Abstract
Ribavirin was discovered nearly 40 years as a broad-spectrum anti-viral drug. Recent data suggest that ribavirin may also be an effective cancer therapy. In this case, ribavirin targets an oncogene, the eukaryotic translation initiation factor eIF4E, elevated in approximately 30% of cancers including many leukemias and lymphomas. Specifically, ribavirin impedes eIF4E mediated oncogenic transformation by acting as an inhibitor of eIF4E. In a Phase II clinical trial, ribavirin treatment led to substantial clinical benefit in poor prognosis acute myeloid leukemia (AML) patients. Here molecular targeting of eIF4E correlated with clinical response. Ribavirin also targets a key enzyme in the guanosine biosynthetic pathway, inosine monophosphate dehydrogenase (IMPDH), and also, modulates immunity. Parallels with known anti-viral mechanisms could be informative; however after 40 years, these are not entirely clear. The anti-viral effects of ribavirin appear cell type specific. This variation likely arises for many reasons including cell specific variations in ribavirin metabolism as well as virus specific factors. Thus, it seems that the mechanisms for ribavirin action in cancer therapy may also vary in terms of the cancer/tissue under study. Here we review the anticancer activities of ribavirin and discuss the possible utility of incorporating ribavirin into diverse cancer therapeutic regimens.
Keywords: eIF4E, leukemia, translation, mRNA export
Introduction
Ribavirin (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide) has been widely used for the treatment of virus infections. Its activities against RNA and DNA viruses were first described nearly 40 years ago[1]. In combination with interferon, it has become part of the standard of care for the treatment of Hepatitis C infection (reviewed in [2,3]). Initially, it was hoped that ribavirin would be clinically beneficial in a wide variety of virus infections. However, outside of Hepatitis C, its clinical utility is limited. The precise mechanisms by which ribavirin exerts its antiviral effects are still not fully known [2,4,5].
Recently there has been renewed interest in ribavirin and its effects in cancer cells. Clinical and pre-clinical studies demonstrated that ribavirin inhibits the activities of an oncogene, the eukaryotic translation initiation factor eIF4E[6-9]. In this setting, ribavirin treatment led to significant clinical improvement in poor prognosis acute myeloid leukemia (AML) patients, including complete and partial remissions[8]. The success of this trial has led to the opening of a second trial combining ribavirin with low dose cytarabine in the same patient population (ClinicalTrials.gov NTC01056523). Our analysis of cancers characterized by elevated eIF4E (Table 1), particularly in light of the 2009 Cancer Statistic report [10], suggests that approximately 30% of cancers have elevated eIF4E. This is consistent with previous estimates of dysregulated translation factors [11]. This observation suggests that ribavirin may lead to clinical benefit beyond AML [12]. The mechanism of action of ribavirin as an antiviral drug has been discussed in many comprehensive reviews and so will not be covered here [2,4,5]. Rather, we will focus on the mechanisms of action of ribavirin against cancer cells. In particular, we will review the effects of ribavirin on eIF4E, on inosine monophosphate dehydrogenase (IMPDH) and on immunity. The effects of specific features of ribavirin metabolism and the potential impact on the modes of action of ribavirin are also discussed. We will start with a brief introduction to eIF4E.
Table I.
Cancers characterized by elevated eIF4E
| Type | Incidence and fold of eIF4E over-expression | Reference |
|---|---|---|
|
Leukemia-AML M4/M5
and bcCML |
eIF4E protein and mRNA were highly elevated in all analyzed samples (in patients 3-8 fold) |
[13] [8] |
| Hodgkin lymphoma | By immunohistochemistry eIF4E is elevated in 69% of nodular sclerosis HL, 75% mixed cellularity HL, and 91% lymphocyte predominant HL |
[67] |
|
Non- Hodgkin
lymphoma 1 |
>2 fold (by immunohistochemistry) | [68] |
| High eIF4E expression was found in 40% mantle cell lymphoma (MCL) tumors by immunocytochemistry. |
[69] | |
| Head and Neck cancers 2 | Tumor: 4-24x increased protein levels (also showing gene amplification 2-7x) |
[70,71] |
| Tumor: 3-22x increased protein levels in all of analyzed samples |
[72] | |
| Surgical margin: 3-10x increased protein levels | [73] | |
| Breast carcinoma 3 | 3-23x increased protein levels (also showing gene amplification 2-6x) |
[74] |
| >14x increased protein levels | [75] | |
| 9.5±6.1x increased protein levels for node- negative breast cancers |
[76] | |
| 12.5±7.6x increased protein levels | [77] | |
| 3-30x increased protein levels in invasive infiltrating ductal carcinoma, and 2.5x for ductal carcinoma in situ |
[78] | |
| 1.9-30.6x increased protein levels | [79] | |
| 2.4-34.3x increased protein levels for node positive breast cancers |
[80] | |
|
Colorectal adenomas
and carcinomas |
2-6x increased protein levels in tumor samples, and even more in tumor margins. |
[81] |
| Gastric adenocarcinoma | 2-38x increased protein levels | [82] |
|
Pancreatic ductal
adenocarcinoma 4 |
85% of samples showed high eIF4E staining in cancer tissue |
[83] |
| Lung carcinomas 5 | Bronchioalveolar carcinoma: 3-8x higher levels estimated by immunohistochemistry. |
[84] |
| Atypical adenomatous hyperplasia and peripheral lung adenocarcinomas showed 3.4- 7.4 fold protein elevation |
[85] | |
| 54% of lung adenocarcinoma samples showed high eIF4E expression by immunostaining. Analyses of mRNA and protein from tumor tissues showed 6-10x elevation compared to surrounding normal tissues. |
[86] | |
| 81% of non small cell lung cancer (NSCLC) samples from tissue microarray showed elevated eIF4E immunostaining. |
[87] | |
| 91% of NSCLC samples showed stronger eIF4E staining than adjacent normal bronchial mucosa. According to subtypes eIF4E was positive in 88% of adenocarcinoma and 100% cases of squamous cell carcinomas. |
[88] | |
| Bladder cancers | 4-10x increased protein and mRNA levels. | [89] |
|
Brain tumors
(oligodendroglial, astrocytomas and meningiomas) |
>3x increased protein levels, being highest in oligodendroglial tumors. |
[90] |
|
Glioblastoma
multiforme |
In tissue microarray 48% of samples showed elevated eIF4E immunohistainig. |
[87] |
|
Prostatic
adenocarcinomas |
>3x increased protein levels | [91] |
| 78% of tissue microarray samples showed elevated eIF4E. |
[87] | |
| Thyroid carcinoma | Elevated immunostaining especially in aggressive types. |
[92] |
| Cervical cancers 6 | 2-4x (by immunohistochemistry) | [93] |
| 7x increased mRNA levels. | [94] | |
| Strong immunostaining of eIF4E found in 21.1% low grade cervical interepitelial neoplasias (CIN) and in 89.5% of high grade CIN and none in the normal squamous epithelium of control cases. 100% of invasive squamous cell carcinoma showed strong eIF4E immunostaining, while mRNA was 2-4x elevated comparing to normal samples. |
[95] | |
| Ovarian cancers | In tissue microarray 50% of samples showed elevated eIF4E |
[87] |
| Increased p-eIF4E in 56% analyzed samples | [96] | |
| Melanoma | In tissue microarray 59% of samples showed elevated eIF4E |
[87] |
eIF4E is an independent predictor of clinical outcome in MCL patients treated with the R-hyper CVAD regimen.
Patients with eIF4E elevation in surgical margins have higher cancer recurrence [72].
eIF4E is independent prognostic marker for cancer recurrence (also independent of nodal status). Patients with 7-14x elevated eIF4E had 4x higher risk for cancer recurrence, and patients with more than 14x elevated eIF4E had 7.2 higher risk. As for HNC, patients with eIF4E elevation in tumor-free surgical margins had a cancer recurrence of 56%, comparing to eIF4E negative group where it was 6.9% [75,79]. There were no correlation between node stage and the degree of 4E overexpression [80].
There was no significant correlation between eIF4E expression and age, gender, histopathological grading, lymphatic invasion or lymph node metastasis. Also, there were no significant differences between the high eIF4E expressing group and either the low or moderate eIF4E expressing groups [83].
Patients with eIF4E had more than 3 times risk of death than those with negative eIF4E [88],
No significant difference was found between HPV+ and HPV-negative, single or double infected samples [93].
AML, acute myeloid leukemia; bcCML, blast crisis chronic myeloid leukemia; HL, Hodgkin lymphoma; MCL, mantle cell lymphoma; R-hyperCVAD, rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine.
eIF4E and cancer
eIF4E is a potent oncogene which is elevated in approximately 30% of human cancers including Hodgkin and non-Hodgkin Lymphomas, in blast crisis (but not chronic phase) chronic myelogenous leukemia (CML), and in M4 and M5 subtypes of AML as well as cancers of the breast, prostate, lung, head and neck, and colon (see Table I). In many cases, eIF4E levels are elevated only in a subset of each type of cancer, e.g. in about 50% of breast cancers. In contrast, it is elevated in nearly 100% of head and neck cancers. For M4/M5 AML, eIF4E levels have been elevated in all of the specimens we examined (44/44) ([6,8,13,14] and unpublished observations). We also examined eIF4E levels in primary specimens from other hematological malignancy with the following results: 2/22 elevated in M1/M2; 7/7 blast crisis CML; 0/2 chronic phase CML (patients matched to two of the preceding blast crisis); 3/3 multiple myeloma; 3/3 MDS; 0/3 APL; 1/8 in B-ALL and 0/5 CD34+ or PBMCs isolated from healthy volunteers ([6,8,13,14] and unpublished observations). In all cases elevation was approximately 3-12+ fold. In general, elevated eIF4E levels are correlated with poor prognosis[8].
Elevated levels of eIF4E likely contribute to the oncogenic process [8]. In transgenic mouse models, eIF4E overexpression leads to the generation of tumours of distinct histological origins [15,16]. In xenograft mouse models, eIF4E overexpression leads to increased number of tumours and increased invasion [17-19]. Overexpression of eIF4E in cell lines leads to oncogenic transformation and also permits evasion of apoptosis in several contexts [20-22].
Biochemically, eIF4E acts at two (at least) levels of gene expression: mRNA translation and mRNA export (reviewed in [8,23]). In the cytoplasm, eIF4E binds the 7-methyl guanosine cap (m7G cap) moiety on the 5′ end of mRNA and recruits the mRNA to the translation machinery. eIF4E requires its ability to bind the m7G cap in order to transform cells[24]. Importantly, not all transcripts are equally sensitive to eIF4E. For instance, eIF4E overexpression does not lead to increased translation of all proteins, but only those with complex 5′ UTRs. These transcripts are referred to as eIF4E sensitive. Many of these encode proteins involved in proliferation and angiogenesis such as c-myc and VEGF. In the nucleus, eIF4E promotes the export of a subset of mRNAs that contain a 50-nucleotide element known as the eIF4E sensitivity element (4E-SE) in the 3′ UTR. Here, eIF4E must also associate with the m7G cap on the transcript. Transcripts controlled at this level also encode for genes associated with enhanced proliferation including cyclin D1 and mdm2. Some transcripts, such as c-myc and ODC, are sensitive to eIF4E at both the translation and mRNA export levels. The localization of eIF4E can change dramatically depending on the subcellular context [25], and thus the effects of eIF4E on gene expression will also be sensitive to such effects. Thus, the final pattern of gene expression changes occurring upon eIF4E overexpression will depend on many factors including cell type, time from response, localization, presence/absence of proteins that modify eIF4E function such as BP1, PML, HoxA9, PRH/Hex etc [14,26-28].
Ribavirin and the inhibition of eIF4E
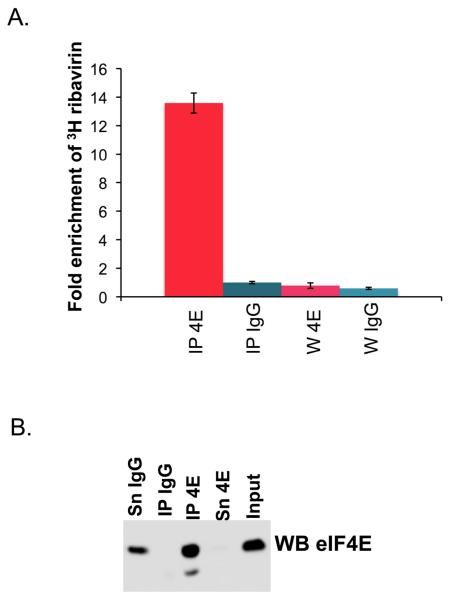
There are a wide variety of cellular mechanisms to inhibit eIF4E activity [12]. One such mechanism includes a nearly 100-fold reduction of the affinity of eIF4E for the m7G cap by the promyelocytic leukemia protein PML or by the arenaviral protein Z [24,28-30]. Further, mutation of the cap binding site abrogates the transformation potential of eIF4E [24]. Thus, the use of competitive inhibitors for eIF4E function seemed a reasonable approach. Previous biophysical studies demonstrated that ribavirin, and its active metabolite ribavirin triphosphate (RTP), bind eIF4E in or around the m7G cap-binding site[6,7]. A battery of biophysical methods demonstrated binding including: mass spectrometry, fluorescence, cap competition chromatography and NMR. The affinity is estimated to be around 10 uM for ribavirin and 0.2 uM for RTP. These assays were done in vitro, and it is equally important to show that ribavirin or RTP can associate with eIF4E in cells. Note that low micromolar levels of RTP are readily achievable intracellularly (see below). Ribavirin treatment led to reduced association of endogenous eIF4E with endogenous export target mRNAs in the nuclear lysates of NIH 3T3 cells [6]. Direct evidence that ribavirin binds eIF4E in living cells comes from immunoprecipitation studies (Figure 1). Here, 0.7 uM 3H ribavirin was incubated with cells for 24 hours, then cells were briefly formaldehyde cross-linked, lysed and immunoprecipitated using an anti-eIF4E antibody. Results show a 12 fold enrichment of 3H ribavirin in the eIF4E immunoprecipitated fraction versus the IgG control. Together, these experiments strongly suggest that ribavirin directly binds eIF4E, in or near the cap binding site, successfully competing for cap binding in vitro and in living cells.
Figure 1. 3H ribavirin associates with eIF4E in living cells.
A. 0.7 uM 3H ribavirin was incubated with eIF4E for 24 hours, FaDu cells were washed, crosslinked with formaldehyde (1% formaldehyde for 15 minutes), lysed and immunoprecipitated (IP) with IgG or anti-eIF4E antibody according to [66]. The ribavirin was tritiated at the 5 position of the triazole ring (Moravek Pharmaceuticals). The extent of ribavirin binding was assessed by scintillation counting. Note that IPs were washed six times prior to scintillation counting and the sixth wash (W) was also examined for 3H ribavirin content. B. Western blot analysis confirmed that eIF4E was present in the eIF4E IP but not in the IgG control. These are taken from the same cells used for scintillation counting.
The biophysical underpinnings of the eIF4E-RTP interaction remain to be determined. Traditionally, ligands that bind eIF4E carry a positive charge so that they can intercalate between two tryptophan residues [31]. However, a recent study reported the crystal structure of eIF4E complexed to glycerol, a neutral molecule [32] suggesting that eIF4E can bind small molecules via other modalities. Thus, the biophysical forces driving interactions of ligands with eIF4E appear more diverse than previously thought. We hypothesized that N4 in the triazole ring of RTP can be protonated leading to a positively (or partially positively) charged moiety and thus can act as a physical mimic of the m7G cap [6,7]. Additional biophysical and structural studies will be necessary to determine the precise molecular interactions between eIF4E-RTP and thus, what underpins complex formation.
The biological effects of ribavirin mirror those of eIF4E [6,8,33]. In other words, the effects of ribavirin are generally paralleled by genetic knockdown of eIF4E [33,34]. Ribavirin impedes growth of eIF4E dependent xenografts using FaDu cells [35] and in another model, impedes the number and size of liver metastases when colon 38 adenocarcinoma cells were injected into the spleen [35]. Ribavirin impairs eIF4E mediated apoptotic rescue of serum-deprived fibroblasts [6, 33] and leads to cell cycle arrest, rather that apoptosis, at least in the contexts examined thus far. eIF4E promotes Akt phosphorylation (and thus signalling) via its effects on gene expression. Consistently, ribavirin treatment leads to reduced Akt phosphorylation and reduced expression of eIF4E target genes, similar to results observed when eIF4E is genetically knocked-down [8,33,34]. Re-localization of nuclear eIF4E to the cytoplasm as a function of treatment with either ribavirin or the m7G cap was observed in cell lines and patients specimens, and is associated with inhibition of the nuclear functions of eIF4E [6,24]. Biochemically, ribavirin reduces the translation efficiency of transcripts that are enhanced by eIF4E such as VEGF and ODC and inhibits eIF4E dependent nuclear mRNA export [6]. All of these effects are readily observable with low micromolar ribavirin which is within the range for its dissociation constant for eIF4E and achievable clinically [6,7].
M4/M5 AML specimens are characterized by elevated levels of eIF4E [8,13,14]. Note that monocytes from healthy individuals do not have higher eIF4E levels than other cell types such as granulocytes, PBMCs or CD34+ cells [13]. Treatment of M4/M5 AML specimens with 1-10 uM ribavirin led to significant impairment of colony growth in methylcellulose [6]. Importantly, ribavirin, in this concentration range, did not substantially affect the growth of normal CD34+ cells or blasts isolated from M1/M2 AML patients which had normal eIF4E levels as verified by western and/or RNA analysis. Ribavirin did effect growth of these latter groups only in the 100+ micromolar range [6]. These studies strongly suggest that M4/M5 AML specimens have developed an oncogene addiction to, or dependency on, eIF4E [6]. This is consistent with more recently reported findings that prostate cancer cells with elevated eIF4E were more sensitive to knockdown of eIF4E than normal cells [36].
Ribavirin treatment targets eIF4E and leads to clinical benefit in poor prognosis AML patients
The above findings led us to investigate whether ribavirin treatment could beneficially impact M4 and M5 AML patients or other subtypes with high eIF4E levels. Thus, a phase II clinical trial was carried out to monitor the response to ribavirin monotherapy in refractory patients, relapsed patients or patients unable to undergo traditional chemotherapy regimens [8]. Patients received oral ribavirin daily starting at doses of 1000 mg/day with escalation to 2800 mg/day as necessary. One complete remission, two partial remissions, two blast responses and four stable diseases out of 11 evaluable patients were observed [8]. Two patients rapidly progressed. Best responses were observed at around 28 days. Responding patients typically became resistant by 4 months treatment; however, one patient continued response for 9 months. Previous reports suggested that ribavirin treatment could induce hemolytic anemia. This was never observed in this patient population [8]. Further, no other therapy related toxicities were observed for any patients in the trial, even after 9 months of treatment [8].
Molecular analyses indicated that ribavirin targeted eIF4E activity in patients [8]. Specimens were isolated at 28-day intervals, blasts and early blood progenitors isolated by flow cytometry, and eIF4E activity was monitored. Prior to therapy, specimens were characterized by not only elevated levels of eIF4E, but also a strong nuclear localization of eIF4E. This is consistent with previous observations in tissue bank specimens [13,14]. As expected, ribavirin treatment led to a dramatic re-localization of eIF4E in specimens from patients that responded [8] which will impact eIF4E activity. After 28 days of treatment, eIF4E was substantially re-localized to the cytoplasm, consistent with cell line data. For most patients, this effect is most striking at 56 days of treatment. Further, there was striking reduction in eIF4E RNA and protein levels after 28 days [8]. Both the reduction of eIF4E and its re-localization led to a phenotype nearly indistinguishable from normal cells (in terms of eIF4E) [8]. Continuous culturing of FaDu cell lines in ribavirin for over 200 days does not lead to any reduction in eIF4E levels (our unpublished observations) nor was this observed in shorter treatment times in FaDu or other cell lines tested [6]. Thus, the reduction of eIF4E was surprising but likely yielded an unexpected clinical benefit. It seems that ribavirin targets a subset of leukemic blasts that rely more heavily on expression of eIF4E, and that a new population emerges after treatment. We observed that flow cytometric markers for the blast population were altered during treatment, supporting this notion.
We also assessed eIF4E activity during the course of treatment [8]. We observe a substantial reduction in cyclin D1 and NBS1 proteins levels, which is consistent with the inhibition of eIF4E activity and reduction in its levels. Further, we observed reduced phospho-Akt levels at 28 days, consistent with our previous preclinical studies [33]. In one patient we had sufficient material to analyze mRNA export and observed that both NBS1 and cyclin D1 mRNA export were inhibited after 28 days of treatment by about 9 fold (Supplemental Figure 1 in [8]). We did not have sufficient material to directly monitor translational efficiency in any patient, as this assay requires approximately 500 mg of blast cells, but given our preclinical data would expect this to be impaired for eIF4E sensitive transcripts. In summary, our molecular analyses indicated that ribavirin targeted eIF4E within the first 28 days of treatment and that this correlated with clinical response. This is the first example of targeting RNA export or eIF4E in patients [8].
Ribavirin resistance and non-responding patients
For responding patients, a loss of clinical response around 4 months of treatment was observed, with the exception of one patient who continued to respond for 9 months [8]. At the molecular level, loss of response correlated with re-entry of eIF4E into the nucleus; however, eIF4E RNA and protein levels did not increase relative to the initial drops observed in the first 28 days (Supplemental Table 1 in [8]). Thus, resistance was associated with re-entry of eIF4E into the nucleus, rather than up-regulation of eIF4E protein levels.
Two patients had rapidly progressing disease [8]. One of these patients died during the first 28 days and thus no specimen was obtained. For the other patient, eIF4E levels were unexpectedly elevated at 28 days of treatment relative to the before treatment specimen. Further, eIF4E protein was not re-distributed to the cytoplasm. Thus, at least in this one case, it appears that lack of clinical response is correlated with no reduction in eIF4E levels and no redistribution of eIF4E [8].
Ribavirin combination therapy for AML treatment
Although there was a substantial clinical benefit to ribavirin monotherapy in these patients; it is important to increase the frequency and duration of clinical responses. Thus, we are examining the clinical benefit of combining ribavirin with known chemotherapy regimens (BC and KLBB, in preparation). Our preliminary studies indicate that ribavirin in combination with low dose cytarabine leads to reduced colony growth in primary AML specimens (KLBB and BC, in preparation). This led to the opening of a Phase I/II clinical trial to investigate the efficacy of this treatment combination in poor prognosis M4/M5 AML (Clinicaltrials.gov NTC01056523; www.ribatrial.com). Ongoing studies of combination therapies suggest that ribavirin will cooperate with a wide variety of commonly used agents (our unpublished observations). Thus, ribavirin may become a commonly added adjuvant to many treatment regimens.
Inosine monophosphate dehydrogenase inhibition and ribavirin
IMPDH catalyzes a key enzymatic reaction in the guanosine biosynthesis pathway where it converts inosine monophosphate to xanthine monophosphate [37]. IMPDH is the rate-limiting step of de novo GTP biosynthesis [37]. Ribavirin monophosphate (RMP) was observed to inhibit IMPDH activity [38,39]. Importantly, the generally more abundant ribavirin metabolite RTP (see metabolism section) cannot bind IMPDH. It is generally accepted that the effects of ribavirin on IMPDH are unlikely to play a major role in its anti-viral effects. This is based, in part, on the finding that analogues of ribavirin such as tiazofurin are potent IMPDH inhibitors but have no broad spectrum anti-viral activity [4]. However, there are some viruses, such as Orthopox and Sindbis virus that are sensitive to the IMPDH inhibitor mycophenolic acid, suggesting that in some circumstances, IMPDH inhibition may have an anti-viral effect [5].
The early studies examining the cytotoxic/cytostatic effects of ribavirin on uninfected mammalian cells focused on IMPDH activity. Ribavirin concentrations of 65 μM were required for 50% reduction in P388 cell growth [40]. However, the Ki for RMP mediated inhibition of IMPDH was approximately 2-4 μM in these cells [40]. Thus, there seems to be no relationship between cytotoxicity and IMPDH inhibition in these cells. Alternatively, these cells may produce very little RMP relative to RTP. Ribavirin also inhibits growth of mouse T-lymphoma (S-49) cells [41]. 10 μM ribavirin was required to achieve 50% growth inhibition. Counter-intuitively, guanosine addition, which should reverse the effects of IMPDH inhibitors, increased the cytotoxicity of ribavirin [41]. This strongly suggests that there are important cell type specific differences that sensitize cells to inhibition by ribavirin and that there are IMPDH independent mechanisms of ribavirin activity.
A chemical analogue of ribavirin, tiazofurin, is a potent IMPDH inhibitor [4]. Tiazofurin inhibits IMPDH activity in blast crisis CML patients leading to transient clinical benefit [42-44]. Interestingly, tiazofurin treatment also leads to increases in IMPDH RNA levels in these patients, suggesting some sort of compensatory mechanism is activated due to the inhibition of IMPDH [42-44]. Unfortunately, tiazofurin treatment is associated with significant treatment toxicity [43,44]. Given that blast crisis CML is characterized by elevated eIF4E levels [13] and given the chemical similarity between ribavirin and tiazofurin, studies to determine if there is a link between the inhibition of eIF4E and the inhibition of IMPDH were carried out [33]. First, a comparison of the effects of ribavirin and tiazofurin on eIF4E-mediated transformation was carried out. 1 μM Tiazofurin failed to inhibit anchorage dependent growth of fibroblasts overexpressing eIF4E or in a head and neck (FaDu) cell line with elevated eIF4E levels; whereas 1 μM ribavirin substantially inhibited these. Further, 1 μM ribavirin was sufficient to impair export of model eIF4E sensitive RNAs and to reduce phospho-Akt activation, whereas 1 μM tiazofurin did not. In addition, at 1 μM ribavirin, very little inhibition of IMPDH was observed in these cells, whereas substantial inhibition of IMPDH by tiazofurin was observed. 10 μM tiazofurin treatment increased IMPDH mRNA levels in THP-1 cells whereas 10 μM ribavirin did not affect these levels (unpublished observations). Although these compounds have chemically similar structures, tiazofurin and ribavirin are metabolized in distinct ways. Tiazofurin is converted to tiazofurin adenine dinucleotide (TAD) whereas ribavirin is metabolized to RTP (see below). These differences likely underlie their distinct activities. Further, ribavirin and tiazofurin synergize to inhibit cell growth [45] and to reduce experimental autoimmune encephalomyelitis [46], suggesting that they target different cellular machinery.
In summary, the ability to inhibit IMPDH was not sufficient to inhibit eIF4E activity. It is still possible that ribavirin can beneficially target eIF4E in AML patients because of a combination of its effects on eIF4E and IMPDH. Further, the extent to which the IMPDH versus eIF4E mechanisms contribute to ribavirin activity likely relies on cell type- and species- specific effects on ribavirin metabolism.
Immune modulation by ribavirin
The role of ribavirin in immune modulation is covered in detail elsewhere (in [2,4]) and thus will only be briefly described here. The immune-modulatory effects of ribavirin are observed in the low micromolar concentration range [2,4]. The role of immune modulation was first explored in the HCV clinical setting. A comparison of patients receiving ribavirin and interferon rather than interferon alone indicated that there was a switching from a Th2 to a Th1 cytokine bias [47]. Ribavirin caused a shift from Th2 to Th1 cytokine profile in vivo and in mast cell mediator release stimulated by IgE [2,4]. Ribavirin treatment leads to increased IL-12 synthesis and reduced IL-10 production [48]. The DCs modulated by ribavirin reduced Th2 type response and alleviated airway inflammation [48]. This is consistent with a repression of Th2 and enhancement of Th1 mediated pathways. Together with other similar studies, these findings could position ribavirin as an adjuvant in immunotherapies. Further, in RSV infected A549 cells, ribavirin was shown to decrease RSV mediated NFκB activation [49]. RSV induces IL-8 release whereas ribavirin partially reverts these effects. Here, background levels of NFκB activation were reduced by ribavirin in uninfected cells[49]. eIF4E, via its affects on Akt signalling [34] or through other pathways, could impact on NFκB signalling. Although discussed separately, clearly the molecular effects of ribavirin on eIF4E and/or IMPDH could underpin its effects on immunity. Alternatively, these effects could be based on a yet undescribed activity.
Uptake and metabolism of ribavirin
Plasma levels of ribavirin were generally observed to be between 10-20 uM when AML patients received in the range of 2000 mg/day of oral ribavirin [8]. HCV patients which receive about 1000 mg/day have plasma levels in the 9 uM range [50]. No therapy related toxicity was noted at these ribavirin concentrations in AML patients [8]. Oral ribavirin is readily absorbed into the circulation by gastrointestinal sodium nucleoside purine (N1) transporters in the jejunum [51].
Ribavirin is likely transported into nearly all human cell types as it is transported via nitrobenzylthioinosine-sensitive (es) nucleoside transporters, which appear widely expressed [52]. Initial studies focused on uptake by the ENT1 receptor but more recent findings suggest that ribavirin is also taken up by concentrative nucleoside transporters and at low ribavirin concentrations, sodium co-transport could be important [52,53]. Once in the cell, ribavirin is metabolized into RMP, ribavirin diphosphate (RDP) and RTP [4]. The initial phosphorylation event is through adenosine kinase [54] with cytosolic 5′-nucleotidase II also playing a role [55]. Given the levels of adenosine kinase vary amongst tissues [56], one might expect variation in phosphorylation efficiency and thus ratio of phosphate metabolites. Importantly, no phosphorylated forms of ribavirin are observed in plasma, urine or excreted by cells in culture [57,58]. Thus, ribavirin must be dephosphorylated in order to leave the cell. Although RTP is generally considered the active metabolite of ribavirin, it is not cell permeable.
Intracellular levels of ribavirin and its metabolites depend on several factors including (but not limited to): levels of ribavirin in the extracellular media, time of incubation, tissue and species origin of cells. For instance, human fibroblasts have an RMP:RDP:RTP ratio of 4.6:1:40 human fibroblasts, human lymphoblasts 2.7:1:7.8 and human erythrocytes 1:5.1:17 [57]. Human erythrocytes do not have the phosphatases required for dephosphorylating RTP, and thus RTP can accumulate in these cells leading to lysis and consequently to hemolytic anemia [57]. In contrast, rat erythrocytes do not accumulate RTP and thus seem to have the appropriate phosphatase(s) for its conversion [58,59]. Presumably this is why hemolytic anemia is not observed in rats. Further highlighting tissue specific differences, the half-life for ribavirin is tissue specific with the longest in the spleen followed by kidney, liver, and lung in rats [58,60].
To determine the mechanisms of action most likely to contribute to ribavirin’s anticancer activities, it is imperative to know the intracellular levels of the ribavirin metabolites and the kinetics of uptake and extrusion. In the presence of 30 micromolar extracellular concentrations of ribavirin, maximal RTP concentrations were achieved at approximately 8 hours and fell off dramatically after 24 hours in MA-104 cells [61]. Catabolism of RTP was rapid with it being almost entirely absent by 4 hours after drug was removed from the media [61]. This has important implications for experimental designs that include changing the cell media as this may lead to depletion of intracellular drug.
Many reports have focused on determining the intracellular concentrations of ribavirin. At 30 μM extracellular ribavirin in MA-104 cells, one study reports concentrations of 20 μM for RMP, 7 μM RDP and 156 μM RTP after 8 hours of incubation [61]. Incubation of 35 μM ribavirin with human skin fibroblasts, lymphoblasts and erythrocytes for 1 to 6 hours concluded that the maximum intracellular concentrations of RTP was 1-3% of cellular ATP [57]. Given that intracellular ATP levels are in the low millimolar range [60], this suggests approximately 10-20 μM intracellular RTP. In another study, 1 hour incubation of L5178 cells with 1 mM ribavirin suggested that more than 500 uM ribavirin was present [62] whereas 8 hour incubation of MA-104 cells with 300 uM ribavirin led to RTP levels in the 7 mM range [61]. However, given the way in which equilibrative transporters work, it is impossible to have intracellular ribavirin concentrations higher than extracellular concentrations in cells that can dephosphorylate ribavirin [50]. Importantly, extracellular concentrations over 100 μM ribavirin, and probably over 50 μM, are not clinically relevant. The highest plasma levels of ribavirin we observed in a leukemia patient were 36 μM, when the patient received 2800 mg/day of oral ribavirin [8]. Thus differences in measurement methodologies, incubation times, extracellular concentrations and cell types likely all contribute to the broad range of intracellular RTP concentrations reported.
It is unknown how the metabolism of ribavirin will be altered in the context of cancer cells. Certainly, cancer cells have many metabolic differences versus their normal counterparts. One would also anticipate that there could be large variation amongst different types of cancer cells in this regard. Further, metabolism of ribavirin could be modulated by the presence of other chemotherapeutic drugs. This will be an important future direction of investigation.
Differences between viral and human effects of ribavirin
Clearly, the extensive viral literature is positioned to aide in the understanding of the mode of action of ribavirin in uninfected cells. It is unlikely that ribavirin will have one general mechanism of viral inhibition, but rather that there will be different activities that effect viruses in distinct manners [5]. Consistent with this is the observation that the anti-viral effects of ribavirin against the same virus can be cell type dependent [63]. One general viral mechanism of action proposed for ribavirin is its mis-incorporation into viral RNA leading to error catastrophe. This mechanism is covered by many comprehensive reviews (e.g. [2,4]) and we only mention the relevance to cellular RNAs here. Importantly, ribavirin is not significantly incorporated into the RNA of human cells. For instance, there is no ribavirin incorporation into RNA of L5178Y cells even when incubated with 250 μM ribavirin for 3 hours [62]. These and other reports suggest that ribavirin and RTP are not substrates for RNA polymerase I or II or polyA-polymerase [5]. Further, ribavirin is not incorporated into cellular DNA [62]. At 30 μM extracellular concentrations and short incubation times, ribavirin treatment did not lead to a reduction in DNA or RNA synthesis [41]. Examination of RNA levels suggests that ribavirin treatment, even at high levels, does not globally modulate the transcriptome in PBMCs, at least after short incubation times (24 hours and 40 μM ribavirin) [64].
Early studies suggest that ribavirin can be misincorporated as the m7G cap on viral RNAs [65]. Here, millimolar levels of RMP or ribavirin were required for complete incorporation whereas 50-75 μM RTP was required for approximately 45% incorporation of radiolabelled ribavirin into vaccinia transcript caps in mouse L929 cell lysates [65]. However, in human cells, no radiolabelled ribavirin is incorporated into cellular RNA in L5178Y cells suggesting that ribavirin is not incorporated into the cap of these transcripts, at least at physiologically relevant concentrations of ribavirin [62]. Finally, whether or not the ability of RTP to inhibit eIF4E activity plays a role in its anti-viral activities remains to be examined. In summary, ribavirin treatment does not appear to lead to error catastrophe, misincorporation of RTP as the mRNA cap, impair RNA or DNA synthesis, or inhibit RNA or polyA polymerases in uninfected mammalian cells.
Conclusion
Like many small molecules, the effects of ribavirin are expected to be somewhat pleiotropic. Ribavirin targets at least two distinct biochemical entities: eIF4E and IMPDH. Ribavirin clearly targets the oncogenic activity of eIF4E in cell lines, in animal models, and in AML patients. Ribavirin monotherapy led to objective clinical benefit in many of these patients. The effects of ribavirin on the immune system, and whether these are mediated through eIF4E and/or IMPDH, may also play a role in its anti-cancer activities in AML patients. Given that eIF4E is up-regulated in over 30% of cancers, the hope is that ribavirin will become an important component in a wide variety of treatment regimens. Many tissue specific factors will likely profoundly influence the utility of ribavirin as a more broadly used anti-cancer drug. These factors include rate of ribavirin influx/efflux in given tissues as well as the steady state levels of RTP. Indeed, these may also influence the anti-viral properties of ribavirin. Further, the modified energetic state of the cancer cell may modulate these processes and vary amongst cancer types in unexpected ways. The development of most effective combination strategies, determination of whether ribavirin can be used as a frontline adjuvant and the means by which to overcome resistance to ribavirin will likely also be tissue and cancer specific. These will be areas of intense future work.
Acknowledgements
The authors thank Alex Kentsis, Maria Salvato and Nadeem Siddiqui for helpful comments on the manuscript.
Declaration of interest: Research was supported by NIH R01 80728, RO1 98571 and a grant from the Translational Research Program of the Leukemia and Lymphoma Society. IRIC receives infrastructure support from the CIHR and FRSQ. K.L.B.B. holds a Canada Research Chair.
References
- 1.Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–6. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 2.Wohnsland A, Hofmann WP, Sarrazin C. Viral determinants of resistance to treatment in patients with hepatitis C. Clin Microbiol Rev. 2007;20:23–38. doi: 10.1128/CMR.00010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixit NM, Perelson AS. The metabolism, pharmacokinetics and mechanisms of antiviral activity of ribavirin against hepatitis C virus. Cell Mol Life Sci. 2006;63:832–42. doi: 10.1007/s00018-005-5455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong Z, Cameron CE. Pleiotropic mechanisms of ribavirin antiviral activities. Prog Drug Res. 2002;59:41–69. doi: 10.1007/978-3-0348-8171-5_2. [DOI] [PubMed] [Google Scholar]
- 5.Parker WB. Metabolism and antiviral activity of ribavirin. Virus Res. 2005;107:165–71. doi: 10.1016/j.virusres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0406927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kentsis A, Volpon L, Topisirovic I, Soll CE, Culjkovic B, Shao L, Borden KL. Further evidence that ribavirin interacts with eIF4E. Rna. 2005;11:1762–6. doi: 10.1261/rna.2238705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, Caplan S, Leber B, Roy DC, Miller WH., Jr. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114:257–60. doi: 10.1182/blood-2009-02-205153. others. [DOI] [PubMed] [Google Scholar]
- 9.Borden KL. Pondering the puzzle of PML (promyelocytic leukemia) nuclear bodies: Can we fit the pieces together using an RNA regulon? Biochim Biophys Acta. 2008;1783:2145–54. doi: 10.1016/j.bbamcr.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 11.Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nat Rev Cancer. 2003;3:179–92. doi: 10.1038/nrc1015. [DOI] [PubMed] [Google Scholar]
- 12.Culjkovic B, Borden KL. Understanding and Targeting the Eukaryotic Translation Initiation Factor eIF4E in Head and Neck Cancer. J Oncol. 2009;2009:981679. doi: 10.1155/2009/981679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topisirovic I, Guzman ML, McConnell MJ, Licht JD, Culjkovic B, Neering SJ, Jordan CT, Borden KL. Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol Cell Biol. 2003;23:8992–9002. doi: 10.1128/MCB.23.24.8992-9002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topisirovic I, Kentsis A, Perez JM, Guzman ML, Jordan CT, Borden KL. Eukaryotic translation initiation factor 4E activity is modulated by HOXA9 at multiple levels. Mol Cell Biol. 2005;25:1100–12. doi: 10.1128/MCB.25.3.1100-1112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 16.Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–6. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 17.Graff JR, Zimmer SG. Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis. 2003;20:265–73. doi: 10.1023/a:1022943419011. [DOI] [PubMed] [Google Scholar]
- 18.Oridate N, Kim HJ, Xu X, Lotan R. Growth inhibition of head and neck squamous carcinoma cells by small interfering RNAs targeting eIF4E or cyclin D1 alone or combined with cisplatin. Cancer Biol Ther. 2005;4:318–23. doi: 10.4161/cbt.4.3.1504. [DOI] [PubMed] [Google Scholar]
- 19.Dong K, Wang R, Wang X, Lin F, Shen JJ, Gao P, Zhang HZ. Tumor-specific RNAi targeting eIF4E suppresses tumor growth, induces apoptosis and enhances cisplatin cytotoxicity in human breast carcinoma cells. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-9956-x. [DOI] [PubMed] [Google Scholar]
- 20.Tan A, Bitterman P, Sonenberg N, Peterson M, Polunovsky V. Inhibition of Myc-dependent apoptosis by eukaryotic translation initiation factor 4E requires cyclin D1. Oncogene. 2000;19:1437–47. doi: 10.1038/sj.onc.1203446. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Takasu T, Perlman DM, Peterson MS, Burrichter D, Avdulov S, Bitterman PB, Polunovsky VA. Translation factor eIF4E rescues cells from Myc-dependent apoptosis by inhibiting cytochrome c release. J Biol Chem. 2003;278:3015–22. doi: 10.1074/jbc.M208821200. [DOI] [PubMed] [Google Scholar]
- 22.Polunovsky VA, Rosenwald IB, Tan AT, White J, Chiang L, Sonenberg N, Bitterman PB. Translational control of programmed cell death: eukaryotic translation initiation factor 4E blocks apoptosis in growth-factor-restricted fibroblasts with physiologically expressed or deregulated Myc. Mol Cell Biol. 1996;16:6573–81. doi: 10.1128/mcb.16.11.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Culjkovic B, Topisirovic I, Borden KL. Controlling gene expression through RNA regulons: the role of the eukaryotic translation initiation factor eIF4E. Cell Cycle. 2007;6:65–9. doi: 10.4161/cc.6.1.3688. [DOI] [PubMed] [Google Scholar]
- 24.Cohen N, Sharma M, Kentsis A, Perez JM, Strudwick S, Borden KL. PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. Embo J. 2001;20:4547–59. doi: 10.1093/emboj/20.16.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strudwick S, Borden KL. The emerging roles of translation factor eIF4E in the nucleus. Differentiation. 2002;70:10–22. doi: 10.1046/j.1432-0436.2002.700102.x. [DOI] [PubMed] [Google Scholar]
- 26.Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–75. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- 27.Topisirovic I, Culjkovic B, Cohen N, Perez JM, Skrabanek L, Borden KL. The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. Embo J. 2003;22:689–703. doi: 10.1093/emboj/cdg069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kentsis A, Dwyer EC, Perez JM, Sharma M, Chen A, Pan ZQ, Borden KL. The RING Domains of the Promyelocytic Leukemia Protein PML and the Arenaviral Protein Z Repress Translation by Directly Inhibiting Translation Initiation Factor eIF4E. J Mol Biol. 2001;312:609–23. doi: 10.1006/jmbi.2001.5003. [DOI] [PubMed] [Google Scholar]
- 29.Kentsis A, Gordon RE, Borden KL. Control of biochemical reactions through supramolecular RING domain self-assembly. Proc Natl Acad Sci U S A. 2002;99:15404–9. doi: 10.1073/pnas.202608799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volpon L, Osborne MJ, Capul AA, de la Torre JC, Borden KL. Structural characterization of the Z RING-eIF4E complex reveals a distinct mode of control for eIF4E. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0909877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Der Haar T, Ball PD, McCarthy JE. Stabilization of eukaryotic initiation factor 4E binding to the mRNA 5′-Cap by domains of eIF4G. J Biol Chem. 2000;275:30551–5. doi: 10.1074/jbc.M004565200. [DOI] [PubMed] [Google Scholar]
- 32.Brown CJ, Verma CS, Walkinshaw MD, Lane DP. Crystallization of eIF4E complexed with eIF4GI peptide and glycerol reveals distinct structural differences around the cap-binding site. Cell Cycle. 2009;8:1905–11. doi: 10.4161/cc.8.12.8742. [DOI] [PubMed] [Google Scholar]
- 33.Tan K, Culjkovic B, Amri A, Borden KL. Ribavirin targets eIF4E dependent Akt survival signaling. Biochem Biophys Res Commun. 2008;375:341–5. doi: 10.1016/j.bbrc.2008.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Culjkovic B, Tan K, Orolicki S, Amri A, Meloche S, Borden KL. The eIF4E RNA regulon promotes the Akt signaling pathway. J Cell Biol. 2008;181:51–63. doi: 10.1083/jcb.200707018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeney A, Kenessey I, Timar F, Olah J, Pogany G, Babo I, Harisi R. Study of drugs against neoplastic metastasis. Magy Onkol. 2006;50:93–100. [PubMed] [Google Scholar]
- 36.Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, Schwier P, Capen A, Goode RL, Dowless MS. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117:2638–48. doi: 10.1172/JCI32044. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber G. Metabolic regulation and chemotherapy. Adv Enzyme Regul. 2006;46:26–32. doi: 10.1016/j.advenzreg.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Streeter DG, Witkowski JT, Khare GP, Sidwell RW, Bauer RJ, Robins RK, Simon LN. Mechanism of action of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci U S A. 1973;70:1174–8. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada Y, Natsumeda Y, Weber G. Action of the active metabolites of tiazofurin and ribavirin on purified IMP dehydrogenase. Biochemistry. 1988;27:2193–6. doi: 10.1021/bi00406a057. [DOI] [PubMed] [Google Scholar]
- 40.Gebeyehu G, Marquez VE, Van Cott A, Cooney DA, Kelley JA, Jayaram HN, Ahluwalia GS, Dion RL, Wilson YA, Johns DG. Ribavirin, tiazofurin, and selenazofurin: mononucleotides and nicotinamide adenine dinucleotide analogues. Synthesis, structure, and interactions with IMP dehydrogenase. J Med Chem. 1985;28:99–105. doi: 10.1021/jm00379a018. [DOI] [PubMed] [Google Scholar]
- 41.Lee HJ, Pawlak K, Nguyen BT, Robins RK, Sadee W. Biochemical differences among four inosinate dehydrogenase inhibitors, mycophenolic acid, ribavirin, tiazofurin, and selenazofurin, studied in mouse lymphoma cell culture. Cancer Res. 1985;45:5512–20. [PubMed] [Google Scholar]
- 42.Wright DG, Boosalis MS, Waraska K, Oshry LJ, Weintraub LR, Vosburgh E. Tiazofurin effects on IMP-dehydrogenase activity and expression in the leukemia cells of patients with CML blast crisis. Anticancer Res. 1996;16:3349–51. [PubMed] [Google Scholar]
- 43.Wright DG, Boosalis M, Malek K, Waraska K. Effects of the IMP-dehydrogenase inhibitor, Tiazofurin, in bcr-abl positive acute myelogenous leukemia. Part II. In vitro studies. Leuk Res. 2004;28:1137–43. doi: 10.1016/j.leukres.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Malek K, Boosalis MS, Waraska K, Mitchell BS, Wright DG. Effects of the IMP-dehydrogenase inhibitor, Tiazofurin, in bcr-abl positive acute myelogenous leukemia. Part I. In vivo studies. Leuk Res. 2004;28:1125–36. doi: 10.1016/j.leukres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Natsumeda Y, Yamada Y, Yamaji Y, Weber G. Synergistic cytotoxic effect of tiazofurin and ribavirin in hepatoma cells. Biochem Biophys Res Commun. 1988;153:321–7. doi: 10.1016/s0006-291x(88)81225-7. [DOI] [PubMed] [Google Scholar]
- 46.Lavrnja I, Stojkov D, Pekovic S, Subasic S, Mostarica-Stojkovic M, Stosic-Grujicic S, Nedeljkovic N, Medic-Mijacevic L, Rakic L, Stojiljkovi M. Combination of nucleoside analogues tiazofurin and ribavirin downregulates experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 2005;1048:392–5. doi: 10.1196/annals.1342.047. [DOI] [PubMed] [Google Scholar]
- 47.Cramp ME, Rossol S, Chokshi S, Carucci P, Williams R, Naoumov NV. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–55. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 48.Chiang DJ, Ye YL, Chen WL, Lee YL, Hsu NY, Chiang BL. Ribavirin or CpG DNA sequence-modulated dendritic cells decrease the IgE level and airway inflammation. Am J Respir Crit Care Med. 2003;168:575–80. doi: 10.1164/rccm.2205005. [DOI] [PubMed] [Google Scholar]
- 49.Fiedler MA, Wernke-Dollries K, Stark JM. Inhibition of viral replication reverses respiratory syncytial virus-induced NF-kappaB activation and interleukin-8 gene expression in A549 cells. J Virol. 1996;70:9079–82. doi: 10.1128/jvi.70.12.9079-9082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glue P. The clinical pharmacology of ribavirin. Semin Liver Dis. 1999;19(Suppl 1):17–24. [PubMed] [Google Scholar]
- 51.Patil SD, Ngo LY, Glue P, Unadkat JD. Intestinal absorption of ribavirin is preferentially mediated by the Na+-nucleoside purine (N1) transporter. Pharm Res. 1998;15:950–2. doi: 10.1023/a:1011945103455. [DOI] [PubMed] [Google Scholar]
- 52.Jarvis SM, Thorn JA, Glue P. Ribavirin uptake by human erythrocytes and the involvement of nitrobenzylthioinosine-sensitive (es)-nucleoside transporters. Br J Pharmacol. 1998;123:1587–92. doi: 10.1038/sj.bjp.0701775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto T, Kuniki K, Takekuma Y, Hirano T, Iseki K, Sugawara M. Ribavirin uptake by cultured human choriocarcinoma (BeWo) cells and Xenopus laevis oocytes expressing recombinant plasma membrane human nucleoside transporters. Eur J Pharmacol. 2007;557:1–8. doi: 10.1016/j.ejphar.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 54.Willis RC, Carson DA, Seegmiller JE. Adenosine kinase initiates the major route of ribavirin activation in a cultured human cell line. Proc Natl Acad Sci U S A. 1978;75:3042–4. doi: 10.1073/pnas.75.7.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu JZ, Larson G, Walker H, Shim JH, Hong Z. Phosphorylation of ribavirin and viramidine by adenosine kinase and cytosolic 5′-nucleotidase II: Implications for ribavirin metabolism in erythrocytes. Antimicrob Agents Chemother. 2005;49:2164–71. doi: 10.1128/AAC.49.6.2164-2171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakowicz M, Grden M, Pawelczyk T. Expression level of adenosine kinase in rat tissues. Lack of phosphate effect on the enzyme activity. Acta Biochim Pol. 2001;48:745–54. [PubMed] [Google Scholar]
- 57.Page T, Connor JD. The metabolism of ribavirin in erythrocytes and nucleated cells. Int J Biochem. 1990;22:379–83. doi: 10.1016/0020-711x(90)90140-x. [DOI] [PubMed] [Google Scholar]
- 58.Miller JP, Kigwana LJ, Streeter DG, Robins RK, Simon LN, Roboz J. The relationship between the metabolism of ribavirin and its proposed mechanism of action. Ann N Y Acad Sci. 1977;284:211–29. doi: 10.1111/j.1749-6632.1977.tb21953.x. [DOI] [PubMed] [Google Scholar]
- 59.Lin CC, Luu K, Lourenco D, Yeh LT. Pharmacokinetics and metabolism of [14C]viramidine in rats and cynomolgus monkeys. Antimicrob Agents Chemother. 2003;47:2458–63. doi: 10.1128/AAC.47.8.2458-2463.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 61.Smee DF, Matthews TR. Metabolism of ribavirin in respiratory syncytial virus-infected and uninfected cells. Antimicrob Agents Chemother. 1986;30:117–21. doi: 10.1128/aac.30.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimmerman TP, Deeprose RD. Metabolism of 5-amino-1-beta-Dribofuranosylimidazole-4-carboxamide and related five-membered heterocycles to 5′-triphosphates in human blood and L5178Y cells. Biochem Pharmacol. 1978;27:709–16. doi: 10.1016/0006-2952(78)90508-7. [DOI] [PubMed] [Google Scholar]
- 63.Kirsi JJ, North JA, McKernan PA, Murray BK, Canonico PG, Huggins JW, Srivastava PC, Robins RK. Broad-spectrum antiviral activity of 2-beta-Dribofuranosylselenazole-4-carboxamide, a new antiviral agent. Antimicrob Agents Chemother. 1983;24:353–61. doi: 10.1128/aac.24.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor MW, Grosse WM, Schaley JE, Sanda C, Wu X, Chien SC, Smith F, Wu TG, Stephens M, Ferris MW. Global effect of PEG-IFN-alpha and ribavirin on gene expression in PBMC in vitro. J Interferon Cytokine Res. 2004;24:107–18. doi: 10.1089/107999004322813354. others. [DOI] [PubMed] [Google Scholar]
- 65.Goswami BB, Borek E, Sharma OK, Fujitaki J, Smith RA. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem Biophys Res Commun. 1979;89:830–6. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- 66.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol. 2006;175:415–26. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenwald IB, Koifman L, Savas L, Chen JJ, Woda BA, Kadin ME. Expression of the translation initiation factors eIF-4E and eIF-2* is frequently increased in neoplastic cells of Hodgkin lymphoma. Hum Pathol. 2008;39:910–6. doi: 10.1016/j.humpath.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 68.Wang S, Rosenwald IB, Hutzler MJ, Pihan GA, Savas L, Chen JJ, Woda BA. Expression of the eukaryotic translation initiation factors 4E and 2alpha in non-Hodgkin’s lymphomas. Am J Pathol. 1999;155:247–55. doi: 10.1016/s0002-9440(10)65118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inamdar KV, Romaguera JE, Drakos E, Knoblock RJ, Garcia M, Leventaki V, Medeiros LJ, Rassidakis GZ. Expression of eukaryotic initiation factor 4E predicts clinical outcome in patients with mantle cell lymphoma treated with hyper-CVAD and rituximab, alternating with rituximab, high-dose methotrexate, and cytarabine. Cancer. 2009;115:4727–36. doi: 10.1002/cncr.24506. [DOI] [PubMed] [Google Scholar]
- 70.Sorrells DL, Jr., Ghali GE, De Benedetti A, Nathan CO, Li BD. Progressive amplification and overexpression of the eukaryotic initiation factor 4E gene in different zones of head and neck cancers. J Oral Maxillofac Surg. 1999;57:294–9. doi: 10.1016/s0278-2391(99)90676-6. [DOI] [PubMed] [Google Scholar]
- 71.Sorrells DL, Ghali GE, Meschonat C, DeFatta RJ, Black D, Liu L, De Benedetti A, Nathan CO, Li BD. Competitive PCR to detect eIF4E gene amplification in head and neck cancer. Head Neck. 1999;21:60–5. doi: 10.1002/(sici)1097-0347(199901)21:1<60::aid-hed8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 72.Nathan CO, Franklin S, Abreo FW, Nassar R, De Benedetti A, Glass J. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J Clin Oncol. 1999;17:2909–14. doi: 10.1200/JCO.1999.17.9.2909. [DOI] [PubMed] [Google Scholar]
- 73.Nathan CA, Liu L, Li BD, Abreo FW, Nandy I, De Benedetti A. Detection of the proto-oncogene eIF4E in surgical margins may predict recurrence in head and neck cancer. Oncogene. 1997;15:579–84. doi: 10.1038/sj.onc.1201216. [DOI] [PubMed] [Google Scholar]
- 74.Sorrells DL, Black DR, Meschonat C, Rhoads R, De Benedetti A, Gao M, Williams BJ, Li BD. Detection of eIF4E gene amplification in breast cancer by competitive PCR. Ann Surg Oncol. 1998;5:232–7. doi: 10.1007/BF02303778. [DOI] [PubMed] [Google Scholar]
- 75.Li BD, Gruner JS, Abreo F, Johnson LW, Yu H, Nawas S, McDonald JC, DeBenedetti A. Prospective study of eukaryotic initiation factor 4E protein elevation and breast cancer outcome. Ann Surg. 2002;235:732–8. doi: 10.1097/00000658-200205000-00016. discussion 738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holm N, Byrnes K, Johnson L, Abreo F, Sehon K, Alley J, Meschonat C, Md QC, Li BD. A prospective trial on initiation factor 4E (eIF4E) overexpression and cancer recurrence in node-negative breast cancer. Ann Surg Oncol. 2008;15:3207–15. doi: 10.1245/s10434-008-0086-9. [DOI] [PubMed] [Google Scholar]
- 77.Byrnes K, White S, Chu Q, Meschonat C, Yu H, Johnson LW, Debenedetti A, Abreo F, Turnage RH, McDonald JC. High eIF4E, VEGF, and microvessel density in stage I to III breast cancer. Ann Surg. 2006;243:684–90. doi: 10.1097/01.sla.0000216770.23642.d8. others. discussion 691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li BD, McDonald JC, Nassar R, De Benedetti A. Clinical outcome in stage I to III breast carcinoma and eIF4E overexpression. Ann Surg. 1998;227:756–6l. doi: 10.1097/00000658-199805000-00016. discussion 761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li BD, Liu L, Dawson M, De Benedetti A. Overexpression of eukaryotic initiation factor 4E (eIF4E) in breast carcinoma. Cancer. 1997;79:2385–90. [PubMed] [Google Scholar]
- 80.McClusky DR, Chu Q, Yu H, Debenedetti A, Johnson LW, Meschonat C, Turnage R, McDonald JC, Abreo F, Li BD. A prospective trial on initiation factor 4E (eIF4E) overexpression and cancer recurrence in node-positive breast cancer. Ann Surg. 2005;242:584–90. doi: 10.1097/01.sla.0000184224.55949.90. discussion 590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenwald IB, Chen JJ, Wang S, Savas L, London IM, Pullman J. Upregulation of protein synthesis initiation factor eIF-4E is an early event during colon carcinogenesis. Oncogene. 1999;18:2507–17. doi: 10.1038/sj.onc.1202563. [DOI] [PubMed] [Google Scholar]
- 82.Chen CN, Hsieh FJ, Cheng YM, Lee PH, Chang KJ. Expression of eukaryotic initiation factor 4E in gastric adenocarcinoma and its association with clinical outcome. J Surg Oncol. 2004;86:22–7. doi: 10.1002/jso.20037. [DOI] [PubMed] [Google Scholar]
- 83.Mishra R, Miyamoto M, Yoshioka T, Ishikawa K, Matsumura Y, Shoji Y, Ichinokawa K, Itoh T, Shichinohe T, Hirano S. Adenovirusmediated eukaryotic initiation factor 4E binding protein-1 in combination with rapamycin inhibits tumor growth of pancreatic ductal adenocarcinoma in vivo. Int J Oncol. 2009;34:1231–40. others. [PubMed] [Google Scholar]
- 84.Rosenwald IB, Hutzler MJ, Wang S, Savas L, Fraire AE. Expression of eukaryotic translation initiation factors 4E and 2alpha is increased frequently in bronchioloalveolar but not in squamous cell carcinomas of the lung. Cancer. 2001;92:2164–71. doi: 10.1002/1097-0142(20011015)92:8<2164::aid-cncr1559>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 85.Seki N, Takasu T, Mandai K, Nakata M, Saeki H, Heike Y, Takata I, Segawa Y, Hanafusa T, Eguchi K. Expression of eukaryotic initiation factor 4E in atypical adenomatous hyperplasia and adenocarcinoma of the human peripheral lung. Clin Cancer Res. 2002;8:3046–53. [PubMed] [Google Scholar]
- 86.Wang R, Geng J, Wang JH, Chu XY, Geng HC, Chen LB. Overexpression of eukaryotic initiation factor 4E (eIF4E) and its clinical significance in lung adenocarcinoma. Lung Cancer. 2009;66:237–44. doi: 10.1016/j.lungcan.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 87.Yang SX, Hewitt SM, Steinberg SM, Liewehr DJ, Swain SM. Expression levels of eIF4E, VEGF, and cyclin D1, and correlation of eIF4E with VEGF and cyclin D1 in multi-tumor tissue microarray. Oncol Rep. 2007;17:281–7. [PubMed] [Google Scholar]
- 88.Khoury T, Alrawi S, Ramnath N, Li Q, Grimm M, Black J, Tan D. Eukaryotic initiation factor-4E and cyclin D1 expression associated with patient survival in lung cancer. Clin Lung Cancer. 2009;10:58–66. doi: 10.3816/CLC.2009.n.009. [DOI] [PubMed] [Google Scholar]
- 89.Crew JP, Fuggle S, Bicknell R, Cranston DW, de Benedetti A, Harris AL. Eukaryotic initiation factor-4E in superficial and muscle invasive bladder cancer and its correlation with vascular endothelial growth factor expression and tumour progression. Br J Cancer. 2000;82:161–6. doi: 10.1054/bjoc.1999.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tejada S, Lobo MV, Garcia-Villanueva M, Sacristan S, Perez-Morgado MI, Salinas M, Martin ME. Eukaryotic initiation factors (eIF) 2alpha and 4E expression, localization, and phosphorylation in brain tumors. J Histochem Cytochem. 2009;57:503–12. doi: 10.1369/jhc.2009.952929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graff JR, Konicek BW, Lynch RL, Dumstorf CA, Dowless MS, McNulty AM, Parsons SH, Brail LH, Colligan BM, Koop JW. eIF4E activation is commonly elevated in advanced human prostate cancers and significantly related to reduced patient survival. Cancer Res. 2009;69:3866–73. doi: 10.1158/0008-5472.CAN-08-3472. others. [DOI] [PubMed] [Google Scholar]
- 92.Wang S, Lloyd RV, Hutzler MJ, Rosenwald IB, Safran MS, Patwardhan NA, Khan A. Expression of eukaryotic translation initiation factors 4E and 2alpha correlates with the progression of thyroid carcinoma. Thyroid. 2001;11:1101–7. doi: 10.1089/10507250152740939. [DOI] [PubMed] [Google Scholar]
- 93.Matthews-Greer J, Caldito G, de Benedetti A, Herrera GA, Dominguez-Malagon H, Chanona-Vilchis J, Turbat-Herrera EA. eIF4E as a marker for cervical neoplasia. Appl Immunohistochem Mol Morphol. 2005;13:367–70. doi: 10.1097/01.pai.0000170625.98446.3e. [DOI] [PubMed] [Google Scholar]
- 94.Van Trappen PO, Ryan A, Carroll M, Lecoeur C, Goff L, Gyselman VG, Young BD, Lowe DG, Pepper MS, Shepherd JH. A model for co-expression pattern analysis of genes implicated in angiogenesis and tumour cell invasion in cervical cancer. Br J Cancer. 2002;87:537–44. doi: 10.1038/sj.bjc.6600471. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee JW, Choi JJ, Lee KM, Choi CH, Kim TJ, Lee JH, Kim BG, Ahn G, Song SY, Bae DS. eIF-4E expression is associated with histopathologic grades in cervical neoplasia. Hum Pathol. 2005;36:1197–203. doi: 10.1016/j.humpath.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 96.Noske A, Lindenberg JL, Darb-Esfahani S, Weichert W, Buckendahl AC, Roske A, Sehouli J, Dietel M, Denkert C. Activation of mTOR in a subgroup of ovarian carcinomas: correlation with p-eIF-4E and prognosis. Oncol Rep. 2008;20:1409–17. [PubMed] [Google Scholar]