Abstract
Survival and death of lymphocytes is regulated by the balance between pro- and anti-apoptotic members of the Bcl-2 family, and this is coordinated with the control of cell cycling and differentiation. Bim, a pro-apoptotic BH3-only member of the Bcl-2 family, can be regulated by MEK/ERK-mediated phosphorylation, which affects its binding to pro-survival Bcl-2 family members and its turnover. We investigated Bim modifications in mouse B and T lymphoid cells after exposure to apoptotic stimuli and during mitogenic activation. Treatment with ionomycin or cytokine withdrawal caused an elevation in BimEL, the most abundant Bim isoform. In contrast, in mitogenically stimulated T and B cells, BimEL was rapidly phosphorylated and its levels declined. Pharmacological inhibitors of MEK/ERK-signaling prevented both of these changes in Bim, reduced proliferation and triggered apoptosis of mitogen-stimulated T and B cells. Remarkably, loss of Bim prevented this cell killing but did not restore cell cycling. These results show that during mitogenic stimulation of T and B lymphocytes MEK/ERK signaling is critical for two distinct processes, cell survival, mediated (at least in part) through phosphorylation and consequent inhibition of Bim, and cell cycling, which proceeds independently of Bim inactivation.
Keywords: T cells, B cells, cell activation, apoptosis, signal transduction
Introduction
The rates of cell proliferation, cell death and cell differentiation are coordinated with respect to each other to ensure proper development and functioning of an organism (1). During differentiation in primary lymphoid organs and after activation in the periphery, B and T lymphocytes undergo successive rounds of cell division and growth arrest followed by the death of cells that are useless or potentially dangerous (2). Whether, a cell lives or dies, is regulated to a large extent by the balance between pro- and anti-apoptotic members of the Bcl-2 protein family. The anti-apoptotic members Bcl-2, Bcl-xL, Bcl-w, A1 and Mcl-1 share up to four regions of homology (Bcl-2 Homology or BH regions) and gene targeting experiments have shown that they are critical for cell survival and function in a cell type-specific manner (3). Bax, Bak and Bok/Mtd have three BH regions and despite extensive structural similarity to their anti-apoptotic relatives, they are essential for activation of the downstream apoptosis effector processes, including mitochondrial outer membrane permeabilization (MOMP) and unleashing of the caspase cascade (4). The BH3-only proteins (Bad, Bik/Blk/Nbk, Hrk/DP5, Bid, Bim/Bod, Bmf, Noxa, Puma/Bbc3) only contain the BH3 domain and they are required to initiate apoptosis signaling (5). Biochemical and genetic experiments have indicated that BH3-only proteins trigger apoptosis by binding to pro-survival Bcl-2 family members thereby unleashing the Bax/Bak-like proteins (6), but direct activation of Bax/Bak has also been proposed (4).
Bim, a pro-apoptotic BH3-only Bcl-2 family member (7), is expressed in a broad range of tissues, including hemopoietic, epithelial, neuronal and germ cells (8). Alternative splicing generates several Bim isoforms (including BimEL, BimL and BimS) (7) of which BimEL is the most abundantly expressed (8). Studies with gene-targeted mice have shown that Bim is required for developmentally programmed cell death as well as cytokine deprivation or stress-induced apoptosis of T as well as B lymphocytes, myeloid cells, neurons and epithelial cells (9–13).
The pro-apoptotic activity of Bim can be regulated by several transcriptional as well as post-translational mechanisms (14). For example, cytokine withdrawal causes shutdown of the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway, resulting in the activation of the transcription factor FOXO3A, which induces Bim mRNA synthesis (15). In response to cytokine withdrawal, the levels and pro-apoptotic activity of Bim can also be controlled by post-translational mechanisms (14). In growth factor-stimulated cells, Bim is phosphorylated by ERK1/2 on multiple sites, which is thought to reduce its binding to Mcl-1 and Bcl-xL (16) and was reported to also target it for ubiquitination and proteasomal degradation (17, 18).
We investigated the control of Bim in mouse T and B cells during transition from the resting to the cycling state after mitogenic stimulation. We found that in naïve, resting T and B cells Bim exists in a hypo-phosphorylated form but is expressed at relatively low levels. Upon mitogenic stimulation, Bim is rapidly phosphorylated in a MEK/ERK-dependent manner and subsequently declines in level. Studies using pharmacological inhibitors and gene-targeted mice showed that MEK/ERK-mediated phosphorylation of Bim is required for survival of mitogen stimulated B and T cells but cell cycling proceeds by a MEK/ERK-dependent mechanism that is independent of Bim inactivation
Materials and Methods
Experimental Animals
All experiments with animals were performed according to the guidelines of The Walter and Eliza Hall Institute Animal Ethics Committee. Wistar rats and C57BL/6 mice were obtained from our Institute’s breeding facility at Kew (Victoria, Australia). The Bim−/− (9), vav-bcl-2 transgenic (19), Bad−/− (20), Bid−/− (21), Bim−/−Bad−/− (22) and Bim−/−Bid−/− (6) mice have all been previously described. The Bim−/− and Bad−/− mice were both originally produced on a mixed C57BL/6x129SV background using 129SV-derived ES cells, but have been backcrossed for >10 generations with C57BL/6 mice before they were used for these studies. The vav-bcl-2 transgenic mice, expressing constitutively high levels of (human) Bcl-2 in all hemopoietic cell types, were generated on an inbred C57BL/6 background. The Bid−/− mice were produced on an inbred C57BL/6 background using C57BL/6-derived ES cells.
B and T Cell Purification
B Lymphocytes were purified from spleen and lymph nodes of mice by negative sorting after staining of unwanted cell types - T cells, macrophages, granulocytes and erythroid cells - with FITC-conjugated surface marker-specific rat monoclonal antibodies (mAbs) (RB6-8C5: anti-Gr-1, F4/80: anti-macrophage marker, MI/70: anti-Mac-1, H129.1: anti-CD4, YTS169: anti-CD8, T24.3.21: anti-Thy-1 and Ter119: anti-erythroid marker). Viable B cells that were not stained with FITC-labeled mAbs or the vital dye propidium iodide (PI, Sigma at 2 µg/mL) (FITC-PI-) were purified on a MoFlo (Cytomation) or FACSstar+ (Becton Dickinson) high-speed cell sorter. Staining with antibodies to CD19, CD45R-B220, IgM and/or IgD followed by FACS analysis revealed that B cell purity was typically >95%.
T lymphocytes were purified from lymph nodes by depletion of all other cell types (B cells, macrophages, granulocytes and erythroid cells) by staining with cell surface marker-specific rat mAbs (RA3-6B2: anti-CD45R-B220, 5.1: anti-IgM, 11-26C: anti-IgD, RB6-8C5: anti-Gr-1, M1/70: anti-Mac-1, Ter119: anti-erythroid marker) and magnetic beads coupled with goat anti-rat IgG antibodies (Qiagen). Staining with antibodies to Thy-1, CD4 and CD8 revealed that T cell purity was typically >95%.
B and T Cell Stimulation
Purified B lymphocytes from spleen and lymph nodes were stimulated for 24–48 h in culture with 20 µg/mL Fab2 goat anti-mouse IgM antibody fragments (Jackson Immunoresearch) plus saturating concentrations of recombinant mouse interleukins, IL-2, -4 and -5, produced by X63/0 hybridoma cells transfected with the relevant expression vectors (23).
Purified T lymphocytes from lymph nodes were stimulated for 24–48 h in culture with 2 ng/mL phorbol-12-myristate-acetate (PMA) plus 0.1 µg/mL ionomycin (both from Sigma) and IL-2 or with plate-bound hamster mAbs to CD3 (145-2C11) and CD28 (37N51) (both at 10 µg/mL in the coating solution, PBS) plus IL-2 or with 0.1–1.0 µg/mL ionomycin alone. Mitogen stimulated B and/or T cells were also treated with either JNK inhibitor 1 (L-stereoisomer; Axxora) at 1-25 µg/mL, U0126 MEK1/2 inhibitor (Cell Signaling) at 10 or 20 µg/mL (with drugs replenished after 24 h), the proteasomal inhibitors PS341/Velcade (Janssen-Cilag) at 10–50 µg/mL or MG132 (CalBiochem) at 10–50 µg/mL, both added 2 h prior to and during mitogen stimulation.
Cytoplasmic Immunofluorescent Staining and Cell Cycle Analysis
Thymocytes, spleen cells, purified mature T cells or thymocyte subpopulations were fixed for 10 min at RT with PBS containing 1% paraformaldehyde (BDH), permeabilized and stained for 30 min at 4°C in PBS/0.3% saponin (Sigma) plus 2% FCS containing rat anti-Bim mAbs (3C5 or 10B12 at 5 µg/mL, Alexis) or an isotype-matched control mAb (rat IgG2a, Pharmingen), followed by secondary staining with biotin-conjugated mouse anti-rat IgG2a antibody (BD Pharmingen) and detected with streptavidin FITC (Caltag) as detailed (24) and analyzed in a FACScan (Becton Dickinson).
For cell cycle analysis, cells were fixed (>8 h at 4°C) in 70% ethanol and then treated for 20 min at 37°C with 0.5 µg/mL DNase-free RNAse A (Promega) with 69 mM propidium iodide (PI, Sigma) in 0.1 M sodium citrate (pH 7.4). Flow cytometric analysis (10’000 cells per sample) was performed on a FACScan and cell cycle distribution determined by manual gating. Statistical analysis was performed using 2-sided student’s t-test with equal variance.
Western Blotting and λPPase Treatment
Cell lysates were prepared, proteins size-fractionated on polyacrylamide gels (Novex) and transferred to nitrocellulose membranes (Amersham Pharmacia) as described previously (8). Non-specific binding of antibodies to membranes was blocked by overnight incubation in PBS, 5% skimmed milk, 1% casein, 0.05% Tween-20.
Membranes were then probed with the following antibodies: rat anti-Bim mAbs (clones 3C5 or 10B12, Alexis), rat isotype-matched control antibody (IgG2a/κ, Pharmingen), rabbit polyclonal anti-Bim antibody (Stressgen), hamster anti-mouse Bcl-2 mAb (3F11), rabbit polyclonal anti-Mcl-1 antibody (Rockland) or rabbit anti-phospho-p44/42 MAP kinase (ERK1/2) antibody (Cell Signaling). To control for the concentration and integrity of proteins in the tissue lysates, blots were probed with mouse anti-HSP70 mAb N6 (gift from Drs R Anderson, Peter MacCallum Cancer Research Institute, Melbourne, Australia and W Welch, UCSF, San Francisco, USA) or a mouse anti-actin antibody (Sigma). Bound antibodies were visualized with goat anti-rat IgG (Southern Biotechnology), sheep anti-rabbit Ig (Chemicon) or sheep anti-mouse IgG antibodies (Chemicon), all conjugated to HRP, followed by enhanced chemiluminescence (ECL; Amersham Pharmacia). Quantitative Western blotting was performed by comparing the strength of the Bim signal to that of the HSP70 or actin signal after staining with secondary goat anti-rat IgG or goat anti-rabbit IgG antibodies conjugated to IR dye 800 (Rockland) or Alexa Fluor 680 or 800 dye (Molecular Probes), using the Odyssey infrared imaging system according to the manufacturer’s instructions (Li-Cor Biosciences).
Cell lysates (prepared as above without NaF or Na3VO4) were incubated for 1 h at 30°C with λ-PPase (NEB) (15 µg/200U) according to the manufacturer’s recommendations. λ-PPase activity was inhibited by the inclusion of NaF (5 mM) and Na3VO4 (2 mM).
Online Supplemental Material
Supplementary Figures 1 and 2 and accompanying Figure legends are available online.
Results
A Broad Range of Apoptotic Stimuli Cause an Increase in Bim Expression Levels
Several studies have indicated that Bim is expressed at low levels in resting lymphoid cells (7, 8). We confirmed that thymocytes and spleen cells express only low levels of Bim by staining such cells from wt and, as a negative control, those from Bim−/− mice with novel anti-Bim mAbs (3C5 or 10B12) that we developed (Supplementary Figure 1) followed by flow cytometric analysis (Figure 1A). As a further control, we stained these cells with an Ig isotype-matched control mAb. Upon exposure to a Bim-dependent apoptotic stimulus, such as treatment with the calcium ionophore ionomycin (9), both wt thymocytes and splenic T cells showed a marked increase in anti-Bim antibody staining intensity in comparison to cells from Bim−/− mice or staining with an isotype-matched control antibody (Figure 1A). Quantitative Western blot analysis using the Odyssey infrared imaging system revealed a 3.8-fold elevation in BimEL levels in thymocytes after 24 h exposure to ionomycin (Figure 1B). In contrast, treatment with the protein kinase C (PKC) activator, PMA, did not cause an increase in Bim protein levels in thymocytes (Figures 1B, C). This is consistent with the observation that PMA kills thymocytes by a mechanism that is independent of Bim (9) but instead requires the BH3-only protein Puma (25).
Figure 1.
Effects of apoptotic stimuli on Bim expression levels. (A) Flow cytometric analysis of Bim expression (rat mAb 10B12; solid black line) in thymocytes and spleen cells from wt and Bim−/− (negative control; red line) mice that had been left untreated or treated for 22 h with ionomycin (2 µg/mL) or PMA (2 ng/mL). Staining with an isotype-matched control mAb was used as an additional control (black dotted line). (B) Bim protein expression was investigated by Western blot analysis (rat mAb 3C5) of lysates from unstimulated thymocytes (T0) and thymocytes cultured for 24 h in the presence of PMA (2 ng/mL), ionomycin (0.1 or 1 µg/mL), medium lacking FCS or in normal medium (control). (C) Expression of Bim (rabbit polyclonal Ab from Stressgen), Bcl-2, Mcl-1, pERK and HSP70 (loading control) was determined by Western blot analysis of lysates from untreated thymocytes or thymocytes that had been treated for 0.5, 1, 6 or 24 h with either PMA (P; 2 ng/mL) or ionomycin (I; 2 µg/mL). (D) Expression of Bim (rat mAb 3C5), Bcl-2, Mcl-1 and HSP70 (loading control) was determined by Western blotting of lysates from spleen cells that had been stimulated for 48 h with ConA (2 µg/mL) plus IL-2, followed by incubation with IL-2 for a further 48 h. These activated T cells were then deprived of IL-2 for the times indicated. The numbers underneath the anti-Bim blots in B, C and D indicate Bim protein counts/mm2 relative to the loading control ((B) total Bim, (C and D) BimEL).
Next, we performed a time course analysis to examine the differing effects of PMA or ionomycin on Bim expression levels in both thymocytes and mature T cells. Treatment with ionomycin caused a gradual (up to ~3.5-fold) increase in BimEL levels peaking at 24 h. In contrast, within 1 to 6 h of treatment with PMA we observed an upward electrophoretic mobility shift in both BimEL and BimL (with BimEL being the predominant isoform; Figure 1C), suggestive of increased phosphorylation. This was accompanied by a gradual decline in the levels of Bim (Figure 1C). The levels of Bcl-2 remained relatively constant after exposure to PMA or ionomycin, whereas the levels of Mcl-1 had declined significantly by 24 h (Figure 1C). Activation of p44 and p42 MAP kinases (ERK1 and ERK2), revealed by staining with phospho-ERK-specific antibodies, was evident within 0.5 h of treatment with PMA (Figure 1C).
Bim has been shown to be required for cytokine deprivation induced apoptosis of activated T lymphocytes (9). Accordingly, we found that withdrawal of IL-2 from ConA activated splenic T cell blasts resulted in a ~3- to 4-fold increase in BimEL expression (Figure 1D), but no upward mobility shift on SDS-PAGE was evident. Growth factor deprivation did not cause an obvious change in the levels of Bcl-2 or Mcl-1 during the period of observation. Collectively, these results show that some but not all apoptotic stimuli cause an increase in Bim (particularly BimEL) expression in immature as well as mature T lymphoid cells.
Mitogenic Stimulation Induces Bim Phosphorylation in T Cells
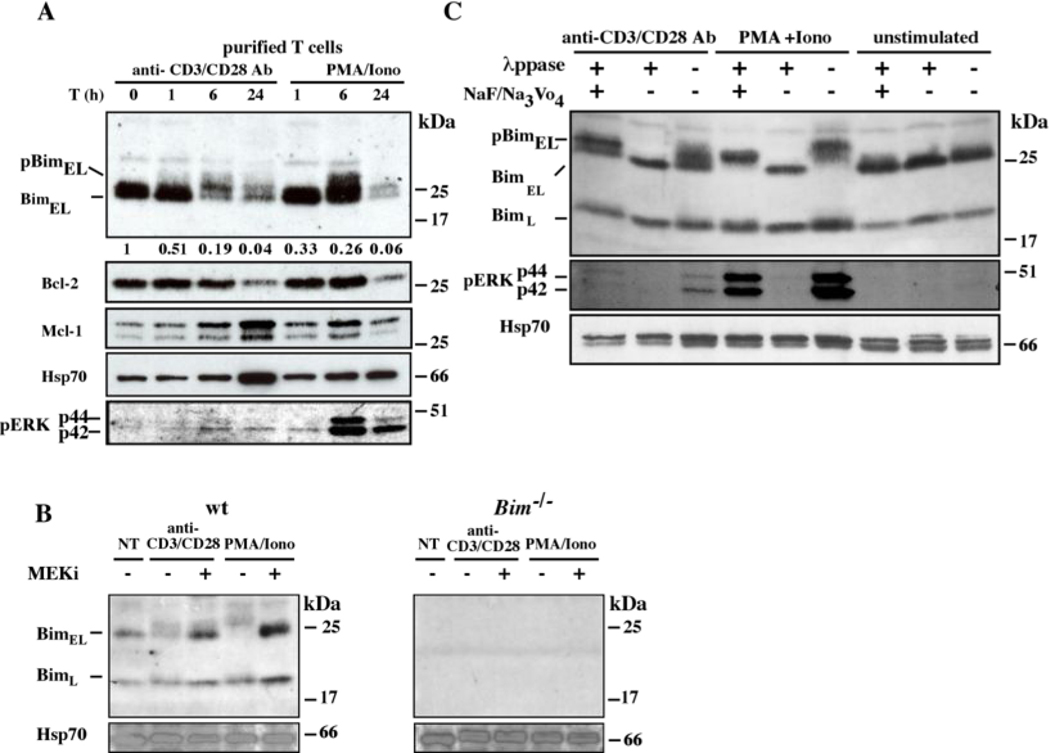
During the induction of an immune response the control of cell death and cell cycling must be coordinated (26). We therefore investigated whether Bim was modified in mitogenically activated mouse T cells during the transition from the resting (G0) to the cycling state. A time course analysis of T cells stimulated with PMA plus ionomycin plus IL-2, or anti-CD3 plus anti-CD28 mAbs plus IL-2 showed an upward electrophoretic mobility shift of BimEL, detectable from 1 h (Figure 2A). The slower migrating band detected with the anti-Bim antibodies clearly represents a modified form of Bim and not a cross-reacting protein as it was not observed in lysates from resting or mitogenically stimulated Bim−/− T cells (Figure 2B). Beyond 6 h of stimulation, there was a gradual decline in Bim to levels below those found in resting T cells (Figure 2A). Activation of ERK1/2, as visualized by staining with phospho-ERK specific antibodies (Figure 2A), was evident from 1 h and was maximal at 6 h, paralleling the upward electrophoretic mobility shift in BimEL. All of these changes, particularly ERK1/2 activation, were more pronounced for stimulation with PMA plus ionomycin compared to treatment with antibodies to CD3 plus CD28, consistent with a previous report (27). Mitogenic stimulation also resulted in a gradual decline in Bcl-2 levels over 24 h, while the levels of Mcl-1 remained constant (Figure 2A).
Figure 2.
Effects of mitogenic stimuli on the levels of Bim expression. (A) Expression of Bim (rabbit polyclonal), Bcl-2, Mcl-1 and pMAPK (pERK1/2) was examined by Western blot analysis of lysates from purified T cells at 0, 1, 6 or 24 h after stimulation with anti-CD3 plus anti-CD28 mAbs (both used at 10 µg/mL to coat plates) plus IL-2 or PMA (2 ng/mL) plus ionomycin (0.1 µg/mL) plus IL-2. Figures underneath the Bim blot indicate BimEL protein counts/mm2 relative to loading control (anti-HSP70 mAb blot). (B) Lysates from purified T cells from wt or Bim−/− mice that had been stimulated with mitogens plus or minus the MEK inhibitor (MEKi -U0126) were Western blotted and probed with anti- Bim or anti-HSP70 (loading control) antibodies. (C) Western blot analysis of lysates from purified wt T cells that were left untreated (NT) or had been stimulated with mitogens plus or minus in vitro treatment of the protein lysates with λ-Ppase. Blots were probed with antibodies to Bim, pMAPK (pERK1/2) or HSP70 (loading control).
To examine whether the modification of Bim (upward electrophoretic mobility shift) was due to phosphorylation, lysates from mitogen-activated (wt) T cells were incubated with lambda phosphatase (λ-PPase), which de-phosphorylates modified serine, threonine and tyrosine residues. This resulted in the disappearance of the slower migrating form of Bim and increased abundance of the non-modified form (Figure 2C). Addition of the λ-PPase inhibitors NaF or Na3VO4 prevented the appearance of the more rapidly migrating (dephosphorylated) form of Bim (Figure 2C). Collectively, these results show that Bim is phosphorylated during mitogenic activation of T lymphocytes.
Mitogen-Induced Bim Phosphorylation Is Prevented by MEK1/2 Inhibitors but not by JNK Inhibitors
The MEK/ERK pathway has been shown to be critical for cell cycle progression and survival (28) and is known to inhibit the pro-apoptotic activity of Bim (18). Therefore and since ERK activation paralleled the changes in Bim mobility on SDS-PAGE (Figures 1, 2), we examined the impact of the MEK/ERK pathway on the phosphorylation of Bim during mitogenic stimulation of T cells. Treatment with U0126, an inhibitor of MEK1/2, the upstream activators of ERK1/2, abrogated the Bim phosphorylation that was observed in mitogenically stimulated T cells (Figure 3A, lanes 2, 6), so that only the non-phosphorylated form of BimEL could be detected (Figure 3A, lanes 3, 7). As a control, treatment with DMSO (vehicle control) had no effect on the migration of Bim on SDS-PAGE (Figure 3A, lanes 4 and 8).
Figure 3.
MEK1/2 inhibition but not JNK inhibition prevents Bim phosphorylation in mitogen-stimulated T cells and proteasomal inhibitors inhibit the reduction in Bim levels. (A) Western blot analysis of lysates from purified T cells that were left untreated (lane 1) or had been stimulated for 6 h with anti-CD3 plus anti-CD28 mAbs plus IL-2, lanes 2–5; or PMA plus ionomycin plus IL-2, lanes 6–9), plus or minus the addition of 10 µM MEK1/2 inhibitor (lanes 3, 7), the proteasome inhibitor MG132 (lanes 5, 9) or DMSO (control, lanes 4, 8). Blots were probed with anti-Bim or anti-HSP70 (loading control) antibodies. (B) Western blot analysis of lysates from purified T cells that were left untreated (NT) or had been stimulated for 6 h with anti-CD3 plus anti-CD28 mAbs plus IL-2 plus or minus addition of the JNK inhibitor (0–2.5 µM). (C and D) Western blot analysis of lysates from purified T cells that had been incubated for 2 h with the proteasomal inhibitor PS341 (Velcade) 0–50 µM prior to no stimulation (NT) or 6 h stimulation with the T cell mitogens (anti-CD3 plus anti-CD28 mAb in (C) and PMA plus ionomycin in (D). Blots were probed with antibodies to Bim, pMAPK (ERK1/2) or HSP70 (loading control). (E) Western blot analysis of lysates from purified T cells that were left untreated (NT) or had been stimulated for 6 h with anti-CD3 plus anti-CD28 mAbs plus IL-2, plus or minus the addition of 0, 10 or 20 µM of the MEK1/2 inhibitor U0126.
It has been reported that Bim can also be phosphorylated by JNK on Thr-112 (29), which is thought to activate the pro-apoptotic of Bim (30, 31), as does ERK-mediated phosphorylation on Thr-112, whereas ERK-mediated phosphorylation on Ser-55/65/73 has been reported to inhibit Bim pro-apoptotic activity and promote cell survival (18). Regardless, addition of the JNK inhibitor (cell permeable JNK Inhibitor 1 specific for JNK1, JNK2, JNK3) did not diminish Bim phosphorylation, but rather resulted in an accumulation of the phosphorylated form of Bim with increasing dosage of the JNK inhibitor (Figure 3B). These results show that the MEK/ERK signaling pathway is required for the changes in Bim phosphorylation during mitogenic stimulation of T lymphocytes.
Treatment with Proteasome Inhibitors Cause an Increase in Bim Levels in Mitogenically Stimulated T Cells
Protein phosphorylation was reported to prime Bim for ubiquitination and proteasomal degradation (18). We therefore investigated whether the phosphorylation of Bim seen in mitogenically stimulated T cells primes this BH3-only protein for ubiquitination and proteasomal degradation. Addition of the proteasome inhibitors MG132 (Figure 3A, lane 9) or PS341 (bortezomide) did not block phosphorylation of Bim in mitogen-activated T cells but inhibited the decline in Bim levels, resulting in an accumulation of the phosphorylated form (Figures 3C, D). This protein band was not seen in lysates of similarly treated Bim−/− T cells (not shown), demonstrating that it represents a post-translationally modified form of BimEL. These results demonstrate that ERK1/2-mediated phosphorylation promotes a decline in Bim levels in mitogen activated T lymphocytes that involves proteasomal degradation.
ERK-Mediated Inactivation of Bim Is Required for Survival of Mitogenically Stimulated T Cells
The MEK/ERK pathway is required for both survival and entry into the cell cycle during mitogenic stimulation (28). We examined the role of ERK1/2-mediated inactivation of Bim in the control of survival of mitogen activated T cells by using the MEK1/2-specific inhibitor U0126 at a dose determined to be suitable in exploratory studies (Supplementary Figure 2). Purified T cells from wt or Bim−/− mice were stimulated with mitogens (anti-CD3/anti-CD28 mAbs plus IL-2 or PMA plus ionomycin plus IL-2) with further addition of the MEK1/2 inhibitor U0126 or DMSO (vehicle control) and cell viability was determined after 24 or 48 h by flow cytometric analysis. Figure 4A shows that treatment with the MEK inhibitor significantly increased apoptosis of mitogen stimulated wt T cells (24 h: 20+/−3% (UO126) vs 9+/−6% (DMSO); 48 h: 32+/−6% (UO126) vs 5+/−1% (DMSO)). In contrast, treatment with UO126 did not cause a significant increase in apoptosis of mitogen-stimulated Bim−/− T cells (24 h: 8+/−5% (UO126) vs 6+/−3% (DMSO); 48 h: 11+/−6% (UO126) vs 6+/−4% (DMSO)). Treatment with the MEK inhibitor reduced the fraction of cycling (% cells residing in S phase) cells in mitogen stimulated T lymphocytes from wt (48 h: 11+/−3% (UO126) vs 25+/−2% (DMSO)) or Bim−/− mice (48 h: 8+/−1% (UO126) vs 19+/−5.0% (DMSO)) to a similar extent (Figures 4B, C). Collectively, these results show that ERK1/2-mediated inactivation of Bim is critical for the survival of mitogen stimulated T lymphocytes but does not play a role in controlling cell cycle entry.
Figure 4.
MEK/ERK-mediated inhibition of Bim is critical for the survival but not the cycling of mitogenically stimulated T cells. Graphs show mean % apoptosis of all cells (A) or mean % cells in the S phase as a proportion of total live cells (B) of purified T cells from wt (black bars) or Bim−/− mice (white bars) at 0, 24 or 48 h after stimulation with anti-CD3 plus anti-CD28 mAbs plus IL-2, plus or minus the addition of the MEK1/2 inhibitor UO126 (10 µM). Data represent mean +/−SD of 3 independent mice of each genotype from 3 independent experiments. (C) Representative examples of cell cycle analyses by FACS analysis are shown for purified T cells from wt or Bim−/− T cells 48 h after stimulation with anti-CD3 plus anti-CD28 mAbs plus IL-2, plus or minus the addition of the MEK1/2 inhibitor UO126 (10 µM) or DMSO (diluent control).
Mitogenic Stimuli Trigger ERK1/2-Dependent Bim Phosphorylation in B Cells
Phosphorylation of BimEL has previously been observed in B lymphoma-derived cell lines stimulated with PMA or BCR cross-linking (32, 33). Therefore, we investigated whether the Bim phosphorylation that we observed in mitogen stimulated T cells during the transition from the resting (G0) to the cycling state did also occur when B cells are stimulated through their BCR. A time course analysis revealed a readily detectable increase in the electrophoretic mobility shift on SDS-PAGE in both BimEL and BimL in B cells within 1 to 3 h of stimulation with Fab2 goat anti-mouse IgM antibody fragments plus interleukins (IL-2, IL-4, IL-5) (Figure 5A). Activation of ERK1/2, visualized by staining with phospho-ERK specific antibodies, was evident within 1 h of mitogenic activation (Figure 5A), paralleling the electrophoretic mobility shift in BimEL and BimL. Treatment with the MEK inhibitor UO126 abrogated the BCR crosslinking induced mobility shift in BimEL and BimL (Figure 5A), reminiscent of previous observations in B lymphoma derived cell lines (32, 33). Addition of the proteasome inhibitors MG132 (Figure 5B) or PS341 (bortezomide) did not block phosphorylation of Bim in mitogen-activated B cells but inhibited the decline in Bim levels, resulting in an accumulation of the phosphorylated form (Figure 5B). These results show that in B cells, like in T cells, mitogenic stimulation causes ERK1/2-mediated phosphorylation of BimEL and BimL.
Figure 5.
MEK1/2 is critical for Bim phosphorylation in mitogenically stimulated B cells and proteasomal inhibitors inhibit the reduction in Bim levels. (A) Western blot analysis of lysates from purified (wt) B cells that were left untreated (lane 1) or had been stimulated with Fab2 anti-IgM antibody fragments plus IL-2, IL-4, IL-5 for 1 h (lanes 2–4) or 3 h (lanes 5–7), plus (lanes 3, 6) or minus (lanes 2, 5) the addition of the MEK1/2 inhibitor UO126 (10 µM) or the carrier, DMSO (lanes 4, 7). (B) Western blot analysis of lysates from purified (wt) B cells that were left untreated (lanes 1, 7) or had been stimulated for 3 h with Fab2 anti-IgM antibody fragments plus IL-2, IL-4, IL-5 (lanes 2–6 and 8–12) plus (lanes 3–5 and 9–11) or minus (lanes 2 and 8) the addition of PS341 (lanes 3–5) or MG132 (lanes 9–11) or the carrier, DMSO (lanes, 6 and 12).
Bim Contributes to the MEK Inhibition Induced Death of Mitogenically Stimulated B Cells
Since ERK1/2-mediated phosphorylation of Bim is required for survival of mitogen stimulated T cells, we examined the role of Bim in the survival of activated B cells. Purified B cells from wt or Bim−/− mice were stimulated with Fab2 goat anti-mouse IgM antibody fragments plus interleukins (IL-2, IL-4, IL-5) with addition of the MEK1/2 inhibitor U0126 or DMSO (vehicle control) and measured apoptosis and cell cycle distribution by flow cytometric analysis. Figure 6A shows that MEK1/2 inhibition caused substantial apoptosis in mitogen stimulated wt B cells (48 h: 32+/−2% (UO126) vs 16+/−6% (DMSO)). Mitogen stimulated B cells from Bim−/− mice were less sensitive to MEK1/2 inhibition (48 h: 22+/−3% (UO126) vs 5+/−1% (DMSO); p=0.01 wt vs bim−/− 48 h with MEK inhibition) but protection was not complete and, in fact, less prominent than the protection seen in mitogenically activated T cells. Flow cytometric analysis revealed that MEK1/2 inhibition reduced cycling (% of cells in S phase) of mitogen stimulated wt (48 h: 3+/−1% (UO126) vs 18+/−4% (DMSO)) and Bim−/− (48 h: 2+/−0.5% (UO126) vs 12+/−3% (DMSO)) B cells to a similar extent (Figures 6B, C). Collectively, these results show that Bim contributes to MEK inhibition induced killing of mitogen stimulated B lymphocytes but appears to play no role in cell cycle entry.
Figure 6.
MEK/ERK-mediated inhibition of Bim is critical for the survival but not the cycling of mitogenically stimulated B cells. (A) The graphs show mean % apoptosis of purified B cells as a proportion of all B cells or (B) the mean percentage of B cells residing in the S phase of the cell cycle as a proportion of total live B cells from wt (black bars) or Bim−/− mice (white bars) at 0, 24, 48 h after stimulation with Fab2 anti-IgM antibody fragments plus IL-2, IL-4, IL-5, plus or minus the addition of the MEK1/2 inhibitor UO126 (10 µM). Data represent mean +/−SD of cells from 3 mice of each genotype analyzed in 3 independent experiments. (C) Representative examples of cell cycle analyses by flow cytometry are shown for purified B cells from wt or Bim−/− B cells at 48 h after stimulation with Fab2 anti-IgM antibody fragments plus IL-2, IL-4, IL-5, plus or minus the addition of the MEK1/2 inhibitor UO126 (10 µM) or DMSO (diluent control). * P<0.05, t-test.
Bad and Bid Do not Contribute to MEK/ERK Inhibition Induced Apoptosis of Mitogen Activated B Cells although Bcl-2 Over-Expression Blocks this Death Completely
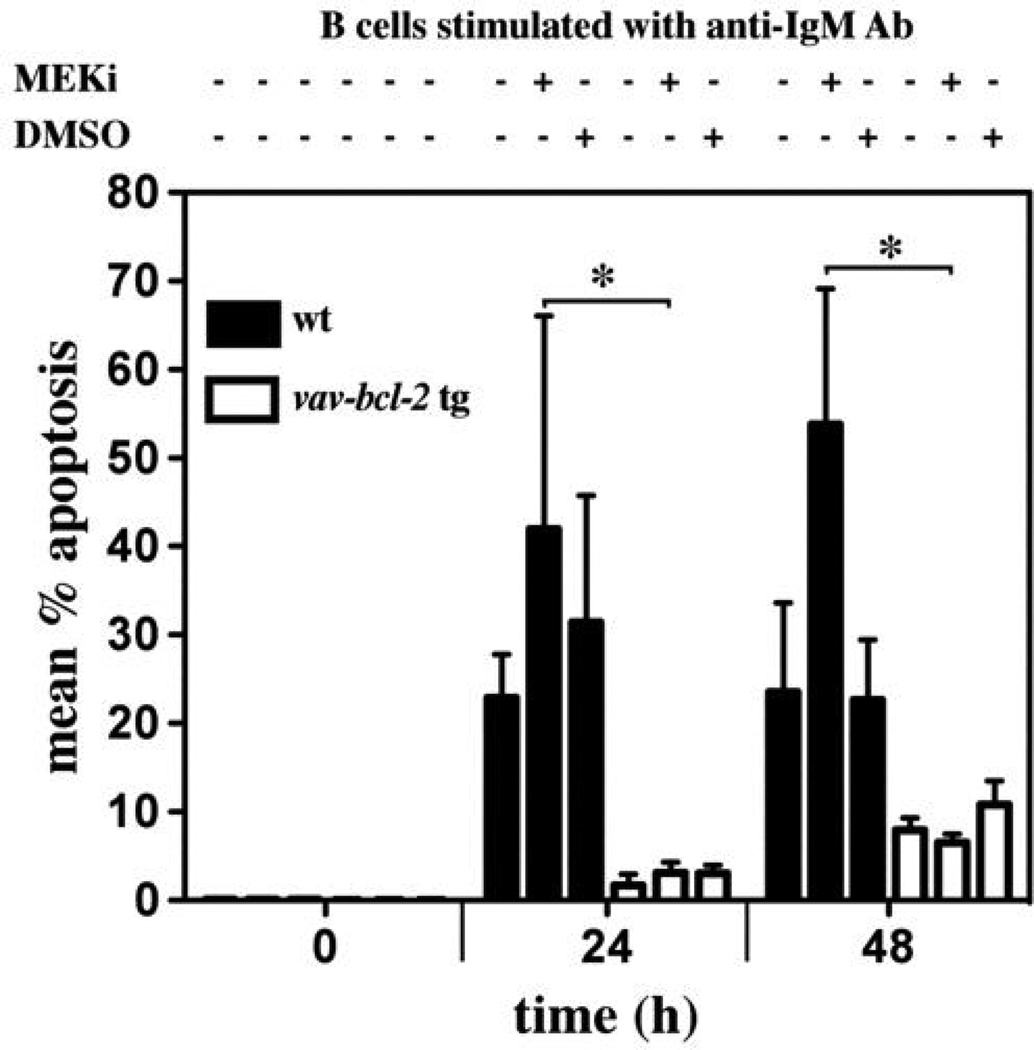
Since loss of Bim afforded mitogen stimulated B cells only with partial protection against MEK/ERK inhibition, whereas Bcl-2 over-expression inhibited this apoptosis completely (24 h: 42+/−24.% (wt) vs 3+/−1% (vav-bcl-2 tg), p<0.05; 48 h: 54+/−15% (wt) vs 6+/−1% (vav-bcl-2 tg), p<0.05) (Figure 7), we reasoned that one or more additional BH3-only proteins may contribute to this B cell death. The MEK/ERK signaling pathway has been reported to also promote cell survival through the activation of RSK-mediated phosphorylation of Bad (34), which inhibits the pro-apoptotic activity of this BH3-only protein by sequestration from Bcl-2 pro-survival family members. We therefore investigated whether mitogen-stimulated B cells lacking both Bim and Bad are more resistant to MEK/ERK inhibition than those lacking only Bim. However, cell survival analysis revealed that loss of Bad did not augment resistance of anti-IgM antibody stimulated Bim-deficient B cells to treatment with UO126 (Figure 8A) and also had no impact on the rate of cycling of these cells (Figure 8B). These results indicate that Bad does not play a major role in MEK/ERK inhibition induced apoptosis of mitogen stimulated B cells. In addition we found that Bim−/−Bid−/− B cells were similarly resistant to MEK inhibition as Bim−/− B cells and that Bid−/− B cells were normally sensitive to this treatment (Figures 8C, D). Collectively, these results indicate that BH3-only proteins other than Bad and Bid are likely to cooperate with Bim in MEK/ERK inhibition induced killing of mitogen stimulated B cells.
Figure 7.
Bcl-2 over-expression completely prevents MEK/ERK inhibition induced apoptosis in mitogen stimulated B cells. The graph shows mean % apoptosis of purified B cells as a proportion of all B cells from wt (black bars) or vav-bcl-2 transgenic mice (white bars) at 0, 24 or 48 h after stimulation with Fab2 anti-IgM antibody fragments plus IL-2, IL-4, IL-5, plus or minus the addition of the MEK1/2 inhibitor UO126 (10 µM). Data represent mean +/−SD of cells from 3 mice of each genotype analyzed in 3 independent experiments. * P<0.05, t-test.
Figure 8.
Bad and Bid are not critical for MEK inhibition induced apoptosis of mitogenically stimulated B cells. Graphs show mean % apoptosis of purified B cells as a proportion of all cells (A and C) or mean % cells in the S phase as a proportion of total live cells (B and D) of purified B lymphocytes from wt (black bars in A, B, C and D), Bim−/−Bad−/− (grey bars in A and B), Bid−/− (grey bars in C and D) or Bim−/−Bid−/− mice (white bars in C and D) at 0, 24, 48 h after stimulation with Fab2 anti-IgM antibody fragments plus IL-2, IL-4, IL-5, plus or minus the addition of the MEK1/2 inhibitor UO126 (10 µM) or DMSO (diluent control). Data represent mean +/−SD of B cells from 3 mice of each genotype analyzed in 3 independent experiments. * P<0.05, t-test.
Discussion
Connections between the pathways that control cell survival and cell cycling are critical to ensure normal development and function of a multi-cellular organism (1). In this study we have investigated the roles of MEK/ERK signaling and the pro-apoptotic BH3-only proteins Bim, Bad and Bid in the regulation of cell survival and proliferation in mitogen stimulated T and B lymphocytes.
Many apoptotic stimuli have been shown to cause an increase in Bim levels in a broad range of cell types, such as NGF withdrawal from sympathetic neurons (35), serum deprivation from fibroblasts (36), cAMP-induced apoptosis in S49 T lymphoma cells (37) or antigen receptor stimulation in T (11, 38) or B lymphoid cells (12, 32, 33). We found that treatment with the calcium ionophore ionomycin, which kills thymocytes via a Bim-dependent process (9), increases BimEL levels by ~3–4 fold. Increased expression of BimEL was also observed in T cell blasts after withdrawal of IL-2 and it has previously been reported that de-phosphorylation of BimEL correlates with apoptosis induction in this setting (39) and that Bim is critical for this pathway to cell death (9).
Since Bim plays a critical role in controlling survival of T (40, 41) and B lymphocytes (42) during shutdown of immune responses, we examined the regulation of Bim expression after mitogenic stimulation. We found that Bim is rapidly phosphorylated in mitogen-stimulated T and B lymphocytes. Experiments with pharmacological inhibitors indicated that this phosphorylation was mediated by a MEK/ERK-dependent process, but did not require JNK, another kinase implicated in the phosphorylation and regulation of Bim (18). Following phosphorylation, Bim levels declined in mitogen stimulated B and T cells and treatment with proteasome inhibitors prevented this decline. This is consistent with previous observations that phosphorylation by ERK targets Bim for ubiquitination and proteasomal degradation (17, 43). ERK-mediated phosphorylation has also been shown to inhibit the pro-apoptotic activity of Bim by reducing its binding to the anti-apoptotic Bcl-2 family members Mcl-1 and Bcl-xL (16). The relative contributions of these two processes to the pro-survival effects of MEK/ERK signalling were examined by the generation of gene-targeted mice that carry mutant alleles expressing phosphorylation-defective Bim proteins. Mutation of phosphorylation site Thr-112 causes decreased binding of Bim to Bcl-2 and thus increased survival, while in contrast mutation of Ser-55/65/73 caused increased apoptosis, due to reduced proteasomal degradation (29). The ERK signaling pathway is critical for ensuring cell survival and proliferation during mitogen-induced T cell proliferation (28). Our experiments using pharmacological inhibitors (UO126) and lymphocytes from wt or Bim−/− mice indicate that phosphorylation of Bim and its subsequent reduction in level are critical to allow the survival of mitogen stimulated T and B cells. Presumably, the stresses associated with transition from the quiescent (G0) state into the cell cycle augment Bim synthesis and this has to be counteracted by TCR or BCR stimulation induced ERK activation. Interestingly, MEK/ERK mediated phosphorylation of BimEL has recently been observed in FGF-stimulated fibroblasts and macrophages undergoing mitosis (44, 45), indicating that Bim phosphorylation may also play a critical role in inhibiting apoptosis at that stage of the cell cycle. Although loss of Bim greatly reduced MEK/ERK inhibition induced apoptosis of mitogen stimulated T and B cells, it had no effect on the reduction in cell cycling. This is consistent with the view that MEK/ERK signalling promotes survival and cell proliferation through distinct effector pathways.
Interestingly, loss of Bim protected mitogen activated T cells better against treatment with the MEK inhibitor than activated B cells. The observation that Bcl-2 over-expression could completely block MEK inhibitor induced killing of mitogen-stimulated B cells indicates that BH3-only proteins in addition to Bim must be inactivated to allow their optimal survival. Our experiments indicate that Bad, which like Bim can also be regulated by phosphorylation (14, 46), and Bid are unlikely to perform this function. With respect to other BH3-only proteins, Puma is an attractive candidate for several reasons: (1) it is expressed in both T and B cells, (2) its loss has been shown to increase resistance of both T and B lymphocytes to a range of apoptotic stimuli (25, 47, 48) and (3) Bim and Puma have been shown to have overlapping function in apoptosis initiation (49).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Drs S Cory, JM Adams, N Danial and A Ranger for gifts of gene targeted mice and reagents; Dr M Cragg for advice; K Vella, G Siciliano, A Naughton, K Pioch, N Iannarella and J Allen for expert animal care; K Scalzo for technical assistance, B Helbert and M Robati for genotyping; Dr F Battye, V Milovac, C Tarlinton, C Young and J Garbe for cell sorting.
Footnotes
The authors have no conflicting financial interests.
Author contributions: Experiments were performed mostly by LAO’R and some by EK. HP, PB, PNK and TK contributed essential reagents. DCSH contributed to anti-Bim antibody production. Data were interpreted mainly by LAO’R and AS with contributions by PB and DCSH. LAO’R and AS conceived the study and wrote the manuscript.
References
- 1.Raff MC. Size control: the regulation of cell numbers in animal development. Cell. 1996;86:173–175. doi: 10.1016/s0092-8674(00)80087-2. [DOI] [PubMed] [Google Scholar]
- 2.Marsden V, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Ann Rev of Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 3.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Ann Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 4.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 5.Huang DCS, Strasser A. BH3-only proteins - essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 6.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DCS. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Reilly LA, Cullen L, Visvader J, Lindeman GJ, Print C, Bath ML, Huang DC, Strasser A. The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am J Pathol. 2000;157:449–461. doi: 10.1016/S0002-9440(10)64557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim 21 required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 10.Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev. Cell. 2001;1:645–653. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 11.Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 12.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreative B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 15.Dijkers PF, Medema RH, Lammers JJ, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Current Biology. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 16.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, Degenhardt K, White E, Cook SJ. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. Embo J. 2007;26:2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, Fukuda A, Hikita A, Seto H, Okada T, Inaba T, Sanjay A, Baron R, Kawaguchi H, Oda H, Nakamura K, Strasser A, Tanaka S. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. Embo J. 2003;22:6653–6664. doi: 10.1093/emboj/cdg635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–1014. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- 19.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci U S A. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK, Johnstone RW, Dixit VM, Strasser A. The BH3-Only Protein Bid Is Dispensable for DNA Damage- and Replicative Stress-Induced Apoptosis or Cell-Cycle Arrest. Cell. 2007;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, Kimura S, Ottmann OG, Druker BJ, Villunger A, Roberts AW, Strasser A. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci U S A. 2006;103:14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. European Journal of Immunology. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 24.Liston A, Lesage S, Gray DHD, O'Reilly LA, Strasser A, Fahrer AM, Boyd RL, Wilson J, Baxter AG, Gallo E, Crabtree GR, Peng K, Wilson SR, Goodnow CC. Generalized resistance to thymic deletion in the NOD mouse: a polygenic trait characterized by defective induction of Bim. Immunity. 2004;21:817–830. doi: 10.1016/j.immuni.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 26.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev. Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 27.DeSilva DR, Jones EA, Feeser WS, Manos EJ, Scherle PA. The p38 mitogen-activated protein kinase pathway in activated and anergic Th1 cells. Cell Immunol. 1997;180:116–123. doi: 10.1006/cimm.1997.1182. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Yan S, Zhou T, Terada Y, Erikson RL. The MAP kinase pathway is required for entry into mitosis and cell survival. Oncogene. 2004;23:763–776. doi: 10.1038/sj.onc.1207188. [DOI] [PubMed] [Google Scholar]
- 29.Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates bim stability and apoptotic activity. Mol Cell. 2008;30:415–425. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann T, Tai L, Gugasyan R, Ekert PG, Huang DCS, Gerondakis S, Pellegrini M, Hakem R, Mak TW, Norris F, Cretney E, Smyth MJ, Dixit V, Strasser A. Submitted. Pro-apoptotic Bid contributes to pathological hepatocyte killing induced by soluble but not membrane-bound TNF and is dispensable for DNA damage-induced apoptosis and cell cycle arrest. Cell [Google Scholar]
- 32.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 33.Craxton A, Draves KE, Gruppi A, Clark EA. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med. 2005;202:1363–1374. doi: 10.1084/jem.20051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 35.Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JM, Strasser A, Johnson EMJ. Induction of Bim, a proapoptotic BH3-only Bcl-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 36.Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated "Bim(EL) kinases" that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J Biol Chem. 2004;279:8837–8847. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Insel PA. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem. 2004;279:20858–20865. doi: 10.1074/jbc.M310643200. [DOI] [PubMed] [Google Scholar]
- 38.Sandalova E, Wei CH, Masucci MG, Levitsky V. Regulation of expression of Bcl-2 protein family member Bim by T cell receptor triggering. Proc Natl Acad Sci U S A. 2004;101:3011–3016. doi: 10.1073/pnas.0400005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seward RJ, von Haller PD, Aebersold R, Huber BT. Phosphorylation of the pro-apoptotic protein Bim in lymphocytes is associated with protection from apoptosis. Molecular Immunology. 2003;39:983–993. doi: 10.1016/s0161-5890(03)00047-6. [DOI] [PubMed] [Google Scholar]
- 40.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 41.Pellegrini M, Belz G, Bouillet P, Strasser A. Shut down of an acute T cell immune response to viral infection is mediated by the pro-apoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci U S A. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer SF, Bouillet P, O'Donnell K, Light A, Tarlinton DM, Strasser A. Pro-apoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody forming cells. Blood. 2007;110:3978–3984. doi: 10.1182/blood-2007-05-091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 44.Graos M, Almeida AD, Chatterjee S. Growth-factor-dependent phosphorylation of Bim in mitosis. Journal of Biochemistry. 2005;388:185–194. doi: 10.1042/BJ20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hacker G, Suttner K, Harada H, Kirschnek S. TLR-dependent Bim phosphorylation in macrophages is mediated by ERK and is connected to proteasomal degradation of the protein. Int Immunol. 2006;18:1749–1757. doi: 10.1093/intimm/dxl109. [DOI] [PubMed] [Google Scholar]
- 46.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. 1996. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not Bcl-xL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 47.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 48.Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L, Adams JM, Strasser A, Villunger A. BH3-only proteins Puma and Bim are rate-limiting for {gamma} -radiation and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 2005;106:4131–4138. doi: 10.1182/blood-2005-04-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erlacher M, Laabi V, Manzl C, Bock G, Tzankov A, Haecker G, Strasser A, Villunger A. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. Journal of Experimental Medicine. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.