Abstract
Elbow contractures, a frequent problem after injury, can be treated by excision of the joint capsule. However, the underlying changes in the joint capsule are poorly understood. Based on skin healing work, we examined the hypotheses that myofibroblast numbers and expression of a myofibroblast marker α-smooth muscle actin, are elevated in patients with posttraumatic joint contractures. Anterior capsules were obtained from six patients who had operative release of posttraumatic contractures greater than 5 months after injury and six elbows of organ donors free of contractures. Immunohistochemical studies revealed that myofibroblast numbers and percentage of total cells that were myofibroblasts were significantly elevated in the joint capsules from patients with contractures (326 ± 61 cells per field, 36% ± 4% total cells) when compared with similar tissues of the organ donors (69 ± 41 cells per field, 9% ± 4% total cells). Western blot analysis showed that protein levels of α-smooth muscle actin were significantly elevated in patients with posttraumatic joint contractures. However, analysis with reverse transcription-polymerase chain reaction determined that messenger ribonucleic acid levels for smooth muscle actin normalized to the housekeeping gene glyceral-dehyde-3-phosphate dehydrogenase were not significantly different between the two groups. An association between increased numbers of myofibroblasts and posttraumatic joint contractures has been established in the human elbow capsule. Additional work is required to determine whether myofibroblast regulators may be targets for adjuvant therapies of posttraumatic contractures.
Elbow injuries, such as fractures, dislocations, or both, frequently are associated with posttraumatic loss of motion or joint contractures. Cooney reported complete loss of motion after 5% of elbow injuries.3 If traditional postinjury modalities such as physiotherapy and bracing are unsuccessful in achieving a functional range of motion (ROM), operative intervention to improve joint motion often is considered. Operative treatments, whether open or arthroscopic, focus on excising or dividing the joint capsule surrounding the elbow to improve joint motion.27,30 However, the mechanisms underlying the change in the joint capsule after joint injury are poorly understood.
Nonarticular processes can lead to contractures and deformity. Dupuytren’s contracture of the hand, and hypertrophic scarring or keloid formation of the skin are examples.1,4,21,29 In these conditions, an association has been drawn with a particular cell type, the myofibroblast. Myofibroblasts first were described in healing skin wounds where the edges that were left unapposed contracted until the wound was closed.10,26,28 Myofibroblasts also have been seen in nonmusculoskeletal pathologic fibrotic conditions characterized by contractures such as liver cirrhosis, pulmonary fibrosis, and corneal fibrosis.14,26,28 A contractile smooth muscle protein, α-smooth muscle actin (α-SMA), is expressed by myofibroblasts and has been used as a marker of myofibroblasts.26,28 There is evidence that this cytoskeletal protein can interact with the extracellular matrix through cell membrane integrins at the fibronexus, and therefore influence matrix organization.28 Myofibroblasts have the ability to contract collagen gels and cellular α-SMA expression is required for this attribute.28 Therefore, one proposed mechanism to explain at least some of the altered capsular properties subsequent to elbow injury is an increase in myofibroblast numbers. Regulators of myofibroblast numbers and α-SMA expression include growth factors such as transforming growth factor-beta 1 (TGF-β1) and endothelin 1 (ET-1). Understanding cellular changes in the joint capsule may lead to adjuvant nonoperative treatments, such as modulating growth factor influences on α-SMA expression and myofibroblast numbers in this case.
The objective of the current study was to evaluate myofibroblast numbers and α-SMA expression in patients with posttraumatic joint contractures. Three hypotheses were tested: (1) myofibroblast numbers and percentage of total cells, (2) α-SMA protein levels, and (3) α-SMA mRNA levels are elevated in anterior joint capsules obtained from patients requiring operative intervention for posttraumatic elbow contractures when compared with similar tissues obtained from age-matched organ donors free of elbow contractures.
MATERIALS AND METHODS
Tissue Processing
Anterior capsules of human elbows were obtained from six patients (33 ± 13 years old, mean ± standard deviation) at the time of contracture release between 5 and 25 months after injury. The original injuries were intraarticular fractures (three distal humerus, three radial head). Control anterior capsules were obtained from six elbows of organ donors (26 ± 15 years old) that were free of contractures. The tissues from the patients either were placed in Optimal Cutting Temperature (OCT) embedding material (Sakura Finetek, Torrance, CA) for immunohistochemical examination or immediately frozen for protein and mRNA analysis. The tissues from the organ donors were obtained within 5 to 18 hours of death (body stored at 4° C) and prepared as above. In cases where rigor mortis had set in, clinical history was used to determine that the joints were free of contractures. All tissues were stored at −80°C. Institutional review board approval from our institution and a donor program was obtained before the study.
Immunolocalization Analysis
Sections (8-μm thickness) of the samples were cut using a cryostat, mounted on precoated glass slides, and pretreated with hyaluronidase (Seikogaku Corporation, Code no. 100740, Tokyo, Japan) for 20 minutes at 37° C. The sections were washed in 0.1 mol/L phosphate-buffered saline, three times for 5 minutes each. Endogenous peroxidases were quenched using 0.3% hydrogen peroxide in methanol for 20 minutes at room temperature. The sections were rinsed once in phosphate-buffered saline for 5 minutes and 10% normal goat serum in phosphate-buffered saline was applied for 20 minutes at 37° C as a blocking agent. The monoclonal α-SMA antibody (clone asm-1, Röche Molecular Biochemicals, Laval, QC, Canada) was applied (5 μg/mL), incubated for 60 minutes at 37° C, and the sections subsequently were washed in phosphate-buffered saline three times for 5 minutes each. The secondary antibody, a sheep antimouse immunoglobulin (IgG) horseradish conjugate (Röche), was applied to the sections (dilution 1:50) for 60 minutes at room temperature. The sections were washed three times for 5 minutes each, followed by application of the diaminobenzidine (DAB)/peroxide substrate (Röche) for 13 minutes. The reaction was stopped by emersion in phosphate-buffered saline, for three washes of 5 minutes each.
Alpha-smooth muscle actin is used as a marker for myofibroblasts; however, it also is expressed abundantly in smooth muscle cells (walls of blood vessels) and pericytes around blood vessels.26 Markers of blood vessels, such as laminin, were used to differentiate α-SMA associated with the vasculature or myofibroblasts.6,17,23,25 Therefore, these sections then had primary antibodies to laminin (rabbit affinity purified antibodies, Sigma, St Louis, MO) applied at a dilution of 1:25. The sections were stored at 4° C overnight and then washed in phosphate-buffered saline three times for 5 minutes each. Subsequently, the sections had the secondary antibody, a goat antirabbit IgG antibody conjugated with Alexa Fluor 488 (Molecular Probes, Eugene, OR) applied for 60 minutes at a dilution of 1:1000. The slides were maintained in darkness for all secondary antibody reactions. The sections were washed in phosphate-buffered saline three times for 5 minutes each, 4′-6-diamidine-2-phenyl indole, or DAPI, (Vector Laboratories, Burlington, ON, Canada) was applied and cover slips were placed on the sections. The sections were stored at 4° C in darkness.
Appropriate controls were assessed in all experimental series. Positive controls for α-SMA and laminin were blood vessels in capsular tissue and human uterus. Negative controls included substituting primary antibodies with 3% bovine serum albumin. A second negative control was sections of human articular cartilage from the age-matched organ donor elbows free of contractures or arthritis on inspection.
The sections were viewed under a light microscope (Zeiss, Axioskop 2 plus, Toronto, ON, Canada). Images were captured (×200) from three randomly selected areas from each of six sections for each specimen with a digital camera (Zeiss, Axiocam). Image-Pro Plus (Media Cybernetics, Silver Spring, MD) was used to analyze cell numbers. Cell nuclei associated with α-SMA and not laminin were counted as myofibroblasts. Total cell counts also were obtained. The data were collected as absolute myofibroblast numbers and also expressed as a ratio of myofibroblast cells to total cells.
Western Blot Studies
The frozen anterior capsule tissue was powdered at liquid N2 temperatures. Two milliliters of sample buffer (0.08 mol/L Tris-HCl, pH 6.8; 2% sodium dodecyl sulfate; 0.1 mol/L Dithiothreitol; 0.001 mol/L phenylmethylsulfonyl fluoride; 2 mmol/L ethylenediaminetetraacetic acid; 10% glycerol) were placed in the dismembrator vessel while the powder still was frozen. After thawing, the slurry was transferred to a clean tube and rotated for 1 hour at 4° C. The sample then was centrifuged at 21,600 rpm for 15 minutes at 4° C and the supernatant fraction removed. The collected supernatant was placed in a 10,000 microconcentrator (Gelman Laboratory, Ann Arbor, MI) and concentrated 20-fold to 100 μL by centrifuging at 7000 × g for 70 minutes using a Sorvall RC-5B refrigerated superspeed centrifuge (Mandel Scientific Co, Guelph, ON, Canada). Total protein content of the sample was determined with a Bio-Rad Protein assay (Bio-Rad Laboratory, Mississauga, ON, Canada). The protein sample was boiled for 5 minutes in sodium dodecyl sulfate sample buffer and 2.5 μg protein per lane was electrophoretically resolved on a 12.5% polyacrylamide precast gel (Owl Scientific, Ports-mouth, NH). Fractionated proteins were transferred to nitro-cellulose paper overnight at 4° C. Nonspecific binding sites were blocked using 5% skimmed milk in Tris buffered saline, (10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl) for 1 hour at 4° C. Primary anti-α-SMA monoclonal antibody (clone asm-1), diluted to 2.5 μg/mL in Tris buffered saline, was incubated for 2 hours at room temperature. After three washes with Tris buffered saline with Tween, the blots were incubated with sheep antimouse IgG conjugated with horseradish peroxidase (Röche) at 1:1000 dilution in Tris buffered saline with Tween with 5% skimmed milk powder for 1 hour at room temperature. The nitrocellulose sheets were washed three times for 15 minutes with Tris buffered saline with Tween. Peroxidase activity was revealed with enhanced chemiluminescent assay detection reagents (Amersham Pharmacia Biotech, Baie d’Urfe, QC, Canada). A film of the peroxidase reaction was scanned using the MasterScan Interpretive Densitometer (CSPI, Billerica, MA), and the density of α-SMA was calculated using Scanalytics Software (CSPI). Extracts of human uterus served as the positive control, and the negative control was extracts of human articular cartilage from the age-matched organ donor elbows free of contractures and arthritis on inspection.
REVERSE TRANSCRIPTION-POLYMERASE CHAIN REACTION (RT-PCR)
The protocol followed methods described previously.19,28 Ribonucleic acid isolation (TRI-spin method) began by reducing the frozen tissue to a powder at liquid N2 temperatures. TRIzol reagent (Life Technologies, Gaithersburg, MD) was added (1 mL TRIzol/50 mg tissue) and the mixture then was warmed to room temperature. Chloroform was added, and the samples were centrifuged. The upper aqueous phase containing the RNA then was removed, and one volume of 70% ethanol was added. Total RNA was extracted and the RNA was purified using a RNeasy Total RNA kit (Qiagen, Chatsworth, CA). The RNA yield was quantified fluorometrically (Perkin-Elmer, Branchburg, NJ) using Sybr Green II (FMC BioProducts, Rockland, MN) and was compared with standards obtained with calf liver ribosomal RNA.
Semiquantitative RT-PCR for mRNA analysis was done on all samples at the same time. Reverse transcription was done using 1-μg total RNA aliquots and the first step of the RT-PCR kit (Stratagene, La Jolla, CA). Random primers (300 ng) were added to the RNA and first-strand cDNA was synthesized by adding ×10 first-strand buffer, RNase block ribonuclease inhibitor, d-nucleoside triphosphates, and Maloney murine leukemia virus reverse transcriptase. Reactions were incubated at 37° C for 1 hour; this was followed by a 5-minute incubation at 90° C and subsequent cooling on ice for 5 minutes. Polymerase chain reaction was done using the Life Technologies kit. Aliquots of cDNA were amplified using ×10 PCR buffer, 10 mmol/L d-nucleoside triphosphate mixture, 50 mmol/L MgCl2, 0.5 μmol/L of each primer, and 2.5 U Taq DNA polymerase. Human primers specific for α-SMA (Strata-gene: Forward 5′-GCT CAC GGA GGC ACC CCT GAA-3′, Reverse 5′-CTG ATA GGA CAT TGT TAG CAT-3′; amplified product size 591 bp) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene, (Genbank Accession # BC029618: Forward 5′-TCA CCA TCT TCC AGG AGC GA- 3′, Reverse 5′-AGT GAT GGC ATG GAC TGT GG - 3′; amplified product size 326 bp) were used.18 Primer sets were optimized for PCR cycles, and linear amplification curves were plotted. The optimal cycle number was selected to be in the linear part of the amplification curve. All RT controls were negative ensuring that no genomic DNA was present in the RNA samples. All samples used in this study were reverse-transcribed at least twice, and all PCR experiments were done at least twice.
Polymerase chain reaction products were observed by electrophoresis using a 2% agarose gel. After electrophoresis, the gels were stained with ethidium bromide, destained in distilled water, and photographed using Polaroid number 55 film (Technicare Inc, Calgary, AB, Canada). Comparison with the standard 1-kb DNA Ladder (Life Technologies) ensured that the PCR products were the proper size. Relative band intensities were quantified by densitometric scanning of negatives (Masterscan Interpretive Densitometer; CSPI). The α-SMA mRNA levels were normalized to the mRNA levels of the housekeeping gene GAPDH. Human uterus served as the positive control.6 The negative control was human articular cartilage from the age-matched organ donor elbows free of contractures and arthritis on inspection.19
Statistical Analysis
Data are presented as mean ± standard deviation. Statistical analysis consisted of t tests for group means. Regression analysis was used to investigate relationships between myofibroblast numbers (absolute, percentage), α-SMA protein levels, or α-SMA mRNA levels and age of subject, chronicity of contracture, and elbow motion arc. Significance levels were set at P ≤ 0.05.
RESULTS
Comparison of clinical characteristics of the patients and organ donors showed a similar average age, obvious motion loss with the patients’ elbows, and the average duration of the contractures was 14 ± 9 months. The average age for the two groups (patients, 33 ± 13 years; organ donors, 26 ± 15 years) was within 7 years, which was not significantly different (P = 0.39). The average arc of elbow extension to flexion motion for the patients was 58° ± 15°; all patients had less than 85° total motion. In comparison, the donor elbows all had extension to flexion plane arcs of motion greater than 130°.
Myofibroblasts were increased significantly in the joint capsules of patients when compared with the organ donors whereas total cell counts were similar in the two groups (Table 1; Figs 1, 2). Absolute myofibroblast numbers were elevated significantly (P < 0.001) in the joint capsules of the patients with posttraumatic contractures (326 ± 61 cells per field) when compared with the joint capsules of the organ donors free of joint contractures (69 ± 41 cells per field). In addition, the percentage of total cells that were myofibroblasts was significantly elevated (P < 0.001) in the joint capsules from patients with posttraumatic contractures (36% ± 4%) when compared with the capsules of the organ donors free of contractures (9% ± 4%). There was no significant difference (P = 0.66) between the two groups comparing the total number of cells per field. Positive internal controls were the presence of blood vessels (α-SMA and laminin colabeling) in the same image (Fig. 1). Human uterus was positive for α-SMA and laminin (data not shown). Negative controls tested appropriately (Fig. 2).
TABLE 1.
Data Summary
| Contractures | Age (Years) | Chronicity (Months) | Total Motion (°) | Myofibroblasts | Cell Counts % Myofibroblasts | Total Cells | Protein | mRNA |
|---|---|---|---|---|---|---|---|---|
| 18 | 20 | 50 | 275 | 34 | 854 | 28 | 1.8 | |
| 48 | 25 | 56 | 329 | 33 | 1031 | 25 | 1.9 | |
| 32 | 9 | 60 | 430 | 39 | 1060 | 16 | 1.7 | |
| 42 | 5 | 85 | 294 | 37 | 794 | 34 | 2.0 | |
| 17 | 22 | 39 | 272 | 42 | 642 | 29 | 1.5 | |
| 41 | 6 | 57 | 357 | 32 | 1134 | 20 | 1.7 | |
| Mean | 33 | 14 | 58 | 326* | 36† | 919 | 25‡ | 2.5 |
| Standard deviation | 13 | 9 | 15 | 61 | 0.04 | 187 | 7 | 0.6 |
| Controls | ||||||||
| 52 | — | >130 | 140 | 9 | 1371 | 6 | 1.6 | |
| 35 | — | >130 | 58 | 16 | 385 | 4 | 1.8 | |
| 15 | — | >130 | 36 | 4 | 976 | 15 | 3.3 | |
| 15 | — | >130 | 91 | 9 | 1040 | 5 | 3.1 | |
| 21 | — | >130 | 55 | 8 | 718 | 5 | 2.6 | |
| 16 | — | >130 | 32 | 6 | 581 | 7 | 0.9 | |
| Mean | 26 | — | — | 69* | 9† | 845 | 7‡ | 3.1 |
| Standard deviation | 15 | — | — | 41 | 0.04 | 335 | 4 | 1.7 |
Chronicity: time from injury to contracture release.
Total motion: extension and flexion plane.
Myofibroblasts: number of cells/field.
% Myofibroblasts: myofibroblast numbers/total cell numbers.
Total cells: number of cells/field.
Protein: optical density of scanned Western blots for α-SMA.
mRNA: optical density of scanned semiquantitative RT-PCR α-SMA/GAPDH.
— not done.
Significantly different (P < 0.05).
Significantly different (P < 0.05).
Significantly different (P < 0.05).
FIGURE 1.
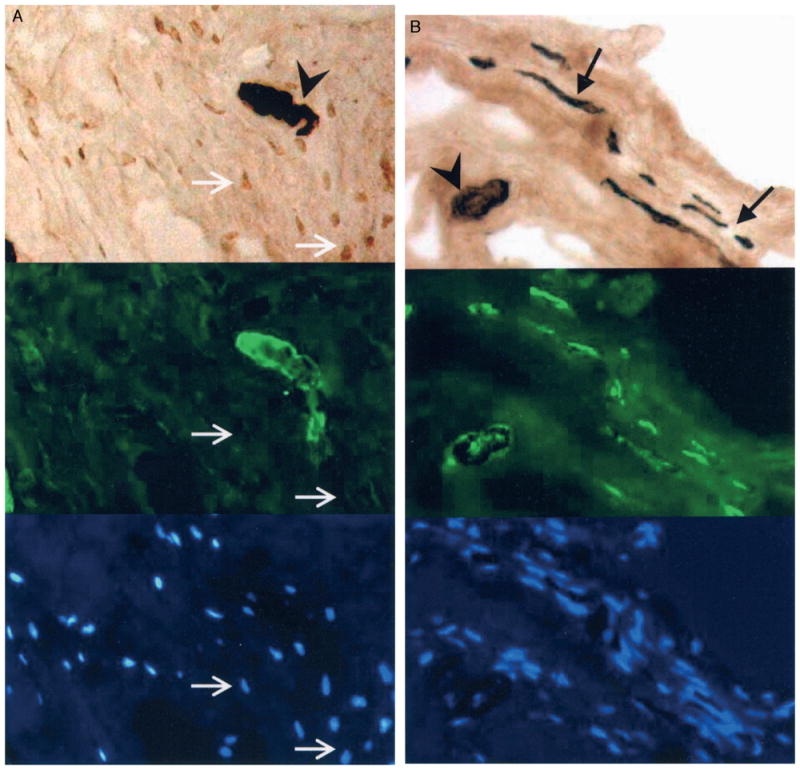
A–B. Immunolocalization analysis is shown of the anterior joint capsule from (A) a patient with a contracture, and (B) from an organ donor (control) free of contractures. All images came from the same area of the same section for each subject; only the light and filters were adjusted as necessary. Alpha-smooth muscle actin is at the top, laminin is in the middle, and 4′-6-diamidine-2-phenyl indole (DAPI) is at the bottom. The triangles indicate vascular structures in cross section and the black arrows indicate vascular structures in longitudinal section. Examples of α-SMA positive areas without laminin colabeling representing myofibroblasts (white arrows) are shown in Figure 1A. There are no myofibroblasts in Figure 1B. (Original magnification, ×200).
FIGURE 2.
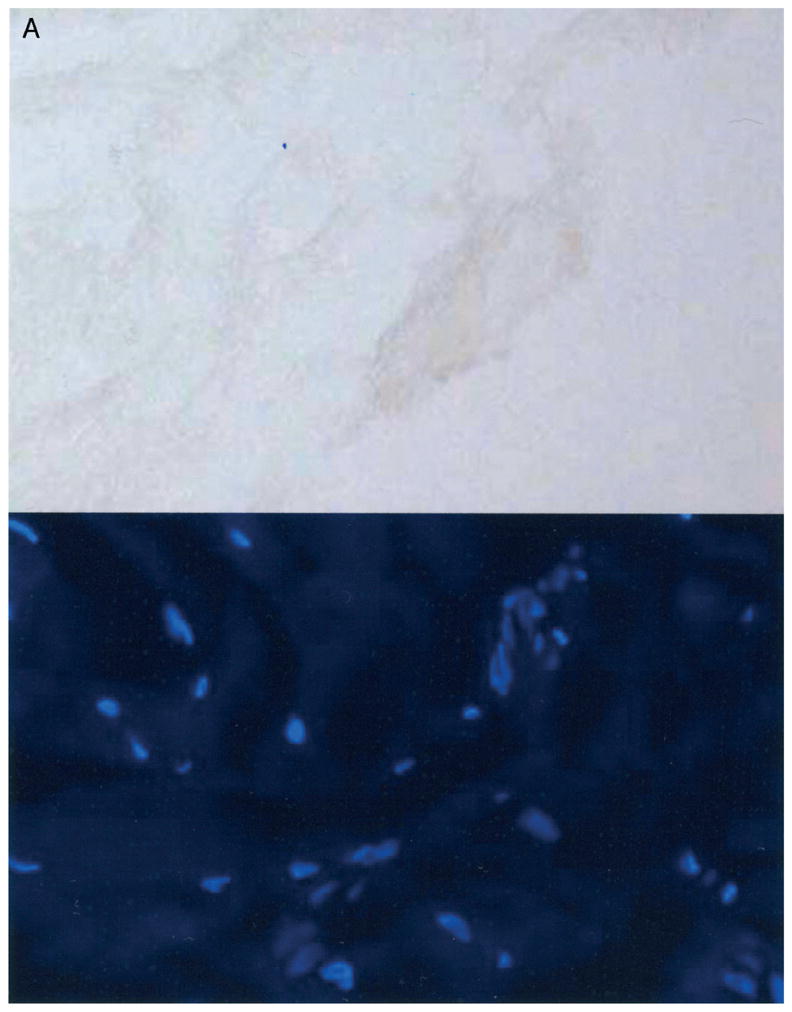
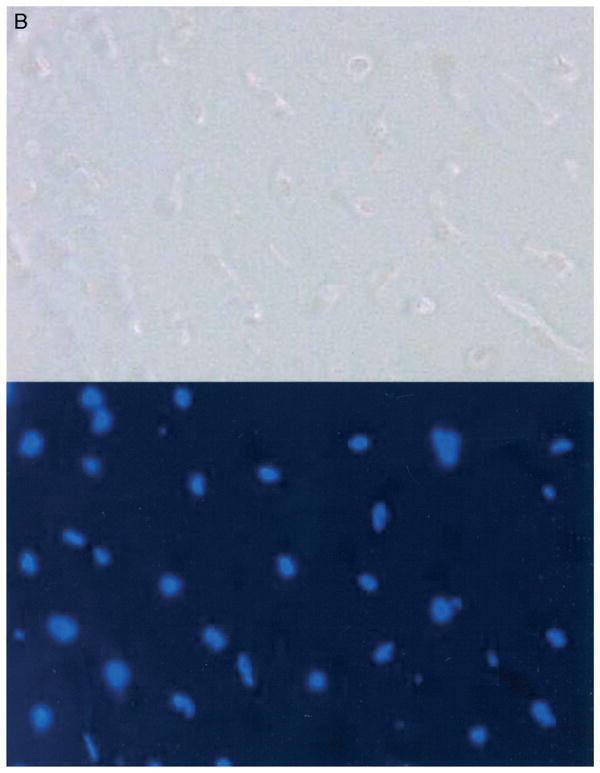
A–B. Negative controls are shown for immunohistochemistry. (A) A joint capsule from a patient with contractures, (substitution of 1° antibodies with 3% bovine serum albumin) and (B) articular cartilage (radial head) from an organ donor free of elbow contractures, arthritis or fracture are shown. Alpha-smooth muscle actin is at the top and 4′-6-diamidine-2-phenylindole (DAPI), is at the bottom. (Original magnification, ×200.)
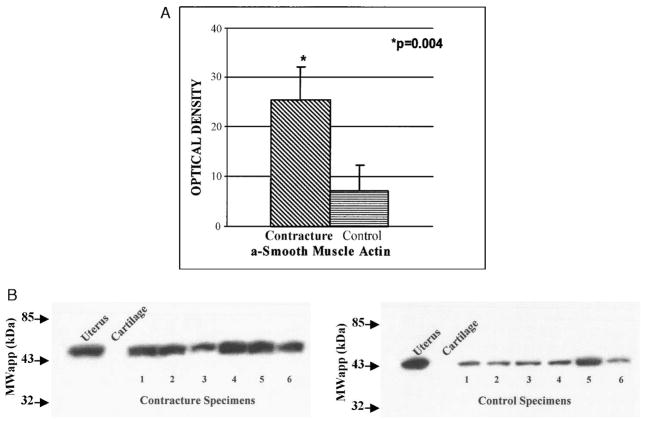
Protein levels of α-SMA were elevated significantly in the joint capsules of the patients when compared with similar tissues from controls, in concert with the myofibroblast numbers (Table 1; Fig. 3). There were statistically significant increases (P = 0.004) in α-SMA protein levels of the anterior capsules obtained from patients with joint contractures (optical density, 25 ± 7) when compared with similar tissues from donor elbows (optical density, 7 ± 4; Fig. 3A). Positive (uterus) and negative (normal articular cartilage) controls tested appropriately (Fig. 3B).
FIGURE 3.
A–B. (A) A comparison of optical density measures for contracture (n = 6) and control (n = 6) specimens is shown. (B) A representative Western Blot (protein) analysis of α-SMA is shown. Positive (uterus, lane 1) and negative controls (articular cartilage, lane 2) are included. There are six specimens for each group.
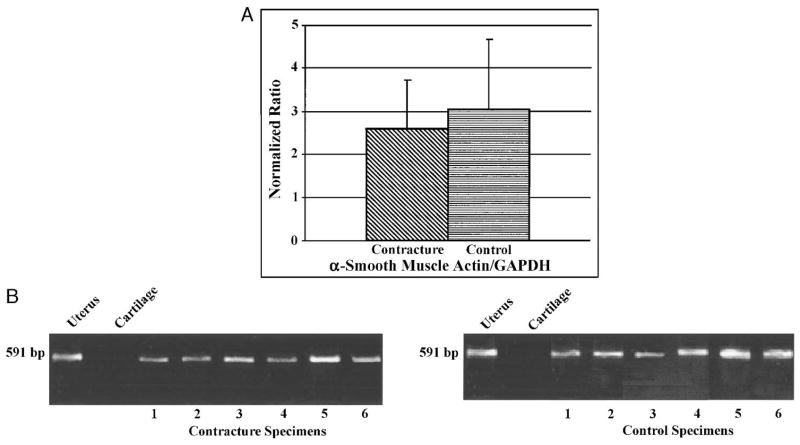
Normalized semiquantitative α-SMA mRNA comparisons showed no differences between the patients’ and the organ donors’ joint capsules (Table 1; Fig. 4). There was no statistically significant difference between the two groups for α-SMA expression (P = 0.59; Fig. 4A). The normalized mRNA levels (optical density) were 2.6 ± 1.1 for the contracture specimens and 3.1 ± 1.6 for the control specimens (Fig. 4A). Positive (uterus) and negative (normal articular cartilage) controls tested appropriately (Fig. 4B).
FIGURE 4.
A–B. (A) A comparison of optical density measures for contracture (n = 6) and control (n = 6) specimens is shown. (B) A representative semiquantitative RT-PCR analysis of α-SMA (normalized to GAPDH) is shown. Positive (uterus, lane 1) and negative controls (articular cartilage, lane 2) are included. There are six specimens for each group.
Regression analysis of pooled patient and organ donor data revealed significant correlations between joint range of motion (total extension and flexion arc, and extension) and myofibroblast numbers, percentage of myofibroblasts to total cells and α-SMA protein levels (P < 0.05). The r2 values ranged from 0.65 to 0.89. As expected, there were inverse relationships between total extension and flexion motion arc and the myofibroblasts or α-SMA whereas there were direct relationships between the extension and the myofibroblasts or α-SMA. There were no significant correlations between age and any parameters, total cell numbers and any parameters, or α-SMA mRNA levels and any parameters. For the six patients, there were no significant correlations between the chronicity of the contracture and any parameters.
DISCUSSION
Elbow contractures after injury can be treated by surgical excision of the joint capsule.3,27,30 Other soft tissue extremity disorders such as Dupuytren’s contracture of the hand, and hypertrophic or keloid skin wound healing have been associated with myofibroblasts.1,4,21,29 In the current study, there were significantly elevated myofibroblast numbers and percentage of myofibroblasts to total cells in elbow capsules of patients with posttraumatic joint contractures when compared with similar tissues from organ donors free of joint contractures. Protein levels of α-SMA, a myofibroblast marker, also were significantly increased in the joint capsules of the patients with posttraumatic contractures whereas the mRNA levels of α-SMA were not significantly different between the joint capsules of the two groups. The myofibroblast numbers and α-SMA protein levels had significant correlations with total elbow extension and flexion motion arc, and extension motions.
The sources of α-SMA in the joint capsule can be vascular or nonvascular. Laminin, a component found in the basement membrane of blood vessels, was used to differentiate α-SMA expression associated with blood vessels from α-SMA expression associated with myofibroblasts on immunohistochemical examination.17,25 The immunohistochemical studies showed elevated myofibroblast numbers suggesting that the increased α-SMA protein levels were not solely attributable to increased vasculature in the joint capsules of the patients with posttraumatic contractures. However, with our techniques we cannot comment on whether vascularity was different between the two groups.
Normal articular cartilage was selected as a negative control because it does not contain blood vessels or nerves,19 and at the start of this study chondrocytes were not known to express α-SMA. Therefore, it was expected that α-SMA would not be evident. The cartilage tissue obtained from the age-matched donor elbows free of arthritis, contractures, and fractures showed no detectable levels of α-SMA mRNA and protein, nor were α-SMA positive cells detected on immunohistochemical analysis. However, authors of two studies reported α-SMA positive cells expressing Type II collagen in human articular cartilage obtained at total joint arthroplasty.15,16 Therefore, chondrocytes from diseased joints can express α-SMA, but the extent that cells in tissues obtained at total joint arthroplasty are normal is uncertain, and it is possible that unique stimuli induce α-SMA expression in osteoarthritic human cartilage.
The measures of α-SMA protein (immunohistochemistry, Western blots) were elevated in the patients’ joint capsules, but the relative mRNA levels were not significantly different. Proteins have a slower turnover than mRNA. Total cell numbers also were similar between the two groups, in keeping with the literature on skin and ligament healing where cellularity decreases with time toward normal levels.7,11,21 The fact that total cell numbers in the capsules of patients with posttraumatic contractures between 5 to 24 months after elbow fractures were comparable to organ donor capsules suggests the patients’ tissues were sampled at more chronic periods. The discordance between protein and mRNA measures of α-SMA may reflect the more chronic nature of the contracture process.
The detection of α-SMA in association with myofibroblasts in pathologic and control human elbow capsules supports similar findings in other musculoskeletal and extraskeletal tissues.1,2,5,12–15,20,22,24,26 Myofibroblasts have been described in pathologic tissue obtained during surgery for frozen shoulder and Dupuytren’s contracture.1,2 Fibrosis developing after muscle laceration in mice also has been reported to be associated with α-SMA expression and myofibroblast formation.9 When considering normal tissue, several organs have been reported to contain myofibroblasts expressing α-SMA.1,2,5,12–15 For musculoskeletal tissue, the reports of myofibroblasts in normal tissues are tissue and species-specific. Normal rabbit Achilles tendon, from neonates to skeletally mature animals (age, 4 years), had cytoskeletal proteins that reacted with antibodies that recognized SMA and the tenocytes had features of myofibroblasts on electron microscopy.12,13 Normal rabbit medial collateral ligaments did not contain detectable myofibroblasts.5 However, assessment of human anterior cruciate ligaments obtained at total joint arthroplasty provided evidence of α-SMA positive cells.20 In the current study, myofibroblasts were seen in pathologic and control human elbow anterior capsules. It remains to be determined whether the role of myofibroblasts in normal tissue is the same as in the pathologic condition, but if one assumes they are the same, then the increased numbers in the joint contracture tissue could play an active role in the aberrant properties of this tissue, at least in the chronic phase.
Although this is the first direct, controlled comparison of pathologic and normal human musculoskeletal tissue showing elevated myofibroblast numbers in joint capsules in association with posttraumatic contractures, cause and effect have not been established. Several investigators have studied the roles of myofibroblasts in contraction of collagen gels and the expression of cytoskeletal proteins and adhesion proteins in myofibroblasts in the context of collagen gel contraction.16,28 Such investigations will provide mechanistic insights that will assist in assigning cause and effect relationships as the clinical condition develops. The regression analysis showed significant relationships between myofibroblast numbers and α-SMA protein levels and the clinical measures of joint motion, but the relationship was not 1 to 1. Other yet to be determined factors (matrix organization and composition) may modify the association of myofibroblasts and joint contractures.
Myofibroblasts were elevated in human elbow capsules obtained from patients with chronic posttraumatic contractures. Certain growth factors and matrix molecules, such as TGF-β, decorin, and the ED-A domain of fibronectin, have been reported to modulate α-SMA expression and myofibroblast formation and function in some tissues and cells.8,9,26 These modulating molecules are the focus of current directions of research in the area of human elbow contractures looking for clues on treatment and prevention of contractures, a condition which affects the quality of life for many individuals.
Acknowledgments
We thank Carla Gronau for preparation of the manuscript and Dr. Stuart Patterson, University of Western Ontario, for contributing patients.
This work was supported by grants from the Alberta Heritage Foundation for Medical Research.
References
- 1.Berndt A, Kosmehl H, Katenkamp D, Tauchmann V. Appearance of the myofibroblastic phenotype in Dupuytren’s disease is associated with a fibronectin, laminin, collagen type IV and tenascin extracellular matrix. Pathobiology. 1994;62:55–58. doi: 10.1159/000163879. [DOI] [PubMed] [Google Scholar]
- 2.Bunker TD, Anthony PP. The pathology of frozen shoulder. J Bone Joint Surg. 1995;77:677–683. [PubMed] [Google Scholar]
- 3.Cooney WP., III . Contractures of the Elbow. In: Morrey BF, editor. The Elbow and its Disorders. 2. Philadelphia: WB Saunders Company; 1993. pp. 464–475. [Google Scholar]
- 4.Dave SA, Banducci DR, Graham WPI, Allison GM, Ehrlich HP. Differences is α smooth muscle expression between fibroblasts derived from Dupuytren’s nodules or cords. Exp Mol Pathol. 2001;71:147–155. doi: 10.1006/exmp.2001.2385. [DOI] [PubMed] [Google Scholar]
- 5.Faryniarz DA, Chaponnier C, Gabbiani G, Yannas IV, Spector M. Myofibroblasts in the healing lapine medial collateral ligament: Possible mechanisms of contraction. J Orthop Res. 1996;14:228–237. doi: 10.1002/jor.1100140210. [DOI] [PubMed] [Google Scholar]
- 6.Fawcett DW. Epithelium. A Textbook of Histology. 11. Philadelphia: WB Saunders Company; 1986. pp. 57–82. [Google Scholar]
- 7.Frank CB, Woo SL-Y, Amiel D, et al. Medial collateral ligament healing: A multidisciplinary assessment in rabbits. Am J Sports Med. 1983;11:379–389. doi: 10.1177/036354658301100602. [DOI] [PubMed] [Google Scholar]
- 8.Fukui N, Nakajima K, Tashiro T, Oda H, Nakamura K. Neutralization of fibroblast growth factor-2 reduces intraarticular adhesions. Clin Orthop. 2001;383:250–258. doi: 10.1097/00003086-200102000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima K, Badlani N, Usas A, et al. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- 10.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand KA, Woo SLY, Smith DW, et al. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. Am J Sports Med. 1998;26:549–554. doi: 10.1177/03635465980260041401. [DOI] [PubMed] [Google Scholar]
- 12.Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C. Ultrastructural and immunochemical evidence of actin in the tendon cells. Clin Orthop. 1977;126:282–284. [PubMed] [Google Scholar]
- 13.Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C. Morphological, immunochemical and biochemical study of rabbit Achilles tendon at various ages. J Bone Joint Surg. 1980;62A:583–598. [PubMed] [Google Scholar]
- 14.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: The role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 15.Kim AC, Spector M. Distribution of chondrocytes containing α-smooth muscle actin in human articular cartilage. J Orthop Res. 2000;18:749–755. doi: 10.1002/jor.1100180511. [DOI] [PubMed] [Google Scholar]
- 16.Kinner B, Spector M. Smooth muscle actin expression by human articular chondrocytes and their contraction of a collagen-glycosaminoglycan matrix in vitro. J Orthop Res. 2001;19:233–241. doi: 10.1016/S0736-0266(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 17.Leeson CR, Leeson TS, Paparo AA. Connective Tissue Proper. Textbook of Histology. 11. Philadelphia: WB Saunders Company; 1985. pp. 97–107. [Google Scholar]
- 18.Lo IKY, Marchuk L, Hart DA, Frank CB. Comparison of mRNA levels for matrix molecules in normal and disrupted human anterior cruciate ligaments using reverse transcription-polymerase chain reaction. J Orthop Res. 1998;16:421–428. doi: 10.1002/jor.1100160405. [DOI] [PubMed] [Google Scholar]
- 19.Mankin HJ, Mow VC, Buckwalter JA, Ratcliffe A. Articular cartilage structure, composition and function: Orthopaedic basic science biology and biomechanics of the musculoskeletal system. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1999. pp. 443–470. [Google Scholar]
- 20.Murray MM, Spector M. Fibroblast distribution in the anteromedial bundle of the human anterior cruciate ligament: The presence of α-smooth muscle actin-positive cells. J Orthop Res. 1999;17:18–27. doi: 10.1002/jor.1100170105. [DOI] [PubMed] [Google Scholar]
- 21.Nedelec B, Shankowsky H, Scott PG, Ghahary A, Tredget EE. Myofibroblasts and apoptosis in human hypertrophic scars: The effect of interferon-α2b. Surgery. 2001;130:798–808. doi: 10.1067/msy.2001.116453. [DOI] [PubMed] [Google Scholar]
- 22.Nodder S, Martin P. Wound healing in embryos: A review. Anat Embryol. 1997;195:215–228. doi: 10.1007/s004290050041. [DOI] [PubMed] [Google Scholar]
- 23.Peterson JJ, Rayburn HB, Lager DJ, et al. Expression of thrombomodulin and consequences of thrombomodulin deficiency during healing of cutaneous wounds. Am J Pathol. 1999;155:1569–1575. doi: 10.1016/S0002-9440(10)65473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Premdas J, Tang J-B, Warner JP, Murray MM, Spector M. The presence of smooth muscle actin in fibroblasts in the torn human rotator cuff. J Orthop Res. 2001;19:221–228. doi: 10.1016/S0736-0266(00)90011-1. [DOI] [PubMed] [Google Scholar]
- 25.Ross MH, Reith EJ, Romprell LJ. The Cardiovascular System. In: Kist K, editor. Histology, A Text and Atlas. Baltimore: Williams & Wilkins; 1989. pp. 283–304. [Google Scholar]
- 26.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 27.Timmerman L, Andrews J. Arthroscopic treatment of posttraumatic elbow pain and stiffness. Am J Sports Med. 1994;22:230–235. doi: 10.1177/036354659402200213. [DOI] [PubMed] [Google Scholar]
- 28.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechanoregulation of connective tissue remodelling. Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 29.Tredget EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids and contractures: The cellular and molecular basis for therapy. Wound Healing. 1997;77:701–730. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- 30.Urbaniak JR, Hansen PE, Beissinger SF, Aitken MS. Correction of post-traumatic flexion contracture of the elbow by anterior capsulotomy. J Bone Joint Surg. 1985;67A:1160–1164. [PubMed] [Google Scholar]