MK. Ithaca, New York: Cornell University Press; 2008. Urban Ants of North America and Europe: Identification, Biology, and Management. [Google Scholar]
- 31.Alonso LE. Ants as indicators of biodiversity. In: Agosti D, Majer J, Alonso E, Schultz T, editors. Ants: Standard Methods for Measuring and Monitoring Biodiversity. Washington DC: Smithsonian Institution Press; 2000. pp. 80–88. [Google Scholar]
- 32.Frankie GW, Ehler LE. Ecology of insects in urban environments. Annual Review of Entomology. 1978;23:367–387. [Google Scholar]
- 33.Andersen AN, Hoffman BD, Muller WJ, Griffiths AD. Using ants as bioindicators in land management: simplifying assessment of ant community responses. Journal of Applied Ecology. 2002;39:1–17. [Google Scholar]
- 34.Lobry de Bruyn LA. Ants as bioindicators of soil function in rural environments. Agriculture, Ecosystems and Environment. 1999;74:425–441. [Google Scholar]
- 35.Peck SL, Betty M, Campbell LC. Using Ant Species (Hymenoptera: Formicidae) as a Biological Indicator of Agroecosystem Condition. Environmental Entomology. 1998;27:1102–1110. [Google Scholar]
- 36.MacArthur RH, Wilson EO. Princeton, NJ: Princeton University Press; 1967. The Theory of Island Biogeography.230 [Google Scholar]
- 37.Bierregaard RO, Jr, Lovejoy TE, Kapos V, Augusto dos Santos A, Hutchings RW. The biological dynamics of tropical rainforest fragments. BioScience. 1992;42:859–866. [Google Scholar]
- 38.Simberloff D. Experimental zoogography of islands: Effects of island size. Ecology. 1976;57:629–648. [Google Scholar]
- 39.Brühl CA, Eltz T, Linsenmair KE. Size does matter - effects of tropical forest fragmentation on the leaf litter ant community in Sabah, Malaysia. Biodiversity and Conservation. 2003;12:1371–1389. [Google Scholar]
- 40.Whittaker R. Oxford, NY: Oxford University Press; 1998. Island Biogeography: Ecology, Evolution, and Conservation. [Google Scholar]
- 41.Lassau SA, Hochuli DF. Effects of habitat complexity on ant assemblages. Ecography. 2004;27:157–164. [Google Scholar]
- 42.Terborgh J. Bird Species Diversity on an Andean Elevational Gradient. Ecology. 1977;58:1007–1019. [Google Scholar]
- 43.Friedrich R, Philpott SM. Nest-site limitation and nesting resources of ants (Hymenoptera: Formicidae) in urban green spaces. Environmental Entomology. 2009;38:600–607. doi: 10.1603/022.038.0311. [DOI] [PubMed] [Google Scholar]
- 44.Tschinkel WR. Distribution of the Fire Ants Solenopsis invicta and S. geminataI (Hymenoptera: Formicidae) in Northern Florida in Relation to Habitat Disturbance. Annals of the Entomological Society of America. 1988;81:76–81. [Google Scholar]
- 45.Plowes RM, Dunn JG, Gilbert LE. The urban fire ant paradox: native fire ants persist in an urban refuge while invasive fire ants dominate natural habitats. Biological Invasions. 2006;9:825–836. [Google Scholar]
- 46.Bestelmayer BR, Agosti D, Alonso LE, Brandao CRF, Brown WLJ, et al. Field Techniques for the Study of Ground-Dwelling Ants. In: Agosti D, Majer JD, Alonso LE, Schultz TR, editors. Ants: Standard Methods for Measuring and Monitoring Biodiversity. Washington, DC: Smithsonian Institution Press; 2000. pp. 122–144. [Google Scholar]
- 47.McIntyre NE. Ecology of Urban Arthropods: A Review and a Call to Action. Ecology and Population Biology. 2000;93:825–835. [Google Scholar]
- 48.Nuhn TP, Wright CG. An Ecological Survey of Ants (Hymenoptera: Formicidae) in a Landscaped Suburban Habitat. American Midland Naturalist. 1979;102:353–362. [Google Scholar]
- 49.Smith RM, Gaston KJ, Thompson K, Warren PH. Urban domestic gardens (VIII): environmental correlates of invertebrate abundance. Biodiversity and Conservation. 2006;15:2515–2545. [Google Scholar]
- 50.Sokal RR, Rohlf FJ. New York, NY: Freeman; 1981. Biometry.880 [Google Scholar]
- 51.SAS . Cary, NC: SAS Institute Inc; 1989. JMP 7. [Google Scholar]
- 52.Crawley MJ. Chichester, UK: John Wiley and Sons Ltd; 2002. Statistical Computing: An Introduction to Data Analysis Using S-plus.772 [Google Scholar]
- 53.Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP. Why do we still use stepwise modelling in ecology and behaviour? Journal of Animal Ecology. 2006;75:1182–1189. doi: 10.1111/j.1365-2656.2006.01141.x. [DOI] [PubMed] [Google Scholar]
- 54.Anderson DR. New York, NY: Springer; 2008. Model Based Inference in the Life Sciences: A Primer on Evidence.184 [Google Scholar]
- 55.Atmar W, Patterson BD. University Park, NM: AICS Research, Inc and Chicago, IL: The Field Museum; 1995. The nestedness temperature calculator: a visual basic program, including 294 presence-absence matrices. [Google Scholar]
- 56.Atmar W, Patterson BD. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia. 1993;96:373–382. doi: 10.1007/BF00317508. [DOI] [PubMed] [Google Scholar]
- 57.Lessard J-P, Buddle CM. The effects of urbanization on ant assemblages (Hymenoptera: Formicideae) associated with the Molson Reserve, Quebec. Canadian Entomologist. 2005;137:215–225. [Google Scholar]
- 58.Talbot M. Distribution of Ant Species in the Chicago Region with Reference to Ecological Factors and Physiological Toleration. Ecology. 1934;15:416–439. [Google Scholar]
- 59.LaPolla JS, Brady SG, Shattuck SO. Phylogeny and taxonomy of the Prenolepis genus-group of ants (Hymenoptera: Formicidae). Systematic Entomology. 2010;35:118–131. [Google Scholar]
- 60.Dunn RR, Guénard B, Wieser MD, Sanders NJ. Geographic Gradients. In: Lach L, Parr CL, Abbott KL, editors. Ant Ecology. New York: Oxford University Press; 2010. [Google Scholar]
- 61.Kaspari M, O'Donnel S, Kercher JR. Energy, Density, and Constraints to Species Richness: Ant Assemblages along a Productivity Gradient. The American Naturalist. 2000;155:280–293. doi: 10.1086/303313. [DOI] [PubMed] [Google Scholar]
- 62.Lessard JP, Dunn RR, Sanders NJ. Temperature-mediated coexistence in temperate forest ant communities. Insectes Sociaux. 2009;56:149–156. [Google Scholar]
- 63.Plowes N. Self organized conflict in territorial ants: University of Connecticut 2008.
- 64.Ivanov K, Milligan J. Paratrechina flavipes (Smith) (Hymenoptera: Formicidae), a new exotic ant for Ohio. Proceedings of the Entomological Society of Washington. 2008;110:439–444. [Google Scholar]
- 65.Collingwood CA, Tigar BJ, Agosti D. Introduced Ants in the United Arab Emirates. Journal of Arid Environments. 1997;37:505–512. [Google Scholar]
- 66.Hölldobler B, Wilson EO. Cambridge, MA: Belknap; 1990. The Ants.752 [Google Scholar]
- 67.Schultz TR, McGlynn TP. The interactions of ants with other organisms. In: Agosti D, Majer J, Alonso E, Schultz T, editors. Ants: Standard Methods for Measuring and Monitoring Biodiversity. Washington, DC: Smithsonian Institution Press; 2000. pp. 35–44. [Google Scholar]
- 68.Hölldobler B, Wilson EO. Cambridge, Massachusetts: Belknap Press of Harvard University Press; 1994. Journey to the Ants.304 [Google Scholar]
- 69.Werner FG, Olson CMS. Tucson, Arizona: Fisher Books; 1994. Insects of the Southwest: How to Identify Helpful, Harmful and Venomous Insects.162 [Google Scholar]
- 70.Nelder MP, Paysen ES, Zungoli PA, Benson EP. Emergence of the introduced ant Pachyondyla chinensis (Formicidae: Ponerinae) as a public health threat in the southeastern United States. Journal of Medical Entomology. 2006;43:1094–1098. doi: 10.1603/0022-2585(2006)43[1094:eotiap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 71.Guénard B, Dunn RR. A new (old), invasive ant in mature forests of Eastern North America and its impacts. PLoS ONE. 2010;5:e11614. doi: 10.1371/journal.pone.0011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cope R. Understanding the spread of the invasive “Giant needle ant” in order to mitigate its spread 2006.
- 73.Clarke KM, Fisher BL, LeBuhn G. The influence of urban park characteristics on ant (Hymenoptera, Formicidae) communities. Urban Ecosystems. 2008;11:317–334. [Google Scholar]
- 74.Menke SB, Booth W, Dunn RR, Schal C, Vargo EL, et al. Is it easy to be urban? Convergent success in urban habitats among lineages of a widespread native ant. PLoS ONE. 2010;5:e9194. doi: 10.1371/journal.pone.0009194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buczkowski G. Extreme life history plasticity and the evolution of invasive characteristics in a native ant. Biological Invasions. 2010:1387–3547. [Google Scholar]
- 76.Colwell RK. Estimate S: Statistical estimation of species richness and shared species from samples. 2009. 8.2 ed.
- 77.Guénard B, Dunn RR. A New (Old), Invasive Ant in the Hardwood Forests of Eastern North America and Its Potentially Widespread Impacts. PLoS ONE. 2010;5:e11614. doi: 10.1371/journal.pone.0011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The correlation and scatter plot matrix for pairwise comparisons among all possible variables. (% high canopy = percent of canopy cover above 2 m, % mulch = percent of ground covered by mulch, % high canopy = percent of canopy cover below 2 m, percent of ground covered by ground-covering plants, trees>2 m = number of trees higher than 2 m, plant sp. = number of plant species)
(1.73 MB TIF)