Abstract
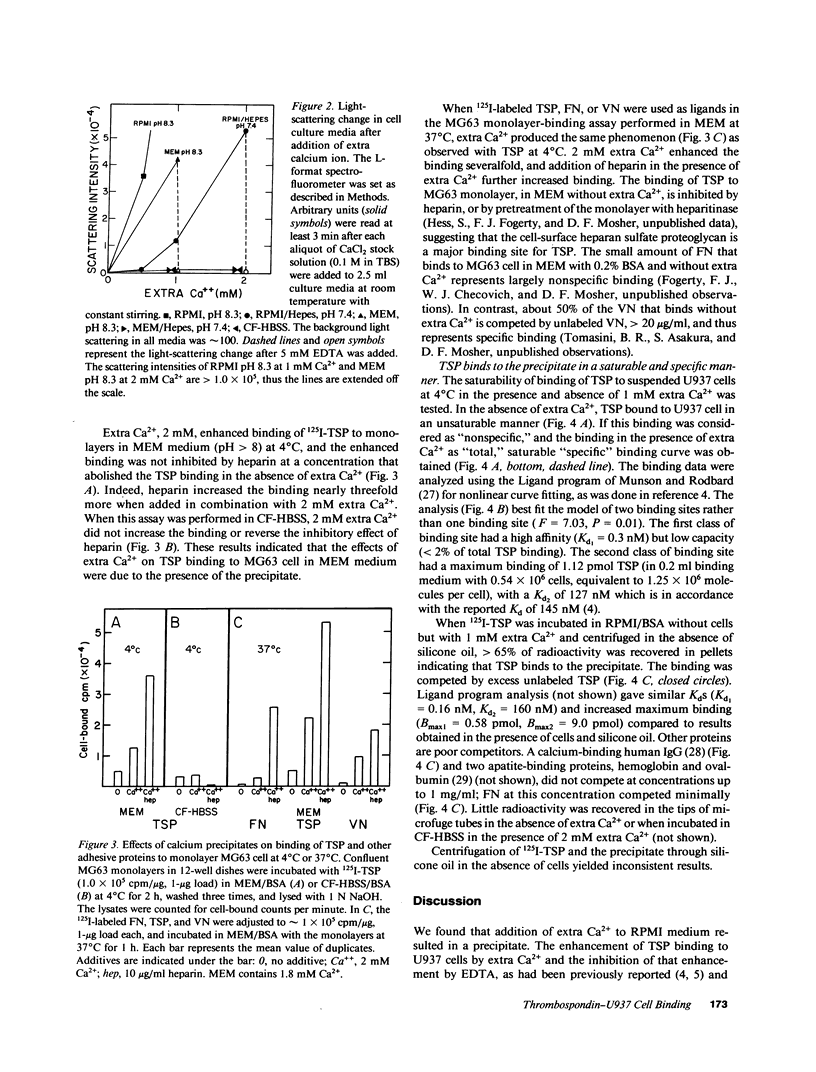
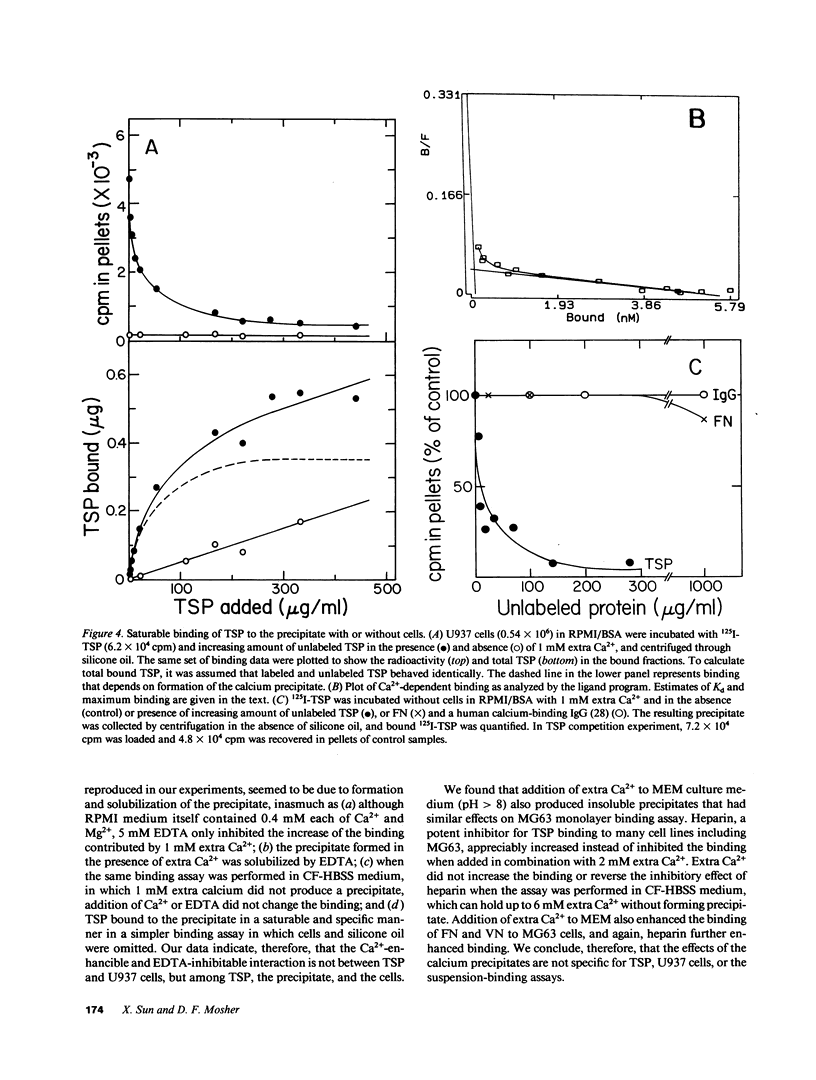
Thrombospondin (TSP) binds to U937 monocytic cells in a Ca2(+)-enhancible and EDTA-inhibitable manner (Silverstein, R. L., and R. L. Nachman. 1987. J. Clin. Invest. 79:867-874; Silverstein, R. L., A. S. Asch, and R. L. Nachman. 1989. J. Clin. Invest. 84:546-552). We reproduced the results when RPMI cell culture medium, but not when HBSS was used as binding medium. Addition of 1 mM Ca2+ to RPMI medium increased the binding of TSP to suspended U937 cells more than eightfold; the increase was blocked by EDTA but not by heparin. Further studies showed that addition of 1 mM Ca2+ to RPMI medium resulted in an insoluble precipitate, which did not form when EDTA was present or when 1 mM extra Ca2+ was added to HBSS. TSP bound to the precipitate in a saturable and specific manner. The precipitate enhanced binding of TSP to MG63 osteosarcoma cells in a monolayer binding assay. Enhancement of binding in the monolayer assay was observed for fibronectin and vitronectin as well. Our data indicate that there is not a specific Ca2(+)-dependent TSP receptor on U937 cell surface. Instead, the extra binding enhanced by Ca2+ is due to the formation of insoluble salts in the medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. J., Detwiler T. C. Quantitative adsorption of platelet glycoprotein G (thrombin-sensitive protein, thrombospondin) to barium citrate. Biochem J. 1984 Jan 1;217(1):67–71. doi: 10.1042/bj2170067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell J. W., Ockenhouse C. F., Knowles D. M., 2nd Monoclonal antibody OKM5 inhibits the in vitro binding of Plasmodium falciparum-infected erythrocytes to monocytes, endothelial, and C32 melanoma cells. J Immunol. 1985 Nov;135(5):3494–3497. [PubMed] [Google Scholar]
- Dahlbäck B., Podack E. R. Characterization of human S protein, an inhibitor of the membrane attack complex of complement. Demonstration of a free reactive thiol group. Biochemistry. 1985 Apr 23;24(9):2368–2374. doi: 10.1021/bi00330a036. [DOI] [PubMed] [Google Scholar]
- Gailit J., Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988 Sep 15;263(26):12927–12932. [PubMed] [Google Scholar]
- Gorbunoff M. J., Timasheff S. N. The interaction of proteins with hydroxyapatite. III. Mechanism. Anal Biochem. 1984 Feb;136(2):440–445. doi: 10.1016/0003-2697(84)90241-0. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Akama T., Kono I., Kashiwagi H. Activation of vitronectin (serum spreading factor) binding of heparin by denaturing agents. J Biochem. 1985 Oct;98(4):1135–1138. doi: 10.1093/oxfordjournals.jbchem.a135363. [DOI] [PubMed] [Google Scholar]
- Jaffe J. P., Mosher D. F. Calcium binding by a myeloma protein. Am J Med. 1979 Aug;67(2):343–346. doi: 10.1016/0002-9343(79)90412-1. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C., D'Agati V., Grimes M., Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984 May;132(5):2170–2173. [PubMed] [Google Scholar]
- Kolset S. O., Ivhed I., Overvatn A., Nilsson K. Differentiation-associated changes in the expression of chondroitin sulfate proteoglycan in induced U-937 cells. Cancer Res. 1988 Nov 1;48(21):6103–6108. [PubMed] [Google Scholar]
- Kolset S. O. Proteoglycans in normal and neoplastic monocytes. Exp Cell Res. 1987 Feb;168(2):318–324. doi: 10.1016/0014-4827(87)90004-8. [DOI] [PubMed] [Google Scholar]
- Larsen E., Celi A., Gilbert G. E., Furie B. C., Erban J. K., Bonfanti R., Wagner D. D., Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989 Oct 20;59(2):305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Simons E. R. Cooperative binding of calcium to thrombospondin. The effect of calcium on the circular dichroism and limited tryptic digestion of thrombospondin. J Biol Chem. 1983 Oct 25;258(20):12098–12101. [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol. 1985 Sep;101(3):1059–1070. doi: 10.1083/jcb.101.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor J. L., Catimel B., Parmentier S., Clezardin P., Dechavanne M., Leung L. L. Rapid purification and partial characterization of human platelet glycoprotein IIIb. Interaction with thrombospondin and its role in platelet aggregation. J Biol Chem. 1989 Jan 5;264(1):501–506. [PubMed] [Google Scholar]
- McKeown-Longo P. J., Hanning R., Mosher D. F. Binding and degradation of platelet thrombospondin by cultured fibroblasts. J Cell Biol. 1984 Jan;98(1):22–28. doi: 10.1083/jcb.98.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Mosher D. F. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983 Aug;97(2):466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Johnson R. B. In vitro formation of disulfide-bonded fibronectin multimers. J Biol Chem. 1983 May 25;258(10):6595–6601. [PubMed] [Google Scholar]
- Mosher D. F. Physiology of thrombospondin. Annu Rev Med. 1990;41:85–97. doi: 10.1146/annurev.me.41.020190.000505. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Mosher D. F. Interactions of thrombospondin with endothelial cells: receptor-mediated binding and degradation. J Cell Biol. 1987 Oct;105(4):1603–1611. doi: 10.1083/jcb.105.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Mosher D. F. Localization of thrombospondin in clots formed in situ. Blood. 1985 Nov;66(5):1098–1104. [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Westrick L. G., Esko J. D., Mosher D. F. Altered metabolism of thrombospondin by Chinese hamster ovary cells defective in glycosaminoglycan synthesis. J Biol Chem. 1988 May 5;263(13):6400–6406. [PubMed] [Google Scholar]
- Oquendo P., Hundt E., Lawler J., Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989 Jul 14;58(1):95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Ostreiko K. K., Tumanova I. A., Sykulev YuK Production and characterization of heat-aggregated IgG complexes with pre-determined molecular masses: light-scattering study. Immunol Lett. 1987 Aug;15(4):311–316. doi: 10.1016/0165-2478(87)90134-9. [DOI] [PubMed] [Google Scholar]
- Persson E., Selander M., Linse S., Drakenberg T., Ohlin A. K., Stenflo J. Calcium binding to the isolated beta-hydroxyaspartic acid-containing epidermal growth factor-like domain of bovine factor X. J Biol Chem. 1989 Oct 5;264(28):16897–16904. [PubMed] [Google Scholar]
- Przysiecki C. T., Staggers J. E., Ramjit H. G., Musson D. G., Stern A. M., Bennett C. D., Friedman P. A. Occurrence of beta-hydroxylated asparagine residues in non-vitamin K-dependent proteins containing epidermal growth factor-like domains. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7856–7860. doi: 10.1073/pnas.84.22.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Argraves S., Suzuki S., Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- Roberts D. D. Interactions of thrombospondin with sulfated glycolipids and proteoglycans of human melanoma cells. Cancer Res. 1988 Dec 1;48(23):6785–6793. [PubMed] [Google Scholar]
- Robey P. G., Young M. F., Fisher L. W., McClain T. D. Thrombospondin is an osteoblast-derived component of mineralized extracellular matrix. J Cell Biol. 1989 Feb;108(2):719–727. doi: 10.1083/jcb.108.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A., Frazier W. A. Isolation and characterization of thrombospondin. Methods Enzymol. 1987;144:438–446. doi: 10.1016/0076-6879(87)44193-1. [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Asch A. S., Nachman R. L. Glycoprotein IV mediates thrombospondin-dependent platelet-monocyte and platelet-U937 cell adhesion. J Clin Invest. 1989 Aug;84(2):546–552. doi: 10.1172/JCI114197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. L., Nachman R. L. Thrombospondin binds to monocytes-macrophages and mediates platelet-monocyte adhesion. J Clin Invest. 1987 Mar;79(3):867–874. doi: 10.1172/JCI112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenflo J., Ganrot P. O. Vitamin K and the biosynthesis of prothrombin. I. Identification and purification of a dicoumarol-induced abnormal prothrombin from bovine plasma. J Biol Chem. 1972 Dec 25;247(24):8160–8166. [PubMed] [Google Scholar]
- Sun X., Mosher D. F., Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J Biol Chem. 1989 Feb 15;264(5):2885–2889. [PubMed] [Google Scholar]
- Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983 May;78(1):83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Raugi G. J., Mumby S. M., Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. J Histochem Cytochem. 1985 Apr;33(4):295–302. doi: 10.1177/33.4.3884704. [DOI] [PubMed] [Google Scholar]



