Abstract
The joint capsule is a key component in posttraumatic joint contractures. The capsule is described as thickened, but little data exist supporting the observation. Our hypotheses were that mRNA levels of (1) collagen; (2) decorin and biglycan; (3) matrix metalloproteinases; and (4) tissue inhibitors of matrix metalloproteinases were significantly elevated in anterior joint capsules obtained from 11 patients having surgery for posttraumatic contractures when compared with nine elbows, from organ donors, that were free of contractures. Reverse transcription-polymerase chain reaction was used to evaluate mRNA expression normalized to a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase. In the joint capsules of the patients with elbow contractures, relative mRNA levels were increased for: collagen Types I, III, and V (1.5–2.5 times); biglycan (1.5 times); and matrix metalloproteinases-1, -2, -9, -13, and -15 (1.6–3.9 times). In contrast, expression of tissue inhibitors of matrix metalloproteinases-1, -2, and -4 were decreased (⅓–¾times) in the capsules of patients with contractures. There was no difference between the groups in relative mRNA expression for decorin, matrix metalloproteinases-8, -14 and -16, and tissue inhibitor of matrix metalloproteinase-3. The results indicate that joint capsule matrix molecule mRNA levels are altered in the chronic stages of posttraumatic elbow contractures in humans, potentially creating an environment with high matrix turnover rates.
Posttraumatic contractures after elbow injuries are common occurrences.3 Operative treatments, whether open or arthroscopic, focus on excising or dividing the joint capsule surrounding the elbow to improve joint motion.17,18 The joint capsule of the elbow has been described as thickened, and study of cellular changes in joint anterior capsules has shown increased numbers of myofibroblasts in patients with posttraumatic contractures.8 However, reports on the changes in the matrix are lacking.3
Animal studies have determined that collagen turnover and reducible collagen cross-links increase whereas glycosaminoglycans and water content decrease in the joint capsules of rabbit knees immobilized for 9 weeks.1 Studies on the shoulder capsule in patients with adhesive capsulitis show increased expression of collagen Type III whereas expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) were increased in the rotator interval of patients with frozen shoulders.2,15 From these studies, it would seem that the joint capsule has altered regulation of matrix metabolism and turnover in these nontraumatic conditions.
We tested the hypotheses that mRNA levels for collagen Types I, III, and V; the small-leucine rich proteoglycans decorin and biglycan; MMP -1, -2, -8, -9, -13, -14, -15, and -16; and TIMP -1, -2, -3, and -4 will be increased in anterior joint capsules obtained from patients requiring operative intervention for posttraumatic elbow contractures when compared with similar tissues obtained from age-matched organ donors free of elbow contractures.
MATERIALS AND METHODS
Anterior capsules of human elbows were obtained from 11 patients, nine males and two females, with a mean age of 31 years [standard deviation (SD) ± 12 years; range, 14–48 years] at the time of contracture release and an average 15 ± 7 months (range, 5–25 months) after injury. The average preoperative range of motion (ROM) in the flexion-extension arc was 46° ± 22° (range, 10°–85°). The original injuries all were intraarticular fractures; five patients had distal humerus fractures, one patient had a radial head fracture, two patients had proximal ulna fractures, one patient had proximal radius and ulna fractures, and two patients had radial head fractures associated with elbow dislocations. Control anterior capsules were obtained from nine elbows of organ donors (23 ± 13 years old; seven males and two females) that were free of contractures. There were no significant differences comparing the average age and the gender distribution of the patient and control groups. The tissues from the patients were immediately frozen for mRNA analysis using liquid nitrogen. The tissues from the organ donors were obtained 5 to 18 hours after death (body stored at 4°C) and prepared as above. Marchuk et al stated that mRNA is stable in periarticular tissues for as much as 96 hours after death.13 In cases in which rigor mortis had set in, clinical history was used to determine that the joints were free of contractures. All tissues were stored at −80°C. Institutional review board approval from the authors’ institution and organ donor program was obtained before the study.
The protocol followed the methods previously described in Hildebrand et al8 and Lo et al.10 Ribonucleic acid isolation (TRI-spin method) began by reducing the frozen tissue to a powder at liquid nitrogen temperatures. TRIzol reagent (Life Technologies, Gaithersburg, MD) was added (1 mL TRIzol/50 mg tissue), and the mixture was warmed to room temperature. Chloroform was added, and the samples were centrifuged. The upper aqueous phase containing the RNA was removed, and one volume of 70% ethanol was added. Total RNA was extracted, and the RNA was purified using an RNeasy Total RNA kit (Qiagen, Chatsworth, CA). The RNA yield was quantified fluorometrically (Perkin-Elmer, Branchburg, NJ) using Sybr Green II (FMC BioProducts, Rockland, MN) and was compared with standards obtained with calf liver ribosomal RNA.
All samples were analyzed by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) for mRNA analysis at the same time to avoid any potential variation in efficiency of the procedures. One microgram total RNA aliquots and the Qiagen Omniscript RT kit (Qiagen, GmbH, Hilden, Germany) were used. Random primers (0.5 μmol/L) were added to the RNA, and first-strand cDNA was synthesized by adding ×10 first-strand buffer, RNase block ribonuclease inhibitor, d-nucleoside triphosphates, and Maloney murine leukemia virus reverse transcriptase. Reactions were incubated at 37°C for 1 hour before a 5-minute incubation at 93°C and subsequent cooling on ice for 5 minutes. Aliquots of cDNA were amplified in a total volume of 50 μL containing ×10 PCR buffer, 10 mmol/L d-nucleoside triphosphate mixture, 50 mmol/L MgCl2, 0.5 μmol/L of each primer, and 1.25 U of Taq DNA polymerase (Rose Scientific Ltd, Edmonton, Canada). Specific human primers were used as indicated (Table 1).10,11 Primer sets were optimized for PCR cycles, and linear amplification curves were plotted. The optimal cycle number was selected to be in the linear part of the amplification curve. All reverse-transcribed controls were negative, ensuring that no detectable genomic DNA was present in the RNA samples. All samples used in this study were reverse transcribed at least twice, and all PCR experiments were done at least twice, with nearly identical results obtained each time.
TABLE 1.
The Primers Used
| Primer | Sequence | bp | Temperature (°C) | Cycles | Source |
|---|---|---|---|---|---|
| α-SMA | 5′-GCT CAC GGA GGC ACC CCT GAA 3′-CTG ATA GGA CAT TGT TGA CAT |
591 | 60 | 30 | NM-001613 |
| Biglycan | 5′-GAT GGC CTG AAG CTC AA 3′-GGT TGT TGA AGA GGC TG |
406 | 55 | 30 | Lo et al11 |
| Collagen I | 5′-AAC ATA ATC CGC AGT GGC CT 3′-CTC CTG TTG CGT TGC TCC TT |
509 | 65 | 30 | Lo et al11 |
| Collagen III | 5′-CCC AGA ACA TCA CAT ATC AC 3′-CAA GAG GAA CAC ATA TGG AG |
366 | 58 | 30 | Lo et al11 |
| Collagen V | 5′-AGA AGT GGA GGC AGC ATT CT 3′-AGA ATG AGG AGA GGT CTT GC |
253 | 55 | 30 | M58529 |
| GAPDH | 5′-TCA CCA TCT TCC AGG AGC GA 3′-AGT GAT GGC ATG GAC TGT GG |
326 | 65 | 25 | BC023632 |
| Decorin | 5′-CTG TGC TCA CTG TAT TGT GG 3′-ATT AGG TGG CAC TGT CTC TC |
455 | 55 | 30 | Lo et al11 |
| MMP-1 | 5′-CGA CTC TAG AAA CAC AAG AGC AAG A 3′-AAG GTT AGC TTA CTG TCA CAC GCT T |
786 | 65 | 35 | NM-002421 |
| MMP-2 | 5′-GTG CTG AAG GAC ACA CTA AAG AAG A 3′-TTG CCA TCC TTC TCA AAG TTG TAG G |
605 | 58 | 33 | NM-004530 |
| MMP-8 | 5′-GCT GCT TAT GAA GAT TTT GAC AGA G 3′-ACA GCC ACA TTT GAT TTT GCT TCA G |
435 | 51 | 38 | NM-002424 |
| MMP-9 | 5′-CAC TGT CCA CCC CTC AGA GC 3′-GCC ACT TGT CGG CGA TAA GG |
243 | 58 | 35 | NM-004994 |
| MMP-13 | 5′-GCA AGA CTC TCC TGT TCT CA 3′-TCC AGC CAC GCA TAG TCA TA |
410 | 60 | 40 | NM-002427 |
| MMP-14 | 5′-CGC TAC GCC ATC CAG GGT CTC AAA 3′-CGG TCA TCA TCG GGC AGC ACA AAA |
497 | 62 | 30 | NM-004995 |
| MMP-15 | 5′-ACA ACC ACC ATC TGA CCT TTA GCA 3′-AGC TTG AAG TTG TCA ACG TCG TTC |
454 | 62 | 35 | NM-002428 |
| MMP-16 | 5′-TTA CTT CTG GCG GGG CTT GCC TCC TAG TAT 3′-ACA GTA CAG TAT GTG GCG GGG TGT TCC TTT |
652 | 58 | 38 | NM-005941 |
| TIMP-1 | 5′-TGC AAT TCC GAC CTC GTC AT 3′-TGC ATT CCT CAC AGC CAA CA |
404 | 65 | 25 | Lo et al11 |
| TIMP-2 | 5′-CTC TGG AAA CGA CAT TTA TGG 3′-AGA TGT AGC ACG GGA TCA TGG |
332 | 55 | 22 | NM-003255 |
| TIMP-3 | 5′-GTG CAA CTT CGT GGA GAG 3′-GGT CTG TGG CAT TGA TGA |
281 | 55 | 22 | NM-000362 |
| TIMP-4 | 5′-ACA GCC AGA AGC AGT ATC 3′-CCA GAG GTC AGG TGG TAA |
446 | 55 | 30 | NM-003256 |
bp = length of primer product in base pairs; temperature = annealing temperature; source = Genbank access number or journal article reference; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; MMP = matrix metalloproteinase; TIMP = tissue inhibitors of matrix metalloproteinase; SMA = smooth muscle actin
Polymerase chain reaction products were separated by electrophoresis of 20 μL of each PCR product using a 2% agarose gel at 100 V/cm in 1× TAE buffer. After electrophoresis, the gels were stained with ethidium bromide, destained in distilled water, and photographed using Polaroid number 55 film (Technicare Inc., Calgary, Canada). Comparison with the standard 1-kb DNA Ladder (Life Technologies) ensured that the PCR products were the proper size. Relative band intensities were quantified by densitometric scanning of negatives (Masterscan Interpretive Densitometer; CSPI, Billerica, MA). The mRNA levels of the various collagen, proteoglycan, MMP, and TIMP molecules were normalized to the mRNA levels of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The optical density measures for GAPDH mRNA determinations in the contracture capsules (0.62 ± 0.14) was similar to the control capsule values (0.63 ± 0.13), supporting the use of this housekeeping gene for the subsequent analysis.
Data are presented as mean ± SD. Statistical analysis consisted of a Student’s t test for group means assuming unequal variance. Significance levels were set at p ≤ 0.05.
RESULTS
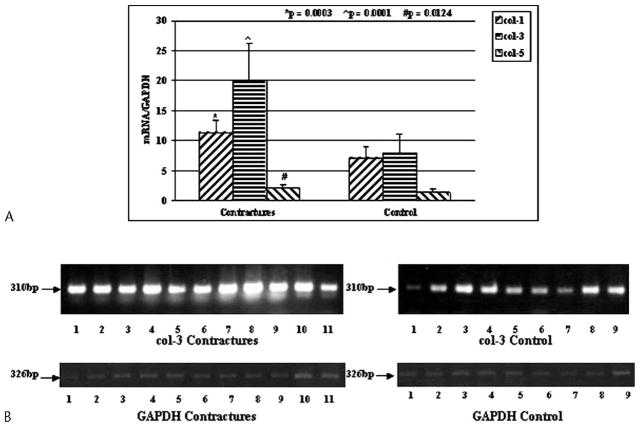
The relative expression of mRNA for collagen Types I, III, and V were elevated (p = 0.0003, 0.0001, and 0.0124, respectively) in the joint capsules of the patients with contractures compared with the tissues from the organ donor elbows free of contractures (Fig 1). The mean mRNA values in the capsules of the patients for collagen Types I, III, and V were 1.6 times, 2.5 times, and 1.5 times greater than, respectively, the values for the organ donor controls.
Fig 1.
A–B. (A) The mRNA expression of collagen Types I, III, and V (col-1, col-3, and col-5, respectively) normalized to GAPDH mRNA expression is shown. (B) An example of the intensity of collagen Type III and GAPDH on gel analysis is shown (bp = length of primer product in base pairs).
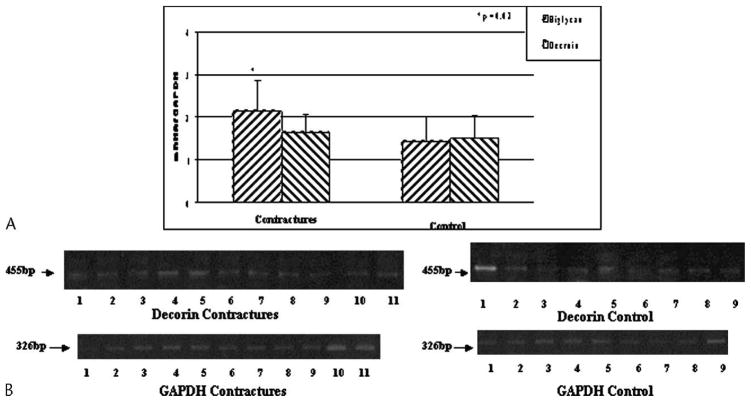
The relative mRNA levels for biglycan were elevated (p = 0.02) in the joint capsule of the patients with contractures, although there was no difference in decorin mRNA levels between the two groups (Fig 2). The mean mRNA value in the capsules of the patients for biglycan was 1.5 times greater than the value for the organ donor controls.
Fig 2.
A–B. (A) The mRNA expression of biglycan and decorin normalized to GAPDH mRNA expression is shown. (B) The intensity of decorin and GAPDH expression on gel analysis is shown (bp = length of primer product in base pairs).
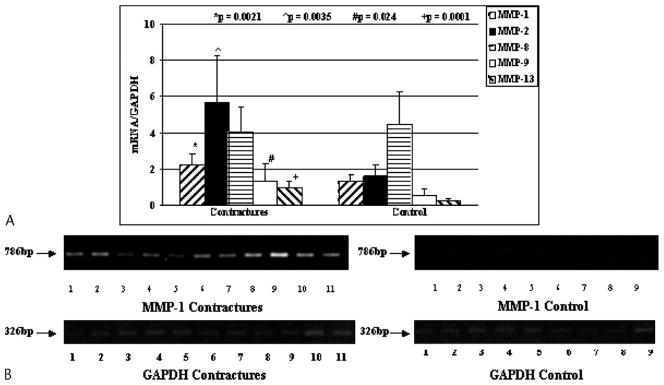
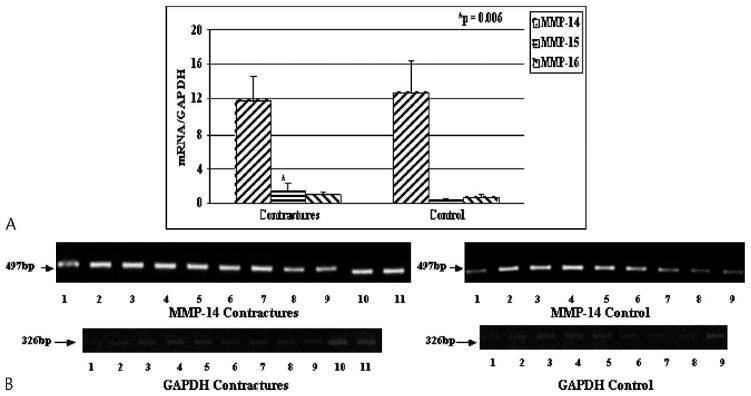
The mRNA levels for many MMPs were greater in the joint capsules of patients (Figs 3, 4). For MMPs usually found in the matrix, there were elevations of MMP-1, -2, -9, and -13 (p = 0.0021, 0.0035, 0.024, and 0.0001, respectively) in the joint capsules of patients with contractures when compared with the tissues from the organ donor controls (Fig 3). There was no difference between the groups with respect to MMP-8 mRNA levels in the joint capsule (Fig 3). The mean mRNA values in the capsules of the patients for MMP-1, -2, -9, and -13 were 1.6 times, 3.5 times, 2.4 times, and 3.9 times greater than, respectively, the values for the organ donor controls. Cell membrane-bound MMP-15 mRNA levels were elevated (p = 0.006) in the joint capsules of patients with contractures whereas mRNA expression for membrane-bound MMP-14 and MMP-16 were not different between the groups (Fig 4). The mean mRNA value in the capsules of the patients for MMP-15 was 3.0 times greater than the value for the organ donor controls.
Fig 3.
A–B. (A) The mRNA expression of MMP-1, -2, -8, -9, and -13 to GAPDH mRNA expression is shown on this graph. (B) An example of the intensity of MMP-1 and GAPDH is shown on gel analysis (bp = length of primer product in base pairs).
Fig 4.
A–B. (A) The mRNA expression of MMP-14, -15, and -16 normalized to GAPDH mRNA expression is shown. (B) The intensity of MMP-14 and GAPDH on gel analysis is shown (bp = length of primer product in base pairs).
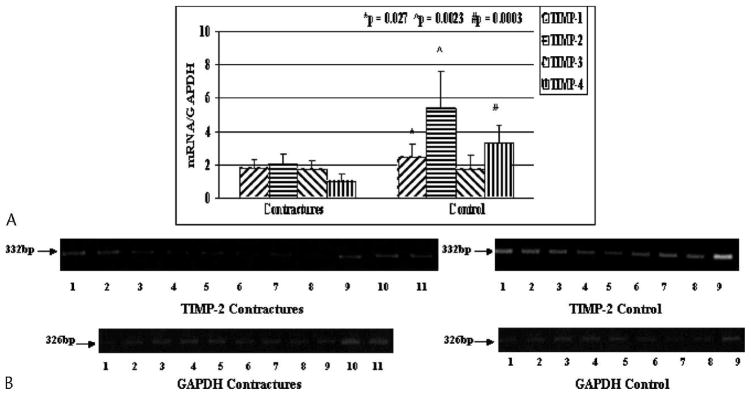
Tissue inhibitors of matrix metalloproteinase mRNA levels were selectively decreased in the joint capsules from the patients with contractures, which is in the opposite direction of those for most of the MMPs and for collagen expression (Fig 5). For TIMP-1, -2, and -4, mRNA expression was decreased (p = 0.027, 0.0023, and 0.0003, respectively) in the joint capsule of the patients with elbow contractures when compared with similar tissues of the control organ donor elbows (Fig 5). However, no difference in the mRNA levels for TIMP-3 was detected when comparing the two groups (Fig 5). The mean mRNA values in the capsules of the patients for TIMP-1, -2, and -4 were 0.73 times, 0.38 times, and 0.31 times the values for the organ donor controls.
Fig 5.
A–B. (A) The mRNA expression of TIMP-1, -2, -3, and -4 normalized to GAPDH mRNA expression is shown. (B) An example of the intensity of TIMP-2 and GAPDH is shown on gel analysis.
DISCUSSION
The joint capsule is a critical component leading to the development of posttraumatic contractures, and it is described as thickened and presumed to have a fibrotic response. Little data exist regarding the changes in expression of matrix molecules or enzymes with the potential to affect matrix composition and turnover. We describe changes in the anterior joint capsule of patients with chronic posttraumatic elbow contractures in relation to anterior joint capsules of age-matched organ donors free of elbow contractures. Normalized expression of mRNA of collagen Types I, III, and V; the small-leucine rich proteoglycan, biglycan; and MMP-1, -2, -9, -13, and -15 was significantly increased, whereas normalized expression of mRNA of TIMP-1, -2, and -4 was significantly decreased in the joint capsules of patients with chronic contractures when compared with the tissues of the organ donor controls. There were no significant differences in the normalized mRNA levels between the two groups for decorin; MMP-8, -14, and -16; and TIMP-3. The relatively small numbers in the contracture (n = 11) and control (n = 9) groups may have prevented detection of significant differences for these five selected molecules, but significant differences were detected for the 12 other molecules studied. Although protein levels were not determined, the selective modification of mRNA expression (increases and decreases in the contracture group) in these matrix molecules and enzymes would support the concept of prolonged matrix turnover and the observation of thickened and fibrotic joint capsules in posttraumatic human contractures.
Elevated collagen expression in human elbow capsules is consistent with the work of Akeson et al,1 who reported an increased collagen turnover in joint capsules of rabbit knees with contractures 9 weeks after immobilization was instituted to produce the contractures (Fig 1). Although results of studies in animal models are from relatively early in the contracture process, results of other studies on medial collateral ligament healing in rabbit knees show collagen and matrix remodeling still was evident at least 2 years after the injury.5,6 Results of both studies are consistent with the current findings of altered collagen expression in anterior joint capsules of patients with chronic elbow contractures.5,12 Similarly, in a descriptive study on adhesive capsulitis of the shoulder, it was shown, with immunohistochemical techniques, that collagen Type III expression was increased in the joint capsule when compared with similar tissues from patients with localized synovitis of the shoulder.15
The small leucine-rich proteoglycans biglycan and decorin were expressed in the joint capsule based on mRNA levels (Fig 2). Studies on human anterior cruciate ligament healing has shown that expression of these molecules is altered chronically, which is consistent with the prolonged alteration in mRNA expression in posttraumatic elbow capsules from humans.11 These proteoglycans are known to have effects on collagen fibril assembly and availability of growth factors, such as transforming growth factor-beta (TGF-β), in the matrix and could influence matrix organization and the anabolic environment in the tissue.14,20
The mRNA expression of many MMPs was elevated in the capsules of patients with elbow contractures (Figs 3, 4). The matrix-bound MMPs (MMPs-1, -2, -9, and -13) are especially effective in the turnover of the major collagens of the joint capsule, including Types I, III, and V, and elevations of their expression would be consistent with the findings of increased collagen turnover in the joint capsules of patients with posttraumatic contractures.16 In a report regarding tissue obtained from the rotator interval of patients with frozen shoulders, mRNA expression was detected for MMP-1 in 64% of patients, MMP-2 in 92% of patients, MMP-3 in 35% of patients, MMP-9 in 57% of patients, and MMP-14 in 0% of patients using RT-PCR methods.2 However in our study, mRNA expression was detected for all MMPs in the anterior joint capsules of elbows from all patients and all controls. Therefore, there may be some differences potentially related to tissue-specific or process-specific variables in the two clinical situations.
Tissue inhibitors of matrix metalloproteinase mRNA levels were decreased in the capsules of the patients with elbow contractures for TIMP-1, -2, and -4, or there was no significant difference between the groups (TIMP-3; Fig 5). In a previous study, TIMP-1 mRNA expression was detected in the rotator interval of all patients with frozen shoulders, which is consistent with the finding in our study of expression of mRNA for TIMP-1, -2, -3, and -4 in all elbow capsules in the contracture and control (organ donor) groups.2 In the study on frozen shoulders, normal control tissue was not available to determine if expression in the tissue from the frozen shoulders was decreased, as in our study.2
Altered MMP:TIMP ratios seem to be associated with clinical conditions of contracture. Hutchinson et al reported that frozen shoulder and Dupuytren’s contracture of the hand developed in six of 12 patients with inoperable gastric carcinoma who were given a synthetic MMP inhibitor for more than 1 month.9 The MMP inhibitors would be expected to give a relative decrease in MMP and relative increase in TIMP activity, which is opposite our findings of increased MMP expression and decreased TIMP expression in human elbow capsules in posttraumatic contractures. We are unable to reconcile the discrepancies of potential MMP:TIMP ratios when comparing results of our study on elbow contractures with results of the study9 on other contracture conditions while taking synthetic MMP inhibitors. Several possibilities may confound the observations on MMP:TIMP ratios or impact the understanding of the roles MMPs and TIMPs are playing in the contracture process. Regarding confounded observations, Hutchinson et al did not evaluate the pathologic contracture tissues to confirm a decreased MMP:TIMP ratio.9 In our study, we report relative mRNA levels (normalized to a housekeeping gene, GAPDH), and there is no comparison of absolute mRNA levels of MMP and TIMP to determine if the ratios are truly decreased. In terms of understanding the roles of MMPs and TIMPs, these molecules have multiple functions. The original functions described were their regulation of collagen turnover or degradation or both. However, both molecule types have other properties, including regulation of noncollagenous matrix proteins and enzymes, growth factors, and cellular proliferation, chemotaxis, and apoptosis.4,19 It may be that these other functions (noncollagenous) of the MMPs and TIMPs are what contribute to the contracture process. The synthetic MMP inhibitors also may have other cellular or matrix effects that contribute to the contracture conditions.
Messenger RNA levels for a subset of matrix molecules and enzymes are altered in anterior elbow capsules of patients with chronic posttraumatic contractures. It is unknown whether these mRNA alterations in a chronic stage of the process are a reaction to, or part of the cause for, the process. Future study will require characterization of protein expression for these molecules. This baseline information is critical for correlation with animal models of posttraumatic joint contractures.7 The animal model will allow evaluation of the contracture process in its initial and earlier stages to answer the question if these chronic findings are a cause for, or a reaction to, the process. An understanding of the process leading to development of contractures is critical if we are to discover new methods of treatment, to evaluate the effects of current treatments (eg, early motion) on joint capsule properties, or to understand the genetic and environmental risk for this abnormal healing phenotype.
Acknowledgments
We thank Carla Gronau and Karen Anderson for preparation of the manuscript, and Dr. Stuart Patterson and Dr. Graham King from the University of Western Ontario for contributing samples for some of the patients.
One or more of the authors has received funding from the Alberta Heritage Foundation for Medical Research (KAH), the Health Research Foundation (KAH), the Canadian Institutes of Health Research (KAH, DAH) and the Calgary Foundation–Grace Glaum Professorship (DAH).
Footnotes
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Akeson W, Amiel D, Woo SL. Immobility effects on synovial joints the pathomechanics of joint contracture. Biorheology. 1980;17:95–110. doi: 10.3233/bir-1980-171-212. [DOI] [PubMed] [Google Scholar]
- 2.Bunker TD, Reilly J, Baird KS, Hamblen DL. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J Bone Joint Surg. 2000;82B:768–773. doi: 10.1302/0301-620x.82b5.9888. [DOI] [PubMed] [Google Scholar]
- 3.Cooney WP., III . Contractures of the elbow. In: Morrey BF, editor. The elbow and its disorders. Philadelphia: WB Saunders Company; 1993. pp. 464–475. [Google Scholar]
- 4.Crocker SJ, Pagenstecher A, Campbell I. The TIMPs tango with MMPs and more in the central nervous system. J Neurosci Res. 2004;75:1–11. doi: 10.1002/jnr.10836. [DOI] [PubMed] [Google Scholar]
- 5.Frank CB, Amiel D, Akeson WH. Healing of the medial collateral ligament of the knee. Acta Orthop Scand. 1983;54:917–923. doi: 10.3109/17453678308992934. [DOI] [PubMed] [Google Scholar]
- 6.Frank CB, McDonald D, Shrive NG. Collagen fibril diameters in the rabbit medial collateral ligament scar: A longer term assessment. Connect Tissue Res. 1997;36:261–269. doi: 10.3109/03008209709160226. [DOI] [PubMed] [Google Scholar]
- 7.Hildebrand KA, Sutherland C, Zhang M. Rabbit knee model of post-traumatic joint contractures: The long-term natural history of motion loss and myofibroblasts. J Orthop Res. 2004;22:313–320. doi: 10.1016/j.orthres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Hildebrand KA, Zhang M, van Snellenberg W, King GJ, Hart DA. Myofibroblast numbers are elevated in human elbow joint capsules after trauma. Clin Orthop. 2004;419:189–197. doi: 10.1097/00003086-200402000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchinson JW, Tierney GM, Parsons SL, Davis TR. Dupuytren’s disease and frozen shoulder induced by treatment with a matrix metalloproteinase inhibitor. J Bone Joint Surg. 1998;80B:907–908. doi: 10.1302/0301-620x.80b5.8464. [DOI] [PubMed] [Google Scholar]
- 10.Lo IK, Marchuk L, Hart DA, Frank CB. Comparison of mRNA levels for matrix molecules in normal and disrupted human anterior cruciate ligaments using reverse transcription-polymerase chain reaction. J Orthop Res. 1998;16:421–428. doi: 10.1002/jor.1100160405. [DOI] [PubMed] [Google Scholar]
- 11.Lo IK, Marchuk L, Hart DA, Frank CB. Messenger ribonucleic acid levels in disrupted human anterior cruciate ligaments. Clin Orthop. 2003;407:249–258. doi: 10.1097/00003086-200302000-00034. [DOI] [PubMed] [Google Scholar]
- 12.Loitz-Ramage B, Frank CB, Shrive NG. Injury size affects long term strength of the rabbit medial collateral ligament. Clin Orthop. 1997;337:272–280. doi: 10.1097/00003086-199704000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Marchuk L, Sciore P, Reno C, Frank CB, Hart DA. Postmortem stability of total RNA isolated from rabbit ligament, tendon and cartilage. Biochim Biophys Acta. 1998;1379:171–177. doi: 10.1016/s0304-4165(97)00094-9. [DOI] [PubMed] [Google Scholar]
- 14.Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- 15.Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickiewicz TL. Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder. J Orthop Res. 1997;15:427–436. doi: 10.1002/jor.1100150316. [DOI] [PubMed] [Google Scholar]
- 16.Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: Old dogs with new tricks. Genome Biol. 2003;4:216.1–216.11. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmerman L, Andrews J. Arthroscopic treatment of posttraumatic elbow pain and stiffness. Am J Sports Med. 1994;22:230–235. doi: 10.1177/036354659402200213. [DOI] [PubMed] [Google Scholar]
- 18.Urbaniak JR, Hansen PE, Beissinger SF, Aitken MS. Correction of post-traumatic flexion contracture of the elbow by anterior capsulotomy. J Bone Joint Surg. 1985;67A:1160–1164. [PubMed] [Google Scholar]
- 19.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]