Abstract
The human breast cancer resistance protein (BCRP/ABCG2) is the second member of the G subfamily of the large ATP-binding cassette (ABC) transporter superfamily. BCRP was initially discovered in multidrug resistant breast cancer cell lines where it confers resistance to chemotherapeutic agents such as mitoxantrone, topotecan and methotrexate by extruding these compounds out of the cell. BCRP is capable of transporting non-chemotherapy drugs and xenobiotiocs as well, including nitrofurantoin, prazosin, glyburide, and 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine. BCRP is frequently detected at high levels in stem cells, likely providing xenobiotic protection. BCRP is also highly expressed in normal human tissues including the small intestine, liver, brain endothelium, and placenta. Therefore, BCRP has been increasingly recognized for its important role in the absorption, elimination, and tissue distribution of drugs and xenobiotics. At present, little is known about the transport mechanism of BCRP, particularly how it recognizes and transports a large number of structurally and chemically unrelated drugs and xenobiotics. Here, we review current knowledge of structure and function of this medically important ABC efflux drug transporter.
Keywords: Breast cancer resistance protein, BCRP, ATP-binding cassette transporter, ABCG2, multidrug resistance, drug disposition, homology model, mutation analysis
1. INTRODUCTION
The human breast cancer resistance protein (BCRP) is the second member of the G subfamily of the ATP-binding cassette (ABC) efflux transporter superfamily, and hence also designated as ABCG2. BCRP is an approximately 75 kDa polytopic plasma membrane protein first identified in 1998 in a multidrug resistant human breast cancer cell line MCF-7/AdrVp which does not express other known multidrug efflux transporters such as P-glycoprotein (P-gp) or the multidrug resistance protein 1 (MRP1) [1]. Two almost identical proteins as BCRP with only a few amino acid differences were later discovered independently by other laboratories from mitoxantrone-resistant human cancer cell lines (so named as MXR) [2] and human placenta (so named as ABCP) [3]. Despite the fact that there is low protein sequence identity in nucleotide binding domains (NBDs) (~20%) and essentially no protein sequence identity in membrane spanning domains (MSDs) between BCRP and P-gp or MRP1, transfection of cells with BCRP cDNA confirmed its ability to confer resistance to a variety of chemotherapeutic agents such as mitoxantrone and topotecan [1–2]. Like P-gp or MRP1, BCRP performs ATP hydrolysis-dependent efflux transport of a large number of structurally and chemically unrelated compounds that also include non-chemotherapy drugs and xenobiotics [4–6].
Various clinical studies have demonstrated BCRP expression and a possible role of the transporter in drug resistance in leukemia [7–9]. In addition, numerous studies have documented that BCRP is highly enriched in a population of primitive stem cells, the so-called side population (SP), of human bone marrow and other organs, and may provide xenobiotic protection for stem cells [10–14]. Similar to P-gp, BCRP is also highly expressed in organs important for the absorption (the small intestine), elimination (the liver and kidney), and distribution (the blood-brain and placental barriers) of drugs and xenobiotics [15], it is therefore increasingly recognized for its important role in drug disposition and tissue protection [4–5, 16–17] An unique feature is that BCRP expression in the mammary gland of mice, cows and humans was found to be strongly induced during lactation and responsible for the secretion and concentration of clinically and toxicologically important substances such as the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and the chemotherapeutic agent topotecan into milk [18]. BCRP is a highly polymorphic transporter with over 80 single-nucleotide polymorphisms in the BCRP gene [19]. Of these, Q141K is most extensively studied and has been found to be associated with inter-individual variations in the pharmacokinetics, response or toxicity of drugs [20–22] as well as genetic diseases such as gout [23]. The physiological roles of BCRP are likely to provide tissue protection against endogenous toxins or xenobiotics and regulate cellular homeostasis of physiologically important endogenous compounds such as heme, porphyrins, riboflavin, and estrogens [24–26]. Despite its significant medical importance, our understanding of the transport mechanism of BCRP is still limited. In this mini-review, we summarize current knowledge of structure and function of human BCRP.
2. TRANSPORT, INHIBITION, DRUG BINDING, AND ATP HYDROLYSIS
2.1. BCRP Transport
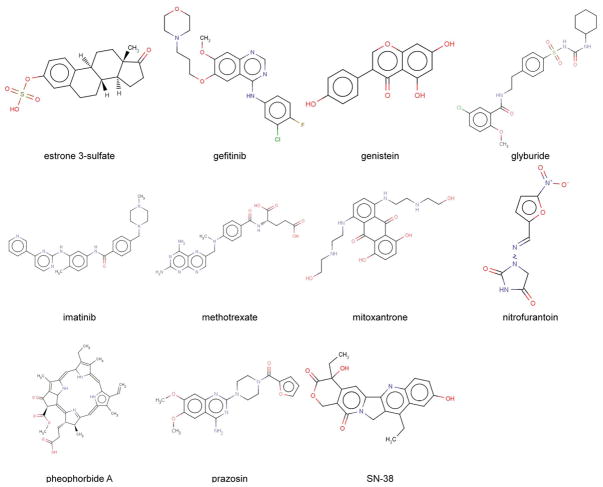
BCRP transports a highly diverse range of substrates. The list of BCRP substrates has been rapidly expanding since its discovery. BCRP substrates include not only chemotherapeutic agents such as mitoxantrone, methotrexate, topotecan, irinotecan and its active analog SN-38, and tyrosine kinase inhibitors imatinib and gefitinib, but non-chemotherapy drugs such as prazosin, glyburide, nitrofurantoin, dipyridamole, statins, and cimetidine as well as non-therapeutic compounds such as the dietary flavonoids, porphyrins, estrone 3-sulfate (E1S), and the carcinogen PhIP. This has been extensively reviewed elsewhere [4–6, 16]. Chemical structures of representative BCRP substrates are shown in Fig. (1). There is a considerable overlap in substrate specificity between BCRP and other ABC transporters. For example, of the BCRP substrates listed in Fig. (1) glyburide, imatinib, methotrexate, mitoxantrone, prazosin, and SN-38 are also P-gp substrates, although some of them such as methotrexate are poor substrates of P-gp [27–29]. Notably, in addition to hydrophobic substrates such as mitoxantrone, BCRP can also transport hydrophilic conjugated organic anions, particularly the sulfated conjugates with high affinity, whereas P-gp generally transports hydrophobic compounds. The overlap in substrate specificity between BCRP and P-gp can lead to a synergistic effect of the transporters in limiting drug penetration across tissue barriers such as the blood-brain barrier [30–31]. The transport kinetic parameters (Km and Vmax) of BCRP for several hydrophilic substrates have been determined using plasma membrane vesicle transport assays and are summarized in Table 1. Except for methotrexate, most of the BCRP substrates have the affinity constants (Km) in a low μM range. For hydrophobic substrates, the apparent Km value of only one substrate, glyburide, was estimated using the cellular accumulation assay [32]. Understanding transport kinetics is of important value in predicting the contribution of BCRP to the disposition of substrate drugs in vivo. Lipid membrane environment could affect transport kinetics of BCRP. For example, the addition of cholesterol dramatically increased Vmax of BCRP for methotrexate without affecting Km [33]. At present, what determines the substrate specificity of BCRP remains elusive. To date, there appears to be only one structure-activity relationship (SAR) study for BCRP substrates, namely SN-38 and its analogs [34]. These authors found that SN-38 analogues with high polarity were good BCRP substrates. The high polarity of SN-38 analogues appears to be attributed to the presence of hydroxyl or amino groups at positions 10 and 11 on the A ring of SN-38. Hydrogen bonds formed through a hydroxyl or amino group could contribute to SN-38 recognition by BCRP. Based on such SAR analysis, novel SN-38 analogues that are not BCRP substrates have been developed to bypass BCRP-mediated SN-38 resistance [35].
Fig. 1.
Chemical structures of representative BCRP substrates.
Table 1. Reported Kinetic Parameters for Transport of BCRP Substrates.
Km and Vmax are the transport affinity constant and the maximal rate of BCRP-mediated transport, respectively. Transport studies were carried out using BCRP-enriched plasma membrane vesicles unless otherwise indicated.
| Substrate | Expression system | Km (μM) | Vmax (nmol/min/mg protein) | Reference |
|---|---|---|---|---|
| Estrone 3-sulfate | K562 human myelogenous leukaemia cells | 6.8 ± 1.4 | 1.4 ± 0.3 | [132] |
| The yeast Pichia pastoris | 3.6 ± 0.3 | 0.055 ± 0.002 | [69] | |
| SN-38 | PC-6 human small cell lung cancer cells | 4.0 | 0.714 | [133] |
| SN-38-glucuronide | PC-6 human small cell lung cancer cells | 26 | 0.833 | [133] |
| Methotrexate | HEK293 cells | 1340 ± 180 | 0.687 ± 0.087 | [122] |
| Sf9 insect cells | 6000 | N/R | [134] | |
| E217βG | HEK293 cells | 44.2 ± 4.3 | 0.103 ± 0.017 | [122] |
| 4-MUS | P388 mouse lymphoma cells | 12.9 ± 2.1 | N/R | [135] |
| E3040S | P388 mouse lymphoma cells | 26.9 ± 4.0 | N/R | [135] |
| Hematoporphyrin | Sf9 insect cells | 17.8 | 0.654 | [120] |
| Glyburidea | HEK293 cells | 13.1 ± 1.2 | 0.101 ± 0.034 | [32] |
| Sulfasalazine | Mammalian cells | 0.70 ± 0.03 | 0.052 ± 0.001 | [136] |
The Km and Vmax values for glyburide are apparent kinetic parameters estimated in cellular accumulation experiments. SN-38, 7-ethyl-10-hydroxycamptothecin; E217βG, 17β-estradiol 17-(β-D-glucuronide); 4-MUS, 4-methylumbelliferone sulfate; E3040, 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole; E3040S, E3040 sulfate; N/R, not reported.
2.2. BCRP Inhibition
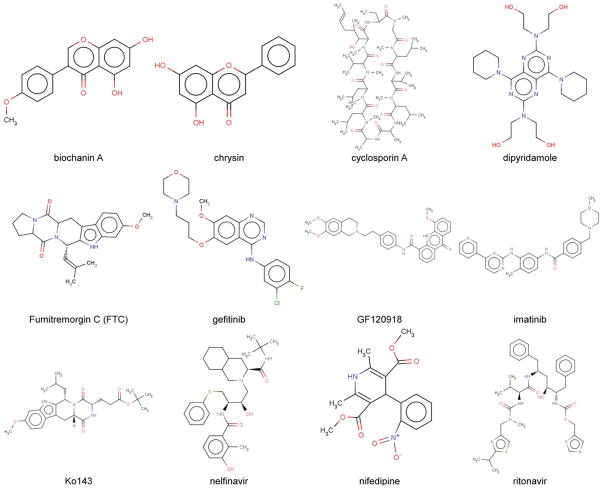
A wide variety of compounds that can inhibit BCRP have been extensively reviewed [4–6, 16, 36]. BCRP inhibitors include, among others, fumitremorgin C (FTC), the FTC analogue Ko143, the acridone carboxamide derivative GF120918, anti-HIV protease inhibitors nelfinavir and ritonavir, the dietary flavonoids chrysin and biochanin A, the tyrosine kinase inhibitors gefitinib and imatinib [5, 16, 36], and herb extracts [37]. Chemical structures of representative BCRP inhibitors are shown in Fig. (2). Some BCRP inhibitors such as Ko143 [38–39], GF120918 [40], gefitinib [41], and imatinib [42] are highly potent with IC50 values in a nM range. Of these, FTC and Ko143 are highly selective for BCRP with no or little inhibition for P-gp and MRP1 [38–39]. However, others such as cyclosporin A and anti-HIV protease inhibitors seem to be general inhibitors of ABC transporters. GF120819 is a potent inhibitor for both BCRP and P-gp.
Fig. 2.
Chemical structures of representative BCRP inhibitors.
SAR and quantitative SAR (QSAR) analyses for structurally related or unrelated BCRP inhibitors have been performed to understand BCRP-inhibitor interactions. This topic has been extensively reviewed elsewhere [43–46], and hence is only briefly discussed here. SAR analysis has been reported for taxane-based reversal agents [47], flavonoids [48–51], tamoxifen analogues [52], cyclin-dependent kinase inhibitors [53], tariquidar analogues [54], and FTC analogues [39]. Structural features of these compounds contributing to BCRP inhibition were investigated in these studies. For example, hydroxyl groups at positions 3, 7, and 4′ of flavonoids are detrimental to BCRP inhibition, whereas the hydroxyl group at position 5 has a positive contribution [50]. QSAR models have also been developed to identify pharmacophores in BCRP inhibitors which were analyzed either as structurally related compounds such as flavonoids [49–50] and tariquidar analogues [54] or as groups of structurally diverse compounds [45, 55–57]. Various modeling methods were used, including classical 2D-QSAR [49], comparative molecular field analysis (CoMFA) or comparative molecular similarity indices analysis (CoMSIA) [54], 3D-QSAR method based on VolSurf descriptors of molecular-interaction fields (MIFs) related to hydrophobic interaction forces, polarizability, and hydrogen bonding potential [56], classification methods based on molecular descriptors including logD and polarizability [57], and generation of chemical fragmentation codes coupled with regression analysis [45, 55]. According to the QSAR models reported so far [55, 57], some descriptors such as lipophilicity and polarizability appear to be significant determinants for BCRP inhibition. On the other hand, depending on compounds, some descriptors such as planar structure, amine bonded to carbon of a heterocyclic ring, and hydrogen bonding potential may also be important. QSAR models could seemingly help predict BCRP inhibition for certain inhibitors. For example, Saito et al. [55] selected 49 compounds representing 9 different classes of BCRP inhibitors and developed a QSAR model using chemical fragmentation codes and multiple linear regression analysis. This model well predicted BCRP inhibition by gefitinib, a compound that was not used in the original model development. Because of the structural diversity, none of the existing QSAR models can work for all BCRP inhibitors. Also, a QSAR model developed using one structural set of compounds cannot be used for another structural set of compounds. Even for compounds with similar chemical structures such as flavonoids, contradicting results were obtained in different laboratories with respect to which substitutions would have favorable or detrimental effects on BCRP inhibition. Note that all the current QSAR models were developed through indirect modeling based on chemical structures of inhibitors because a high resolution 3D structure of BCRP has not been available. Hence, exactly how inhibitors interact with BCRP is essentially not known. Moreover, owning to multiple drug binding sites in BCRP as discussed below, the inhibition profile used in QSAR analysis that was determined with one substrate may not be the same as that obtained with a different substrate.
2.3. Drug Binding in BCRP
An earlier study demonstrated that two different substrates such as mitoxantrone and rhodamine 123, topotecan or daunorubicin did not reciprocally inhibit the efflux of each other [58], suggesting that there are multiple drug binding sites in BCRP that are not or only partially overlapping. To prove drug binding in BCRP, direct drug binding interactions with BCRP has been investigated using photo-affinity labeling [59–62]. The prazosin analogue [125I]-IAAP is the photo-active compound that is most frequently used. Labeling of BCRP with [125I]-IAAP was achieved [59, 61–62] and could be prevented by the addition of other BCRP substrates or inhibitors in a concentration-dependent manner [61–63]. [125I]-IAA-rhodamine 123 [59] and [3H]-azidopine [62] have also been successfully used to photo-label BCRP and again the labeling could be prevented by increasing concentrations of several known BCRP substrates. Since both [125I]-IAAP and [3H]-azidopine are BCRP substrates [62], their ability to photo-label BCRP suggests a direct interaction of the two compounds with the transporter. Photo-affinity labeling of purified BCRP combined with trypsin digestion and mass spectrometry analysis of peptide fragments may provide valuable information for the drug-binding sites in BCRP. At present, the residues potentially involved in interactions of the photo-active substrates with BCRP have not been identified. BCRP substrates or inhibitors can displace the binding of photo-affinity analogues, indicating that photo-affinity labeling studies could serve to screen BCRP substrates or inhibitors. Unfortunately, [3H]-azidopine is no longer commercially available.
There are only a few equilibrium or kinetic binding studies for substrates or inhibitors of BCRP [64–66]. Clark et al. examined the kinetics of association and dissociation of [3H]-daunomycin with the BCRP mutant R482G which can effectively transport the drug [67] using plasma membranes isolated from insect cells, and the ability of several other drugs to displace [3H]-daunomycin binding [64]. They showed that the binding affinity (Kd) of [3H]-daunomycin to BCRP was approximately 0.6 μM. Doxorubicin, prazosin and non-radioactive daunomycin nearly completely displaced [3H]-daunomycin binding, whereas mitoxantrone and Hoechst 33342 only had a partial displacement. Rhodamine 123 and methotrexate had no any effects on [3H]-daunomycin binding. These results again suggest that there are multiple drug binding sites in BCRP with doxorubicin, prazosin and daunomycin binding clustered in one region and rhodamine 123 and methotrexate binding in different regions. The binding sites for mitoxantrone and Hoechst 33342 may partially overlap with the binding sites for doxorubicin, prazosin and daunomycin. The drug binding study monitored by quenching of intrinsic fluorescence of purified BCRP by Pozza et al. revealed that there seem to be two binding sites for each substrate or inhibitor in BCRP, with one high affinity (Kd in a low μM range) and one low affinity sites [66]. The inhibitor GF120918 did not displace mitoxantrone binding, suggesting distinct binding sites for substrate and inhibitor.
2.4. ATPase Activity of BCRP
For ABC proteins, the transport process is supposed to be coupled with ATP binding and hydrolysis which provide the energy for substrate translocation. ATP hydrolysis (ATPase) activity of BCRP has been reported in various studies [68]. ATP hydrolysis by BCRP was confirmed by photo-labeling of BCRP under hydrolytic conditions with 8-azido [α-32P]ATP in the presence of vanadate and Mg2+ or Co2+ [69–70]. Most of the studies used BCRP-enriched insect cell plasma membranes, although plasma membranes from other expression systems such as HEK cells, Pichia pastoris, and Lactococcus lactis or purified BCRP were also utilized. In general, BCRP exhibits basal ATPase activity that is considerably lower than that of P-gp, but much greater than that of MRP1. Unlike P-gp, whose basal ATPase activity can be strongly stimulated several-fold by its substrates, basal ATPase activity of BCRP could only be stimulated up to 2-fold by its substrates such as prazosin [33, 70–73]. It has been suggested that this insensitivity of drug-stimulation of BCRP ATPase activity may reflect the presence of endogenous substrates or a partially uncoupled form of BCRP [74]. Note that, basal AT-Pase activity of MRP1 could not be strongly stimulated by its substrates either [75]. The addition of cholesterol can significantly potentiate ATPase activity of BCRP and its substrate-stimulation characteristics [33]. How ATP binding and/or hydrolysis is coupled with drug transport in BCRP is not known. Only one study showed that ATP binding alone could convert the drug binding site from a high to a low affinity state [65], suggesting that it is ATP binding, not ATP hydrolysis, that appears to initiate drug transport by BCRP.
3. OLIGOMERIZATION
3.1. Homodimer/Homooligomer of BCRP
A unique feature that distinguishes BCRP from other ABC proteins such as P-gp and MRP1 is in its basic structure. P-gp or MRP1 possesses two repeated halves which contain two MSDs each followed by one NBD. In contrast, BCRP contains only one NBD preceding one MSD [1–3]. It has been widely accepted that a functional ABC transporter requires two MSDs and two NBDs which form a central substrate translocation pathway. Thus, BCRP may function as a homodimer with evidence provided in several earlier studies [76–78]. The earlier study by Kage et al. revealed that BCRP migrated as a 70 kDa band on SDS-PAGE under reducing condition, but as a 140 kDa complex in the absence of reducing agents, suggesting that BCRP could form a homodimer bridged by disulfide bonds [76]. Litman et al. [77] also observed a molecular mass shift of BCRP from 72 kDa to 180 kDa after treatment with chemical cross-linking agents. Bhatia et al. [78] showed that chimeric fusion proteins containing two BCRP monomers fused with or without a linker peptide were properly targeted to the plasma membrane and retained drug transport activity. Several recent studies demonstrated that BCRP may exist as a higher order oligomer [55, 66, 73]. Using sucrose density gradient sedimentation and non-denaturing gel electrophoresis, Xu et al. [79] provided evidence that detergent-solubilized BCRP was capable of formation of homotetramers. Likewise, the electron microscopy (EM) analysis of BCRP protein particles in detergent solutions identified an octameric state that was organized as a tetramer of dimers [61]. The recent cryo-EM analysis of 2D crystals revealed that BCRP could form a tetrameric complex (two BCRP dimers) in one unit cell of the projection maps [72]. These data suggest that BCRP can form higher order oligomeric states in vitro. Most recently, BCRP with GFP/YFP attached at its N-terminus was expressed in HEK cells and, dimer/oligomer formation of BCRP was determined by measuring the FRET efficiencies between GFP and YFP in intact cells using fluorescence resonance energy transfer (FRET) microscopy [80]. The FRET efficiencies of GFP/YFP-tagged BCRP were significantly higher than the GFP/YFP pair control, suggesting that BCRP likely forms a homodimer or homooligomer in vivo in intact cells [80]. At present, the role of oligomerization in BCRP function is not clear. It has been suggested that the function of BCRP could be regulated by the dynamic association/dissociation of BCRP monomers in the dimeric/oligomeric complex via protein-protein interactions [81]. Therefore, disruption of the contact interfaces between BCRP monomers by small peptides or small molecules, thus preventing the formation of active higher order oligomers, may represent a promising strategy for inhibition of BCRP. Studies performed to identify residues or regions in BCRP potentially involved in oligomerization are discussed below.
3.2. Residues or Regions in BCRP Potentially Involved in Oligomerization
Several studies suggest that dimer/oligomer formation of BCRP may involve intermolecular disulfide bonds formed between extracellular Cys residues [78, 82–85]. These studies showed that mutations of Cys603 in the extracellular loop connecting transmembrane (TM) segments 5 and 6 resulted in the appearance of BCRP monomers on non-denaturing polyacrylamide gel under non-reducing conditions, whereas wild-type BCRP formed only dimeric complex under the same conditions. Mutations of other Cys residues Cys592 and Cys608 in the same extracellular loop resulted in a mixed population of monomeric and dimeric BCRP [82] or only dimeric BCRP [83] under non-reducing conditions. These data appear to support that Cys603 is involved in intermolecular disulfide bond formation that is responsible for dimer/oligomer formation of BCRP, but Cys509 and Cys608 are not. Nevertheless, several lines of evidence indicate that intermolecular disulfide bond formation involving Cys603 is likely not the sole mechanism of BCRP oligomerization. Various studies have shown that mutations of Cys603 alone do not affect expression and function of BCRP at all [80, 82–83]. Additionally, the studies by Liu et al. [85] and Shigeta et al. [86] have suggested that Cys592 and/or Cys608 are also potentially involved in intermolecular disulfide bond formation. Importantly, Shigeta et al. showed that the triple-mutant C592S/C603S/C608S retained substantial resistance to mitoxantrone, topotecan, and SN-38 [86]. Because intermolecular disulfide bonds via the three extracellular Cys residues are not expected to be formed in the triple-mutant, these authors concluded that BCRP could possibly function as a dimer or an oligomer formed through non-covalent protein-protein interactions. The recent study using FRET and chemical cross-linking also revealed that Ala substitution of Cys603 had no any effect on dimer/oligomer formation of BCRP in vivo in intact cells [80]. Thus, all these studies appear to support the conclusion that, besides intermolecular disulfide bonds formed by Cys603, intermolecular disulfide bonds formed by other Cys residues and/or non-covalent protein-protein interactions could also have a crucial contribution to BCRP oligomerization. Xu et al. [87] showed that the recombinant fragment containing TM5-loop-TM6 was capable of forming homododecamer, suggesting that this region is important for BCRP oligomerization. Phosphorylation of BCRP by the Pim-1 kinase has been shown to promote its oligomerization and drug resistance in human prostate cancer cells; however, the underlying mechanism is unknown [88]. Taken together, despite extensive studies, it is still not clear as to whether the minimal functional unit of BCRP in the plasma membrane is a homodimer or a homooligomer and the effect of the dimeric or oligomeric state of BCRP on drug binding and transport. This requires further investigation into the mechanisms by which BCRP forms homodimers or homooligomers.
4. MEMBRANE TOPOLOGY
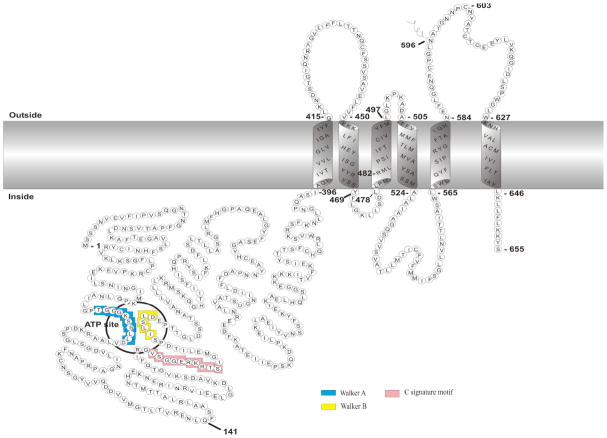
Knowledge in membrane topology of BCRP is important for understanding the structural basis of BCRP action and for homology modeling of the transporter. Hydropathy analysis based on amino acid sequence predicted that BCRP contains one NBD (residues ~1 – 396) followed by one MSD (residues ~397 – 655), and the MSD contains 6 TM α-helices [1–3, 89–90]. The membrane topology of BCRP has recently been determined experimentally [72, 91]. In total of 20 hemagglutinin (HA) tags were inserted in various locations throughout BCRP, and the insertion mutants were expressed in HEK cells by transient transfection. Polarity of the tags with respect to the plasma membrane was determined by immunofluorescence in the presence and absence of membrane-permeabilizing detergent. The study confirmed 6 TM α-helices in BCRP; however, there are major differences between the experimental and the computer-predicted topology models as follows. As shown in Fig. (3) the residues 431 – 450 predicted to be part of TM2 were found to be in an extracellular loop connecting TM1 and TM2. The residues 566 – 583 predicted in the extracellular loop between TM5 and TM6 were determined to form TM5. The residues 460 and 462 predicted in an intracellular loop connecting TM2 and TM3 were now found in the TM2. The computer-predicted TM1, TM3, TM4 and TM6 are in agreement with the experimental data. Such a change in the membrane topology would be expected to alter the composition and shape of the putative substrate translocation pathway which is primarily composed of multiple TM α-helices. Although this experimental topology model awaits further verification by other biochemical and structural experiments, it appears to be consistent with existing biochemical data. For example, Asp590 which is the only N-linked glycosylation site in BCRP [92–93] is located in the extracellular loop connecting TM5 and TM6 Fig. (3). Cys603 responsible for intermolecular disulfide bond formation is also in the extracellular loop, and Arg482 which is crucial for substrate specificity and overall transport activity of BCRP is located in TM3 near the cytosolic membrane interface Fig. (3).
Fig. 3.
Schematic illustration of the membrane topology of BCRP. The boundary of transmembrane α-helices is approximate and based on the experimentally determined membrane topology of BCRP. The ATP site (the Walker A and Walker B motifs), and the C signature motif are indicated. Lys substitution of Gln141 (Q141K), a natural variant with decreased protein expression, causes changes in the pharmacokinetics of BCRP substrate drugs in vivo. Arg482 is critical for substrate specificity and transport activity of BCRP. Asn596 is the N-linked glycosylation site. Cys603 is possibly involved in dimerization/oligomerization of BCRP through intermolecular disulfide bonds.
This topology structure of BCRP was compared with that of mouse P-gp observed in crystal structures [94]. Except for two regions, the overall topology structures of BCRP and P-gp were surprisingly similar [72]. First, the large intracellular loop connecting TM2 and TM3 of P-gp does not exist in the corresponding region of BCRP. Second, the extracellular loop between TM5 and TM6 of BCRP is significantly longer than the corresponding region in P-gp. Notably, the amino acid identity in MSDs between BCRP and P-gp is only ~ 4% based on pairwise alignment [68]; however, according to the experimental topology model of BCRP, the amino acid identity in MSDs between BCRP and the first half of mouse P-gp is increased to 18% (without gaps). Likewise, the amino acid identity in MSDs between BCRP and the bacterial ABC half transporter Sav1866 or the lipid A transporter MsbA was also found to be much improved [72].
5. STRUCTURAL ANALYSES AND HOMOLOGY MODELING
5.1. Current Progress on BCRP Structural Analyses
High yield expression of human BCRP with a His tag attached has been achieved in baculovirus-infected insect cells [73, 95] and the yeast Pichia pastoris [69], facilitating purification of BCRP by Ni2+-affinity chromatography for structural analysis [61, 66, 72]. BCRP could be solubilized from membranes using detergents such as 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) [66], Foscholine derivatives [61], or n-dodecyl-β-D-maltoside (DDM) [72]. Purified BCRP in detergent solution was active in drug binding [61] or ATP hydrolysis [66, 72], and suitable for electron microscopy (EM) analysis of protein particles [61] or 2D crystals [72].
At present, there are only two structural studies for BCRP [61, 72]. McDevitt et al. [61] reported the first EM analysis of single protein particles of BCRP purified from insect cells. These analyses illustrated that BCRP protein particles in a detergent solution formed a higher order oligomeric complex that was organized as a tetramer of BCRP dimers. A 3D structure was reconstructed at 18 Å resolution with a maximum diameter of 180 Å and an overall peak height of 145 Å [61]. At such a low resolution, only the overall shape and oligomeric state of the BCRP complex could be visualized. Most recently, Rosenberg et al. [72] determined the first projection structures of BCRP purified from Pichia pastoris by cryo-EM of well-diffracting 2D crystals. The 2D crystals showed a p121 symmetry and the projection structures were determined to 5 Å resolution. At this relatively high resolution, ring-shaped high density features in the projection maps were clearly visualized, probably representing TM α-helices (and NBDs). The dimension of the asymmetric unit cell of the crystals indicated that there were 4 BCRP monomers (two BCRP dimers) in one unit cell, indicating the existence of an oligomeric complex of BCRP in the 2D crystals. Importantly, the unit cell dimension of the crystals grown in the presence of mitoxantrone was significantly smaller than that in the absence of mitoxantrone in the dimension b, suggesting that BCRP has undergone a significant conformational change upon mitoxantrone binding. Thus, BCRP appears to have a more closed configuration in the presence of mitoxantrone. This is the first experimental evidence showing substrate binding induced conformational changes in BCRP, which has implications for transport mechanism of the transporter. The number of TM α-helices, and their orientations in the membrane and assignment in the BCRP protein sequence cannot be resolved from the projection structures. This will require the calculation of a 3D map at a high resolution.
5.2. BCRP Homology Models
5.2.1. Homology Modeling of BCRP
To further illustrate drug binding and transport mechanism of BCRP, homology models have been developed in different laboratories. The first model of BCRP was constructed using the crystal structure of MsbA from Vibrio cholera (VcMsbA) [90] as the template for TM segments. Unfortunately, this crystal structure has now been retracted. Two additional models of BCRP were developed using the crystal structure of Sav1866 [96] as the template [89, 97]. Although certain features in the models seem to be consistent with experimental data, for example, showing multiple drug binding sites in a large central cavity, these studies were solely based on the computer-predicted BCRP topology. However, we now know that the topology structure of BCRP based on computer prediction is significantly different from the experimental topology structure. Here, we discuss the homology models that were developed based on the experimental topology structure of BCRP [72].
First of all, the sequence alignment for TM segments between BCRP and the templates (MsbA, the first and second halves of mouse P-gp, and Sav1866) was refined manually by comparing the experimentally determined TM segments of BCRP with those of the templates observed in crystal structures [72]. The template used in modeling of the NBD was the ATP-binding subunit of the E. coli maltose transporter MalK, whose crystal structures were resolved at atomic resolutions [98]. Next, the dimeric NBD-MSD structure of BCRP was modeled as a whole in a single step, with no loss of information in the interactions between monomers and between protein domains within the same monomer. The templates were edited to reflect the same domain organization of BCRP, by “cutting” the linker regions between the MSD and the NBD of the templates and changing the order of the MSD and the NBD. The model building was done by the MODELLER package [99].
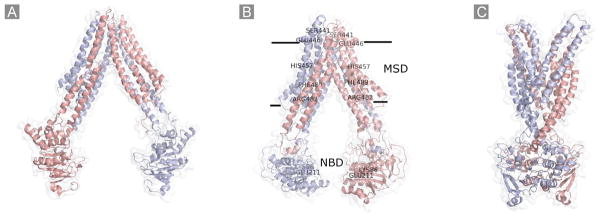
With the same method as described above, three homology models of BCRP representing different conformational states have been generated. The first model using the MsbA structure as template (PDB code 3B5W) [100] represents the substrate-unbound nucleotide-free inward-facing open apo conformation Fig. (4A). The second model using the mouse P-gp structure as template (PDB code 3G60) [94] represents the substrate-bound nucleotide-free inward-facing closed apo conformation Fig. (4B). The third model using the Sav1866 structure as template (PDB code 2HYD) [96] represents the nucleotide-bound outward-facing conformation Fig. (4C). The first two models have been published [72]. Ward et al. [100] reported crystal structures of MsbA trapped in different conformations, two similar nucleotide-bound outward-facing conformations and two different nucleotide-free inward-facing apo conformations. TM segments in the closed apo form of MsbA are much more compact than in the open apo form. These authors proposed that substrate binding to the open apo form promotes the closure of TMs, which in turn, sends a signal to the NBDs, allowing the formation of the ATP sandwich in the outward-facing conformation upon nucleotide binding. The results of homology modeling for BCRP as well as the finding that the projection structures of BCRP display a more closed conformation in the presence of mitoxantrone than in the absence of the drug appear to be consistent with the structural data of MsbA.
Fig. 4.
Schematic representation of the homology models of BCRP. A, the substrate-unbound nucleotide-free inward-facing open apo conformation based on the MsbA structure (PDB code 3B5W); B, the substrate-bound nucleotide-free inward-facing closed apo conformation based on the mouse P-gp structure (PDB code 3G60). The approximate locations of several amino acid residues in the MSD (Ser441, Glu446, His457, Phe489, and Arg482) or the NBD (Lys86 and Glu211) that could be important for substrate specificity and/or overall transport activity are indicated; C, the nucleotide-bound outward-facing conformation based on the Sav1866 structure (PDB code 2HYD). Two monomers in the BCRP dimer are shown in different colors.
5.2.2. Predicted Features of BCRP Homology Models
There are several unique features in the models. First, the intracellular entry of the two inward-facing apo forms Fig. (4A and 4B) is large enough to allow access of a bulk of BCRP substrates from either the inner lipid leaflet of the plasma membrane or cytoplasm. Second, the extracellular loop 1 connecting TM1 and TM2 forms intermolecular contacts with the extracellular loop 3 between TM5 and TM6, thus possibly stabilizing the dimeric structure. Third, there is not a coupling helix 1 in the intracellular loop connecting TM 2 and TM3, which is present in all of the available ABC efflux transporter structures and could play a crucial role in the communications between the NBD and the TMD of the same monomer. Residues 285 – 380 in the putative linker region connecting the NBD to the MSD of BCRP could not be modeled due to the lack of appropriate templates. This linker region in BCRP is much longer than those in other ABC transporters, part of which may serve as the coupling helix 1. As revealed in crystal structures of Sav1866, MsbA, and P-gp, there is a coupling helix 2 that crosses over and associates with the NBD of the opposite monomer. Such a coupling helix 2 also exists in the BCRP models in the intracellular loop connecting TM4 and TM5 which may define the relative orientation of individual domains and allow flexibility in the transporter. Forth, comparing the open and closed inward-facing apo forms Fig. (4A and 4B), there is a rotation of ~30 °C for TM4 and TM5 of BCRP which subtend a narrower angle to the rest of the MSD in the closed apo form. Similar changes in the angle of TM4 and TM5 may also occur in other ABC transporters such as Sav1866 and P-gp as pointed out by Kos and Ford [101]. It is worth noting that it is TM4 and TM5 that mediate the coupling helix 2 to cross over the opposing NBD. The rotation and change in the angle of TM4 and TM5 also force TMs 1 – 3 and 6 to similarly change their positions, resulting in a more closed intracellular entry in the closed apo structure as compared with the open apo structure. Such a domain rearrangement would alter the shape of the substrate-binding cavity which is formed primarily by residues in the TMs by making it more compact in the presence of substrate binding. Fifth, in the outward-facing form of BCRP, there are even more drastic conformational changes, with the intracellular entry completely closed and a wide open V-shaped gap in the extracellular side extending to the lipid bilayer Fig. (4C). This V-shaped gap may allow the release of substrates from BCRP after ATP binding/hydrolysis. Because the intracellular entry in the outward-facing form is completely closed, the two NBDs of the BCRP dimer form the so-called ATP sandwich. Finally, comparing the membrane topology of P-gp and BCRP, the extracellular loop between TM5 and TM6 in BCRP is much larger than the corresponding region in P-gp [72]. Recent studies suggest that this extracellular loop may be critical in modulating substrate binding [102] as well as stability and ubiquitin-mediated degradation of BCRP [103]. In our models, this extracellular loop closely contacts with the extracellular loop between TM1 and TM2. Such contacts may help stabilize the BCRP dimer through non-covalent interactions. Consistent with this, Xu et al. [87] demonstrated that the TM5-loop-TM6 fragment may play a critical role in BCRP oligomerization. A monoclonal antibody 5D3 that recognizes a conformational sensitive epitope in this extracellular loop has been highly useful for biochemical characterization of BCRP in terms of interactions of the transporter with substrates or inhibitors [63, 102, 104] or conformational changes induced by point mutations [105].
Do the homology models interpret existing biochemical data? First of all, docking calculations of various BCRP substrates to the closed apo form did suggest the existence of multiple substrate binding sites in the central cavity which is primarily formed by TM α-helices [105]. Second, Arg482 has been extensively analyzed by site-directed mutagenesis and found to be crucial for substrate specificity and transport activity [106–109]. In the homology models, Arg482 in TM3 is located in the central cavity close to the cytosolic membrane interface with the side chain pointing towards the drug translocation pathway. Docking calculations indicated that Arg482 may directly interact with mitoxantrone and Hoechst33342, but not with prazosin and SN-38. This is consistent with previous studies showing that resistance to mitoxantrone was increased, but resistance to SN-38 or efflux of prazosin was not significantly affected, by the mutations of Arg482 [107, 109]. This also appears to be in good agreement with the studies showing that prazosin binds to a pharmacologically distinct site relative to other BCRP substrates such as mitoxantrone and Hoechst33342 [64] and the binding of a prazosin derivative to BCRP was relatively unaffected by the mutations of Arg482 [59]. Thus, the homology models could be used to interpret existing data. Further biochemical studies are needed to validate these models. If validated, these models may be valuable for the development of pharmacophores of BCRP substrates or inhibitors.
6. MUTAGENESIS STUDIES
6.1. Natural Variants of BCRP
Naturally occurring variants of BCRP caused by single nucleotide polymorphisms in the coding region of BCRP gene may have significant physiological and pharmacological relevance. A large body of data is available [19, 110] and summarized in Table 2. The most important variant is Q141K, which occurs in Japanese and Chinese populations at high allele frequencies (30 – 60%) and in Caucasians and African-American populations at relatively low allele frequencies (5 – 10%) [111–112]. Several studies consistently revealed that Q141K had a lower protein expression level than wild-type BCRP in both transfected cells and human tissues [113–115]. The transport activity of Q141K in vivo would be expected to be decreased compared with wild-type BCRP owing to its lower level of protein expression. Indeed, human subjects carrying the Q141 variant often had higher plasma levels of BCRP substrate drugs than the subjects carrying wild-type BCRP [116–118]. A recent study has revealed that Q141K undergoes increased lysosomal and proteasomal degradations than wild-type BCRP, possibly explaining the lower level of protein expression of the variant [119]. A systematic study of 18 natural variants of BCRP expressed in insect cells showed that the variants Q126stop, F208S, S248P, E334stop, and S441N were defective in porphyrin transport, whereas F489L displayed approximately 10% of the transport activity of wild-type BCRP [120]. These are rare variants with allele frequencies generally less than 2% [111–112, 120–121].
Table 2.
Mutagenesis Studies of BCRP
| Mutation | Position in BCRP | Effect | Reference |
|---|---|---|---|
| Natural variants | |||
| V12Ma | N-terminus | No major effect on membrane expression and function | [114, 120–121] |
| G51Ca | N-terminus | No major effect on membrane expression and function | [120] |
| Q141Ka | NBD | Lower protein expression and transport activity | [112, 114, 121] |
| T153M, Q166E, I206La | NBD | No major effect on expression and function | [120, 137] |
| Q126stop, F208S, E334stopa | NBD | No expression and transport activity | [120] |
| S248Pa | NBD | Well expressed, but with no transport activity | [120] |
| F489La | TM3 | Well expressed, no transport activity for porphyrin and methotrexate | [120] |
| F571Ia | TM5 | Well expressed, slightly decreased activity depending on substrates | [120] |
| N590Y, D620Na | Extracellular loop 3 | Well expressed, slightly decreased activity depending on substrates | [120, 137] |
| Artificial mutants | |||
| K86M, K86I | NBD, Walker A | No transport or ATPase activity, but ATP binding retained No transport or ATPase activity, altered subcellular expression |
[70] [138] |
| E211Q | NBD, Walker B | Completely abolished ATPase and transport activity | [124] |
| The 315–316 deletion | NBD | Impaired BXP-21 antibody recognition, but with no effect on function | [139] |
| R383A, R383G | Linker region | Increased protein degradation and decreased protein strability Altered subcellular distribution and glycosylation |
[126] |
| T402A, T402L, T402R | TM1 | Well expressed, impaired transport activity | [97], b |
| G406L, G410L, G406L/G410L | GXXXG motif in TM1 | Well expressed, impaired transport and ATPase activity | [127] |
| N418Q | Extracellular loop 1 | Well expressed, not a N-linked glycosylation site | [92] |
| R426A | Extracellular loop 1 | Increased protein degradation and decreased protein stability | c |
| E446X | Extracellular loop 1 | Well expressed, but with no drug resistance | [109] |
| K452A, H457A | TM2 | Well expressed, increased transport activity and drug resistance | [105] |
| K453D, R465A | TM2 | Well expressed, lower transport activity and drug resistance | [105] |
| K473A | Intracellular loop 1 | Well expressed, no effect on transport activity and drug resistance | [105] |
| R482G, R482T, R482X | TM3 | Well expressed, with altered substrate specificity. For example, “gain-of- function” for transport of daunorubicin and rhodamine 123; but completely lost transport activity for methotrexate | [106–109] [122, 140] |
| G553L, G553E | Intracellular loop 2 | Impaired trafficking, expression, and N-linked glycosylation | [130] |
| L554P | Intracellular loop 2 | Completely lost resistance to SN-38 and mitoxantrone | [76] |
| N557X | Intracellular loop 2 | Lower resistance to SN-38 for N557H, N557D, and N557E Lower resistance to mitoxantrone for N557H, N557E, but not for N557D |
[109] |
| N596Q | Extracellular loop 3 | N-linked glycosylation site | [92–93] |
| C592A, C608A, C592A/C608A | Extracellular loop 3 | Impaired expression and function for C592A and C608A Partially restored plasma membrane expression for C592A/C608A Transport of mitoxantrone was normal, but transport of BODIPY-prazosin was impaired for C592A/C608A |
[82] |
| C592A/C603A/C608A | Extracellular loop 3 | Decreased expression with no measurable activity | [82] |
| C603A, C608A | Extracellular loop 3 | Involved in BCRP dimerization | [86] |
| C603A | Extracellular loop 3 | No effect on membrane expression and function; disulfide bridge | [82–84] |
| No effect on membrane expression, function and dimerization in vivo | [80] | ||
| C592S, C603S, C608S | Extracellular loop 3 | Impaired expression and function | [86] |
| C592S/C603S/C608S | Extracellular loop 3 | Impaired expression and subcellular localization. Retained significant activity with alteration in substrate specificity |
[86] |
| H630X | TM6 | No major effect on drug resistance | [109] |
| Cys-less BCRP | Well expressed in Sf9 cells, but with no transport activity | [85] | |
The natural variants of BCRP are illustrated in italics. The allele frequencies of individual single nucleotide polymorphisms among different ethnic groups have been detailed in references [120, 141];
Ni et al., manuscript submitted;
Unpublished results. NBD, nucleotide binding domain. TM, transmembrane α-helix. Positions of the residues in BCRP are based on the membrane topology illustrated in Fig. 3.
6.2. Non-Natural BCRP Mutants
Much attention has also been devoted to elucidating the structure and function of BCRP by site-directed mutagenesis of particular residues of interest, and the data is summarized in Table 2. There are basically four categories of non-natural BCRP mutants reported thus far as discussed below. First, mutations do not affect plasma membrane expression, but alter substrate specificity and/or overall transport activity. In this category, Arg482 has been most extensively characterized. Arg482 is an important determinant of substrate specificity and transport activity. For example, wild-type BCRP does not transport daunorubicin, rhodamine 123, and Lyso-Tracker Green; however, the mutants R482T and R482G do [108]. Therefore, for these substrates, the mutations of Arg482 result in “gain-of-function”. On the other hand, mitoxantrone, BODIPY-prazosin, and Hoechst 33342 are substrates of both wild-type BCRP and the two mutants [107–108]. Methotrexate is a substrate only for wild-type BCRP [122]. Mutations of Arg482 generally increased resistance to mitoxantrone, but did not have much effect on resistance to SN-38 [109]. Thus, the effect of Arg482 on transport activity is substrate-dependent. Although Arg482 is likely located in the substrate translocation pathway, exactly how mutations of Arg482 change substrate specificity and transport activity is still unknown. Alqawi et al. [123] showed that wild-type BCRP was more intensely photo-labeled with a photo-active analog of rhodamine 123 than R482T even though wild-type BCRP does not transport rhodamine 123. The positive charge of Arg482 does not seem to be a key determinant because the substitution of Arg482 with Lys of the same charge resulted in a complete loss of drug resistance [109].
Ala substitution of the basic residue His457 within TM2 has been shown to increase transport activity and drug resistance several-fold and caused significant conformational change [105]. Docking calculations suggest that His457 may be directly involved in substrate binding. Miwa et al. [109] reported that amino acid substitutions of Glu446 resulted in complete loss of drug resistance to SN-38 and mitoxantrone. These authors also found that mutations of Asn557 tended to selectively decrease resistance to mitoxantrone and/or SN-38. Similarly, Leu substitution of Phe431 retained full transport activity for porphyrin, but not for methotrexate [120]. According to the membrane topology of BCRP Fig. (1), Phe431, Glu446 and Asn557 are located in the extracellular loop 1 connecting TM1 and TM2 and the intracellular loop 2 connecting TM4 and TM5, respectively. How can amino acid substitutions of residues in the cellular loops between TM α-helices affect transport activity remains to be determined. Mutation of Glu211 adjacent to the Walker B motif of the NBD also belongs to this category. Hou et al. [124] demonstrated that Gln substitution of Glu211 had no effect on protein expression, but completely abolished ATP-dependent transport of methotrexate and ATPase activity. This confirms that Glu211 directly adjacent to Asp210 within the Walker B motif may activate the water molecule to attack the bound Mg·ATP in the ATP hydrolytic cycle as previously suggested for other ABC transporter [125]. This capability would be lost by mutation at Glu211, thus leading to inactivation of BCRP.
In the second category, mutations affect biogenesis with decreased stability, lower expression and/or altered subcellular distribution of BCRP. Arg383 in the linker region has been shown to be crucial for biogenesis of BCRP. Mutations of Arg383 resulted in a significant decrease in the protein level, partial retention in the endoplasm reticulum, and altered glycosylation [126]. The lower levels of Arg383 mutants may be caused by rapid degradation by the proteasome, and the treatment with mitoxantrone assisted in protein maturation [126]. Likewise, Ala substitution of Arg426 in the extracellular loop 1 also caused rapid degradation, leading to lower levels of expression of intact BCRP and transport activity (unpublished results).
Gly406 and Gly410 in the GXXXG dimerization motif in TM1 of BCRP have been investigated for their impact on potential dimerization [97, 127]. Single or double Leu substitutions of Gly406 and Gly410 resulted in lower expression possibly caused by increased degradation, as well as impaired or complete loss of ATP hydrolysis and substrate transport [127]. Some of these mutants were partially retained in the endoplasm reticulum. Triple mutations of Gly406 and Gly410 with Thr402 which is just adjacent to the GXXXG motif resulted in an even more reduced level of expression [97]. These results suggest that mutations within or near the GXXXG motif affect proper folding of BCRP. Since the GXXXG motif and Thr residues in TMs are known to be important for interhelical interactions [128–129], the GXXXG motif and Thr402 in BCRP were suggested to play a role in dimerization [97, 127]. In a Sav1866-based model, the GXXXG motif and Thr402 in TM1 interact with TMs 5 and 6 of the opposite BCRP monomer, contributing to dimerization [97]. However, the double or triple mutants of Thr402, Gly406 and Gly410 could still form dimers by chemical cross-linking [97, 127]. Thus, these data do not conclusively support the role of the GXXXG motif and Thr402 in BCRP dimerization. In the homology models shown in Fig. (2), TM1 interacts with TM2 and/or TM3 of the same BCRP monomer. The exact interaction partners of the GXXXG motif and its role in BCRP dimerization remain to be determined.
Leu or Glu substitution of Gly553 led to rapid degradation and retention in the endoplasm reticulum of BCRP [130], suggesting that Gly553 is critical for plasma membrane targeting and proper folding of the transporter. In the models shown in Fig. (2), Gly553 is located in the intracellular loop connecting TM4 and TM5. This intracellular loop contains a coupling helix interacting with the NBD of the opposite BCRP monomer. Whether mutations of Gly553 affect the communication between the NBD and the MSD and thus the activity and overall integrity of BCRP remains to be investigated.
Ala substitution of Cys592 and Cys608 has been shown to impair plasma membrane targeting and function of BCRP; however, the double mutation (C592A/C608A) partially restored plasma membrane expression [82]. When the three Cys residues Cys592, Cys603 and Cys608 were mutated to Ala simultaneously, the expression level of the triple-mutant in HEK cells was drastically decreased with no measurable activity [82]. In contrast, Liu et al. [85] showed that mutations of the three Cys residues did not affect BCRP expression and transport activity in Sf9 insect cells. The recent study by Shigeta et al. [86] also demonstrated that the triple-mutant C592S/C603S/C608S in PA317 cells retained substantial activity with some alteration in substrate recognition, although its level of expression was significantly decreased and its subcellular distribution was altered. The study by Wakabayashi et al. provided evidence that BCRP lacking the disulfide bond formed by Cys592 and Cys608 is misfolded and the misfolded protein undergoes ubiquitin-mediated protein degradation in proteasomes [103]. Overall, these results, although not all consistent, appear to suggest that these Cys residues are important for structural integrity and proper folding of BCRP. Consistent with this, Cys-less BCRP can be well expressed in Sf9 insect cells, but lacks transport activity [85]. This precludes the use of Cys-less BCRP for structure-function analyses.
In the third category, mutations cause alterations in chemical modifications such as N-linked glycosylation or disulfide bond formation in BCRP. The only N-linked glycosylation site in BCRP is Asn596 which is located in the extracellular loop 3 connecting TM5 and TM6 Fig. (3). Gln substitution of Asn596, but not the other two Asn residues (Asn418 and Asn557), produced a smaller molecular weight BCRP band on SDS-PAGE due to the lack of glycosylation [92–93]. The lack of glycosylation did not appear to affect plasma membrane targeting and transport activity of BCRP; however, glycosylation may stabilize BCRP because disruption of N-linked glycosylation has been shown to enhance ubiquitin-mediated proteasomal degradation of the transporter [131]. Several studies have elucidated the role of Cys603 in dimer/oligomer formation of BCRP [82–84]. Ala substitution of Cys603 led to migration of BCRP bands to a size corresponding to BCRP monomers, but had no effect on plasma membrane expression and function, suggesting that Cys603 participates in intermolecular disulfide bond formation. An earlier mutagenesis study [82] suggests that Cys592 and Cys608 could form an intramolecular disulfide bond; however, the recent studies [85–86] indicate that Cys592 and Cys608 may participate in intermolecular disulfide bond formation. Therefore, contrasting data exist in the present literature with respect to the role of the three extracellular Cys residues in intra- or intermolecular disulfide bond formation.
In the fourth category, mutations do not have major effects on both plasma membrane expression and function of BCRP. Such mutants include K473A and H630X, suggesting that these residues are likely not critical for expression and function of BCRP.
7. CONCLUSION AND FUTURE PERSPECTIVE
Much progress has been made in understanding the in vitro and in vivo function of BCRP, such as the identification of an increasing number of substrates and inhibitors as well as the role of BCRP in the absorption, excretion and distribution of substrate drugs, xenobiotics, and endogenous substrates. BCRP is also highly expressed in the side population of stem cells, likely providing a protective function for stem cells. Although BCRP has long been recognized to play an important role in multidrug resistance of cancer cells, it has not been possible to circumvent clinical drug resistance in cancer patients by the use of BCRP inhibitors. This awaits the further development of more potent and highly selective BCRP inhibitors and the appropriate design of clinical trials in the future. In addition, we believe that a successful translation of scientific findings about BCRP into the clinic would require a full understanding of how BCRP acts to transport drugs and xenobiotics. For example, how BCRP recognizes its substrates and what determines its broad substrate specificity? How a substrate is transported by BCRP and how transport of substrate is coupled with ATP hydrolysis? Is the minimal functional unit of BCRP a homodimer or homooligomer, and how the dimeric or oligomeric state of BCRP might affect its activity? How BCRP monomers are organized to form a functional homodimer or higher order oligomer? At present, we virtually do not know any answers to these questions, and studies to address these questions are desperately needed. Mutagenesis studies in recent years have begun to identify amino acid residues, particularly those in the membrane-spanning domain, that are critical in determining substrate specificity, transport activity, and proper folding of BCRP. Homology models have also been developed to interpret the existing data and may provide guidance for further biochemical studies. Further biochemical studies, such as site-direct mutagenesis of amino acid residues in critical protein domains, identification of residues in the putative drug binding sites by photo-affinity labeling and mass spectroscopy analysis, and mapping the contact interface between BCRP monomers in a dimer or oligomer and between the MSD and the NBD, in turn could help refine and validate these homology models. Computational methods such as 3D-QSAR and molecular dynamic simulations using biochemical data as constraints may further refine the models, and help predict conformational changes associated with the transport process and characterize drug interactions in the putative drug binding cavity. In the absence of high resolution 3D structures of BCRP, the refined models may help rational design of substrate drugs or inhibitors that are highly selective and potent for BCRP. Nevertheless, in order to gain a clear understanding of transport mechanism, the ultimate goal is to resolve the structure of BCRP at atomic resolutions that is supported by most of the biochemical data. This is a big challenge. However, with the 3D structures of P-gp [94] and 2D structures of BCRP [72] resolved at relatively high resolutions, the possibility to achieve this goal is high.
Acknowledgments
We acknowledge financial support from the National Institutes of Health grant GM073715 (to Q.M). M.F.R. was supported by a VIP fellowship and in part by a grant 081406/Z/06/Z from the Wellcome Trust.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- 1.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95(26):15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59(1):8–13. [PubMed] [Google Scholar]
- 3.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58(23):5337–5339. [PubMed] [Google Scholar]
- 4.Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61(1):3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. Aaps J. 2005;7(1):E118–133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86(4):179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 7.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26(1):39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 8.Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP, Legrand O. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10(23):7896–7902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- 9.Wilson CS, Davidson GS, Martin SB, Andries E, Potter J, Harvey R, Ar K, Xu Y, Kopecky KJ, Ankerst DP, Gundacker H, Slovak ML, Mosquera-Caro M, Chen IM, Stirewalt DL, Murphy M, Schultz FA, Kang H, Wang X, Radich JP, Appelbaum FR, Atlas SR, Godwin J, Willman CL. Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood. 2006;108(2):685–696. doi: 10.1182/blood-2004-12-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkadi B, Orban TI, Szakacs G, Varady G, Schamberger A, Erdei Z, Szebenyi K, Homolya L, Apati A. Evaluation of ABCG2 expression in human embryonic stem cells: crossing the same river twice? Stem Cells. 2010;28(1):174–176. doi: 10.1002/stem.262. [DOI] [PubMed] [Google Scholar]
- 11.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99(2):507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 12.Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 2002;99(19):12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huls M, Russel FG, Masereeuw R. The role of ATP binding cassette transporters in tissue defense and organ regeneration. J Pharmacol Exp Ther. 2009;328(1):3–9. doi: 10.1124/jpet.107.132225. [DOI] [PubMed] [Google Scholar]
- 14.An Y, Ongkeko WM. ABCG2: the key to chemoresistance in cancer stem cells? Expert Opin Drug Metab Toxicol. 2009;5(12):1529–1542. doi: 10.1517/17425250903228834. [DOI] [PubMed] [Google Scholar]
- 15.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61(8):3458–3464. [PubMed] [Google Scholar]
- 16.Polgar O, Robey RW, Bates SE. ABCG2: structure, function and role in drug response. Expert Opin Drug Metab Toxicol. 2008;4(1):1–15. doi: 10.1517/17425255.4.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Mao Q. BCRP/ABCG2 in the placenta: expression, function and regulation. Pharm Res. 2008;25(6):1244–1255. doi: 10.1007/s11095-008-9537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, Mesman E, Dale TC, Schinkel AH. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11(2):127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- 19.Tamura A, Onishi Y, An R, Koshiba S, Wakabayashi K, Hoshijima K, Priebe W, Yoshida T, Kometani S, Matsubara T, Mikuriya K, Ishikawa T. In vitro evaluation of photosensitivity risk related to genetic polymorphisms of human ABC transporter ABCG2 and inhibition by drugs. Drug Metab Pharmacokinet. 2007;22(6):428–440. doi: 10.2133/dmpk.22.428. [DOI] [PubMed] [Google Scholar]
- 20.Keskitalo JE, Pasanen MK, Neuvonen PJ, Niemi M. Different effects of the ABCG2 c.421C>A SNP on the pharmacokinetics of fluvastatin, pravastatin and simvastatin. Pharmacogenomics. 2009;10(10):1617–1624. doi: 10.2217/pgs.09.85. [DOI] [PubMed] [Google Scholar]
- 21.Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2009;86(2):197–203. doi: 10.1038/clpt.2009.79. [DOI] [PubMed] [Google Scholar]
- 22.Sissung TM, Baum CE, Kirkland CT, Gao R, Gardner ER, Figg WD. Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010;44(2):152–167. doi: 10.1007/s12033-009-9220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106(25):10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamurthy P, Xie T, Schuetz JD. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol Ther. 2007;114(3):345–358. doi: 10.1016/j.pharmthera.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 25.van Herwaarden AE, Wagenaar E, Merino G, Jonker JW, Rosing H, Beijnen JH, Schinkel AH. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol Cell Biol. 2007;27(4):1247–1253. doi: 10.1128/MCB.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grube M, Reuther S, Meyer Zu Schwabedissen H, Kock K, Draber K, Ritter CA, Fusch C, Jedlitschky G, Kroemer HK. Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab Dispos. 2007;35(1):30–35. doi: 10.1124/dmd.106.011411. [DOI] [PubMed] [Google Scholar]
- 27.Arimori K, Kuroki N, Hidaka M, Iwakiri T, Yamsaki K, Okumura M, Ono H, Takamura N, Kikuchi M, Nakano M. Effect of P-glycoprotein modulator, cyclosporin A, on the gastrointestinal excretion of irinotecan and its metabolite SN-38 in rats. Pharm Res. 2003;20(6):910–917. doi: 10.1023/a:1023847521767. [DOI] [PubMed] [Google Scholar]
- 28.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 29.Dohse M, Scharenberg C, Shukla S, Robey RW, Volkmann T, Deeken JF, Brendel C, Ambudkar SV, Neubauer A, Bates SE. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos. 2010;38(8):1371–1380. doi: 10.1124/dmd.109.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther. 2010;333(3):788–796. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]
- 31.Polli JW, Olson KL, Chism JP, John-Williams LS, Yeager RL, Woodard SM, Otto V, Castellino S, Demby VE. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethy l]amino}methyl)-2-furyl]-4-quinazolinamine, GW572016) Drug Metab Dispos. 2009;37(2):439–442. doi: 10.1124/dmd.108.024646. [DOI] [PubMed] [Google Scholar]
- 32.Pollex EK, Anger G, Hutson J, Koren G, Piquette-Miller M. Breast cancer resistance protein (BCRP)-mediated glyburide transport: effect of the C421A/Q141K BCRP single-nucleotide polymorphism. Drug Metab Dispos. 2010;38(5):740–744. doi: 10.1124/dmd.109.030791. [DOI] [PubMed] [Google Scholar]
- 33.Pal A, Mehn D, Molnar E, Gedey S, Meszaros P, Nagy T, Glavinas H, Janaky T, von Richter O, Bathori G, Szente L, Krajcsi P. Cholesterol potentiates ABCG2 activity in a heterologous expression system: improved in vitro model to study function of human ABCG2. J Pharmacol Exp Ther. 2007;321(3):1085–1094. doi: 10.1124/jpet.106.119289. [DOI] [PubMed] [Google Scholar]
- 34.Yoshikawa M, Ikegami Y, Hayasaka S, Ishii K, Ito A, Sano K, Suzuki T, Togawa T, Yoshida H, Soda H, Oka M, Kohno S, Sawada S, Ishikawa T, Tanabe S. Novel camptothecin analogues that circumvent ABCG2-associated drug resistance in human tumor cells. Int J Cancer. 2004;110(6):921–927. doi: 10.1002/ijc.20216. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa H, Saito H, Ikegami Y, Aida-Hyugaji S, Sawada S, Ishikawa T. Molecular modeling of new camptothecin analogues to circumvent ABCG2-mediated drug resistance in cancer. Cancer Lett. 2006;234(1):81–89. doi: 10.1016/j.canlet.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed-Belkacem A, Pozza A, Macalou S, Perez-Victoria JM, Boumendjel A, Di Pietro A. Inhibitors of cancer cell multidrug resistance mediated by breast cancer resistance protein (BCRP/ABCG2) Anticancer Drugs. 2006;17(3):239–243. doi: 10.1097/00001813-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Tamaki H, Satoh H, Hori S, Ohtani H, Sawada Y. Inhibitory effects of herbal extracts on breast cancer resistance protein (BCRP) and structure-inhibitory potency relationship of isoflavonoids. Drug Metab Pharmacokinet. 2010;25(2):170–179. doi: 10.2133/dmpk.25.170. [DOI] [PubMed] [Google Scholar]
- 38.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1(6):417–425. [PubMed] [Google Scholar]
- 39.van Loevezijn A, Allen JD, Schinkel AH, Koomen GJ. Inhibition of BCRP-mediated drug efflux by fumitremorgin-type indolyl diketopiperazines. Bioorg Med Chem Lett. 2001;11(1):29–32. doi: 10.1016/s0960-894x(00)00588-6. [DOI] [PubMed] [Google Scholar]
- 40.de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146(2):117–126. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- 41.Ozvegy-Laczka C, Hegedus T, Varady G, Ujhelly O, Schuetz JD, Varadi A, Keri G, Orfi L, Nemet K, Sarkadi B. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65(6):1485–1495. doi: 10.1124/mol.65.6.1485. [DOI] [PubMed] [Google Scholar]
- 42.Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, Traxler P. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64(7):2333–2337. doi: 10.1158/0008-5472.can-03-3344. [DOI] [PubMed] [Google Scholar]
- 43.Gandhi YA, Morris ME. Structure-activity relationships and quantitative structure-activity relationships for breast cancer resistance protein (ABCG2) Aaps J. 2009;11(3):541–552. doi: 10.1208/s12248-009-9132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicolle E, Boumendjel A, Macalou S, Genoux E, Ahmed-Belkacem A, Carrupt PA, Di Pietro A. QSAR analysis and molecular modeling of ABCG2-specific inhibitors. Adv Drug Deliv Rev. 2009;61(1):34–46. doi: 10.1016/j.addr.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Saito H, An R, Hirano H, Ishikawa T. Emerging new technology: QSAR analysis and MO Calculation to characterize interactions of protein kinase inhibitors with the human ABC transporter, ABCG2 (BCRP) Drug Metab Pharmacokinet. 2010;25(1):72–83. doi: 10.2133/dmpk.25.72. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa T, Nakagawa H. Human ABC transporter ABCG2 in cancer chemotherapy and pharmacogenomics. J Exp Ther Oncol. 2009;8(1):5–24. [PubMed] [Google Scholar]
- 47.Brooks TA, Kennedy DR, Gruol DJ, Ojima I, Baer MR, Bernacki RJ. Structure-activity analysis of taxane-based broad-spectrum multidrug resistance modulators. Anticancer Res. 2004;24(2A):409–415. [PubMed] [Google Scholar]
- 48.Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64(12):4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S, Yang X, Coburn RA, Morris ME. Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem Pharmacol. 2005;70(4):627–639. doi: 10.1016/j.bcp.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed-Belkacem A, Pozza A, Munoz-Martinez F, Bates SE, Castanys S, Gamarro F, Di Pietro A, Perez-Victoria JM. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 2005;65(11):4852–4860. doi: 10.1158/0008-5472.CAN-04-1817. [DOI] [PubMed] [Google Scholar]
- 51.Katayama K, Masuyama K, Yoshioka S, Hasegawa H, Mitsuhashi J, Sugimoto Y. Flavonoids inhibit breast cancer resistance protein-mediated drug resistance: transporter specificity and structure-activity relationship. Cancer Chemother Pharmacol. 2007;60(6):789–797. doi: 10.1007/s00280-007-0426-7. [DOI] [PubMed] [Google Scholar]
- 52.Sugimoto Y, Tsukahara S, Imai Y, Ueda K, Tsuruo T. Reversal of breast cancer resistance protein-mediated drug resistance by estrogen antagonists and agonists. Mol Cancer Ther. 2003;2(1):105–112. [PubMed] [Google Scholar]
- 53.An R, Hagiya Y, Tamura A, Li S, Saito H, Tokushima D, Ishikawa T. Cellular phototoxicity evoked through the inhibition of human ABC transporter ABCG2 by cyclin-dependent kinase inhibitors in vitro. Pharm Res. 2009;26(2):449–458. doi: 10.1007/s11095-008-9738-5. [DOI] [PubMed] [Google Scholar]
- 54.Pick A, Muller H, Wiese M. Structure-activity relationships of new inhibitors of breast cancer resistance protein (ABCG2) Bioorg Med Chem. 2008;16(17):8224–8236. doi: 10.1016/j.bmc.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 55.Saito H, Hirano H, Nakagawa H, Fukami T, Oosumi K, Murakami K, Kimura H, Kouchi T, Konomi M, Tao E, Tsujikawa N, Tarui S, Nagakura M, Osumi M, Ishikawa T. A new strategy of high-speed screening and quantitative structure-activity relationship analysis to evaluate human ATP-binding cassette transporter ABCG2-drug interactions. J Pharmacol Exp Ther. 2006;317(3):1114–1124. doi: 10.1124/jpet.105.099036. [DOI] [PubMed] [Google Scholar]
- 56.Nicolle E, Boccard J, Guilet D, Dijoux-Franca MG, Zelefac F, Macalou S, Grosselin J, Schmidt J, Carrupt PA, Di Pietro A, Boumendjel A. Breast cancer resistance protein (BCRP/ABCG2): new inhibitors and QSAR studies by a 3D linear solvation energy approach. Eur J Pharm Sci. 2009;38(1):39–46. doi: 10.1016/j.ejps.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Matsson P, Englund G, Ahlin G, Bergstrom CA, Norinder U, Artursson P. A global drug inhibition pattern for the human ATP-binding cassette transporter breast cancer resistance protein (ABCG2) J Pharmacol Exp Ther. 2007;323(1):19–30. doi: 10.1124/jpet.107.124768. [DOI] [PubMed] [Google Scholar]
- 58.Nakanishi T, Doyle LA, Hassel B, Wei Y, Bauer KS, Wu S, Pumplin DW, Fang HB, Ross DD. Functional characterization of human breast cancer resistance protein (BCRP, ABCG2) expressed in the oocytes of Xenopus laevis. Mol Pharmacol. 2003;64(6):1452–1462. doi: 10.1124/mol.64.6.1452. [DOI] [PubMed] [Google Scholar]
- 59.Ejendal KF, Diop NK, Schweiger LC, Hrycyna CA. The nature of amino acid 482 of human ABCG2 affects substrate transport and ATP hydrolysis but not substrate binding. Protein Sci. 2006;15(7):1597–1607. doi: 10.1110/ps.051998406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alqawi O, Bates S, Georges E. Arginine482 to threonine mutation in the breast cancer resistance protein ABCG2 inhibits rhodamine 123 transport while increasing binding. Biochem J. 2004;382(Pt 2):711–716. doi: 10.1042/BJ20040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDevitt CA, Collins RF, Conway M, Modok S, Storm J, Kerr ID, Ford RC, Callaghan R. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure. 2006;14(11):1623–1632. doi: 10.1016/j.str.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45(29):8940–8951. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 63.Shukla S, Robey RW, Bates SE, Ambudkar SV. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos. 2009;37(2):359–365. doi: 10.1124/dmd.108.024612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark R, Kerr ID, Callaghan R. Multiple drug binding sites on the R482G isoform of the ABCG2 transporter. Br J Pharmacol. 2006;149(5):506–515. doi: 10.1038/sj.bjp.0706904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDevitt CA, Crowley E, Hobbs G, Starr KJ, Kerr ID, Callaghan R. Is ATP binding responsible for initiating drug translocation by the multidrug transporter ABCG2? Febs J. 2008;275(17):4354–4362. doi: 10.1111/j.1742-4658.2008.06578.x. [DOI] [PubMed] [Google Scholar]
- 66.Pozza A, Perez-Victoria JM, Sardo A, Ahmed-Belkacem A, Di Pietro A. Purification of breast cancer resistance protein ABCG2 and role of arginine-482. Cell Mol Life Sci. 2006;63(16):1912–1922. doi: 10.1007/s00018-006-6159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113(Pt 11):2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 68.McDevitt CA, Collins R, Kerr ID, Callaghan R. Purification and structural analyses of ABCG2. Adv Drug Deliv Rev. 2009;61(1):57–65. doi: 10.1016/j.addr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Mao Q, Conseil G, Gupta A, Cole SP, Unadkat JD. Functional expression of the human breast cancer resistance protein in Pichia pastoris. Biochem Biophys Res Commun. 2004;320(3):730–737. doi: 10.1016/j.bbrc.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 70.Ozvegy C, Varadi A, Sarkadi B. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation. J Biol Chem. 2002;277(50):47980–47990. doi: 10.1074/jbc.M207857200. [DOI] [PubMed] [Google Scholar]
- 71.Glavinas H, Kis E, Pal A, Kovacs R, Jani M, Vagi E, Molnar E, Bansaghi S, Kele Z, Janaky T, Bathori G, von Richter O, Koomen GJ, Krajcsi P. ABCG2 (breast cancer resistance protein/mitoxantrone resistance-associated protein) ATPase assay: a useful tool to detect drug-transporter interactions. Drug Metab Dispos. 2007;35(9):1533–1542. doi: 10.1124/dmd.106.014605. [DOI] [PubMed] [Google Scholar]
- 72.Rosenberg MF, Bikadi Z, Chan J, Liu X, Ni Z, Cai X, Ford RC, Mao Q. The human breast cancer resistance protein (BCRP/ABCG2) shows conformational changes with mitoxantrone. Structure. 2010;18(4):482–493. doi: 10.1016/j.str.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ozvegy C, Litman T, Szakacs G, Nagy Z, Bates S, Varadi A, Sarkadi B. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun. 2001;285(1):111–117. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- 74.Hegedus C, Szakacs G, Homolya L, Orban TI, Telbisz A, Jani M, Sarkadi B. Ins and outs of the ABCG2 multidrug transporter: an update on in vitro functional assays. Adv Drug Deliv Rev. 2009;61(1):47–56. doi: 10.1016/j.addr.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Mao Q, Leslie EM, Deeley RG, Cole SP. ATPase activity of purified and reconstituted multidrug resistance protein MRP1 from drug-selected H69AR cells. Biochim Biophys Acta. 1999;1461(1):69–82. doi: 10.1016/s0005-2736(99)00150-9. [DOI] [PubMed] [Google Scholar]
- 76.Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, Sugimoto Y. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer. 2002;97(5):626–630. doi: 10.1002/ijc.10100. [DOI] [PubMed] [Google Scholar]
- 77.Litman T, Jensen U, Hansen A, Covitz KM, Zhan Z, Fetsch P, Abati A, Hansen PR, Horn T, Skovsgaard T, Bates SE. Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim Biophys Acta. 2002;1565(1):6–16. doi: 10.1016/s0005-2736(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 78.Bhatia A, Schafer HJ, Hrycyna CA. Oligomerization of the human ABC transporter ABCG2: evaluation of the native protein and chimeric dimers. Biochemistry. 2005;44(32):10893–10904. doi: 10.1021/bi0503807. [DOI] [PubMed] [Google Scholar]
- 79.Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half ABC transporter ABCG2/BCRP/MXR/ABCP in plasma membranes. J Biol Chem. 2004;279(19):19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 80.Ni Z, Mark ME, Cai X, Mao Q. Fluorescence Resonance Energy Transfer (FRET) Analysis Demonstrates Dimer/Oligomer Formation of the Human Breast Cancer Resistance Protein (BCRP/ABCG2) in Intact Cells. Int J Biochem Mol Biol. 2010;1(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- 81.Mo W, Zhang JT. Oligomerization of human ATP-binding cassette transporters and its potential significance in human disease. Expert Opin Drug Metab Toxicol. 2009;5(9):1049–1063. doi: 10.1517/17425250903124371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henriksen U, Fog JU, Litman T, Gether U. Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J Biol Chem. 2005;280(44):36926–36934. doi: 10.1074/jbc.M502937200. [DOI] [PubMed] [Google Scholar]
- 83.Kage K, Fujita T, Sugimoto Y. Role of Cys-603 in dimer/oligomer formation of the breast cancer resistance protein BCRP/ABCG2. Cancer Sci. 2005;96(12):866–872. doi: 10.1111/j.1349-7006.2005.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wakabayashi K, Nakagawa H, Adachi T, Kii I, Kobatake E, Kudo A, Ishikawa T. Identification of cysteine residues critically involved in homodimer formation and protein expression of human ATP-binding cassette transporter ABCG2: a new approach using the flp recombinase system. J Exp Ther Oncol. 2006;5(3):205–222. [PubMed] [Google Scholar]
- 85.Liu Y, Yang Y, Qi J, Peng H, Zhang JT. Effect of cysteine mutagenesis on the function and disulfide bond formation of human ABCG2. J Pharmacol Exp Ther. 2008;326(1):33–40. doi: 10.1124/jpet.108.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shigeta J, Katayama K, Mitsuhashi J, Noguchi K, Sugimoto Y. BCRP/ABCG2 confers anticancer drug resistance without covalent dimerization. Cancer Sci. 2010;101(8):1813–1821. doi: 10.1111/j.1349-7006.2010.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu J, Peng H, Chen Q, Liu Y, Dong Z, Zhang JT. Oligomerization domain of the multidrug resistance-associated transporter ABCG2 and its dominant inhibitory activity. Cancer Res. 2007;67(9):4373–4381. doi: 10.1158/0008-5472.CAN-06-3169. [DOI] [PubMed] [Google Scholar]
- 88.Xie Y, Xu K, Linn DE, Yang X, Guo Z, Shimelis H, Nakanishi T, Ross DD, Chen H, Fazli L, Gleave ME, Qiu Y. The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. J Biol Chem. 2008;283(6):3349–3356. doi: 10.1074/jbc.M707773200. [DOI] [PubMed] [Google Scholar]
- 89.Hazai E, Bikadi Z. Homology modeling of breast cancer resistance protein (ABCG2) J Struct Biol. 2008;162(1):63–74. doi: 10.1016/j.jsb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Li YF, Polgar O, Okada M, Esser L, Bates SE, Xia D. Towards understanding the mechanism of action of the multidrug resistance-linked half-ABC transporter ABCG2: a molecular modeling study. J Mol Graph Model. 2007;25(6):837–851. doi: 10.1016/j.jmgm.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 91.Wang H, Lee EW, Cai X, Ni Z, Zhou L, Mao Q. Membrane topology of the human breast cancer resistance protein (BCRP/ABCG2) determined by epitope insertion and immunofluorescence. Biochemistry. 2008;47(52):13778–13787. doi: 10.1021/bi801644v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diop NK, Hrycyna CA. N-Linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane. Biochemistry. 2005;44(14):5420–5429. doi: 10.1021/bi0479858. [DOI] [PubMed] [Google Scholar]
- 93.Mohrmann K, van Eijndhoven MA, Schinkel AH, Schellens JH. Absence of N-linked glycosylation does not affect plasma membrane localization of breast cancer resistance protein (BCRP/ABCG2) Cancer Chemother Pharmacol. 2005;56(4):344–350. doi: 10.1007/s00280-005-1004-5. [DOI] [PubMed] [Google Scholar]
- 94.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323(5922):1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pozza A, Perez-Victoria JM, Di Pietro A. Overexpression of homogeneous and active ABCG2 in insect cells. Protein Expr Purif. 2009;63(2):75–83. doi: 10.1016/j.pep.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 96.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443(7108):180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 97.Polgar O, Ierano C, Tamaki A, Stanley B, Ward Y, Xia D, Tarasova N, Robey RW, Bates SE. Mutational analysis of threonine 402 adjacent to the GXXXG dimerization motif in transmembrane segment 1 of ABCG2. Biochemistry. 2010;49(10):2235–2245. doi: 10.1021/bi902085q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen J, Lu G, Lin J, Davidson AL, Quiocho FA. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol Cell. 2003;12(3):651–661. doi: 10.1016/j.molcel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 100.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci U S A. 2007;104(48):19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kos V, Ford RC. The ATP-binding cassette family: a structural perspective. Cell Mol Life Sci. 2009;66(19):3111–3126. doi: 10.1007/s00018-009-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ozvegy-Laczka C, Laczko R, Hegedus C, Litman T, Varady G, Goda K, Hegedus T, Dokholyan NV, Sorrentino BP, Varadi A, Sarkadi B. Interaction with the 5D3 monoclonal antibody is regulated by intramolecular rearrangements but not by covalent dimer formation of the human ABCG2 multidrug transporter. J Biol Chem. 2008;283(38):26059–26070. doi: 10.1074/jbc.M803230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wakabayashi K, Nakagawa H, Tamura A, Koshiba S, Hoshijima K, Komada M, Ishikawa T. Intramolecular disulfide bond is a critical check point determining degradative fates of ATP-binding cassette (ABC) transporter ABCG2 protein. J Biol Chem. 2007;282(38):27841–27846. doi: 10.1074/jbc.C700133200. [DOI] [PubMed] [Google Scholar]
- 104.Ozvegy-Laczka C, Varady G, Koblos G, Ujhelly O, Cervenak J, Schuetz JD, Sorrentino BP, Koomen GJ, Varadi A, Nemet K, Sarkadi B. Function-dependent conformational changes of the ABCG2 multidrug transporter modify its interaction with a monoclonal antibody on the cell surface. J Biol Chem. 2005;280(6):4219–4227. doi: 10.1074/jbc.M411338200. [DOI] [PubMed] [Google Scholar]
- 105.Cai X, Bikadi Z, Ni Z, Lee EW, Wang H, Rosenberg MF, Mao Q. Role of basic residues within or near the predicted transmembrane helix 2 of the human breast cancer resistance protein in drug transport. J Pharmacol Exp Ther. 2010;333(3):670–681. doi: 10.1124/jpet.109.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ozvegy-Laczka C, Koblos G, Sarkadi B, Varadi A. Single amino acid (482) variants of the ABCG2 multidrug transporter: major differences in transport capacity and substrate recognition. Biochim Biophys Acta. 2005;1668(1):53–63. doi: 10.1016/j.bbamem.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 107.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE. Mutations at aminoacid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89(10):1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, Litman T, Dean M, Bates SE. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001;61(18):6635–6639. [PubMed] [Google Scholar]
- 109.Miwa M, Tsukahara S, Ishikawa E, Asada S, Imai Y, Sugimoto Y. Single amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectants. Int J Cancer. 2003;107(5):757–763. doi: 10.1002/ijc.11484. [DOI] [PubMed] [Google Scholar]
- 110.Tamura A, Wakabayashi K, Onishi Y, Takeda M, Ikegami Y, Sawada S, Tsuji M, Matsuda Y, Ishikawa T. Re-evaluation and functional classification of non-synonymous single nucleotide polymorphisms of the human ATP-binding cassette transporter ABCG2. Cancer Sci. 2007;98(2):231–239. doi: 10.1111/j.1349-7006.2006.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zamber CP, Lamba JK, Yasuda K, Farnum J, Thummel K, Schuetz JD, Schuetz EG. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13(1):19–28. doi: 10.1097/00008571-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, Miki Y, Sugimoto Y. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1(8):611–616. [PubMed] [Google Scholar]
- 113.Morisaki K, Robey RW, Ozvegy-Laczka C, Honjo Y, Polgar O, Steadman K, Sarkadi B, Bates SE. Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol. 2005;56(2):161–172. doi: 10.1007/s00280-004-0931-x. [DOI] [PubMed] [Google Scholar]
- 114.Kondo C, Suzuki H, Itoda M, Ozawa S, Sawada J, Kobayashi D, Ieiri I, Mine K, Ohtsubo K, Sugiyama Y. Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res. 2004;21(10):1895–1903. doi: 10.1023/b:pham.0000045245.21637.d4. [DOI] [PubMed] [Google Scholar]
- 115.Kobayashi D, Ieiri I, Hirota T, Takane H, Maegawa S, Kigawa J, Suzuki H, Nanba E, Oshimura M, Terakawa N, Otsubo K, Mine K, Sugiyama Y. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33(1):94–101. doi: 10.1124/dmd.104.001628. [DOI] [PubMed] [Google Scholar]
- 116.Li J, Cusatis G, Brahmer J, Sparreboom A, Robey RW, Bates SE, Hidalgo M, Baker SD. Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients. Cancer Biol Ther. 2007;6(3):432–438. doi: 10.4161/cbt.6.3.3763. [DOI] [PubMed] [Google Scholar]
- 117.Sparreboom A, Gelderblom H, Marsh S, Ahluwalia R, Obach R, Principe P, Twelves C, Verweij J, McLeod HL. Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype. Clin Pharmacol Ther. 2004;76(1):38–44. doi: 10.1016/j.clpt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 118.Sparreboom A, Loos WJ, Burger H, Sissung TM, Verweij J, Figg WD, Nooter K, Gelderblom H. Effect of ABCG2 genotype on the oral bioavailability of topotecan. Cancer Biol Ther. 2005;4(6):650–658. doi: 10.4161/cbt.4.6.1731. [DOI] [PubMed] [Google Scholar]
- 119.Furukawa T, Wakabayashi K, Tamura A, Nakagawa H, Morishima Y, Osawa Y, Ishikawa T. Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations. Pharm Res. 2009;26(2):469–479. doi: 10.1007/s11095-008-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tamura A, Watanabe M, Saito H, Nakagawa H, Kamachi T, Okura I, Ishikawa T. Functional validation of the genetic polymorphisms of human ATP-binding cassette (ABC) transporter ABCG2: identification of alleles that are defective in porphyrin transport. Mol Pharmacol. 2006;70(1):287–296. doi: 10.1124/mol.106.023556. [DOI] [PubMed] [Google Scholar]
- 121.Honjo Y, Morisaki K, Huff LM, Robey RW, Hung J, Dean M, Bates SE. Single-nucleotide polymorphism (SNP) analysis in the ABC half-transporter ABCG2 (MXR/BCRP/ABCP1) Cancer Biol Ther. 2002;1(6):696–702. doi: 10.4161/cbt.322. [DOI] [PubMed] [Google Scholar]
- 122.Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, Ross DD, Bates SE, Kruh GD. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63(14):4048–4054. [PubMed] [Google Scholar]
- 123.Alqawi O, Bates S, Georges E. Arginine 482 to threonine 482 mutation in breast cancer resistance protein (ABCG2) inhibits rhodamine123 transport while increasing binding. Biochem J. 2004;382(Pt 2):711–716. doi: 10.1042/BJ20040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hou YX, Li CZ, Palaniyandi K, Magtibay PM, Homolya L, Sarkadi B, Chang XB. Effects of putative catalytic base mutation E211Q on ABCG2-mediated methotrexate transport. Biochemistry. 2009;48(38):9122–9131. doi: 10.1021/bi900675v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell. 2002;10(1):139–149. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Polgar O, Ediriwickrema LS, Robey RW, Sharma A, Hegde RS, Li Y, Xia D, Ward Y, Dean M, Ozvegy-Laczka C, Sarkadi B, Bates SE. Arginine 383 is a crucial residue in ABCG2 biogenesis. Biochim Biophys Acta. 2009;1788(7):1434–1443. doi: 10.1016/j.bbamem.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Polgar O, Robey RW, Morisaki K, Dean M, Michejda C, Sauna ZE, Ambudkar SV, Tarasova N, Bates SE. Mutational analysis of ABCG2: role of the GXXXG motif. Biochemistry. 2004;43(29):9448–9456. doi: 10.1021/bi0497953. [DOI] [PubMed] [Google Scholar]
- 128.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol. 2000;296(3):911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 129.Curran AR, Engelman DM. Sequence motifs, polar interactions and conformational changes in helical membrane proteins. Curr Opin Struct Biol. 2003;13(4):412–417. doi: 10.1016/s0959-440x(03)00102-7. [DOI] [PubMed] [Google Scholar]
- 130.Polgar O, Ozvegy-Laczka C, Robey RW, Morisaki K, Okada M, Tamaki A, Koblos G, Elkind NB, Ward Y, Dean M, Sarkadi B, Bates SE. Mutational studies of G553 in TM5 of ABCG2: a residue potentially involved in dimerization. Biochemistry. 2006;45(16):5251–5260. doi: 10.1021/bi0521590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakagawa H, Wakabayashi-Nakao K, Tamura A, Toyoda Y, Koshiba S, Ishikawa T. Disruption of N-linked glycosylation enhances ubiquitin-mediated proteasomal degradation of the human ATP-binding cassette transporter ABCG2. Febs J. 2009;276(24):7237–7252. doi: 10.1111/j.1742-4658.2009.07423.x. [DOI] [PubMed] [Google Scholar]
- 132.Imai Y, Asada S, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol. 2003;64(3):610–618. doi: 10.1124/mol.64.3.610. [DOI] [PubMed] [Google Scholar]
- 133.Nakatomi K, Yoshikawa M, Oka M, Ikegami Y, Hayasaka S, Sano K, Shiozawa K, Kawabata S, Soda H, Ishikawa T, Tanabe S, Kohno S. Transport of 7-ethyl-10-hydroxy-camptothecin (SN-38) by breast cancer resistance protein ABCG2 in human lung cancer cells. Biochem Biophys Res Commun. 2001;288(4):827–832. doi: 10.1006/bbrc.2001.5850. [DOI] [PubMed] [Google Scholar]
- 134.Ishikawa T, Kasamatsu S, Hagiwara Y, Mitomo H, Kato R, Sumino Y. Expression and functional characterization of human ABC transporter ABCG2 variants in insect cells. Drug Metab Pharmacokinet. 2003;18(3):194–202. doi: 10.2133/dmpk.18.194. [DOI] [PubMed] [Google Scholar]
- 135.Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem. 2003;278(25):22644–22649. doi: 10.1074/jbc.M212399200. [DOI] [PubMed] [Google Scholar]
- 136.Jani M, Szabo P, Kis E, Molnar E, Glavinas H, Krajcsi P. Kinetic characterization of sulfasalazine transport by human ATP-binding cassette G2. Biol Pharm Bull. 2009;32(3):497–499. doi: 10.1248/bpb.32.497. [DOI] [PubMed] [Google Scholar]
- 137.Vethanayagam RR, Wang H, Gupta A, Zhang Y, Lewis F, Unadkat JD, Mao Q. Functional analysis of the human variants of breast cancer resistance protein: I206L, N590Y, and D620N. Drug Metab Dispos. 2005;33(6):697–705. doi: 10.1124/dmd.105.003657. [DOI] [PubMed] [Google Scholar]
- 138.Henriksen U, Gether U, Litman T. Effect of Walker A mutation (K86M) on oligomerization and surface targeting of the multidrug resistance transporter ABCG2. J Cell Sc. 2005;118(Pt 7):1417–1426. doi: 10.1242/jcs.01729. [DOI] [PubMed] [Google Scholar]
- 139.Polgar O, Deeken JF, Ediriwickrema LS, Tamaki A, Steinberg SM, Robey RW, Bates SE. The 315–316 deletion determines the BXP-21 antibody epitope but has no effect on the function of wild type ABCG2 or the Q141K variant. Mol Cell Biochem. 2009;322(1–2):63–71. doi: 10.1007/s11010-008-9940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mitomo H, Kato R, Ito A, Kasamatsu S, Ikegami Y, Kii I, Kudo A, Kobatake E, Sumino Y, Ishikawa T. A functional study on polymorphism of the ATP-binding cassette transporter ABCG2: critical role of arginine-482 in methotrexate transport. Biochem J. 2003;373(Pt 3):767–774. doi: 10.1042/BJ20030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ishikawa T, Tamura A, Saito H, Wakabayashi K, Nakagawa H. Pharmacogenomics of the human ABC transporter ABCG2: from functional evaluation to drug molecular design. Naturwissenschaften. 2005;92(10):451–463. doi: 10.1007/s00114-005-0019-4. [DOI] [PubMed] [Google Scholar]