Abstract
We previously demonstrated that angiotensin II (Ang II) receptor signaling is involved in azoxymethane-induced mouse colon tumorigenesis. To clarify the role of Ang II in COX-2 expression in the intestinal epithelium, the receptor subtype-specific effect on COX-2 expression in a rat intestinal epithelial cell line (RIE-1) has been investigated. Ang II dose- and time-dependently increased the expression of COX-2, but not COX-1 mRNA and protein. This stimulation was completely blocked by the AT1 receptor antagonist but not the AT2 receptor antagonist. Ang II and lipopolysaccharide (LPS) additively induced COX-2 protein in RIE-1 cells, whereas the LPS-induced COX-2 expression was significantly attenuated by low concentrations of Ang II or the AT2 agonistic peptide CGP-42112A only in AT2 over-expressed cells. These data indicate that Ang II bi-directionally regulates COX-2 expression via both AT1 and AT2 receptors. Control of COX-2 expression through the Ang II signaling may have significance in cytokine-induced COX-2 induction and colon tumorigenesis.
Keywords: AT1 receptor, AT2 receptor, lipopolysaccharide, cyclooxygenase-2, intestinal epithelium
Introduction
Prostaglandins (PGs) play an important role in a broad range of physiological processes. Alterations in its production are implicated in pathophysiological processes, including angiogenesis, cancer, acute and chronic inflammation, etc. (1, 2). Cyclooxygenase (COX) is the rate limiting enzyme catalyzing the conversion of arachidonic acid into prostaglandins and others eicosanoids, critically implicated in a variety of physiological and pathophysiological processes, including inflammation, vascular and renal homeostasis, and immune responses (3). Since the early 1990s, the existence of two isoforms of cyclooxygenases (COX-1 and COX-2) has been appreciated. COX-1 and COX-2 are recognized as constitutive and inducible COX isoenzymes, respectively. Conventional findings show that the therapeutics effects of non-steroidal anti-inflammatory drugs (NSAIDs such as aspirin, ibuprofen) are mostly dependent on COX-2 inhibition; therefore, agents that selectively inhibit COX-2 over COX-1 are desirable for treatment of inflammation (4). Increased expression of COX-2 and prostaglandins has been widely observed in human (5) and rodent intestinal tumors (6, 7)] when compared with normal adjacent mucosa, whereas COX-1 expression is not differentially expressed. Among the consequences of elevated COX-2 expression and increased prostaglandin production are stimulation of angiogenesis, inhibition of apoptosis, and stimulation of cellular proliferation, all of which favor tumorigenesis (8).
Colorectal cancer is a major cause of death in developed countries, even though its prognosis has improved yearly due to advances in diagnostic and surgical techniques. Many reports indicate that the intake of NSAIDs decreases the risk of developing colorectal cancer in man (9) and in model animals (10, 11). Inhibitors of COX-2 activity such as aspirin, NSAIDs, and specific COX-2 antagonists significantly reduce the occurrence of colorectal carcinoma in both animal and human studies (12). Studies on the mechanism by which NSAIDs decrease the risk of colorectal cancer suggests that inhibition of prostaglandin synthesis by cyclooxygenase-2 (COX-2) is the major mechanism in this chemoprevention (13, 14). Therefore, the ideal procedure in the prevention of colorectal cancer is to keep prostaglandin levels low. However, few studies have reported on the endogenous negative regulation of prostaglandin production except with the use of nonspecific drugs such as dexamethasone (15)].
It has been suggested that angiotensin-converting enzyme inhibitors which are commonly employed in the treatment of human clinical hypertension also attenuate tumor growth in experimental animals (16–19)] and may reduce the risk of several human cancers (20). Angiotensin-converting enzyme inhibitors block the formation of angiotensin II (Ang II), an octapeptide which exerts the many diverse effects of the renin-angiotensin system through its receptors (21). This suggests that Ang II may have a modulating role in neoplasia. We recently demonstrated that Ang II type 2 receptor (AT2)-mediated signaling is involved in azoxymethane-induced mouse colon tumorigenesis (22). Ang II, the effector of the renin-angiotensin system, has been shown to stimulate the release of prostaglandins in a variety of cells, including intestinal epithelial cells (23, 24)]. Ang II-dependent increase of prostaglandin production has shown to be mediated through its type 1 receptor (AT1) via the stimulation of COX-2 induction in intestinal cells (25). Although AT1 receptor is believed to mediate most of the cardiovascular actions of Ang II (26), expression of AT2 in the intestinal tissue is also noted (27–29). Since AT2 often counteracts the AT1-mediated Ang II signaling (30, 31), if AT2 signaling attenuates COX-2 induction, this might be an endogenous bidirectional regulation mechanism for COX-2 induction and resultant prostaglandin formation. This type of negative regulation is potentially an important target for the chemoprevention procedure of colorectal cancer.
In this study, we investigated whether Ang II regulates COX-2 expression in a rat intestinal cell line (RIE-1). We further examined the Ang II receptor subtype-specific role in this pathway.
Materials and Methods
Chemicals and reagents
Ang II, (Sar1, Ile8)-Ang II, AT2 receptor antagonist PD123319, AT2 receptor agonist CGP-42112A and lipopolysaccharides (LPS) from Escherichia coli were purchased from Sigma-Aldrich (St. Louis, MO). The AT1 receptor antagonist losartan was obtained from the pharmacy at Vanderbilt University Medical Center. The anti-murine COX-2 polyclonal antibody and anti-mouse COX-1 monoclonal antibody were purchased from Cayman Chemical (Ann Arbor, MI). The actin monoclonal antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell culture
Rat intestinal epithelial (RIE-1) cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100,000 U/L penicillin G, and 100,000 µg/L streptomycin sulfate. For all experiments 90–95% confluent cultures were tendered quiescent by incubation in serum-free DMEM for 72 h before Ang II stimulation.
RNA extraction and RT-PCR
Total cellular RNA was extracted from confluent RIE-1 cells cultured in serum-free medium by TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol and was quantified by measurement of absorbance at 260 nm in a spectrophotometer. Two µg of total RNA was subjected to first-strand cDNA synthesis using the oligo-dT primer and Ominiscript RT kit (Qiagen, Valencia, CA). The primer sets for the AT1 receptor were 5'-GGA AAC AGCTTG GTG GTG-3' (sense) and 5'-BCA CAA TCG CCA TAA TTA TCC-3' (antisense), for the AT2 receptor were 5'-CAC CAG CAG AAA CAC CAC-3' (sense) and 5'CCA AAC AAG GGG AAC TAC3' (antisense), for COX-2 were 5'-TTC ACC AGA CAG ATT GCT GGC-3' (sense) and 5'-AGT CTG GAG TGG GAG CCA CTT G-3' (antisense), and for GAPDH were 5'-CGC CTG GTC ACC AGG GCT GC-3' (sense) and 5'-CTT ACT CCT TGG AGG CCA TGT-3' (antisense). GAPDH was used as a control to demonstrate equal total RNA. The PCR cycle consisted of 94°C for 1 min, 60°C for 1 min (GAPDH 58°C, COX-2 62°C), 72°C for 1 min; these were repeated for 19 cycles for GAPDH, 25 cycles for AT1, 36 cycles for AT2 and for COX-2.
Western blot analysis
Total cellular protein was extracted by lysing the cells in 250 µl of lysis buffer (0.25 M sucrose, 30 mM Tris, 1% Triton X-100, 0.4% SDS, 1 mM EDTA, one tablet Complete (Roche Applied Science, Indianapolis, IN) in 10 ml extraction solution. Protein concentration was determined by the bicinchoninic acid protein assay (Pierce, Rockford, IL). Sixty microgram of protein underwent electrophoresis (SDS-PAGE) and were transferred to a nitrocellulose membrane, Protran (Schleicher & Schuell, Keene, NH). Proteins were detected using anti-COX-2 and -COX-1 antibodies, respectively. An actin monoclonal antibody was used as a control to demonstrate equal loading and transfer. ECL (Amersham Biosciences, Pittsburgh, PA) was used to detect the signals. Intensity of each protein band was quantified using Fluor-S multi-imager (Bio-Rad, Hercules, CA) and Quantity One software (Bio-Rad).
Preparation of adenoviral vectors
Overexpression of AT2 in RIE-1 cells was achieved with an adenovirus encoding mouse AT2 cDNA (Ad-AT2). To generate recombinant replication-deficient adenovirus expressing AT2 receptor, EcoRI-XbaI fragment of mouse AT2 receptor cDNA construct (generous gift of Dr. Inagami, Vanderbilt University, Nashville, TN) was subcloned into a recombinant adenovirus shuttle vector pACCMVpLpA which has deletions in the E1A, E1B and E3 genes. For preparation of virus stocks, 293 cells were infected at multiplicity of infection (MOI) 1–5 and harvested after cytopathic effect became visible (72–96 h). Cells were harvested and lysed in 10% FBS containing DMEM, and virus aliquots were stored at −80°C. Titers were determined by either plaque assay on 293 cells for plaque forming units/ml or spectrometer reading at 260 nm for particles/ml. Recombinant replication-deficient adenovirus encoding β-galactosidase (Ad-LacZ), which contains E. Coli LacZ gene, human cytomegalovirus promoter and SV40 polyadenylation signal (pHCMVsp1LacZ, generous gift of Dr. Myers, Vanderbilt University, Nashville, TN) (32)was used as control vector.
Virus Infection
For viral infections, 2 × 106 cells were plated in 60-mm tissue culture dishes and incubate for 48–72 h (90~95% confluent). Cells were infected by incubating with the adenoviral vectors at MOI of 50 in 200 µl of DMEM for 6 h at 37°C. After the infection, cells were washed and incubated with serum-free DMEM for 92 h, and then stimulated with either Ang II or both receptor antagonist and Ang II.
Radioligand-receptor-binding assay
The radioligand-receptor-binding assay was performed by using transfected cultured cells and [125I](Sar1,Ile8)-Ang II in the presence of PD123319 for AT1 or [125I]CGP-42112A for AT2. The [125I]-labeled peptides were separately prepared from (Sar1,Ile8)-Angi II or CGP-42112A and [125I]Na by the lactoperoxidase method. Infected cells were incubated with 0.5 nM radiolabeled peptide with or without 1 µM unlabeled peptide for 2 h at 24°C in the presence of 0.5 mg/ml bovine serum albumin. Unbound labeled ligand was thoroughly washed out with HBSS. Cells were solubilized with 0.5 N NaOH and the remaining radioactivity was counted. Specific binding was estimated by subtracting the non-specific binding obtained in the presence of 1 µM unlabeled ligand from the total binding. An aliquot of the solubilized cells was subjected to the bicinchoninic acid protein assay. Specific binding was normalized by the protein quantity per dish.
Expression of COX-2 in AT2 transfected RIE-1 cell
In this study cells were transfected with AT2 cDNA using the AT2 adenoviral vector as described elsewhere. Briefly, the RIE-1 cells (3 × 105/well) were plated in a 6-well tissue culture plate and incubated for 48 h (90~95% confluent). Cells were infected with an adenovirus vector encoding mouse AT2 (Ad-AT2) or an adenovirus encoding LacZ (Ad-LacZ) with the adenoviral vectors MOI of 50 in 200 µl of DMEM for 6 h at 37°C. After washing with PBS the cells were incubated with 10% FBS-containing DMEM for 18 h and then serum free DMEM for 48–96 h. Cells were stimulated with various stimuli such as Ang II, CGP-42112A or LPS (5 µg/ml) for 6–8 h.. The AT1-specific antagonist losartan (10−6 M) or AT2-specific antagonist PD123319 (10−6 M) were added 30 min prior to the addition of stimuli. After stimulation cells were harvested and subjected to Western blot analysis for COX-2, GAPDH or actin as described above.
Statistical analysis
Data obtained from the RT-PCR and Western blot analysis were presented as means ±S.E (standard error). Statistical significance between groups were evaluated by one-way ANOVA with the Student-Newman-Keuls test. A value of P < 0.05 was considered significant.
Results
Both Ang II receptor subtypes were detected in RIE-1 cells
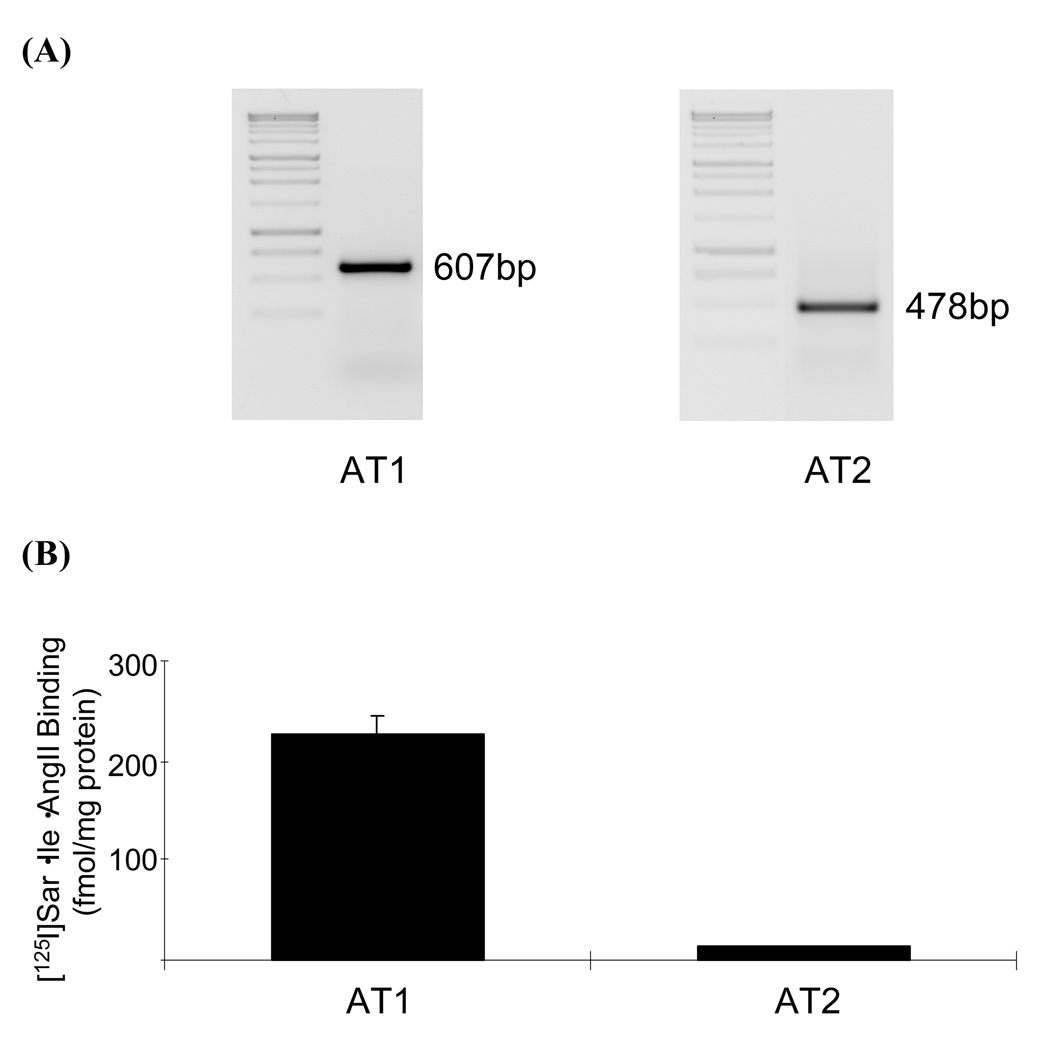
To evaluate the status of Ang II receptor expression in RIE-1-cells, the expression levels of both mRNA and functional proteins of the two receptor subtypes were determined by RT-PCR and radioligand receptor-binding assay, respectively (Fig. 1). Expression level of AT1 mRNA was abundant whereas the level of AT2 mRNA was approximately 10 % of the AT1 expression levels. Expression levels of the functional AT1 and AT2 receptor proteins were revealed to be 226 and 12 fmol/mg protein, respectively. These data indicate that the rat intestinal epithelial cell line RIE-1 cells express a large amount of AT1 but a small amount of AT2. Since the expression level of AT2 in adult tissues is known to be small but it is inducible (33), this cell line appears to be an appropriate cell line to study the effect of Ang II on cyclooxygenase expression.
Fig. 1.
Detection of Ang II type 1 (AT1) and type 2 (AT2) receptors in RIE-1 cell line. A, Total RNA was isolated from confluent RIE-1 cells cultured in serum-free medium as described in the Materials and Methods section. The expression of specific mRNA was detected by RT-PCR. B, Receptor protein expression was determined by ligand receptor–binding assay as described in the Materials and Method section using cultured whole intact cells and [125I](Sar1,Ile8)-Ang II. Data are representative of triplicate determinations.
Ang II stimulated COX-2 but not COX-1 expression through AT1 receptor
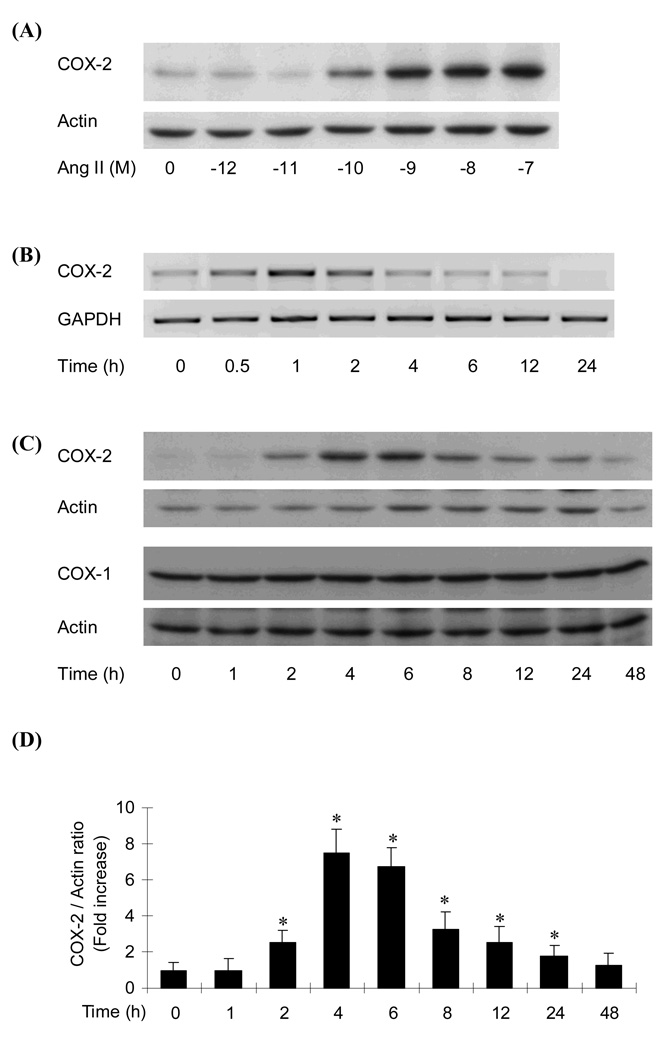
Ang II increased COX-2 protein expression in RIE-1 cells in a concentration dependent manner as evaluated by Western blot analysis (Fig 2 A). Ang II (10−9 M) time-dependently increased COX-2 mRNA and protein level as shown in Fig. 2B and 2C, respectively, but not COX-1 protein level. The COX-2 mRNA response was observed as early as 30 min after Ang II treatment and the maximal increase was observed 1h after treatment. Ang II-induced COX-2 protein expression was first seen 2 h after treatment and the maximal response was observed at 4–6 h after the treatment and lasted until 24 h after treatment (Fig. 2 C and D).
Fig. 2.
Effect of Ang II on COX expression in RIE-1 cells. A. Concentration dependent expression of COX-2 protein in RIE-1 cells by Ang II. B. Time dependent expression of COX-2 mRNA in RIE-1 cells by Ang II (10−9 M). C. Time dependent expression of COX-2 and COX-1 protein by Ang II (10−8 M). The whole homogenates were subjected to SDS-polyacrylamide gel electrophoresis followed by Western blotting with anti-murine COX-1 and COX-2 antibodies. D. Quantification of COX-2 protein in panel C was carried out by scanning densitometry and averages of the expression levels (n=5) normalized by actin levels are displayed in the histogram. *, P≤0.05 as compared to the level of time 0h.
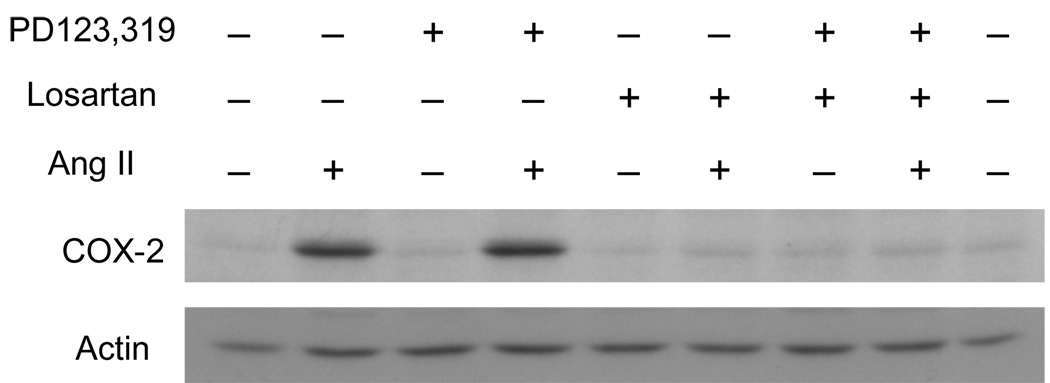
The subtype–specific role of Ang II receptors in COX-2-induction was clarified by utilizing receptor-specific antagonists. Ang II-dependent induction of COX-2 was completely blocked by the AT1-specific antagonist losartan (10−6 M) but not by the AT2-specific antagonist PD123319 (Fig.3). This result clearly indicates that Ang II stimulates COX-2 induction via AT1 because there is no expression of COX-2 in the presence of the AT1-specific antagonist losartan or both AT1 and AT2-specific antagonists (Fig.3).
Fig. 3.
Effect of Ang II and its receptor antagonists on COX-2 protein expression in RIE-1 cells. Cells were pretreated with either AT1-specific antagonist losartan (LOS; 10−6M) or AT2-specific antagonist PD123319 (10−6 M) for 30 min, and then stimulated with Ang II (10−8 M) for 6 h. Western blot analysis of the COX-2 protein was carried out as described in the Materials and Methods section.
Ang II and LPS additively stimulated COX-2 expression
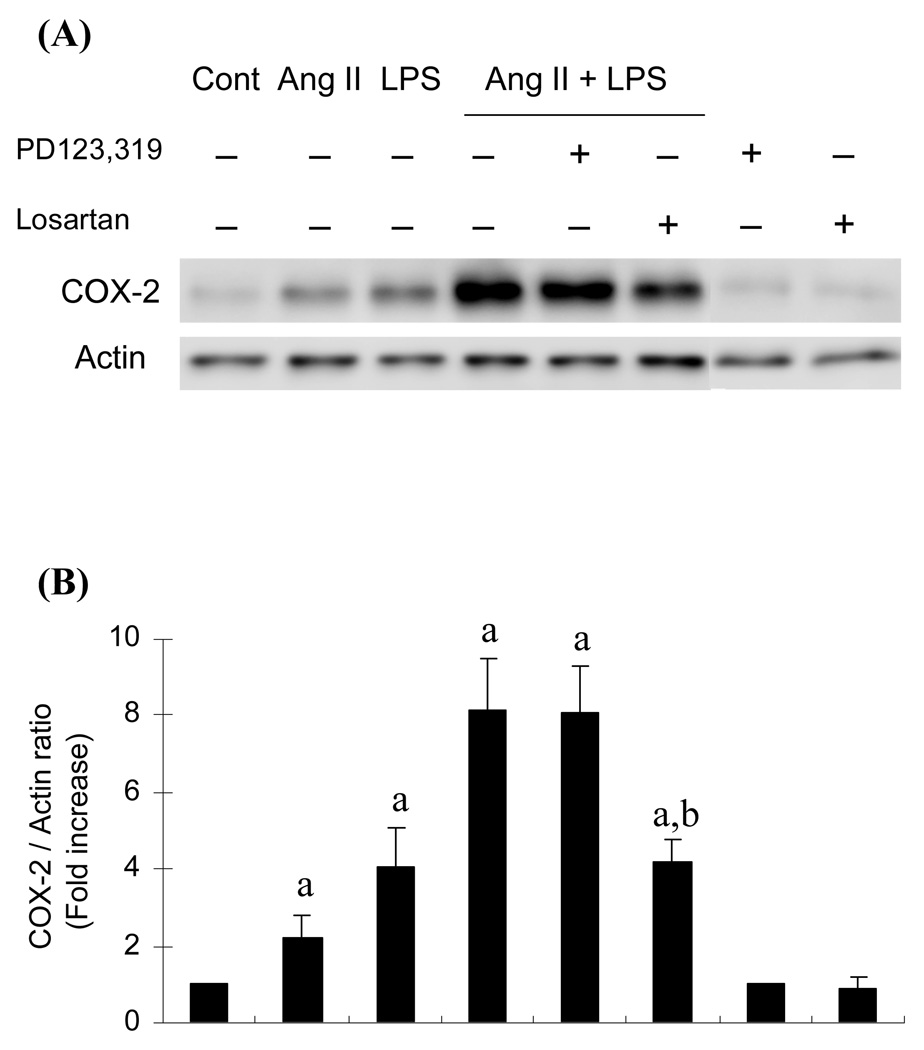
Although we obtained clear-cut results indicating that the Ang II stimulates COX-2 induction through the AT1 in intestinal epithelial cells, the pathophysiological significance of Ang II in COX- 2 induction is still uncertain. In order to address this issue, the effect of Ang II on a typical COX-2 inducer, LPS-stimulated COX-2 induction was studied. Ang II (10−8 M) and LPS (5 µg/ml) additively increased COX-2 expression in RIE-1 cells and this additive effect was completely blocked by the AT1-specific antagonist losartan (Fig.4). These results suggest that Ang II-dependent COX-2 induction in intestinal epithelial cells is likely to have pathophysiological significance.
Fig. 4.
Effect of Ang II and LPS on COX-2 protein expression in RIE-1 cells. Cells were treated with either Ang II (10−8 M), LPS (5 µg/ml) or both for 6 h in the presence or absence of AT1-specific antagonist losartan (10−6M) or AT2-specific antagonist PD123319 (10−6 M). Western blot analysis of the COX-2 protein was carried out as described in the Materials and Methods section. Quantification of COX-2 protein in panel B was carried out by scanning densitometry and averages of the three expression levels normalized by actin levels are displayed in the histogram. a, P≤0.05 as compared to the level in the untreated control. b, P≤0.05 as compared to the level in Ang II and LPS treated cells.
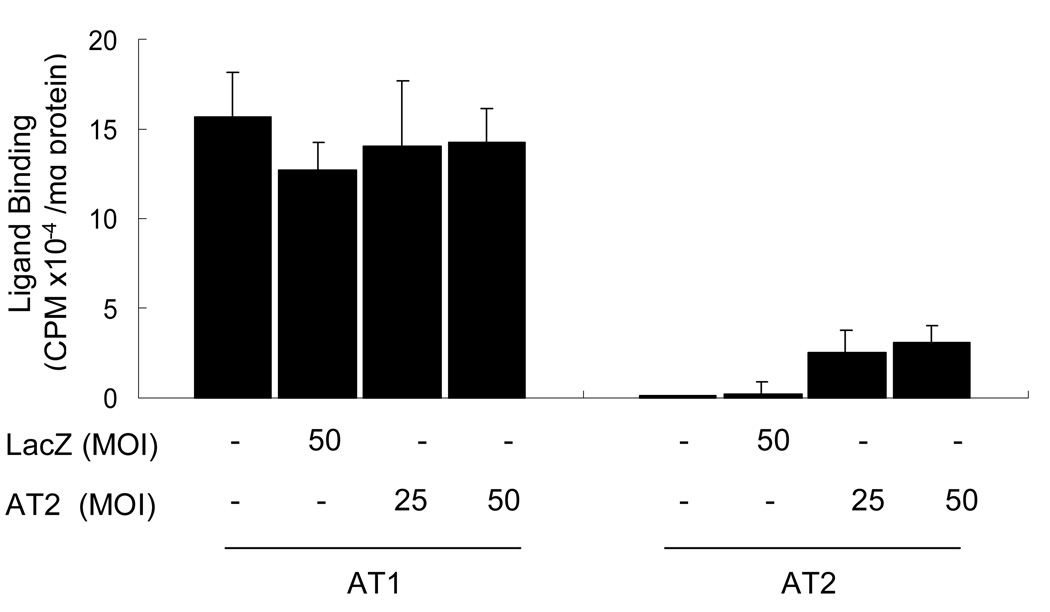
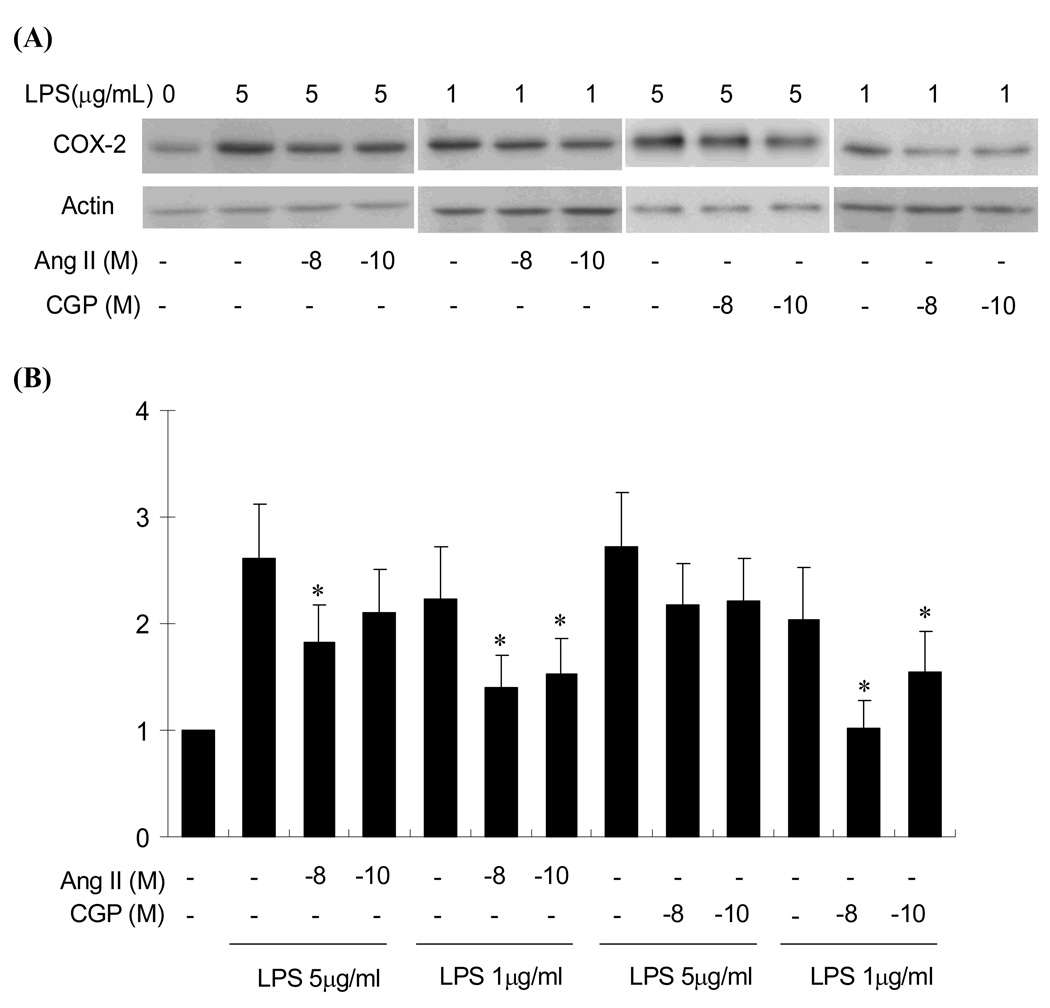
Ang II attenuated LPS-induced COX-2 expression through over-expressed AT2 receptor
Since the AT2 protein expression level in this cultured cell is smaller than those reported (27, 34) the AT2 protein level may not be sufficient to exhibit the AT2-mediated Ang II effect in COX-2 expression. In this study, the AT2 was over-expressed by transfecting cells with AT2 cDNA using the AT2 adenoviral vector. In this study the most efficient AT2 receptor expression was observed at 4 days after transfection, and the expression of AT2 was approximately 22% of the AT1 expression level when 50 MOI vector was transfected (Fig.5). Accordingly, co-stimulation of the cells with LPS and Ang II was performed 4 days after transfection with 50 MOI AT2 vector. Although Ang II (10−8 M) and LPS (5 µg/ml) additively increased COX-2 expression in both untransfected (Fig.4) and LacZ transfected RIE-1 cells (data not shown), low concentrations of Ang II and AT2 agonist CGP-42112A significantly attenuated LPS-induced COX-2 expression when AT2 receptor was transfected (Fig.6). The attenuation effect was more pronounced when lower concentration of LPS was used for the COX-2 induction. In addition, Ang II-dependent attenuation was pronounced in the presence of the AT1 receptor antagonist losartan (1 µg/ml, data not shown). These results suggest that Ang II attenuates LPS-induced COX-2 expression through AT2 mediated signaling. This implies that Ang II bi-directionally regulates COX-2 expression (AT1 stimulates, but AT2 attenuates COX-2 induction).
Fig. 5.
Expression levels of Ang II receptors in the RIE-1 cells transfected with AT2 cDNA were assessed by ligand receptor-binding assay as described in the Materials and Methods section. AT2 protein expression was markedly increased by AT2 cDNA transfection, whereas AT1 expression was not significantly altered as compared to LacZ transfected cells. The averages of three separate experiments are displayed in the histogram.*, P≤0.05 as compared to the level of LacZ transfected cells.
Fig. 6.
Effect of Ang II on LPS-induced COX-2 expression in AT2 over-expressed RIE-1 cells. AT2 transfected RIE-1 cells were pretreated with LPS (1–5 µg/ml) and then stimulated with either Ang II or CGP-42112A (CGP, 10−10 or 10−8 M) for 8 h as indicated in the figure. Top panel, COX-2 protein expression was assessed by Western blot analysis as described in Materials and Methods. Bottom panel, Semi-quantification of COX-2 protein expression was carried out according to the method described in the Materials and Methods using scanning densitometry. The average expression levels normalized by actin level (n=3) are displayed in the histogram. *, P≤0.05 as compared to the level of LPS alone treatments.
Discussion
It has been suggested that Ang II signaling may play important roles in tumorigenesis in several tissues (17, 20, 35–39). However, the specific role of the Ang II receptors in tumorigenesis has not been elucidated. We have previously demonstrated a pro-oncogenic role of the AT2 receptor in chemical carcinogen azoxymethane–induced colon tumorigenesis (37)] and tobacco-specific nitrosamine–induced lung tumorigenesis (39). In the present study, we used cultured normal intestinal epithelial cells to clarify the roles of Ang II and its receptors in cyclooxygenase-induction in intestine. Cyclooxygenases are the rate-limiting enzymes for the prostaglandin formation and have been reported to be associated with human colon cancer development (14, 40, 41) and other chronic bowel diseases (42). Elucidation of the role of Ang II and its receptors in colon tumorigenesis-associated cyclooxygenases are important in understanding the insight of upstream regulation of this enzyme.
First, we examined the Ang II receptor expression status of the rat intestinal epithelial cell line, which was utilized for the present study, using RT-PCR and ligand-receptor-binding assay. The present study demonstrated that the RIE-1 cell line expresses both AT1 and AT2 receptor mRNA’s (Fig 1A). However, the proportion of the AT1 protein was significantly larger than that of the AT2 protein (Fig. 1B). Proportion of the AT2 receptor expression levels seems smaller than the previous measurement of the receptor expression in intestinal mucosa in vivo (27). However this result seems reasonable, since AT2 expression is suppressed during cell culture but is inducible by removing serum from the culture medium (34). Indeed, AT2 protein expression in RIE-1 cells was noticeably increased by serum removal (data not shown). Therefore, the present study with cultured cells of RIE-1 should be a good model system to evaluate the role of Ang II in COX expression in intestinal epithelial cells.
In view of that, we evaluated the effect of Ang II on the expression of the COX-2 expression in the rat intestinal epithelial cell line using RT-PCR and Western blot analysis. The results clearly demonstrated that Ang II, dose- and time-dependently stimulated COX-2 but not COX-1 expression in the RIE-1 cell line (Fig. 2). The present study also indicates that the Ang II AT1-mediated signal plays the primary role in COX-2 induction. These results are in good agreement with a recent report in which Ang II stimulates COX-2 expression in non-transformed rat intestinal cell line (25). Slice et al have shown that Ang II potently stimulated expression of COX-2 mRNA and protein as an immediate-early gene response through the Ang II type 1 receptor.
Although the present study also strongly suggests that AT1 plays a critical role in Ang II-dependent COX-2-induction in intestinal epithelium, involvement of another Ang II receptor subtype in this reaction is unclear since the RIE-1 cell line expresses only a limited amount of AT2 (Fig. 1). Therefore, the effect of Ang II on COX-2 expression was re-evaluated in the RIE-1 cell line expressing higher levels of AT2. As demonstrated in the Fig. 5, the AT2 transfected cells express much higher levels of the AT2 protein in the cells. Although Ang II alone did not alter COX-2 expression in these cells, Ang II significantly attenuated the effect of a most powerful stimulator LPS-induced COX-2 expression (Fig. 6) and this attenuation was also mimicked by AT2 agonist CGP-42112A. These results clearly demonstrated that the presence of the AT2 receptor is potentially important in the regulation of COX-2 expression. This notion is potentially important, since tumor tissue consists of a variety of cell types such as infiltrated lymphocytes, macrophages, fibroblasts and endothelial cells. These cells seem to play an important role in tumor cell growth, invasion, metastasis, angiogenesis and evasion of the host immune system (43). Prostaglandins and various cytokines produced either in tumor cells or stromal cells are involved in many of these biological reactions (14, 43–45).
Although a number of reports have described the role of Ang II in intestinal sodium and water absorption function (46, 47), only a few reports describe subtype-specific Ang II receptor expression in the intestine (27, 29, 47). AT2 receptor has been shown to be involved in absorption/secretion of water and electrolytes (47, 48),and to induce mucosal release of nitric oxide (29). However, this receptor does not influence smooth muscular contractions (49, 50). Since Ang II has similar affinity to both of its receptor subtypes it is suggested in most cases the AT1 receptor counteracts AT2 receptor-mediated effects. Johansson et al. have shown such an antagonistic relationship, in which activation of AT1 receptor contributes to inhibition and AT2 receptor stimulates duodenal mucosal alkaline secretion (51). In terms of the potential mechanism by which AT2 receptor signaling antagonizes AT1 receptor signaling, it has been reported that AT2 receptor signaling stimulates tyrosine phosphatase, thus antagonizes AT1 receptor-mediated actions (52–54). It is also reported that the AT2 receptor directly interacts with the AT1 receptor, thus counteracting its specific function (22, 55). Accordingly, the overall response to Ang II in COX-2 induction is the AT1 receptor-mediated stimulation as shown in figures 2 and 3; this response may be modulated in part by AT2 receptor-mediated signaling via regulation of de-phosphorylation of AT1 receptor-dependent phosphoproteins or direct interaction of both receptor proteins.
On the other hand, it is difficult to speculate a precise mechanism by which AT2 receptor signaling attenuated LPS-induced COX-2 expression. However, since LPS is shown to stimulate multiple kinase activities and regulate multiple gene expression (56–58), AT2 receptor over-expression may have modulated LPS-dependent phosphoproteins via protein tyrosine phosphatase activation and hence attenuated LPS-dependent COX-2 protein expression. In addition, AT2 receptor over-expression has shown to down regulate AT1 receptor expression (22) and this may also be a part of a potential explanation for the AT2 receptor-dependent attenuation of COX-2 protein expression. However, clarification of this mechanism needs to be evaluated by another study.
In summary, the present study demonstrated a bidirectional regulatory function of the Ang II receptors in cyclooxygenase-2 expression in non-transformed rat intestinal epithelial cell line. Since pharmacological control of the Ang II receptor function in vivo is a viable therapeutic technique (59), it is feasible to determine whether AT1 receptor blockers attenuate colorectal tumorigenesis. Increasing AT2 expression in intestinal epithelium, which attenuates COX-2 expression, may also be a potential route for a chemoprevention of colorectal cancer. This may lead to the development of a potential chemoprevention procedure for human colorectal cancer. However, the importance of the Ang II receptor function in human colorectal cancer awaits further study. To the best of our knowledge, the present study is the first to demonstrate that the Ang II AT2 possesses a modulator function in COX-2 expression in intestinal cells.
Acknowledgements
The authors thank Dr. Raymond DuBois (Department of Medicine, Vanderbilt University) for sharing rat intestinal epithelial cell line. We are grateful to Ms. Lara. Pickel (Department of Engineering/Anatomy & Physiology, Kansas State University) for critical reading and constructive comments during the preparation of the manuscript. This work was supported in part by NIH grants RO3 CA091428, P50 CA90949, P30 CA68485, Kansas State University (KSU) Provost’s Fund and KSU College of Veterinary Medicine Dean’s Fund.
References
- 1.De Witt DL. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991;1083:121–134. doi: 10.1016/0005-2760(91)90032-d. [DOI] [PubMed] [Google Scholar]
- 2.Masferrer KML Jaime L, Koki Alane T, Zweifel Ben S, Settle Steven L, Woerner B Mark, Edwards Dorothy A, Flickinger Amy G, Moore Rosalyn J, Seibert Karen. Antiangiogenic and Antitumor Activities of Cyclooxygenase-2 Inhibitors. Cancer Research. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 3.Cuccurullo CFM, Mezzetti A, Cipollone F. COX-2 expression in atherosclerosis: the good, the bad or the ugly? Curr Med Chem. 2007;14:1595–1605. doi: 10.2174/092986707780830998. [DOI] [PubMed] [Google Scholar]
- 4.Iezzi AFC, Mezzetti A, Cipollone F. COX-2: friend or foe? Curr Pharm Des. 2007;13:1715–1721. doi: 10.2174/138161207780831293. [DOI] [PubMed] [Google Scholar]
- 5.Eberhart CECR, Radhik A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 6.Williams CSLC, Radhika A, Zhang T, Lamps LW, Nanney LB, Beauchamp RD, DuBois RN. Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenterology. 1996;111:1134–1140. doi: 10.1016/s0016-5085(96)70083-5. [DOI] [PubMed] [Google Scholar]
- 7.Shao JSH, Aramandla R, Pereira MA, Lubet RA, Hawk E, Grogan L, Kirsch IR, Washington MK, Beauchamp RD, et al. Coordinate regulation of cyclooxygenase-2 and TGF-beta1 in replication error-positive colon cancer and azoxymethane-induced rat colonic tumors. Carcinogenesis. 1999;20:185–191. doi: 10.1093/carcin/20.2.185. [DOI] [PubMed] [Google Scholar]
- 8.Jianguo Du BJ, John Barnard. Differential Regulation of Cyclooxygenase-2 in Nontransformed and Ras-Transformed Intestinal Epithelial Cells. Neoplasia. 2005;7:761–770. doi: 10.1593/neo.04652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 10.Kudo T, Narisawa T, Abo S. Antitumor activity of indomethacin on methylazoxymethanol-induced large bowel tumors in rats. Gann. 1980;71:260–264. [PubMed] [Google Scholar]
- 11.Jacoby RF, Marshall DJ, Newton MA, Novakovic K, Tutsch K, Cole CE, Lubet RA, Kelloff GJ, Verma A, Moser AR, Dove WF. Chemoprevention of spontaneous intestinal adenomas in the Apc Min mouse model by the nonsteroidal anti-inflammatory drug piroxicam. Cancer Res. 1996;56:710–714. [PubMed] [Google Scholar]
- 12.DuBois RN. Cyclooxygenase-2 and colorectal cancer. Prog Exp Tumor Res. 2003;37:124–137. doi: 10.1159/000071370. [DOI] [PubMed] [Google Scholar]
- 13.DuBois RN, Shao J, Tsujii M, Sheng H, Beauchamp RD. G1 delay in cells overexpressing prostaglandin endoperoxide synthase-2. Cancer Res. 1996;56:733–737. [PubMed] [Google Scholar]
- 14.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanayama T, Hara S, Inoue H, Yokoyama C, Tanabe T. Regulation of two isozymes of prostaglandin endoperoxide synthase and thromboxane synthase in human monoblastoid cell line U937. Prostaglandins. 1995;49:371–382. doi: 10.1016/0090-6980(95)00068-l. [DOI] [PubMed] [Google Scholar]
- 16.Volpert OV, Ward WF, Lingen MW, Chesler L, Solt DB, Johnson MD, Molteni A, Polverini PJ, Bouck NP. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J Clin Invest. 1996;98:671–679. doi: 10.1172/JCI118838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hii SI, Nicol DL, Gotley DC, Thompson LC, Green MK, Jonsson JR. Captopril inhibits tumour growth in a xenograft model of human renal cell carcinoma. Br J Cancer. 1998;77:880–883. doi: 10.1038/bjc.1998.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maluccio M, Sharma V, Lagman M, Konijn G, Suthanthiran M. Angiotensin II receptor blockade: a novel strategy to prevent immunosuppressant-associated cancer progression. Transplant Proc. 2001;33:1820–1821. doi: 10.1016/s0041-1345(00)02696-8. [DOI] [PubMed] [Google Scholar]
- 19.Fujita M, Hayashi I, Yamashina S, Fukamizu A, Itoman M, Majima M. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis. 2005;26:271–279. doi: 10.1093/carcin/bgh324. [DOI] [PubMed] [Google Scholar]
- 20.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL, Robertson JW. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 21.Burrell LM, Johnston CI. Angiotensin converting enzyme inhibitors in perspective: an update. Aust Fam Physician. 1993;22:1243–1247. 1251, 1255. [PubMed] [Google Scholar]
- 22.Jin XQ, Fukuda N, Su JZ, Lai YM, Suzuki R, Tahira Y, Takagi H, Ikeda Y, Kanmatsuse K, Miyazaki H. Angiotensin II type 2 receptor gene transfer downregulates angiotensin II type 1a receptor in vascular smooth muscle cells. Hypertension. 2002;39:1021–1027. doi: 10.1161/01.hyp.0000016179.52601.b4. [DOI] [PubMed] [Google Scholar]
- 23.Segawa M, Nakao S, Ogata Y, Sugiya H, Furuyama S. Angiotensin II induces prostaglandin E(2) release in human gingival fibroblasts. Life Sci. 2003;72:795–803. doi: 10.1016/s0024-3205(02)02340-8. [DOI] [PubMed] [Google Scholar]
- 24.Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int. 2005;68:2143–2153. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 25.Slice LW, Chiu T, Rozengurt E. Angiotensin II and epidermal growth factor induce cyclooxygenase-2 expression in intestinal epithelial cells through small GTPases using distinct signaling pathways. J Biol Chem. 2005;280:1582–1593. doi: 10.1074/jbc.M408172200. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb SSDK, Fleck E, Kostis J, Levine B, LeJemtel T, DeKock M. Hemodynamic and neurohumoral effects of the angiotensin II antagonist losartan in patients with congestive heart failure. Circulation. 1993;88:1602–1609. doi: 10.1161/01.cir.88.4.1602. [DOI] [PubMed] [Google Scholar]
- 27.Sechi LA, Valentin JP, Griffin CA, Schambelan M. Autoradiographic characterization of angiotensin II receptor subtypes in rat intestine. Am J Physiol. 1993;265:G21–G27. doi: 10.1152/ajpgi.1993.265.1.G21. [DOI] [PubMed] [Google Scholar]
- 28.Hirasawa K, Sato Y, Hosoda Y, Yamamoto T, Hanai H. Immunohistochemical localization of angiotensin II receptor and local renin-angiotensin system in human colonic mucosa. J Histochem Cytochem. 2002;50:275–282. doi: 10.1177/002215540205000215. [DOI] [PubMed] [Google Scholar]
- 29.Ewert S, Laesser M, Johansson B, Holm M, Aneman A, Fandriks L. The angiotensin II receptor type 2 agonist CGP 42112A stimulates NO production in the porcine jejunal mucosa. BMC Pharmacol. 2003;3:2–9. doi: 10.1186/1471-2210-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munzenmaier DH, Greene AS. Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension. 1996;27:760–765. doi: 10.1161/01.hyp.27.3.760. [DOI] [PubMed] [Google Scholar]
- 31.Horiuchi M, Hayashida W, Akishita M, Tamura K, Daviet L, Lehtonen JY, Dzau VJ. Stimulation of different subtypes of angiotensin II receptors, AT1 and AT2 receptors, regulates STAT activation by negative crosstalk. Circ Res. 1999;84:876–882. doi: 10.1161/01.res.84.8.876. [DOI] [PubMed] [Google Scholar]
- 32.French BA, Mazur W, Geske RS, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 33.Kambayashi Y, Bardhan S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, Inagami T. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem. 1993;268:24543–24546. [PubMed] [Google Scholar]
- 34.Ichiki T, Kambayashi Y, Inagami T. Differential inducibility of angiotensin II AT2 receptor between SHR and WKY vascular smooth muscle cells. Kidney Int Suppl. 1996;55:S14–S17. [PubMed] [Google Scholar]
- 35.Reddy MK, Baskaran K, Molteni A. Inhibitors of angiotensin-converting enzyme modulate mitosis and gene expression in pancreatic cancer cells. Proc Soc Exp Biol Med. 1995;210:221–226. doi: 10.3181/00379727-210-43942. [DOI] [PubMed] [Google Scholar]
- 36.Yoshiji H, Kuriyama S, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factor. Clin Cancer Res. 2001;7:1073–1078. [PubMed] [Google Scholar]
- 37.Takagi T, Nakano Y, Takekoshi S, Inagami T, Tamura M. Hemizygous mice for the angiotensin II type 2 receptor gene have attenuated susceptibility to azoxymethane-induced colon tumorigenesis. Carcinogenesis. 2002;23:1235–1241. doi: 10.1093/carcin/23.7.1235. [DOI] [PubMed] [Google Scholar]
- 38.Egami K, Murohara T, Shimada T, Sasaki K, Shintani S, Sugaya T, Ishii M, Akagi T, Ikeda H, Matsuishi T, Imaizumi T. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest. 2003;112:67–75. doi: 10.1172/JCI16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tani T, Takagi T, Nakano Y, Howard EF, Tamura M. Angiotensin II type 2 receptor gene deficiency attenuates susceptibility to tobacco-specific nitrosamine-induced lung tumorigenesis: involvement of transforming growth factor-beta-dependent cell growth attenuation. Cancer Res. 2005;65:7660–7665. doi: 10.1158/0008-5472.CAN-05-0275. [DOI] [PubMed] [Google Scholar]
- 40.Mann JR, DuBois RN. Cyclooxygenase-2 and gastrointestinal cancer. Cancer J. 2004;10:145–152. doi: 10.1097/00130404-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Kohno H, Suzuki R, Sugie S, Tanaka T. Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer. 2005;5:46–58. doi: 10.1186/1471-2407-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendel J, Nielsen OH. Expression of cyclooxygenase-2 mRNA in active inflammatory bowel disease. Am J Gastroenterol. 1997;92:1170–1173. [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 44.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 45.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 46.Levens NR. Control of intestinal absorption by the renin-angiotensin system. Am J Physiol. 1985;249:G3–G15. doi: 10.1152/ajpgi.1985.249.1.G3. [DOI] [PubMed] [Google Scholar]
- 47.Jin XH, Wang ZQ, Siragy HM, Guerrant RL, Carey RM. Regulation of jejunal sodium and water absorption by angiotensin subtype receptors. Am J Physiol. 1998;275:R515–R523. doi: 10.1152/ajpregu.1998.275.2.R515. [DOI] [PubMed] [Google Scholar]
- 48.Ewert S, Sjoberg T, Johansson B, Duvetorp A, Holm M, Fandriks L. Dynamic expression of the angiotensin II type 2 receptor and duodenal mucosal alkaline secretion in the Sprague-Dawley rat. Exp Physiol. 2006;91:191–199. doi: 10.1113/expphysiol.2005.031401. [DOI] [PubMed] [Google Scholar]
- 49.Schinke M, Doods HN, Ganten D, Wienen W, Entzeroth M. Characterization of rat intestinal angiotensin II receptors. Eur J Pharmacol. 1991;204:165–170. doi: 10.1016/0014-2999(91)90701-q. [DOI] [PubMed] [Google Scholar]
- 50.Ewert S, Spak E, Olbers T, Johnsson E, Edebo A, Fandriks L. Angiotensin II induced contraction of rat and human small intestinal wall musculature in vitro. Acta Physiol (Oxf) 2006;188:33–40. doi: 10.1111/j.1748-1716.2006.01600.x. [DOI] [PubMed] [Google Scholar]
- 51.Johansson BHM, Ewert S, Casselbrant A, Pettersson A, Fandriks L. Angiotensin II type 2 receptor-mediated duodenal mucosal alkaline secretion in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1254–G1260. doi: 10.1152/ajpgi.2001.280.6.G1254. [DOI] [PubMed] [Google Scholar]
- 52.Tsuzuki S, Matoba T, Eguchi S, Inagami T. Angiotensin II type 2 receptor inhibits cell proliferation and activates tyrosine phosphatase. Hypertension. 1996;28:916–918. doi: 10.1161/01.hyp.28.5.916. [DOI] [PubMed] [Google Scholar]
- 53.Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- 54.Feng YH, Sun Y, Douglas JG. Gbeta gamma -independent constitutive association of Galpha s with SHP-1 and angiotensin II receptor AT2 is essential in AT2-mediated ITIM-independent activation of SHP-1. Proc Natl Acad Sci U S A. 2002;99:12049–12054. doi: 10.1073/pnas.192404199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar V, Knowle D, Gavini N, Pulakat L. Identification of the region of AT2 receptor needed for inhibition of the AT1 receptor-mediated inositol 1,4,5-triphosphate generation. FEBS Lett. 2002;532:379–386. doi: 10.1016/s0014-5793(02)03713-4. [DOI] [PubMed] [Google Scholar]
- 56.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki T, Hashimoto S, Toyoda N, Nagai S, Yamazaki N, Dong HY, Sakai J, Yamashita T, Nukiwa T, Matsushima K. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96:2584–2591. [PubMed] [Google Scholar]
- 58.Hirasawa N, Torigoe M, Ohgawara R, Murakami A, Ohuchi K. Involvement of MAP kinases in lipopolysaccharide-induced histamine production in RAW 264 cells. Life Sci. 2006;80:36–42. doi: 10.1016/j.lfs.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Levy BI, Benessiano J, Henrion D, Caputo L, Heymes C, Duriez M, Poitevin P, Samuel JL. Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J Clin Invest. 1996;98:418–425. doi: 10.1172/JCI118807. [DOI] [PMC free article] [PubMed] [Google Scholar]