Abstract
To evaluate changes in matrix molecules of the joint capsule, the right knees of 24 skeletally mature female NZW rabbits were immobilized while the contralateral limb served as an unoperated control. The immobilization was discontinued at 8 weeks and the rabbits were divided among four groups (n = 6) based on the number of weeks the right knees were remobilized: 0, 8, 16, or 32. Three rabbits (six knees) that did not have operations provided normal control joint capsules. The mRNA levels for collagen types I, II, and III, and MMP-1 and -13 were significantly increased in the joint capsules of the contracture knees in all groups when compared to normal and contralateral limb joint capsules. In contrast, the mRNA levels for TIMP-1, -2, and -3 were decreased in the joint capsules of the contracture knees in all groups when compared to normal and contralateral limb joint capsules. The mRNA levels for lumican and decorin were increased in the joint capsules of the contracture knees in all groups when compared to normal capsules. Many of the changes observed in this animal model are similar to those observed in human joint capsules from posttraumatic elbow contractures, supporting the value of this rabbit model.
Keywords: contracture, animal model, capsule, matrix, mRNA
INTRODUCTION
Joint contractures, characterized clinically by loss of joint range of motion, are features of many pathologic conditions including arthritis, posttraumatic contractures, and spinal cord injuries. In posttraumatic elbow contractures, operative treatments, whether open or arthroscopic, focus on excising or dividing the joint capsule surrounding the elbow to improve joint motion.1–3 To study the changes in the joint capsule, our laboratory has recently described a posttraumatic joint contracture animal model where permanent loss of joint motion was produced in rabbit knees that mimics the posttraumatic contractures seen in human joints.4 Using this model, it was determined that myofibroblasts, fibroblasts expressing the contractile smooth muscle protein α-smooth muscle actin, were elevated in the capsules of the knee joints with contractures in chronic stages of the process.4 These animal model results regarding myofibroblasts and α-smooth muscle actin are similar to what we have described in human elbow joint capsules obtained from patients with chronic posttraumatic contractures.4,5 Recent work in our laboratory on human elbow joint anterior capsules has shown altered expression of collagen, small leucine-rich proteoglycans, matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs) in patients with chronic posttraumatic contractures.6
The objectives of the current study were to evaluate collagen, MMP, TIMP, and small leucine-rich proteoglycan mRNA levels in knee joint capsules of the rabbit model of posttraumatic joint contractures in subacute and chronic stages of the process, and to compare the results to findings in joint capsules from chronic human elbow posttraumatic contractures.4,5,7 Based on our work on human elbow joint capsule, the hypotheses were that collagen, MMP, and small leucine-rich proteoglycan mRNA levels are increased, and TIMP mRNA levels are decreased, in the joint capsule of the contracture knees at each period of remobilization when compared to contralateral knee joint capsules and normal knee joint capsules of unoperated rabbits.6 The final hypothesis was that there is a time effect on mRNA levels, with levels decreasing in the contracture knees over time for all molecules considered.
MATERIALS AND METHODS
Skeletally mature New Zealand White female rabbits (12–18 months old, 5.5 ± 0.5 kg) were used (Reimans Furrier, St. Agatha, ON). Institutional Animal Review Committee approval was obtained prior to the animal experiments. Twenty-four rabbits were equally divided into four groups based on the time of remobilization: 0, 8, 16, or 32 weeks (Table 1). All rabbits had the right knee immobilized for 8 weeks prior to remobilization. The left knee served as an unoperated control. The biomechanical description of the joint contracture severity over time for these animals has been published previously.4 Three age- and gender-matched rabbits were the source of normal knee joint capsules (n = 6).
Table 1.
Rabbit Distribution
| Group | Immobilization (Weeks) | Remobilization (Weeks) |
|---|---|---|
| 0-week | 8 | 0 |
| 8-week | 8 | 8 |
| 16-week | 8 | 16 |
| 32-week | 8 | 32 |
N = 6 per group.
Surgical Interventions
Under inhalational general anesthesia (halothane 3–5%), incisions over the lateral thigh and anterior aspect of the mid-tibia were made.4,7 Cortical windows (5 mm2) were removed from the nonarticular cartilage portion of the medial and lateral femoral condyles, taking care not to damage the collateral ligaments. The intrasynovial cortical windows have been validated as a model of fracture healing and produced a hemarthrosis.8 The knee joint was then immobilized with a smooth 1.6 mm-diameter Kirschner wire (K-wire; Zimmer, Mississauga, ON, Canada). The lateral thigh incision was used to expose the femur. The tibial incision allowed the drilling of the K-wire through the tibia; the K-wire was passed posterior (extraarticular) to the knee joint and bent around the femur. The knee was placed at 150° of flexion and then the tibial portion of the wire was bent and cut below the skin.4 The patellofemoral joint was checked to ensure it was reduced. The arthrotomies were not closed. All skin incisions were closed with 3-0 Ethilon (Ethicon, Johnson & Johnson, Peterborough, ON, Canada). The rabbits were allowed free cage activity (0.1 m3) following all operations. The left knees were never surgically manipulated.
Eight weeks after the original procedure, a second inhalational general anesthetic (halothane 3–5%) was administered to cut the K-wires for the 8-, 16-, and 32-week groups. The tibial portion of the K-wires was removed and the rabbits were returned to free cage activity. The 0-week remobilization group was killed after 8 weeks of immobilization with an overdose (1.5 mL) of Euthanyl (MTC, Cambridge, ON, Canada) while the other three groups were killed (Euthanyl overdose) after the prescribed number of weeks of remobilization. Biomechanical measures of knee motion were performed the same day as sacrifice.7 Immediately following joint angle measurements the posterior joint capsule was harvested and frozen using liquid nitrogen.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
The protocol followed methods described previously.5,9 Ribonucleic acid isolation (TRIspin method) began by reducing the frozen tissue to a powder at liquid N2 temperatures. TRIzol reagent (Life Technologies, Gaithersburg, MD) was added (1 mL TRIzol/50 mg tissue) and the mixture then was warmed to room temperature. Chloroform was added, and the samples were centrifuged. The upper aqueous phase containing the RNA then was removed, and 1 volume of 70% ethanol was added. Total RNA was extracted and the RNA was purified using an RNeasy Total RNA kit (Qiagen, Chatsworth, CA). The RNA yield was quantified fluorometrically (Perkin-Elmer, Branchburg, NJ) using Sybr Green II (FMC BioProducts, Rockland, MN), and was compared with standards obtained with calf liver ribosomal RNA.
Semiquantitative RT-PCR for mRNA analysis was performed on all samples at the same time to avoid any potential variation in efficiency of the procedures. Reverse transcription was performed using 1-μg total RNA aliquots and the Qiagen Omniscript RT kit (Qiagen, GmbH, Hilden, Germany). Random primers (0.5 μmol/L) were added to the RNA and first-strand cDNA was synthesized by adding ×10 first-strand buffer, RNase block ribonuclease inhibitor, d-nucleoside triphosphates, and Maloney murine leukemia virus reverse transcriptase. Reactions were incubated at 37°C for 1 h; this was followed by a 5-min incubation at 93°C and subsequent cooling on ice for 5 min. Aliquots of cDNA were amplified in a total volume of 50 μL containing ×10 polymerase chain reaction buffer, 10 mmol/L d-nucleoside triphosphate mixture, 50 mmol/L MgCl2, 0.5 μmol/L of each primer, and 1.25 U of Taq DNA polymerase (Rose Scientific Ltd, Edmonton, AB, Canada). Rabbit specific primers were used as indicated in Table 2.10,11 Primer sets were optimized for PCR cycles, and linear amplification curves were plotted. The optimal cycle number was selected to be in the linear part of the amplification curve. All no-RT controls were negative, ensuring that no detectable genomic DNA was present in the RNA samples. All samples used in this study were reverse-transcribed at least twice, and all PCR experiments were performed at least twice with results indistinguishable from those reported here.
Table 2.
The List of Primers Used in the Study is Displayed
| Primer | Sequence | bp | Temperature(°C) | Cycles | Source |
|---|---|---|---|---|---|
| Biglycan | F -GAT GGC CTG AAG CTC AA R -GGT TGT TGA AGA GGC TG |
406 | 55 | 30 | AF159382 |
| Collagen I | F -GAT GCG TTC CAG TTC GAG TA R -GGT CTT CCG GTG GTC TTG TA |
312 | 55 | 30 | Le Graverand et al. (1999)10 |
| Collagen II | F -GCA CCC ATG GAC ATT GGA GGG R -GAC ACG GAG TAG CAC CAT CG |
366 | 65 | 35 | S83370 |
| Collagen III | F -TTA TAA ACC AAC CTC TTC CT R -TAT TAT AGC ACC ATT GAG AC |
255 | 55 | 30 | S83371 |
| Decorin | F -TGT GGA CAA TGG TTC TCT GG R -CCA CAT TGC AGT TAG GTT CC |
419 | 60 | 31 | S76584 |
| GAPDH | F -TCA CCA TCT TCC AGG AGC GA R -CAC AAT GCC GAA GTG GTC GT |
293 | 55 | 25 | L23961 |
| Lumican | F -CTG CAG TGG CTC ATT CTA R -GAC CTC CAG GTA ATA GTT |
576 | 55 | 35 | Majima et al. (2000)11 |
| MMP-1 | F -TCA GTT CGT CCT CAC TCC AG R -TTG GTC CAC CTG TCA TCT TC |
649 | 55 | 32 | M17821 |
| MMP-13 | F -TTC GGC TTA GAG GTG ACA GG R -ACT CTT GCC GGT GTA GGT GT |
527 | 65 | 40 | AF059201 |
| TIMP-1 | F -GCA ACT CCG ACC TTG TCA TC R -AGC GTA GGT CTT GGT GAA GC |
326 | 60 | 32 | AF 156029 |
| TIMP-2 | F -CTC TGG AAA CGA CAT TTA TGG R -AGA TGT AGC ACG GGA TCA TGG |
416 | 60 | 29 | AF 156030 |
| TIMP-3 | F -GTG CAA CTT CGT GGA GAG R -GGT CTG TGG CAT TGA TGA |
454 | 60 | 29 | AF 156031 |
F = Forward; R = Reverse; bp = length of primer product in base pairs; temperature = annealing temperature; source = Genbank accession number or journal article reference; GAPDH = glyceraldehydes 3-phosphate dehydrogenase; MMP = matrix metalloproteinase; TIMP = tissue inhibitors of matrix metalloproteinase.
Polymerase chain reaction products were separated by electrophoresis of 20 μl of each PCR product using a 2% agarose gel at 100 V/cm in 1× TAE buffer. After electrophoresis, the gels were stained with ethidium bromide, destained in distilled water, and photographed using Polaroid number 55 film (Technicare Inc., Calgary, AB, Canada). Comparison with the standard 1-kb DNA Ladder (Life Technologies) ensured that the PCR products were the proper size. Relative band intensities were quantified by densitometric scanning of negatives (Masterscan Interpretive Densitometer; CSPI, Billerica, MA). The mRNA levels of the various collagen, proteoglycan, MMP, and TIMP molecules were normalized to the mRNA levels of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistical Analysis
Data are presented as mean ± standard deviation. Statistical analysis consisted of a paired t-test to evaluate experimental and contralateral control knees for each group. To evaluate changes over time, an analysis of variance (ANOVA) with post hoc Tukey tests was used. The normal knee joint capsules were included as one group in the ANOVA. Significance levels were set at p ≤ 0.05.
RESULTS
The range of motion measures describing the knee joint contractures over time in the four groups has been published previously.4 Briefly, all K-wires remained intact for the 8 weeks of immobilization and contractures developed in the immobilized knees. The severity of the contractures decreased when comparing the 0-, 8-, and 16-week remobilized groups (means 38°, 33°, and 19°, respectively). Joint motion loss stabilized when considering the 16- and 32-week remobilized groups (means 19° and 18°, respectively), indicating that the joint contractures had reached a plateau. The control contralateral knees had a mean flexion contracture of 8°. The contralateral knee motion is similar to normal knee joint motion measured from skeletally mature NZW female rabbits with our biomechanical methodology.7
The mRNA levels for GAPDH were not significantly different between the contracture and control knees for all four experimental groups, and the right and left knees of the normal rabbits (data not shown). The relative mRNA levels of all capsular matrix molecules and enzymes assessed were not significantly different when comparing right and left knees from the normal rabbits. Thus, the mRNA values of the joint capsules from the right and left knees were pooled for the normal rabbits giving a total of six samples.
Collagen mRNA levels were significantly elevated in the posterior joint capsules of the contracture knees in all groups and the levels tended to decrease with time (Fig. 1). For collagen types I, II, and III, relative mRNA levels for the contracture knee joint capsule were significantly elevated when compared to the control knees for all four groups and when compared to the normal knees (Fig. 1). When considering the effect of time on collagen mRNA levels in the capsules of the contracture knees, the general trend was for a decrease in mRNA levels postremobilization (Fig. 1). The statistically significant differences in collagen I levels were such that the values for the 32-week group were significantly less than the other three time groups (Fig. 1a). For collagen III, statistical analysis showed that mRNA levels in the 0-week group were significantly greater than those in the 32-week group (Fig. 1c). The pattern of the collagen II mRNA levels was somewhat different from those for collagens I and III in that the values peaked in the 8-week group and these values were significantly greater than those of the other three time groups (Fig. 1b). Furthermore, the collagen II mRNA levels in the contracture knee capsules of the 0-week group were significantly greater than the 16- and 32-week groups (Fig. 1b).
Figure 1.
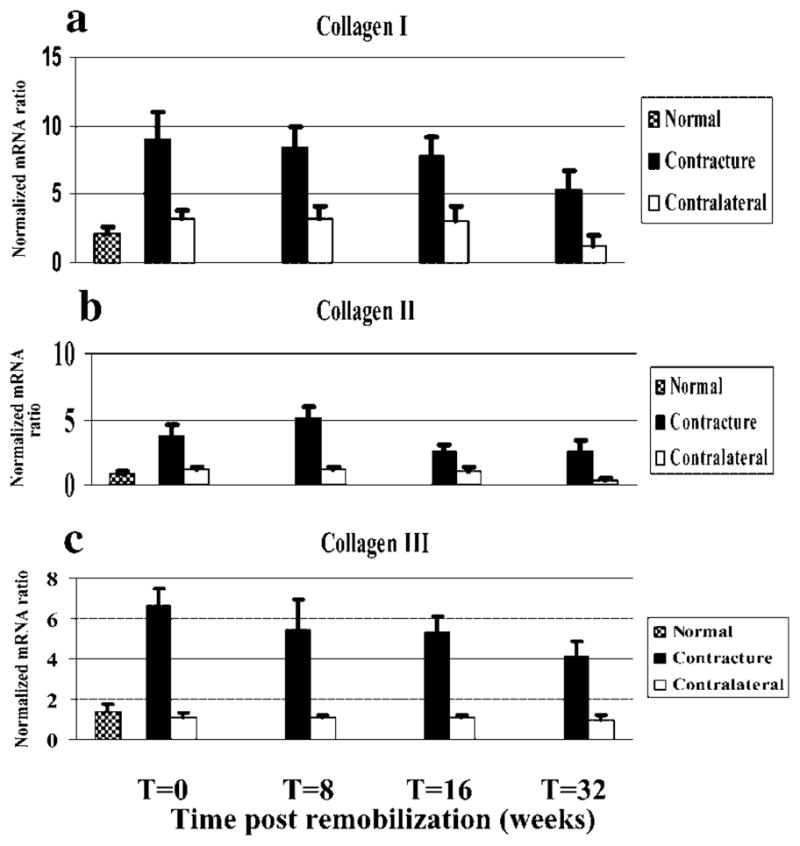
Histograms of the mRNA expression of collagen types I (a), II (b), and III (c) normalized to GAPDH mRNA expression are shown.
The mRNA levels for MMP-1 and -13 were increased in the posterior joint capsules of the contracture knees and levels tended to decrease with time postremobilization (Fig. 2). For MMP-1, the values of the contracture knees for the 0- and 8-week remobilization groups were significantly greater than their respective contralateral knees, and also the normal knees (Fig. 2a). For MMP-13, the contracture knees had significantly increased mRNA levels when compared to the control knees in all groups and when compared to the normal knees (Fig. 2b). When considering the effect of time postremobilization, the trend was for a decrease in mRNA expression in the contracture knees, but the only statistically significant difference was the mRNA levels for MMP-13 of the 8-week group compared with the 16-week group.
Figure 2.
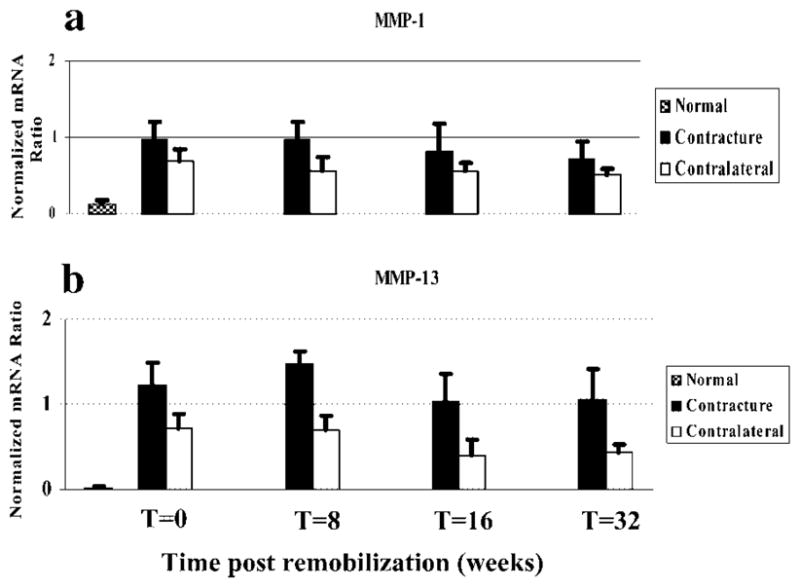
Histograms of the mRNA expression of MMP-1 (a) and MMP-13 (b) normalized to GAPDH mRNA expression are shown.
TIMP mRNA levels were selectively modified in the joint capsules, but in an opposite direction to those of the MMPs, as well as collagen (Fig. 3). The relative mRNA levels in the contracture knees were significantly less than the values in the contralateral control knees at all time periods for TIMP-1, -2, and -3. For all three TIMP molecules assessed, the values for the contractures knees were significantly less than the normal knees for all time periods. The trend for TIMP molecule expression with time postremobilization in the contracture knees was an increase in expression. The only statistically significant differences were the TIMP-3 values; the 32-week group values were significantly greater than the 0- and 8-week values and the 16-week values were significantly greater than the 0-week values (Fig. 3c).
Figure 3.
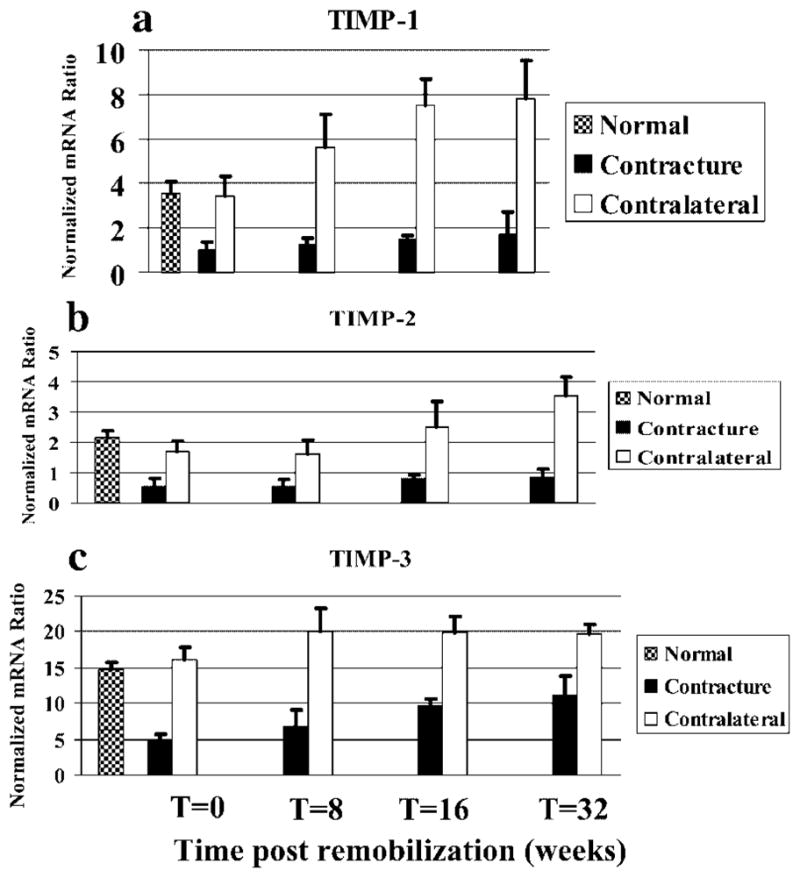
Histograms of the mRNA expression of TIMP-1 (a), -2 (b), and TIMP-3 (c) to GAPDH mRNA expression are shown.
The small leucine-rich proteoglycans displayed unique patterns of mRNA levels (Fig. 4). Decorin and lumican did not exhibit any significant differences between the contracture and control knees for any group (Fig. 4a and b). The capsules from the contracture knees at all time periods had mRNA levels that were significantly greater than the values of the normal knees. There was no effect of time post remobilization. Bigylcan mRNA levels did not exhibit a consistent pattern (Fig. 4c). There were significant differences detected between contracture and control knees in all four groups; expression in the contracture group was significantly less than the control groups at 0 and 8 weeks, but then the contracture group biglycan mRNA levels were significantly greater than the control group values at 16 and 32 weeks. When considering the effect of time postremobilization, there was a bimodal expression pattern for biglycan in the contracture groups with the 8- and 32-week groups having significantly greater expression than the 0-and 16-week groups and the normal knees.
Figure 4.
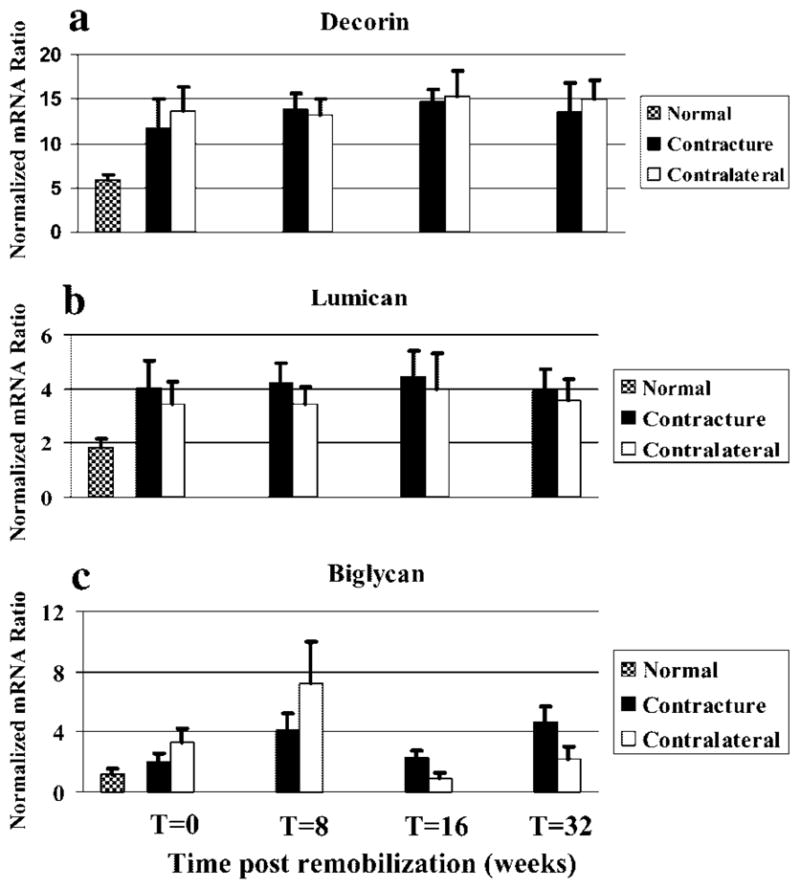
Histograms of the mRNA expression of decorin (a), lumican (b), and biglycan (c) normalized to GAPDH mRNA expression are shown.
DISCUSSION
The results presented in this report indicate that the metabolism of the joint capsule is specifically altered and the alterations persist long after the motion loss changes have stabilized. This rabbit knee model of posttraumatic joint contractures evaluates outcomes 8 to 40 weeks after the injury, representing subacute and more chronic stages of the process.4 As published previously, the initial severity of the contracture decreases with time post remobilization, but then it stabilizes and does not change further. In the current study, evaluation of the posterior knee joint capsules from the rabbits reported in the range of motion study showed an alteration in matrix molecule and enzyme mRNA levels. With a few exceptions (MMP-1 at 16 and 32 weeks), the mRNA levels of collagen types I, II, and III and MMP-1 and -13 were significantly greater, and the mRNA levels of TIMP-1, -2, and -3 were significantly less, in the contracture knee capsules when compared with similar tissues from the contralateral unoperated knees or normal rabbit knees that did not have operations at all time periods evaluated. The small leucine-rich proteoglycans decorin and lumican did not display any statistically significant differences in mRNA levels between contracture and contralateral control knees at any time point, although the contracture knees tissue had significantly greater values than the normal knees at all time points. Biglycan mRNA levels exhibited a biphasic pattern with values in the contracture knee capsules significantly less than the contralateral control knee tissues in the 0- and 8-week groups, while mRNA levels in the contracture knees was significantly greater than in the contralateral control knees in the 16- and 32-week groups. The mRNA levels in the capsules of the contracture knees in the 8- and 32-week groups were significantly greater than for the normal knee joint capsules. Thus, the stabilization of the loss of range of motion in the chronic stages of the contracture does not correlate with the establishment of a new metabolic set point in the tissue, but rather an environment that can be considered hypermetabolic compared to normal age and gender matched tissues.
The effect of time postremobilization on mRNA level profiles in the contracture knee joint capsules manifested themselves in four patterns. For collagen types I, II, and III, and MMP-1 and 13, the trend was for levels to decrease with time but still be elevated compared to control values (Figs. 1 and 2). Except for MMP-1, statistical analysis determined that mRNA levels in those groups at the shorter times postremobilization had significantly greater values than the 32-week group. The trend for TIMP-1, -2, and -3 mRNA levels was to increase over time (Fig. 3). Statistical analysis revealed significant differences only for TIMP-3, where 0- and 8- week postremobilization values were significantly lower than values at 32 weeks postremobilization. Decorin and lumican mRNA expression in the contracture joint capsules did not show any time effect (Fig. 4a and b). Biglycan mRNA expression displayed a bimodal distribution with the 8- and 32-week groups having significantly greater values than the 0- and 16-week groups (Fig. 4c). Thus, mRNA levels for matrix molecules and enzymes in joint capsules appear to be specifically modulated.
The changes in matrix molecule and enzyme mRNA levels in the posterior joint capsules of this rabbit knee model are similar to findings in the anterior joint capsule of humans with chronic posttraumatic elbow joint contractures.6 The human joint capsules expressed significantly increased collagen, MMP, and biglycan mRNA levels and significantly decreased TIMP mRNA levels in contracture patients when compared to age-matched organ donors free of contractures. Thus, chronic stages of the rabbit model reflect the alterations detected for matrix molecules and enzymes observed in the human process.6 Our laboratory has also reported that this animal model parallels the motion loss characteristics and myofibroblast increases observed in chronic human posttraumatic elbow contractures.4,5 The evidence from the present study further strengthens the parallels between the human condition of post-traumatic contractures and the animal model of posttraumatic contractures. A state with chronically altered mRNA levels occurs even though motion changes have stabilized in both the human condition and the animal model of posttraumatic contractures.
Previous work by other authors immobilizing normal rabbit knees have shown alterations in collagen and collagenase expression.12,13 Akeson et al. reported an increased collagen turnover in joint capsules of rabbit knees with contractures 9 weeks after immobilization was instituted to produce the contractures.12 Harper reported that immobilization of a normal rabbit knee for 4 weeks led to decreased collagenase activity in ligaments, but did not report on joint capsule changes. The decreased collagenase reported by Harper et al. is in contrast to the increases in MMP-1 and -13 mRNA detected in the present study. Several differences may potentially explain the discrepancy. The tissues evaluated were different; capsule versus ligament. Harper et al. immobilized normal knees in contrast to the injury plus immobilization in this model. Harper et al. evaluated the measures earlier in the process (4 weeks) in contrast to the later stages (8 weeks +) evaluated in the present study. Finally, Harper et al. measured active enzyme, while MMP mRNA was assessed in the present study. As active enzyme does not account for proenzyme, enzyme–inhibitor complexes, or posttranscriptional controls, finding some differences due to different methodologies may not be unexpected. However, the findings of the present study do indicate alterations of cell metabolism in this rabbit model.
It should be noted that in many instances the mRNA levels in the contralateral control knees were significantly different from the normal control knees. This is true especially for the MMPs, TIMPs, and the small leucine-rich proteoglycans (Figs. 2, 3, and 4). Whether using the contralateral knee or the normal knee as the control, the statistical comparison showed significantly different values from the contracture knee for the collagens, MMPs, and TIMPs, and the difference was consistently increased or decreased levels with either control. Thus, the interpretation of the significance of the results in the contracture knee capsules in relation to control capsules was not affected for these molecules. In contrast, the issue of which control that is used can alter the interpretation of the findings for the small leucine-rich proteoglycans. Lumican and decorin mRNA levels in the contracture knees were significantly greater than the normal knee capsular values but not significantly greater than the contralateral knees. Biglycan levels were even more variable. Evaluation of the mRNA levels of all molecules in this study in the capsules of the normal right and left knees did not reveal any significant differences, suggesting that there is no side-to-side effect in normal rabbit knees. However, a contralateral effect has been described in injury models of the rabbit medial collateral ligament (MCL).14 The authors reported that in rabbits with a unilateral MCL injury, the contralateral unoperated MCL had altered biomechanical properties when compared biomechanical properties of MCLs obtained from age-, gender-, and activity-matched control rabbits. The source of the contralateral effect is unknown, but may include altered biomechanical loading of the contralateral limb, a systemic response due to blood borne mediators, altered neurologic input to the contralateral limb or other unknown factors. The bottom line is that the choice of control is important and may alter the interpretation of the results.
In our model of posttraumatic joint contractures, immobilization is required to produce contractures. Preliminary work in our model with the femoral condyle injury only did not produce contractures.7 Most authors have used various forms of immobilization to produce joint motion loss or contractures.12,15–25 Only a few have reported on what happens to the motion loss after the immobilization is discontinued.22,26 Interestingly, these authors did not combine joint injury with the immobilization and the motion losses disappeared after remobilization of a period equal to the time of immobilization. Our model is unique in that we have combined joint injury with immobilization, followed by remobilization up to four times longer than the immobilization time. Motion loss is permanent in the long-term in our model, suggesting we are studying joint contractures and not the effect of joint immobilization. Future work is required to determine the roles of the injury, hemarthrosis, immobilization, and remobilization in our animal model of joint contractures.
A potential mechanism to explain the clinically observed “thickened” joint capsule in posttraumatic contractures is tissue accumulation. Two processes that can contribute to tissue accumulation are excess tissue production or insufficient degradation in the repair and remodelling processes. The collagen mRNA findings would support an excess of production. However, the MMP and TIMP results could be interpreted to support excessive, or ineffective, degradation depending on the comparison. Excessive degradation would be supported by the contracture and control (contralateral and normal) knee comparisons where MMP mRNA levels were significantly increased, and TIMP mRNA levels were significantly decreased in the contracture knees. Ineffective degradation would be supported by considering the MMP and TIMP expression in the contracture knees with time postremobilization. MMP expression decreases while TIMP expression increases in the contracture knees in this comparison, which implies a decreasing MMP:TIMP ratio. A decreasing MMP:TIMP ratio would support ineffective degradation, and is consistent with a report regarding contracture phenomena and synthetic MMP inhibitors. Hutchinson et al.27 indicated that frozen shoulder and Dupuytren’s contracture of the hand developed in 6 of 12 patients with inoperable gastric carcinoma who were given a synthetic MMP inhibitor for greater than 1 month. The inhibitor treatment would be expected to lead to a reduction in the MMP:TIMP ratio. Caution must be heeded with this observation of the MMP:TIMP ratio. In the present study protein levels of the MMPs and TIMPs were not measured. In addition, Hutchinson et al.27 did not confirm decreased MMP:TIMP ratios (mRNA or protein) in the pathologic tissue. Further work is required to understand which mechanisms may be active. Studies in the immediate and early phases of the contracture process, when the knees are immobilized in this model, will give us further insight into the MMP and TIMP expression. Work is also required to evaluate protein levels for the molecules and enzymes evaluated by mRNA techniques.
In summary, mRNA levels for a subset of matrix molecules and enzymes are specifically altered in joint capsules of this rabbit model of posttraumatic contractures. The alterations in mRNA levels are similar to changes reported in joint capsules of patients with chronic posttraumatic contractures, validating this animal model.6 With this information we can now focus future research on questions related to why the changes occur and persist, as well as how they can be influenced. Such baseline information is critical to evaluate the effects of current treatments (e.g., early motion) on joint capsule properties or to develop new methods of treatment or intervention that must be vetted in animal models prior to progressing to patients.
Acknowledgments
The authors thank Dorothy King for preparation of the manuscript. This work was supported by grants from the Canadian Institutes of Health Research. D.A.H. is supported by the Calgary Foundation–Grace-Glaum Professorship, and K.A.H. is supported by Alberta Heritage Foundation for Medical Research, Health Research Foundation, and Canadian Institutes of Health Research.
References
- 1.Aldridge JM, III, Atkins TA, Gunneson EE, et al. Anterior release of the elbow for extension loss. J Bone Joint Surg. 2004;86A:1955–1960. doi: 10.2106/00004623-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman L, Andrews J. Arthroscopic treatment of posttraumatic elbow pain and stiffness. Am J Sports Med. 1994;22:230–235. doi: 10.1177/036354659402200213. [DOI] [PubMed] [Google Scholar]
- 3.Urbaniak JR, Hansen PE, Beissinger SF, et al. Correction of post-traumatic flexion contracture of the elbow by anterior capsulotomy. J Bone Joint Surg. 1985;67-A:1160–1164. [PubMed] [Google Scholar]
- 4.Hildebrand KA, Sutherland C, Zhang M. Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res. 2004;22:313–320. doi: 10.1016/j.orthres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrand KA, Zhang M, van Snellenberg W, et al. Myofibroblast numbers are elevated in human elbow joint capsules following trauma. Clin Orthop. 2004;419:189–197. doi: 10.1097/00003086-200402000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrand KA, Zhang M, Hart DA. High rate of joint capsule matrix turnover in chronic human elbow contractures. Clin Orthop. 2005;439:228–234. doi: 10.1097/01.blo.0000177718.78028.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrand KA, Holmberg M, Shrive NG. A new method to measure post-traumatic joint contractures in the rabbit knee. J Biomech Eng. 2003;125:887–892. doi: 10.1115/1.1634285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uusitalo H, Rantakokko J, Ahonen M, et al. A metaphyseal defect model of the femur for studies of murine bone healing. Bone. 2001;28:423–429. doi: 10.1016/s8756-3282(01)00406-9. [DOI] [PubMed] [Google Scholar]
- 9.Reno C, Marchuk L, Sciore P, et al. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. BioTechniques. 1996;22:1082–1086. doi: 10.2144/97226bm16. [DOI] [PubMed] [Google Scholar]
- 10.Hellio Le Graverand MP, Reno C, Hart DA. Gene expression in menisci from the knees of skeletally immature and mature female rabbits. J Orthop Res. 1999;17:738–744. doi: 10.1002/jor.1100170518. [DOI] [PubMed] [Google Scholar]
- 11.Majima T, Marchuk LL, Shrive NG, et al. In-vitro cyclic tensile loading of an immobilized and mobilized ligament autograft selectively inhibits mRNA levels for collagenase (MMP-1) J Orthop Sci. 2000;5:503–510. doi: 10.1007/s007760070030. [DOI] [PubMed] [Google Scholar]
- 12.Akeson W, Amiel D, Woo SL-Y. Immobility effects on synovial joints the pathomechanics of joint contracture. Biorheology. 1980;17:95–110. doi: 10.3233/bir-1980-171-212. [DOI] [PubMed] [Google Scholar]
- 13.Harper J, Amiel D, Harper E. Collagenases from periarticular ligaments and tendon: enzyme levels during the development of joint contracture. Matrix. 1989;9:200–205. doi: 10.1016/s0934-8832(89)80051-4. [DOI] [PubMed] [Google Scholar]
- 14.Frank CB, Loitz B, Bray R, et al. Abnormality of the contralateral ligament after injuries of the medial collateral ligament. An experimental study in rabbits. J Bone Joint Surg Am. 1994;76:403–412. doi: 10.2106/00004623-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Akai M, Shirasaki Y, Tateishi T. Viscoelastic properties of stiff joints: a new approach in analyzing joint contracture. Bio-Med Mater Eng. 1993;3:67–73. [PubMed] [Google Scholar]
- 16.Akai M, Shirasaki Y, Tateishi T. Electrical stimulation on joint contracture: an experiment in rat model with direct current. Arch Phys Med Rehabil. 1997;78:405–409. doi: 10.1016/s0003-9993(97)90233-1. [DOI] [PubMed] [Google Scholar]
- 17.Clark DD, Weckesser EC. The influence of triamcinolone acetonide on joint stiffness in the rat. J Bone Joint Surg Am. 1971;53-A:1409–1414. [PubMed] [Google Scholar]
- 18.Fukui N, Fukuda A, Kojima K, et al. Suppression of fibrous adhesion by proteoglycan decorin. J Orthop Res. 2001;19:456–462. doi: 10.1016/S0736-0266(00)90016-0. [DOI] [PubMed] [Google Scholar]
- 19.Furlow LT, Peacock EE. Effect of beta-aminopropionitrile on joint stiffness in rats. Ann Surg. 1967;165:442–447. doi: 10.1097/00000658-196703000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Namba R, Kabo M, Dorey F, et al. Intra-articular corticosteroid reduces joint stiffness after an experimental periarticular fracture. J Hand Surg. 1992;17A:1148–1153. doi: 10.1016/s0363-5023(09)91083-8. [DOI] [PubMed] [Google Scholar]
- 21.Rudolph R, Woodward M, Hurn I. Ultrastructure of active versus passive contracture of wounds. Surg Gynecol Obstet. 1980;151:396–400. [PubMed] [Google Scholar]
- 22.Schollmeier G, Sarkar K, Fukuhara K, et al. Structural and functional changes in the canine shoulder after cessation of immobilization. Clin Orthop. 1996;323:310–315. doi: 10.1097/00003086-199602000-00044. [DOI] [PubMed] [Google Scholar]
- 23.Trudel G, Uhthoff H. Contractures secondary to immobility: is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch Phys Med Rehabil. 2000;81:6–13. doi: 10.1016/s0003-9993(00)90213-2. [DOI] [PubMed] [Google Scholar]
- 24.Ushida T, Willis WD. Changes in dorsal horn neuronal responses in an experimental wrist contracture model. J Orthop Sci. 2001;6:46–52. doi: 10.1007/s007760170024. [DOI] [PubMed] [Google Scholar]
- 25.Wilson CJ, Dahners LE. An examination of the mechanism of ligament contracture. Clin Orthop. 1988;227:286–291. [PubMed] [Google Scholar]
- 26.Akeson W, Woo SL-Y, Amiel D, et al. Biomechanical and biochemical changes in the periarticular connective tissue during contracture development in the immobilized rabbit knee. Connect Tissue Res. 1974;2:315–323. doi: 10.3109/03008207409152261. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson JW, Tierney GM, Parsons SL, et al. Dupuytren’s disease and frozen shoulder induced by treatment with a matrix metalloproteinase inhibitor. J Bone Joint Surg Br. 1998;80-B:907–908. doi: 10.1302/0301-620x.80b5.8464. [DOI] [PubMed] [Google Scholar]


