Abstract
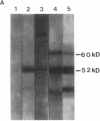
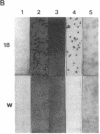
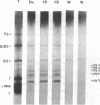
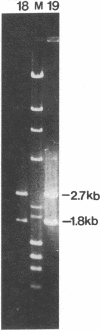
Two cDNA clones encoding the 52-kD form of a protein present in human Ro/SSA ribonucleoprotein complexes were cloned from a lambda gt11 human thymocyte cDNA library. These clones reacted with lupus patient sera which had anti-52-kD Ro/SSA antibodies, and with affinity-purified anti-52-kD Ro/SSA antibodies. Moreover, affinity-purified antibodies isolated from isopropyl-beta-D-thiogalactopyranoside-induced proteins of these clones reacted only with the 52-kD protein of lymphocytes in Western blots and precipitated Ro/SSA hY RNAs, confirming that the clones encode a 52-kD Ro/SSA antigen. The cDNA contains a single open reading frame of 1,425 nucleotides and encodes a predicted 475-amino acid polypeptide with a molecular mass of 54,108 D. This protein appears unique in that both a zinc finger and leucine zipper motif are present on this protein. Surprisingly, no homology was found between the 52-kD Ro/SSA gene or protein and three published 60-kD Ro/SSA sequences. However, significant similarity of the 52-kD Ro/SSA was detected with human rfp and mouse rpt-1. These three proteins each contain similar zinc finger motifs in approximately their first 145 amino acid residues. The cDNA and the protein expressed therefrom are useful in the analysis of the structural and functional properties of this autoantigen.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann M., Mayet W. J., Schröder H. C., Pfeifer K., Meyer zum Büschenfelde K. H., Müller W. E. Identification of the Ro and La antigens in the endoribonuclease VII--ribonucleoprotein complex. Biochem J. 1987 Apr 1;243(1):189–194. doi: 10.1042/bj2430189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D. A., Maddison P. J. Serologic subsets in systemic lupus erythematosus: an examination of autoantibodies in relationship to clinical features of disease and HLA antigens. Arthritis Rheum. 1980 Nov;23(11):1268–1273. doi: 10.1002/art.1780231107. [DOI] [PubMed] [Google Scholar]
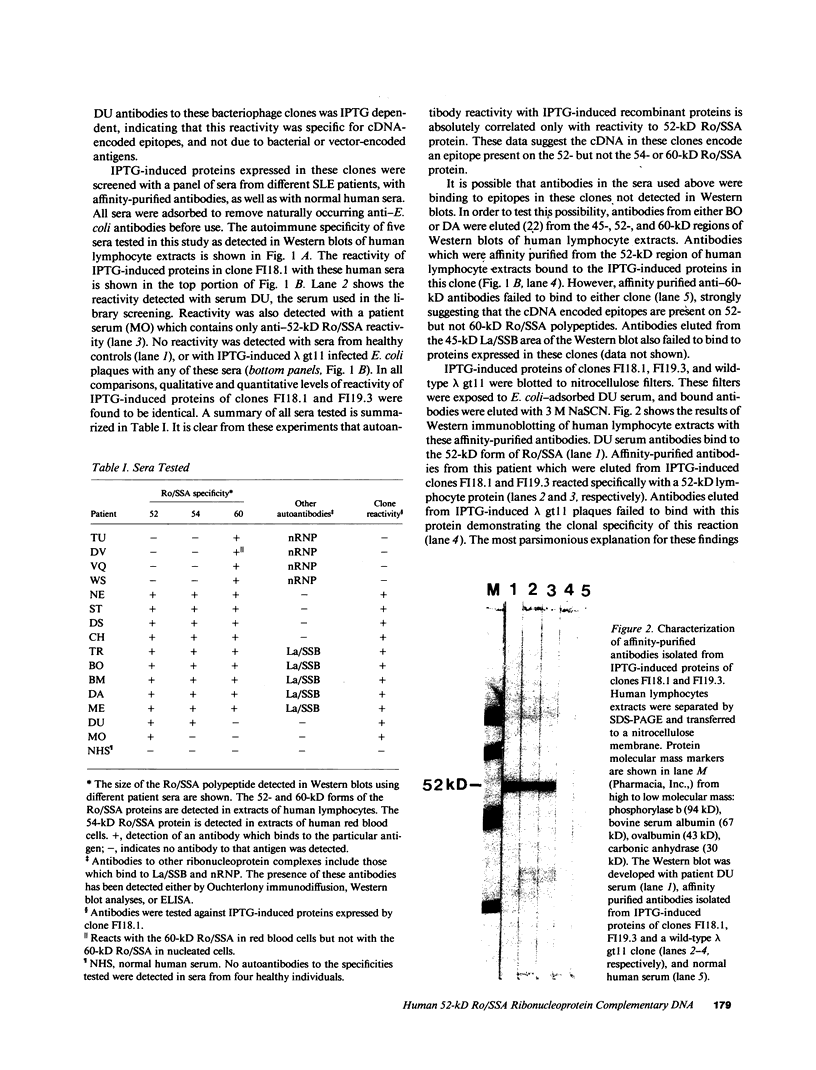
- Ben-Chetrit E., Chan E. K., Sullivan K. F., Tan E. M. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988 May 1;167(5):1560–1571. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Chetrit E., Gandy B. J., Tan E. M., Sullivan K. F. Isolation and characterization of a cDNA clone encoding the 60-kD component of the human SS-A/Ro ribonucleoprotein autoantigen. J Clin Invest. 1989 Apr;83(4):1284–1292. doi: 10.1172/JCI114013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Boire G., Craft J. Biochemical and immunological heterogeneity of the Ro ribonucleoprotein particles. Analysis with sera specific for the RohY5 particle. J Clin Invest. 1989 Jul;84(1):270–279. doi: 10.1172/JCI114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire G., Craft J. Human Ro ribonucleoprotein particles: characterization of native structure and stable association with the La polypeptide. J Clin Invest. 1990 Apr;85(4):1182–1190. doi: 10.1172/JCI114551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. C., Kenan D., Martin B. J., Keene J. D. Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem. 1988 Dec 5;263(34):18043–18051. [PubMed] [Google Scholar]
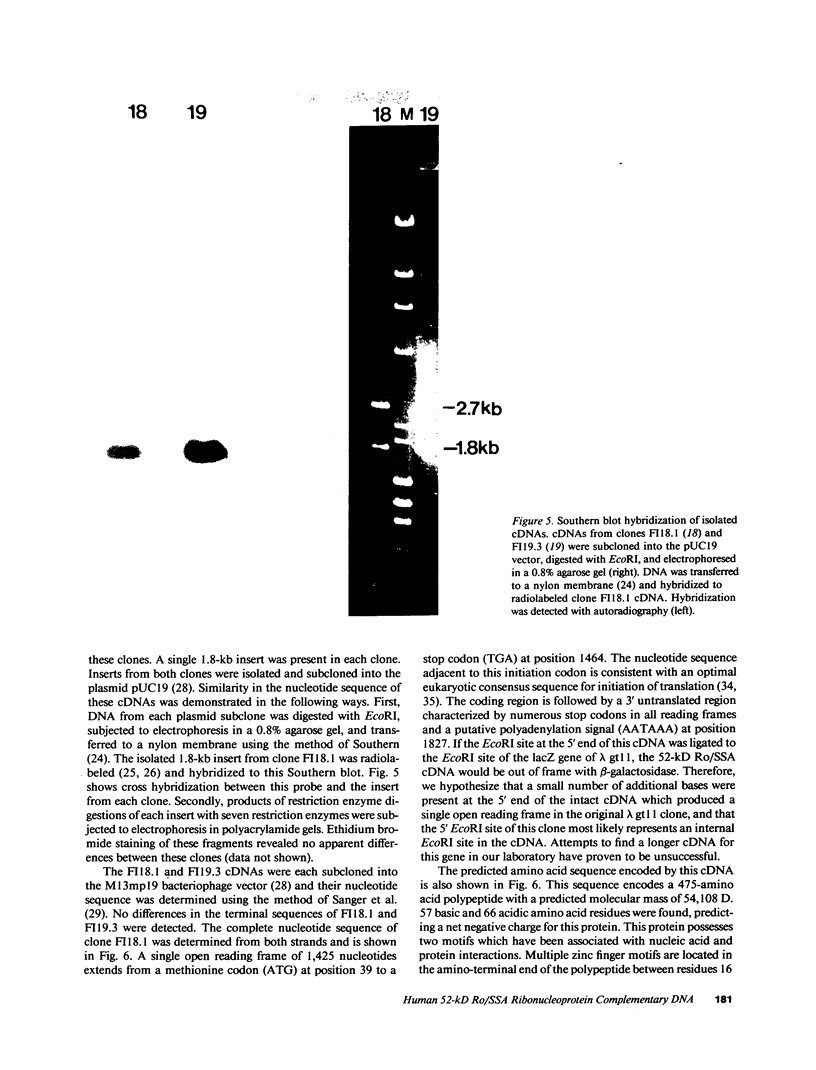
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clark G., Reichlin M., Tomasi T. B., Jr Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythmatosus. J Immunol. 1969 Jan;102(1):117–122. [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Harley J. B., Keene J. D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9479–9483. doi: 10.1073/pnas.85.24.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Swanson M. S., Piñol-Roma S. Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem Sci. 1988 Mar;13(3):86–91. doi: 10.1016/0968-0004(88)90046-1. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. A. Association between the Ro and La antigenic determinants: immunodiffusion analysis of human spleen extract. J Immunol. 1985 Sep;135(3):1707–1713. [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forman M. S., Nakamura M., Mimori T., Gelpi C., Hardin J. A. Detection of antibodies to small nuclear ribonucleoproteins and small cytoplasmic ribonucleoproteins using unlabeled cell extracts. Arthritis Rheum. 1985 Dec;28(12):1356–1361. doi: 10.1002/art.1780281207. [DOI] [PubMed] [Google Scholar]
- Frank M. B., McArthur R., Harley J. B., Fujisaku A. Anti-Ro(SSA) autoantibodies are associated with T cell receptor beta genes in systemic lupus erythematosus patients. J Clin Invest. 1990 Jan;85(1):33–39. doi: 10.1172/JCI114430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaku A., Frank M. B., Neas B., Reichlin M., Harley J. B. HLA-DQ gene complementation and other histocompatibility relationships in man with the anti-Ro/SSA autoantibody response of systemic lupus erythematosus. J Clin Invest. 1990 Aug;86(2):606–611. doi: 10.1172/JCI114751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Burgess R. R. Sigma factors from E. coli, B. subtilis, phage SP01, and phage T4 are homologous proteins. Nucleic Acids Res. 1986 Aug 26;14(16):6745–6763. doi: 10.1093/nar/14.16.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M., Santoro M., Berlingieri M. T., Melillo R. M., Donghi R., Bongarzone I., Pierotti M. A., Della Porta G., Fusco A., Vecchio G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990 Feb 23;60(4):557–563. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- Hamilton R. G., Harley J. B., Bias W. B., Roebber M., Reichlin M., Hochberg M. C., Arnett F. C. Two Ro (SS-A) autoantibody responses in systemic lupus erythematosus. Correlation of HLA-DR/DQ specificities with quantitative expression of Ro (SS-A) autoantibody. Arthritis Rheum. 1988 Apr;31(4):496–505. doi: 10.1002/art.1780310406. [DOI] [PubMed] [Google Scholar]
- Hanas J. S., Hazuda D. J., Bogenhagen D. F., Wu F. Y., Wu C. W. Xenopus transcription factor A requires zinc for binding to the 5 S RNA gene. J Biol Chem. 1983 Dec 10;258(23):14120–14125. [PubMed] [Google Scholar]
- Harley J. B., Alexander E. L., Bias W. B., Fox O. F., Provost T. T., Reichlin M., Yamagata H., Arnett F. C. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren's syndrome. Arthritis Rheum. 1986 Feb;29(2):196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- Harley J. B., Reichlin M., Arnett F. C., Alexander E. L., Bias W. B., Provost T. T. Gene interaction at HLA-DQ enhances autoantibody production in primary Sjögren's syndrome. Science. 1986 May 30;232(4754):1145–1147. doi: 10.1126/science.3458307. [DOI] [PubMed] [Google Scholar]
- Harley J. B., Sestak A. L., Willis L. G., Fu S. M., Hansen J. A., Reichlin M. A model for disease heterogeneity in systemic lupus erythematosus. Relationships between histocompatibility antigens, autoantibodies, and lymphopenia or renal disease. Arthritis Rheum. 1989 Jul;32(7):826–836. [PubMed] [Google Scholar]
- Hochberg M. C., Boyd R. E., Ahearn J. M., Arnett F. C., Bias W. B., Provost T. T., Stevens M. B. Systemic lupus erythematosus: a review of clinico-laboratory features and immunogenetic markers in 150 patients with emphasis on demographic subsets. Medicine (Baltimore) 1985 Sep;64(5):285–295. [PubMed] [Google Scholar]
- Ishida H., Kumagai S., Umehara H., Sano H., Tagaya Y., Yodoi J., Imura H. Impaired expression of high affinity interleukin 2 receptor on activated lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1987 Aug 15;139(4):1070–1074. [PubMed] [Google Scholar]
- Itoh Y., Rader M. D., Reichlin M. Heterogeneity of the Ro/SSA antigen and autoanti-Ro/SSA response: evidence of the four antigenically distinct forms. Clin Exp Immunol. 1990 Jul;81(1):45–51. doi: 10.1111/j.1365-2249.1990.tb05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Stick R., Kleinschmidt J. A., Moll R., Franke W. W., Hausen P. Immunological localization of a major karyoskeletal protein in nucleoli of oocytes and somatic cells of Xenopus laevis. J Cell Biol. 1982 Sep;94(3):749–754. doi: 10.1083/jcb.94.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Snurps and scyrps. Cell. 1981 Aug;25(2):298–300. doi: 10.1016/0092-8674(81)90047-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Robles E., Herrera-Esparza R., Avalos-Diaz E. Cellular localization of the Ro/SS-A antigen. Clin Rheumatol. 1986 Jan;5(1):33–38. doi: 10.1007/BF02030965. [DOI] [PubMed] [Google Scholar]
- Maddison P. J., Reichlin M. Deposition of antibodies to a soluble cytoplasmic antigen in the kidneys of patients with systemic lupus erythematosus. Arthritis Rheum. 1979 Aug;22(8):858–863. doi: 10.1002/art.1780220808. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Silverman E. D., Laxer R. M., Bentur L., Isacovics B., Hardin J. A. Human monoclonal anti-La antibodies. The La protein resides on a subset of Ro particles. J Immunol. 1989 Nov 1;143(9):2923–2928. [PubMed] [Google Scholar]
- McCauliffe D. P., Lux F. A., Lieu T. S., Sanz I., Hanke J., Newkirk M. M., Bachinski L. L., Itoh Y., Siciliano M. J., Reichlin M. Molecular cloning, expression, and chromosome 19 localization of a human Ro/SS-A autoantigen. J Clin Invest. 1990 May;85(5):1379–1391. doi: 10.1172/JCI114582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- Mond C. B., Peterson M. G., Rothfield N. F. Correlation of anti-Ro antibody with photosensitivity rash in systemic lupus erythematosus patients. Arthritis Rheum. 1989 Feb;32(2):202–204. doi: 10.1002/anr.1780320213. [DOI] [PubMed] [Google Scholar]
- Nourse J., Mellentin J. D., Galili N., Wilkinson J., Stanbridge E., Smith S. D., Cleary M. L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990 Feb 23;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- Patarca R., Freeman G. J., Schwartz J., Singh R. P., Kong Q. T., Murphy E., Anderson Y., Sheng F. Y., Singh P., Johnson K. A. rpt-1, an intracellular protein from helper/inducer T cells that regulates gene expression of interleukin 2 receptor and human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2733–2737. doi: 10.1073/pnas.85.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader M. D., O'Brien C., Liu Y. S., Harley J. B., Reichlin M. Heterogeneity of the Ro/SSA antigen. Different molecular forms in lymphocytes and red blood cells. J Clin Invest. 1989 Apr;83(4):1293–1298. doi: 10.1172/JCI114014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin M. Significance of the Ro antigen system. J Clin Immunol. 1986 Sep;6(5):339–348. doi: 10.1007/BF00915372. [DOI] [PubMed] [Google Scholar]
- Rokeach L. A., Haselby J. A., Hoch S. O. Molecular cloning of a cDNA encoding the human Sm-D autoantigen. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4832–4836. doi: 10.1073/pnas.85.13.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Co M. S., Brown E. G., Fields B. N. Distinct binding sites for zinc and double-stranded RNA in the reovirus outer capsid protein sigma 3. Mol Cell Biol. 1988 Jan;8(1):273–283. doi: 10.1128/mcb.8.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindinger W. F., Warner J. R. DNA sequence analysis on the IBM-PC. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):601–604. doi: 10.1093/nar/12.1part2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. S., Maddison P. J., Taylor P. V., Esscher E., Scott O., Skinner R. P. Connective-tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983 Jul 28;309(4):209–212. doi: 10.1056/NEJM198307283090403. [DOI] [PubMed] [Google Scholar]
- Semenzato G., Bambara L. M., Biasi D., Frigo A., Vinante F., Zuppini B., Trentin L., Feruglio C., Chilosi M., Pizzolo G. Increased serum levels of soluble interleukin-2 receptor in patients with systemic lupus erythematosus and rheumatoid arthritis. J Clin Immunol. 1988 Nov;8(6):447–452. doi: 10.1007/BF00916949. [DOI] [PubMed] [Google Scholar]
- Smolen J. S., Morimoto C., Steinberg A. D., Wolf A., Schlossman S. F., Steinberg R. T., Penner E., Reinherz E., Reichlin M., Chused T. M. Systemic lupus erythematosus: delineation of subpopulations by clinical, serologic, and T cell subset analysis. Am J Med Sci. 1985 Apr;289(4):139–147. doi: 10.1097/00000441-198504000-00003. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swanson M. S., Nakagawa T. Y., LeVan K., Dreyfuss G. Primary structure of human nuclear ribonucleoprotein particle C proteins: conservation of sequence and domain structures in heterogeneous nuclear RNA, mRNA, and pre-rRNA-binding proteins. Mol Cell Biol. 1987 May;7(5):1731–1739. doi: 10.1128/mcb.7.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Cooper G. M. ret transforming gene encodes a fusion protein homologous to tyrosine kinases. Mol Cell Biol. 1987 Apr;7(4):1378–1385. doi: 10.1128/mcb.7.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Inaguma Y., Hiai H., Hirose F. Developmentally regulated expression of a human "finger"-containing gene encoded by the 5' half of the ret transforming gene. Mol Cell Biol. 1988 Apr;8(4):1853–1856. doi: 10.1128/mcb.8.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Taylor P. V., Taylor K. F., Norman A., Griffiths S., Scott J. S. Prevalence of maternal Ro (SS-A) and La (SS-B) autoantibodies in relation to congenital heart block. Br J Rheumatol. 1988 Apr;27(2):128–132. doi: 10.1093/rheumatology/27.2.128. [DOI] [PubMed] [Google Scholar]
- Tokano Y., Murashima A., Takasaki Y., Hashimoto H., Okumura K., Hirose S. Relation between soluble interleukin 2 receptor and clinical findings in patients with systemic lupus erythematosus. Ann Rheum Dis. 1989 Oct;48(10):803–809. doi: 10.1136/ard.48.10.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasicek C. A., Reichlin M. Clinical and serological differences between systemic lupus erythematosus patients with antibodies to Ro versus patients with antibodies to Ro and La. J Clin Invest. 1982 Apr;69(4):835–843. doi: 10.1172/JCI110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Harley J. B., Reichlin M. Molecular properties of the Ro/SSA antigen and enzyme-linked immunosorbent assay for quantitation of antibody. J Clin Invest. 1984 Aug;74(2):625–633. doi: 10.1172/JCI111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]