Abstract
BACKGROUND
Substance abuse in women with HIV/AIDS overshadows other priorities, including health care. Substance abuse may cause women to avoid health care systems and not adhere to their medication regimen.
METHODS
A randomized controlled trial tested the efficacy of Structural Ecosystems Therapy (SET) relative to a psychoeducational health group (HG) in 126 HIV+ women in recovery. SET, a 4-month intervention, focused on building family support for relapse prevention and HIV medication adherence. Over 12-month follow-up, women were assessed for drug use and medication adherence every 2-months; CD4 T-cell count and HIV viral load were assessed every 4-months.
RESULTS
Levels of drug use did not differ by condition. There was a significant difference in curvature of the rates of change in drug use with SET increasing and then decreasing and HG decreasing and then increasing. Women in SET were more likely to increase substance abuse services in response to relapse and separate from drug using household members than were women in HG. These two changes explained the decline in drug use observed within SET between 6 and 12 months. SET showed declines in medication adherence but increases in CD4 T-cell count relative to HG. The increase in CD4 T-cell count in SET was related to increasing proportions of women in SET taking antiretroviral medications.
CONCLUSION
The results of the trial were mixed. Women in SET did not show better drug use or medication adherence outcomes, but did show improvement in CD4 T-cell count and theoretical mechanisms of action on drug relapse.
Keywords: FAMILY THERAPY, RELAPSE, HIV INFECTION, MINORITY WOMEN
1. Introduction
Women, particularly women of color, are at an increased risk for HIV infection and AIDS. The Center for Disease Control and Prevention (CDC, 2008) estimates that women account for 26% of all new HIV/AIDS diagnoses. In 2005, HIV infection was the leading cause of death for African American women aged 25–34 years and the fourth leading cause of death for Hispanic women aged 35–44 years (National Institute on Drug Abuse, 2008).
Drug abuse is inextricable from HIV/AIDS (National Institute on Drug Abuse, 2006). Injection drug use underlies 1 in 5 new HIV diagnoses among women (CDC, 2005). Women who use cocaine or other non-injection drugs have higher risk from selling or trading sex for drugs (Edlin et al., 1994). In addition, high-risk behaviors are more likely under the influence of drugs or alcohol (Leigh and Stall, 1993). Physical and emotional problems of HIV are compounded by legal and social consequences of substance use (Boyd and Holmes, 2002). Substance abuse often overshadows other priorities, including health care and adherence to HIV medication regimens (Garcia and Cote, 2003; Lucas et al., 2001; Sherer, 1998; Turner et al., 1998; Williams et al., 2000).
Most interventions targeting HIV medication adherence are individual in nature (Remien et al., 2005). Individual modalities may be less efficacious with poor, inner-city minorities (Markowitz et al., 2000), likely from not considering cultural context (e.g., racial discrimination, poverty-stricken neighborhoods). Family, in particular, is a source of support for African Americans and Hispanics (Boyd-Franklin, 1989; Burns et al., 2005; Smith et al., 2001), who come from a collectivist tradition with strong family influences on decision-making and behavior. But family problems may be related to drug use. Iraurgi-Castillo et al. (2004) linked drug use diagnosis to higher family stress, lower family satisfaction, less communication, and fewer family resources. Conflicts with family and drug-using partners often present unique challenges to sobriety (Grella et al., 2003). Some women may use drugs to cope with painful feelings, stress, and family conflicts. Drug-using partners may increase access to substances, paraphernalia, and other cues, as well as encourage use (Moos, 2007).
We know of no empirically-supported interventions specifically designed for women with HIV/AIDS in recovery. Structural Ecosystems Therapy (SET; Mitrani et al., 2009) is a family based intervention for poor inner-city HIV+ women targeting psychosocial factors (e.g., reduced family stress and increased family support) associated with progressions in HIV symptoms. In a previous randomized clinical trial, SET showed efficacy in reducing psychological distress and family-related irritation (Szapocznik et al., 2004), lowering rates of relapse (Feaster, Burns, et al., 2010), and increasing medication adherence (Feaster, Brincks, et al., 2010). The current study extends previous findings by testing the efficacy of SET for improving HIV medication adherence and reducing relapse as compared to a Health Group (HG) intervention for women in recovery.
Two primary hypotheses were tested. First, women in SET would have lower substance use than women in HG. Second, women in SET would have greater HIV medication adherence than women in HG. Medication adherence combined four measures of both pill-taking behavior and biological consequences of pill-taking behavior: taking antiretroviral medications, self-report percentage of pills taken if taking medications, CD4 T-cell count, and HIV viral load. In addition, we tested potential mediators of treatment effects. Family dissatisfaction, living with a substance user, and drug treatment service utilization were considered as potential mediators of effects on drug use. Family dissatisfaction and medical service utilization were considered as mediators of effects on HIV medication adherence. Finally, the two behavioral components of adherence were considered as mediators of CD4 T-cell count and HIV viral load.
2. Methods
2.1. Participants
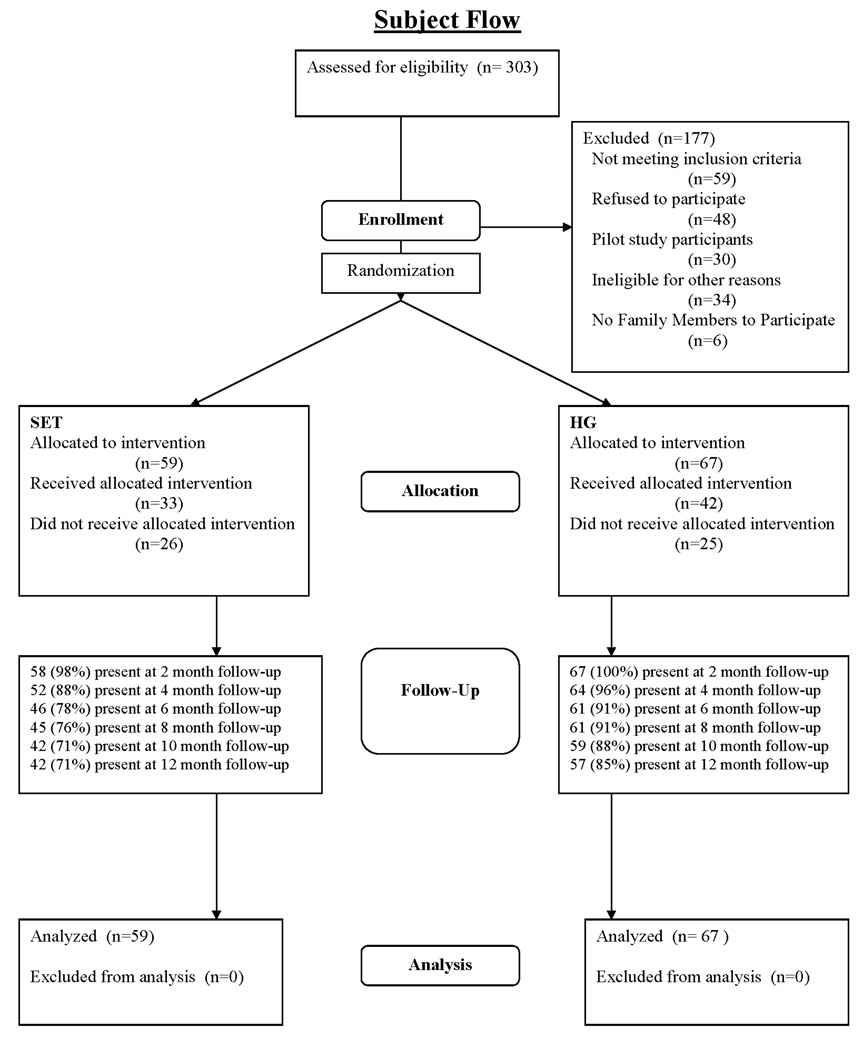
To be eligible for the current clinical trial, women had to have been HIV-1 seropositive and 1) be prescribed antiretroviral medication, 2) have a viral load over 100,000 or CD4 T-cell count under 350, or 3) have a diagnosis of any AIDS-defining disease. Therefore participants were either taking HIV medications or at a stage when HIV medications would be advised. Additional inclusion criteria were: at least 18 years of age, meet the DSM-IV criteria for substance use diagnosis within the last year (with cocaine as either the primary or secondary drug of abuse), willing to disclose their HIV status to at least one health care professional, and have at least one family member agree to enroll in a companion study of family intervention mechanisms. Recruitment for the study was from 2003 until 2007. Figure 1 shows participant flow through the study.
Figure 1.
CONSORT Subject Flow Diagram
2.2. Structural Ecosystems Therapy
SET is a family-ecosystemic intervention that targets the HIV+ woman’s social environment by strengthening adaptive interactions and reducing maladaptive interactions within the family and between the woman, family and other systems (e.g., health care, substance abuse treatment, religious institutions, neighbors) to improve the woman’s psychosocial functioning and health (see Mitrani, et al., 2009). In this application of SET, therapists were instructed to 1) strengthen family support for health care and medication adherence, 2) draw clear boundaries between the woman and any substance-using social contacts, and 3) develop a plan to address potential relapse that included family member assistance with access to drug treatment services to either prevent or respond to a relapse. SET sessions were completed in the home, therapist's offices, or other locations based on client choice. In general, SET sessions were weekly for 50 minutes for up to 4 months after randomization.
2.3. The HIV Health Group (Attention Control)
The HIV health group (HG) was incorporated to control for common factors in therapy, e.g., attention, therapist qualities, or client expectancies. Adapted from Hartfield's Wellness Manual (Baker et al., 2003), HG represented a standard psycheducational intervention for HIV+ women. Topics included medication adherence and HIV transmission risk reduction. All HG sessions were conducted biweekly at the offices of the study for a total of 8, 90-minute sessions.
2.4. Interventionists
There were different therapists in each condition. In the HG, facilitators were one African American female certified addiction counselor and one Hispanic female master’s level social worker with 9.6 (SD = 7.2) years experience. In SET, therapists were two African American women and one Hispanic woman; all were master’s level social workers with 12.0 (SD = 14.0) years experience.
2.5. Procedure
2.5.1 Pilot
Procedures were piloted with 30 women (15 in each condition) without random assignment, i.e., to train intervention and assessment staff, and to finalize modifications to the SET manual to incorporate relapse prevention and medication adherence. No data on pilot subjects are presented.
2.5.2 Randomized Clinical Trial
The IRB approved protocol included manuals for both interventions and all study procedures. Staff described study procedures to potential participants in a private office. After the woman had a chance to ask any clarifying questions, informed consent was obtained. Following an approximately two hour assessment, the study coordinator assigned women to one of the two conditions using computerized urn randomization (Wei and Lachin, 1988), balancing for age (+/− 40 years), ethnicity (Hispanic, African American, and other), HIV medication status (currently taking medications or not taking but appropriate for medications), and level of last drug treatment (residential and day treatment or outpatient). Women could receive up to $330 for participation in assessments ($40 at baseline, $15 at 2-months, $55 at 4-months, $20 at 6-months, $75 at 8-months, $25 at 8-months, and $100 at 12-months). Assessors were blind to treatment assignment. Treatment and assessment staff were in physically separated offices. The General Clinical Research Center completed blood draws and medical histories. To conserve resources depleted by a longer than planned recruitment period, assessments at 2-, 6-, and 8-months were dropped in the last year of the study. Resulting sample sizes were Baseline=126, Month 2=118, Month 4=116, Month 6=90, Month 8=105, Month 10=70, Month 12=99 (See Figure 1). Estimation procedures allowed for unbalanced data and all collected data were utilized in analysis. The study was monitored every six months by a departmental quality assurance unit administered independently of the research study. Monitoring was designed to prevent drift in procedures, ensure full human subjects and regulatory compliance, and verify completion and accuracy of case report forms. Reporting was to the principal investigator and local IRB.
2.6. Measures
2.6.1. Fidelity to Treatment Protocol
A total of 291 randomly selected videotaped sessions (221 from SET and 70 from Health Group) were rated on a 5-point scale, from 1 (not at all/poor) to 5 (extensively/ excellent), for fidelity to therapy protocol. Two raters were initially trained to an inter-rater reliability coefficient of .80 with the rating supervisor (MSR), and retrained every 6 months to prevent drift. Overall inter-rater reliability was .98 for the SET sessions and .96 for the health group sessions. SET sessions were rated on the following five domains: joining, tracking and eliciting diagnostic enactments, creating a context for change, restructuring the family system, and content focus of therapy session. Cronbach’s α of the first four ranged from .76 to .88. Four behaviors (joining, promoting group cohesiveness, acting as a “switchboard,” and wrapping up) were rated for HG sessions. Additionally, the extent that assigned topics were covered was rated separately for each of the eight group sessions. Internal consistency was not anticipated because the HG did not have theoretically prescribed behaviors.
2.6.2. Substance Use
DSM–IV substance use diagnoses were obtained using the Composite International Diagnostic Interview (CIDI 2.3, World Health Organization, 1997). Reported days of use of alcohol and illicit drugs in the past 30 days from the Addiction Severity Index (ASI; McGahan et al., 1986) were summed into a single substance use composite. Self-report substance use was correlated with urine drug screen results at each assessment (rs = .20 to .43). Substance use was analyzed using a negative binomial distribution (Atkins and Gallop, 2007).
2.6.3. Medication Adherence
Self-report adherence
Self-report adherence was measured with the AIDS Clinical Trial Group Adherence Interview Questionnaire (Chesney et al., 2000). Women listed HIV medications, number of pills taken, and the number pills missed in each of the previous four days. For analysis self-report adherence was measured in two ways. First, it was dichotomized as adherent (taking at least 90% of the prescribed dose) and non-adherent (taking less than 90% of prescribed doses) as a compromise between the 95% adherence recommended by the World Health Organization and more recent recommendations of 80–90% adherence for acceptable suppression of viral replication (Bangsberg, 2006; Parienti et al., 2008). Second, it was dichotomized as taking vs. not taking HIV medications to test effects on medication initiation. A binomial distribution was used for both outcomes in analyses.
HIV viral load
HIV Viral Load Blood HIV-1 RNA levels were obtained using reverse transcriptase polymerase chain reaction (RT-PCR) by Roche with a detection range of 400–750,000 copies/ml. Viral load was log-transformed for all analyses, and was approximately normally distributed.
CD4 T-cell count
T-cell Subset (CD4/CD8) lymphocyte phenotypes were obtained using BD Biosciences FACSalibur 4-color flow cytometer and monoclonal antibodies for lympohcytes, T cells, T-helper and suppressor cells. CD4 T-cell count was approximately normally distributed.
2.6.4. Mechanisms of Action
Family and support dissatisfaction was measured using a subscale of the 29-item Feetham Family Functioning Survey (FFFS) (Roberts and Feetham, 1982), which asks respondents to indicate the amount of time they spend and that they would like to spend with family, friends, and healthcare providers on a 7-point Likert scale (1 = Little to 7 = Much). Family dissatisfaction was the gap score, calculated by summing differences between desired and actual interactions. Cronbach’s alpha for family dissatisfaction was .88.
Living with a substance user
The full ASI provided the woman's report of currently living with someone using alcohol or drugs. These two items were combined into an indicator of living with someone that uses substances.
Service use
Dosage of in-study services was collected from study records. Services from outside of the study were assessed by a form that enumerated various potentially supportive services: psychosocial (including drug abuse treatment), social, religious/spiritual, and medical. Analyses focused on the number of medical and drug abuse treatment services.
2.7. Analytic Plan
Each hypothesis was tested using a separate intent-to-treat (ITT) Generalized Estimating Equations (GEE) analysis. For all analyses the auto-regressive (AR1) correlation structure was selected after comparing the fit of alternative structures. Primary analyses were conducted with the full sample for the five outcomes: substance use, taking antiretroviral medications, self-reported medication adherence, CD4 T-cell count, and HIV viral load. Fit of linear and quadratic models for each outcome was tested using the quasi-likelihood under the independence model criterion (QIC), with lower values indicating better fit (Pan, 2001); the best fitting models are presented. For models with more than a linear time trend, time was coded orthogonally to avoid collinearity of polynomial trends (Hedeker & Gibbons, 2006).
Tests of mediation
The second stage of the analysis was to test whether changes in 1) family dissatisfaction, living with a substance user, or outside drug treatment services mediated treatment effects on substance use, and 2) family dissatisfaction and amount of medical services mediated effects on medication adherence. To further understand the relationship between the various HIV medication adherence variables, the two self report variables were examined as potential mediators of effects on CD4 T-cell count and viral load. Initially each of these potential mediators was examined for differences across condition using the GEE methods described above. The test of mediation for variables measured at four timepoints tested the product of the pathways from the intervention to the slope of the hypothesized mediator and from the slope of the mediator to the slope of the outcome. For mediators that were measured at seven timepoints, a cross-lagged model was used. In both cases the statistical test utilized the delta-method standard errors for this product (Muthén and Muthén, 1998–2008). Additionally, due to a small difference in baseline rates across conditions, we tested 1) if living with a substance user moderated treatment effects on substance use, and 2) for moderated-mediation of effects on substance use.
Sample size determination
Sample size was determined using a program described by Hedeker, et al. (1999). Analyses showed that a sample of 134 women would provide over 80% power to uncover a medium-sized difference between conditions (0.50 SD). With N = 126, there was over 80% power to uncover a moderate effect size (0.53 SD; Cohen, 1988).
3. Results
3.1 Sample Characteristics
As shown in Table 1, women were relatively low-income and mostly minority, with relatively severe substance use disorders. There were no significant differences between conditions at the .01 level (30 tests were conducted).
Table 1.
Participant Characteristics at Basline.
| SET (n = 59) | HG (n = 67) | |||
|---|---|---|---|---|
| Characteristic | M or N | SD or % | M or N | SD or % |
| Age | 44.10 | 0.90 | 42.20 | 1.00 |
| Less than High School Education | 41 | 73.2% | 53 | 79.1% |
| Hispanic | 8 | 13.6% | 7 | 10.5% |
| African American | 42 | 71.2% | 58 | 86.6% |
| Never Married, Not Cohabitating | 19 | 33.9% | 34 | 50.8% |
| Household Income | $7,796 | 1025 | $7,551 | 933 |
| CD4 Cell Count | 467.7 | 36.3 | 519.3 | 39.6 |
| Log Viral Load | 3.03 | 0.18 | 2.94 | 0.17 |
| Years Since HIV Diagnosis | 9.40 | 0.80 | 10.40 | 0.70 |
| Years of Substance Abuse | 22.7 | 1.3 | 21.2 | 1.2 |
| Cocaine Dependence | 57 | 96.6% | 62 | 92.5% |
| Alcohol Dependence | 41 | 69.5% | 51 | 76.1% |
| Cannabis Dependence | 19 | 32.2% | 34 | 50.8% |
| Opioid Dependence | 10 | 17.0% | 18 | 26.9% |
| Dependent on Multiple Substances | 44 | 74.8% | 56 | 83.6% |
3.2. Fidelity to Treatment Protocol
Ratings of SET sessions showed fair to average (~ 3) fidelity (joining, M = 3.89, SD = 0.81; eliciting diagnostic enactments, M = 3.72, SD = 0.78; creating context for change, M = 2.58, SD = 1.01; restructuring, M = 2.41, SD = 0.82). Over a third (38.1%) of sessions addressed systems outside of the family at least minimally; 24.0% addressed medication adherence, and 48.9% substance use/relapse. Ratings of HG sessions showed above average fidelity (joining; M = 4.13, SD = 0.68; promoting cohesiveness, M = 3.31, SD = 1.01; acting as a switchboard, M = 4.86, SD = 0.39; wrapping-up, M = 3.17, SD = 1.38; topic coverage, M = 4.20, SD = 0.61).
3.3. Engagement and Dose of Intervention
Engagement was defined as attendance at two or more sessions (Prado et al., 2002; Mitrani et al., 2003). Of the 126 women randomized to SET or HG, 59.5% (n = 75) were successfully engaged. Engagement was not different between SET (55.9%) and HG (62.7%), (χ2 (1, N = 126) = 0.12, p = .44). In SET, 49.2% of cases also had family members engaged. Mean session length in SET was 71.83 min. (SD = 28.02). HG sessions were approximately 90 minutes. For the women engaged, the number of sessions was greater in SET (M = 9.12, SD = 4.11) than HG (M = 5.50, SD = 1.84), F(1,75) = 26.01, p < .001.
3.4. Substance Use
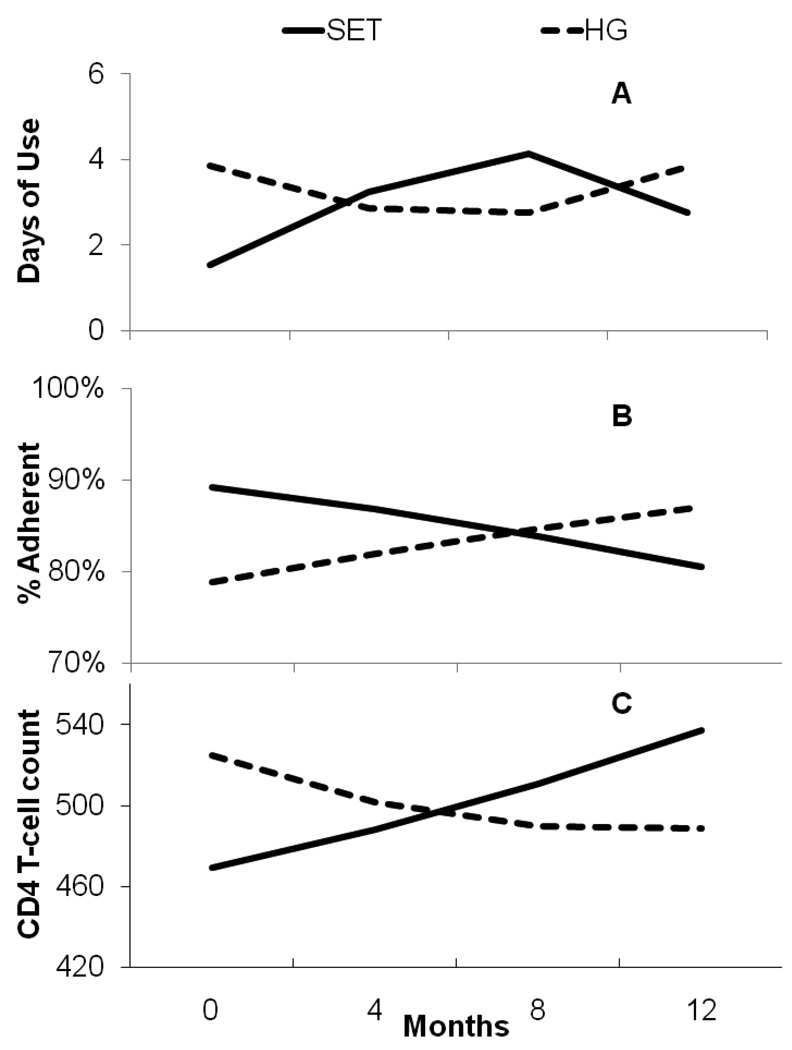
We found a non-significant Time × Treatment interaction, (B = 0.17, SE = 0.13, p < .20). There was a significant Time2 × Treatment, (B = −0.27, SE = 0.11, p < .02), with substance use, shown in Figure 2a.
Figure 2.
Estimated trajectories of substance use days, the proportion of medication adherence, and CD4-Cell count for women in SET and the Health Group.
3.5. Medication Adherence
The probability of taking prescribed HIV antiretroviral medications was not significantly different across conditions. However, the model predicted an increase in SET from 79% taking prescribed medications at baseline to 88% at 12 months whereas HG started at 87% and increased to 88% at 12 months. There was a significant Time × Treatment interaction (B = −1.14, SE = 0.57, p < .05) for % pills taken, shown in Figure 2b. There was also a significant Time × Treatment interaction (B = 77.02, SE = 30.18, p < .05) for CD4 T-cell count, but a non-significant Time2 × Treatment (B = −7.22, SE = 19.04, ns), shown in Figure 2c. We found no significant Time × Treatment effect on HIV viral load, although the direction of change was consistent with CD4 T-cell results.
3.6. Potential Mediators of Substance Abuse Results
3.6.1 Family dissatisfaction
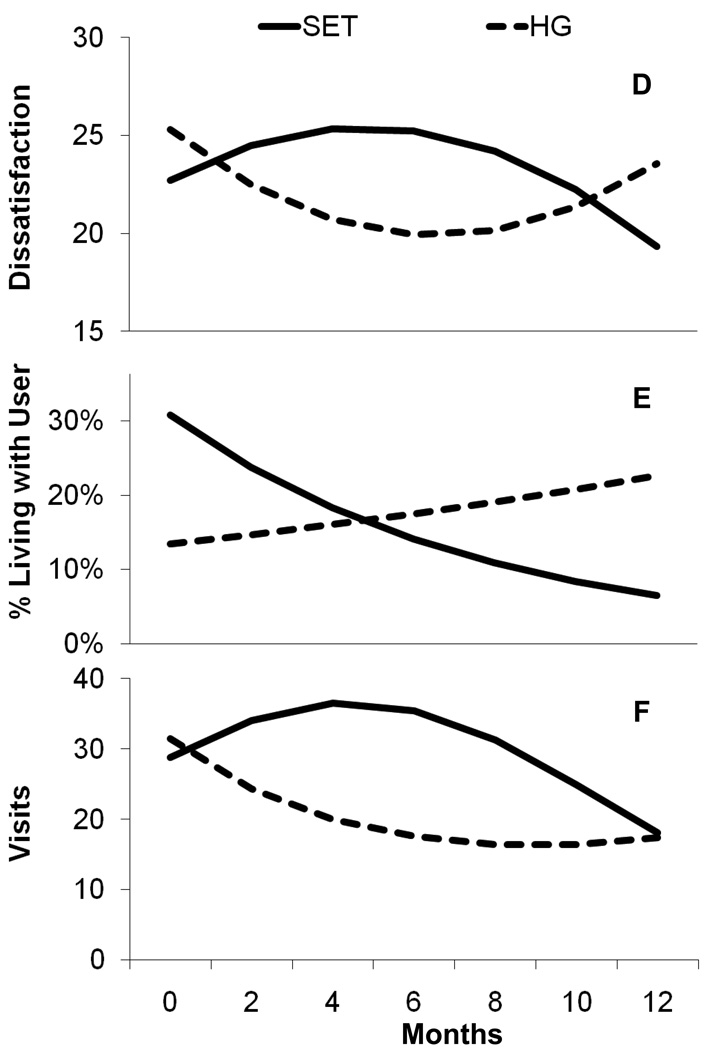
The Time × Treatment interaction was not significant, but the Time2 × Treatment was significant (B = −8.87, SE = 4.12, p < .04), shown in Figure 3a. Family Dissatisfaction was not related to substance use; therefore there was no evidence of mediation. There was no evidence of family dissatisfaction as a mediator of the effect of SET on medication adherence.
Figure 3.
Estimated trajectories of family dissatisfaction, the probability of living with a substance user, and drug treatment visits for women in SET and the Health Group.
3.6.2. Living with a substance user
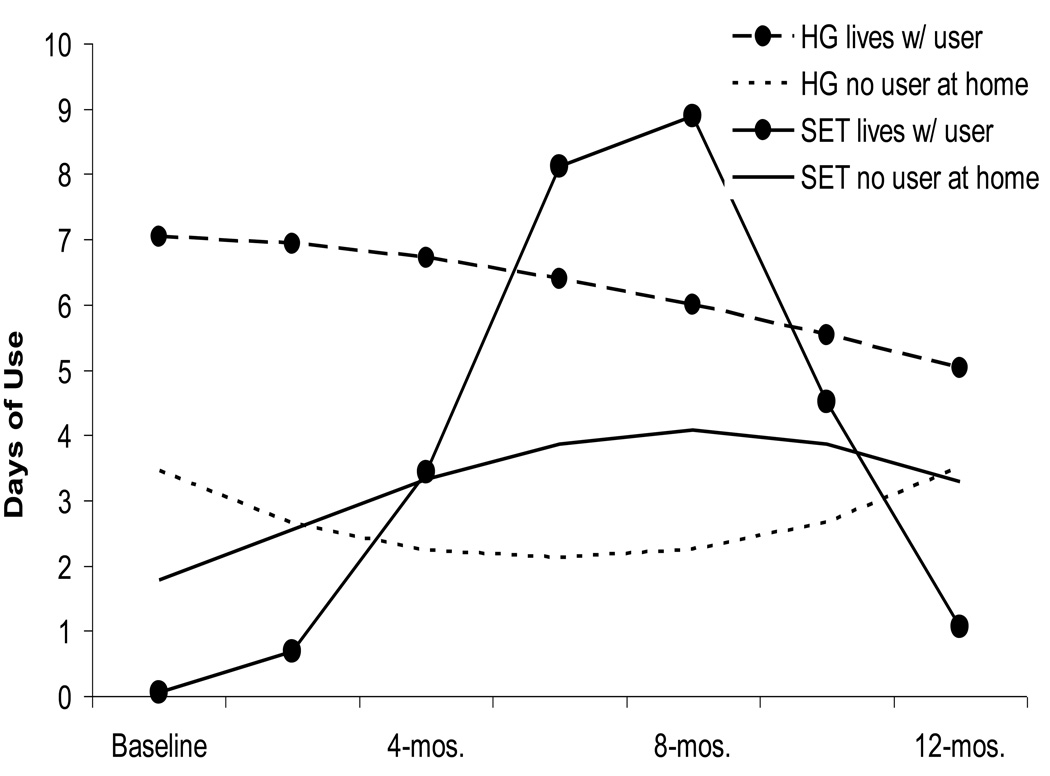
There was a non-significant trend in the probability of living with a user at baseline (B = 0.83, SE = 0.46, p < .08), with more women in the SET condition living with a substance user. As shown in Figure 3b, the probability of living with a substance user declined significantly in SET versus an increase in Health Group (B = −0.35, SE = 0.12, p < .004). The linear trajectory of living with a user was related to the quadratic component of substance use (B = 13.5, SE = 6.5, p < .04), indicating that women with decreased likelihood of living with a user also had decreases in drug use. A test of treatment effects on the quadratic component of drug use mediated by the probability of living with a user was significant (Indirect Effect = −.50, SE = .23, p <. 03), suggesting that decreases in substance use in SET worked through the decreased probability of living with substance users.
3.6.3. Service use
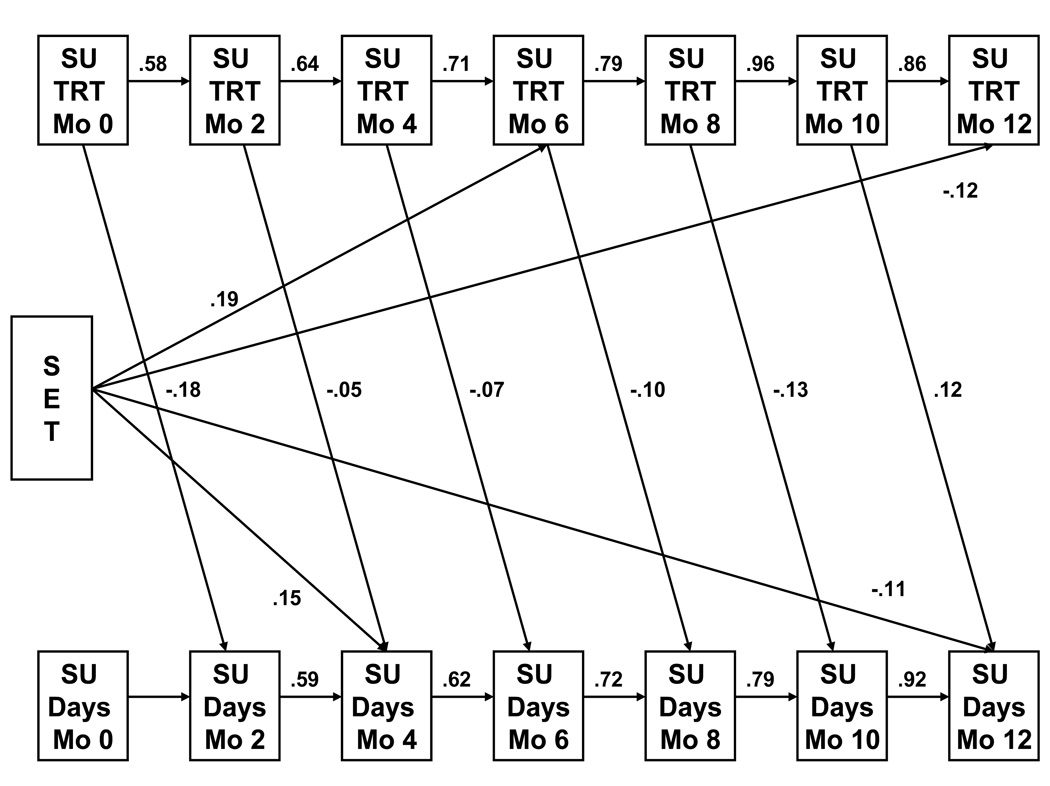
There was a significant difference in the quadratic trajectories of drug treatment services across condition (B = −.74, SE = .25, p < .003), as shown in Figure 3c. As seen in the autoregressive cross-lag model in Figure 4, there was a significant negative effect of SET on drug use at the end of the study that works through SET's increase in participation in outside substance abuse treatment at 6 months (Indirect Effect = −0.22, SE = .11, p < .05). There was no treatment effect on medical services, and therefore no mediation with medication adherence.
Figure 4.
Mediation of SET's decrease in substance use days by engagement with treatment services. Note: only significant standardized pathways are shown; pathways from prior substance use to later substance use treatment were not statistically significant.
3.6.4. Medication Adherence
We found no evidence that % pills taken mediated the treatment effect on CD4 T-cell count. Although treatment differences in the probability of taking medication was not significant, there was weak positive relationship between probability of taking meds to CD4-cell count (B = 21.5, SE = 13.0, p < .10). In addition, the treatment effect on CD4 cell count was no longer significant when the slope of taking medications was controlled. However, the product of pathways was not significantly different from zero suggesting no mediation.
3.6.5 Moderation Analyses
The trend toward baseline differences warranted further examination of whether living with a user at baseline moderated intervention effects. There were significant Time × Treatment × Living with a Substance User, (B = −2.28, SE = 0.96, p < .05), and Time2 × Treatment × Living with a Substance User (B = 2.47, SE = 0.89, p < .01) effects; the moderation effect is shown in Figure 5. Due to the complexity of the resulting model, we did not examine a single moderated-mediation model. However, we examined the relationship between treatment and living with a substance user at baseline on drug treatment services at 6-months. Treatment had a significant effect on drug treatment services for women not living with a user at baseline (B= 1.40, SE = .69, p < .05). The relative effect of SET was larger for women living with a substance user at baseline (B = 5.47, SE = 2.27, p < .02). This suggests that women in SET had greater drug treatment services at 6-months than those in HG, and in SET, women living with a user at baseline also had even greater drug treatment services than those not living with a user.
Figure 5.
Moderation of estimated substance use days by initially living with a substance user for women in SET and the Health Group.
4. Discussion
This trial targeted urban HIV+ woman recently out of drug treatment, a population worthy of intervention for relapse prevention and HIV-associated health issues as shown by pre-intervention characteristics. Over one-third (38.9%) of women either reported substance use or had a positive urinalysis. Almost a fifth (16.8%) were not taking HIV medications, despite laboratory values suggesting the benefit of medications. Of those on medications, 13% reported poor adherence. The results of comparisons of the two interventions are mixed. However, it is important to remember that for ethical reasons both were active interventions directly targeting the outcomes. The remainder of this discussion highlights the results, reviews the issues that need to be addressed to improve aftercare interventions for this population, and concludes with recommendations and limitations.
4.1. Drug Use
Although reported levels of substance use were relatively low throughout the study, relapse was still a major concern for these women. Average reported substance using days were about 4 per month for the Health Group and about 3 per month for SET. However more than half (56%) of women in both conditions either reported substance use or had a positive urine drug screen 12-months post-randomization. These numbers are consistent with the chronic nature of substance use disorders.
There were no significant differences in levels of substance use between conditions. However, there was evidence that theoretical mechanisms of change were active. SET had significant decreases in the proportion of women living with an active substance use user, and drug treatment service utilization was greater in SET than HG. These differences are consistent with the SET protocol’s establishment of firm boundaries with substance using contacts and development of a relapse plan with both the recovering woman and her (non-using) family members. Family-initiated intervention to re-engage the woman in drug treatment may be part of the reason for the slight increase in family dissatisfaction observed in follow-up. Women in SET showed an increase in substance use in the middle of follow-up, then a decline at the end of follow-up. Initial increases in drug use by women in SET were likely driven by those entering the study living with a substance user. For some reason (perhaps because they happened to come from more restrictive drug treatments than women in HG who were initially living with a user), at baseline women in SET living with a user had extremely low levels of substance use relative to HG women. Later declines in drug use of women in SET are likely related to declines in the probability of living with an active substance user. Furthermore, women in SET had increased participation in drug treatment services after a relapse, and those living with a substance user at baseline were even more likely to utilize drug treatment services.
4.2. Medication Adherence
Adherence results seem inconsistent. Reported medication adherence declined for women in SET relative to women in HG, but CD4-cell count increased for women in SET relative to the decline for women in HG. Higher levels of adherence should be associated with lower HIV viral loads and increased immune function. However, falling slightly below 90% adherence may not have much of an effect on viral load (and in turn CD4 cell count). In addition, a number of the women in SET not taking HIV medications at baseline did begin taking them during the study. Although the mediation model was not statistically significant, there was some indication that the increase in the proportion taking medication was related to increased CD4-cell count.
This increase in the numbers taking antiretroviral therapy may be important from a public health perspective. Guidelines for initiation of antiretroviral therapy have changed to starting therapy earlier in the course of infection (Zolopa, 2010). This is in part due to new evidence of health benefits to the HIV+ individual of starting earlier (Kitahata, et al., 2009, Traynor, 2010). Additionally, antiretroviral treatment with adequate adherence is known to decrease the likelihood that an individual will transmit the HIV virus (Quinn, et al, 2003), leading to a movement to 'Seek, Test and Treat' (Hayden, 2010) as an HIV prevention strategy.
4.3. Issues to Address in Future Interventions
Overall low rates of engagement pointed to a need for procedures to enhance engagement for this population. Results of subgroup analyses (Mitrani, et al, under review) suggested that women living with children engaged at higher rates. Thus, targeting family-ecological interventions at women based on family context may facilitate relapse prevention. Another issue related to engagement was the high individual cost to family therapy. Involvement of family members adds an additional burden of organization, as well as the stress of families working through issues. It may be that even for women who might benefit from family involvement, a hybrid approach (family and non-family sessions) could lower these costs. A hybrid approach might also facilitate longer-run therapeutic follow-up as with Recovery Management Check-ups (Dennis et al., 2003; Scott et al., 2005).
The mixed drug use and medication adherence results indicate a need to add potency to the SET intervention, possibly through further integration with drug treatment and HIV care. Integration with drug treatment would allow the intervention to begin at the end of drug treatment. The reality of recruitment for the current trial necessitated a mixed strategy of recruitment from drug treatment, HIV care, and from word of mouth and local outreach. Community implementation of the SET model as a hybrid after-care model could provide a true bridge between treatment and maintenance from a chronic disease perspective. Given the increase in drug use of the women within SET after the four month treatment period, a longer and flexible tapering of drug treatment services may be warranted as successfully implemented by Dennis et al. (2003).
Closer integration with HIV care would also be desirable to improve HIV medication adherence. Many of the women entering the study not taking HIV medications reported that their doctor had told them that they were healthy and did not need medications, despite laboratory values indicating that HIV medications should be seriously considered. This type of triangulation (between therapist, woman, and physician) is one of the interaction patterns that SET aims to change. Embedding the SET intervention in an HIV clinic might facilitate the transformation of this type of communication pattern between the woman, her family and health care. This integration could allow therapists to directly work with resistance to prescribing HIV medications for women in recovery (Bogart et al., 200, Wong et al., 2004). Integrating an aftercare intervention in two different systems—drug treatment and HIV care—is likely to be challenging for clients and agency staff, but there is a growing impetus for integrated care (Calsyn et al., 2004; Mertens, et al., 2008, Sylla, et al., 2007).
4.4. Limitations
The results of this study need to be interpreted in the light of several limitations. First, the study had a relatively low rate of engagement into treatment. Second, the substance use outcomes are self-reports. There was agreement between the urine drug screens and self-report, but the 2-month interval between biological assessments makes it difficult to get a full picture based on biological measures. Although self-reports may bias reports of drug use downward, randomization to condition should balance this across condition. Third, the sample was quite heterogeneous, with a mixture of volunteers recruited from residential and outpatient drug treatment and HIV treatment providers, and self-referrals from word-of-mouth. Fourth, the study mixed women on HIV antiretroviral medication at baseline and women not taking medications (though in the range of HIV infection that medications are to be considered). The original reason for the inclusion of a mixed study design of this nature was the ecological validity of an aftercare program that could be used to target most HIV+ women getting out of drug treatment. This mixed population dilutes the ability to uncover a difference on particular components of adherence. For example, SET increased the number of women on a medication regimen. Despite increasing ecological validity, the fraction of the sample that started the study without medications decreases power for test of treatment effects.
An additional limitation is the use of only self-report of HIV medication adherence. As with the self-report of drug use, this is unlikely to bias treatment comparisons; however, self-reports typically over-estimate actual medication adherence. The study used a Medical Events Monitoring Systems (MEMS) Caps from AARDEX group, medication bottle caps with a computer chip to measure adherence. Unfortunately, use of the caps by the women was sporadic and caps were frequently lost. Even though replacement caps were provided, the extreme amount of missing data precluded analysis.
In conclusion, the SET intervention was not overwhelmingly supported by this trial; however, SET established boundaries with drug using family and friends and facilitated engagement into drug treatment in response to relapse. SET also was associated with an increase in CD4 cell count, likely related to the increased medication initiation of women in SET. Despite this evidence of effects of the intervention, engagement was unacceptably low and future efforts need to explore a more targeted population and enhanced engagement strategies. The lack of an empirically-validated intervention for women dually-diagnosed with HIV and substance abuse/dependence highlights the importance of continuing modifications of SET to increase effectiveness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Atkins DC, Gallop RJ. Rethinking how family researchers model infrequent outcomes: A tutorial on count regression and zero-inflated models. Journal of Family Psychology. 2007;21:726–735. doi: 10.1037/0893-3200.21.4.726. [DOI] [PubMed] [Google Scholar]
- Baker SA, Beadnell B, Stoner S, Morrison DM, Gordon J, Collier C, Knox K, Wickizer L, Stielstra S. Skills training versus health education to prevent STDs/HIV in heterosexual women: A randomized controlled trial utilizing biological outcomes. AIDS Education and Prevention. 2003;15(1):1–14. doi: 10.1521/aeap.15.1.1.23845. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR. Less than 95% adherence to nonnucleoside reverse transcriptase inhibitor therapy can lead to viral suppression. Clinical Infectious Diseases. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- Bogart LM, Kelly JA, Catz SL, Sosman JM. Impact of medical and non-medical factors on physician decision making for HIV/AIDS antiretroviral treatment. Journal of Acquired Immunodeficiency Syndromes. 2000;23:396–404. doi: 10.1097/00126334-200004150-00006. [DOI] [PubMed] [Google Scholar]
- Boyd-Franklin N. Black families in therapy. NY: Guilford Press; 1989. [Google Scholar]
- Boyd C, Holmes C. Women who smoke crack and their family substance abuse problems. Health Care for Women International. 2002;23:576–586. doi: 10.1080/07399330290107340. [DOI] [PubMed] [Google Scholar]
- Boyd-Franklin N, Aleman J, del C, Jean-Gilles MM, Lewis SY. Cultural Sensitivity and Competence: African-American, Latino and Haitian Families with HIV/AIDS. In: Boyd-Franklin, Steiner, Boland, editors. Children, Families and HIV/AIDS: Psychosocial and Therapeutic Issues. NY: The Guilford Press; 1995. [Google Scholar]
- Burns SM, Maniss S, Young L, Gaubatz M. Attributions of control and seropositivity among Latinos: Examining the predictive utility of the locus of control construct. AIDS Care. 2005;17(2):263–269. doi: 10.1080/09540120512331326374. [DOI] [PubMed] [Google Scholar]
- Calsyn RJ, Klinkenberg WD, Morse GA, Miller J, Cruthis R. Recruitment, engagement, and retention of people living with HIV and co-occurring mental health and substance use disorders. AIDS Care. 2004;16(1):56–70. doi: 10.1080/09540120412331315286. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. HIV/AIDS among women. [Retrieved June 15, 2009];2008 August; from CDC/HIV/AIDS fact sheet: http://www.cdc.gov/hiv.
- Center for Disease Control and Prevention. HIV/AIDS Surveillance Report. 2005;17:1–46. [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Dennis ML, Scott CK, Funk R. An experimental evaluation of recovery management checkups (RMC) for people with chronic substance use disorders. Evaluation and Program Planning. 2003;26:339–352. doi: 10.1016/S0149-7189(03)00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin BR, Irwin KL, Faruque S, McCoy CB, Word C, Serrano Y, Inciardi JA, Bowser BP, Schilling RF, Holmberg SD. Intersecting epidemics-crack cocaine use and HIV infection among inner-city young adults. New England Journal of Medicine. 1994;331:1422–1427. doi: 10.1056/NEJM199411243312106. [DOI] [PubMed] [Google Scholar]
- Feaster DJ, Burns MJ, Brincks AM, Prado G, Mitrani VB, Mauer MH, Szapocznik J. Structural ecosystems therapy for HIV+ African American women and drug abuse relapse. Family Process. 2010;49:204–219. doi: 10.1111/j.1545-5300.2010.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaster DJ, Brincks AM, Mitrani VB, Prado G, Schwartz SJ, Szapocznik J. The efficacy of structural ecosystems therapy for HIV medication adherence with African American women. Journal of Family Therapy. 2010;24:51–59. doi: 10.1037/a0017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García PR, Côté JK. Factors affecting adherence to antiretroviral therapy in people living with HIV/AIDS. Journal of the Association of Nurses in AIDS Care. 2003;14:37–45. doi: 10.1177/1055329003252424. [DOI] [PubMed] [Google Scholar]
- Grella CE, Scott CK, Foss MA, Joshi V, Hser YI. Gender differences in drug treatment outcomes among participants in the Chicago Target Cities Study. Evaluation and Program Planning. 2003;26(3):297–310. [Google Scholar]
- Hayden EC. 'Seek, test and treat' slows HIV. [Retrieved on April 10, 2010];Nature. 2010 463:1006. doi: 10.1038/4631006a. doi:10.1038/4631006a. from /2010/100224/full/4631006a.html?s=news_rss http://www.nature.com/news. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hobeken, NJ: Wiley-Interscience; 2006. [Google Scholar]
- Iraurgi-Castillo I, Sanz-Vazquez M, Martinez-Pampliega A. Family functioning and addiction severity in persons that request treatment. Addictions. 2004;16(3):185–195. [Google Scholar]
- Kimerling R, Calhoun KS. Somatic symptoms, social support, and treatment seeking among sexual assault victims. Journal of Consulting & Clinical Psychology. 1994;62:333–340. doi: 10.1037//0022-006x.62.2.333. [DOI] [PubMed] [Google Scholar]
- Kimerling R, Calhoun KS, Forehand R, Armistead L, Morse E, Morse P, Clark R, Clark L. Traumatic stress in HIV-infected women. AIDS Education & Prevention. 1999;11:321–330. [PubMed] [Google Scholar]
- Kitahata M, Gange S, Abraham A. Effect of early versus deferred antretroviral therapy for HIV on survival. New England Journal of Medicine. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau-Stanton J, Clements CD. AIDS, health and mental health: A primary sourcebook. Philadelphia, PA: Brunner/Mazel; 1993. [Google Scholar]
- Leigh BC, Stall R. Substance use and risky sexual behavior for exposure to HIV: Issues in methodology, interpretation, and prevention. American Psychologist. 1993;48:1035–1045. doi: 10.1037//0003-066x.48.10.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas G, Cheever L, Chaisson R, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of Acquired Immune Deficiency Syndrome. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- Markowitz JC, Spielman LA, Sullivan M, Fishman B. An exploratory study of ethnicity and psychotherapy outcome among HIV-positive patients with depressive symptoms. Journal of Psychotherapy Practice and Research. 2000;9:226–231. [PMC free article] [PubMed] [Google Scholar]
- McGahan P, Griffith J, Parente R, McLellan T. Addiction Severity Index Composite Scores Manual. Philadelphia, PA: Treatment Research Institute; 1986. [Google Scholar]
- Mertens JR, Flisher AJ, Satre DD, Weisner CM. The role of medical conditions and primary care services in 5-year substance use outcomes among chemical dependency treatment patients. Drug and Alcohol Dependence. 2008;98:45–53. doi: 10.1016/j.drugalcdep.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrani VB, McCabe BE, Feaster DJ, Weiss-Laxer NS, Robinson C. Psychological outcomes among children of HIV+ mothers in substance abuse recovery: Results of a randomized controlled trial of Structural Ecosystems Therapy. University of Miami; 2009. [Google Scholar]
- Mitrani VB, Weiss-Laxer NS, Ow CE, Burns MJ, Ross S, Feaster DJ. Examining family networks of HIV+ women in drug recovery: Challenges and opportunities. Families, Systems, and Health. 2009;27:267–283. doi: 10.1037/a0017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrani VB, Prado G, Feaster DJ, Robinson-Batista C, Szapocznik J. Relational factors and family treatment engagement among low-income, HIV-positive African American mothers. Family Process. 2003;42(1):31–45. doi: 10.1111/j.1545-5300.2003.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrani VB, Robinson C, Szapocznik J. Structural Ecosystems Therapy (SET) for women with HIV/AIDS. In: Stanton M, Bray J, editors. Handbook of Family Psychology. Blackwell Publishing; 2009. [Google Scholar]
- Moos RH. Theory-based processes that promote the remission of substance use disorders. Clinical Psychology Review. 2007;27:537–551. doi: 10.1016/j.cpr.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 5th ed. Los Angeles, CA: Author; 1998–2007. [Google Scholar]
- National Institute on Drug Abuse. Drug abuse and the link to HIV/AIDS andother infectious diseases. [Retrieved June 15, 2009];2008 from NIDA Info Facts: http://www.drugabuse.gov.
- National Institute on Drug Abuse. HIV/AIDS: How does drug abuse impact the HIV/AIDS epidemic? NIDA Research Report Series, NIH Publication Number 06-5760. 2006 March [Google Scholar]
- Parienti J, Das-Douglas M, Massari V, Guzman D, Deeks SG, Verdon R, Bangsberg DR. Not all missed doses are the same: Sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS ONE. 2008;3(7):e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado G, Szapocznik J, Mitrani VB, Mauer MH, Smith L, Feaster DJ. Factors influencing engagement into interventions for adaptation to HIV in African American women. AIDS and Behavior. 2002;6:141–151. doi: 10.1023/a:1015497115009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T, Wawer M, Sewankambo Rakai Project Study Group. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New England Journal of Medicine. 2003;355:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Remien RH, Stirratt MJ, Dolezal C, Dognin JS, Glenn JW, Carballo-Dieguez A, El-Bassel N, Jung TM. Couple-focused support to improve HIV medication adherence: A randomized controlled trial. AIDS. 2005;19:807–814. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- Roberts CS, Feetham SL. Assessing family functioning across three areas of relationships. Nursing Research. 1982;31(4):231–235. [PubMed] [Google Scholar]
- Scott CK, Dennis ML, Foss MA. Utilizing recovery management checkups to shorten the cycle of relapse, treatment reentry, and recovery. Drug and Alcohol Dependence. 2005;78(3):325–338. doi: 10.1016/j.drugalcdep.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer R. Adherence and Antiretroviral Therapy in Injection Drug Users. Journal American Medical Association. 1998;280(6):567–568. doi: 10.1001/jama.280.6.567. [DOI] [PubMed] [Google Scholar]
- Smith L, Feaster DJ, Prado G, Kamin M, Blaney N, Szapocznik J. The psychosocial functioning of HIV+ HIV- African American mothers. AIDS and Behavior. 2001;5:219–231. [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. The International Journal of Drug Policy. 2007;18:306–312. doi: 10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapocznik J, Hervis O, Schwartz S. Brief strategic family therapy for adolescent drug abuse. Bethesda, Maryland: National Institute on Drug Abuse; 2003. [Google Scholar]
- Szapocznik J, Feaster DJ, Mitrani VB, Prado G, Smith W, Robinson-Batista C, Schwartz SJ, Mauer MH, Robbins MS. Structural Ecosystems Therapy for HIV-seropositive African-American women: Effects on psychological distress, family hassles, and family support. Journal of Consulting and Clinical Psychology. 2004;72(2):288–303. doi: 10.1037/0022-006X.72.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor K. HIV treatment guidelines revised. American Journal of Health-System Pharmacy. 2010;67:94. doi: 10.2146/news100006. [DOI] [PubMed] [Google Scholar]
- Turner JG, Nokes KM, Corless IB, Holzemer WL, Inouye J, Brown MA, Powell-Cope GM. History of drug use and adherence in HIV+ persons; International Conference AIDS; 1998. p. 595. (abstract no. 391/32366) [Google Scholar]
- Wei LJ, Lachin JM. Properties of the Urn Randomization in Clinical Trials. Controlled Clinical Trials. 1988;9:345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- Williams M, Bowen A, Ross M, Freeman R, Elwood W. Perceived compliance with AZT dosing among a sample of African-American drug users. International Journal of STD & AIDS. 2000;11(1):57–63. doi: 10.1258/0956462001914797. [DOI] [PubMed] [Google Scholar]
- Wong MD, Cunningham WE, Shapiro MF, Andersen RM, Cleary PD, Duan N, Liu HH, Wilson IB, Landon BE, Wenger NS HCSUS Consortium. Disparities in HIV treatment and physician attitudes about delaying protease inhibitors for nonadherent patients. Journal of General Internal Medicine. 2004;19:366–374. doi: 10.1111/j.1525-1497.2004.30429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. CIDI-Auto 2.1: Administrator's Guide and Reference. Sydney: World Health Organization; 1997. [Google Scholar]
- Zolopa AR. The evolution of HIV treatment guidelines: Current state-of-the-art of ART. Antiviral Research. 2010;85:241–244. doi: 10.1016/j.antiviral.2009.10.018. [DOI] [PubMed] [Google Scholar]