Abstract
Isolation of mitochondria of high purity and with intact enzymatic activities from malaria parasites has proven to be a major obstacle in characterizing the parasite mitochondrial physiology. We describe here an improved procedure for the isolation of a mitochondrially enriched preparation from the trophozoite stage of erythrocytic P. falciparum, combining disruption by N2 cavitation and differential centrifugation with magnetic removal of hemozoin-associated material. These mitochondrial preparations may be used to assay various mitochondrial enzyme activities, such as succinate and dihydroorotate dehydrogenases, ubiquinol-cytochrome c oxidoreductase, and cytochrome c oxidase. They also exhibit a low level of ATPase activity, which is only marginally inhibited by classical inhibitors. We have used this preparation to determine the susceptibility of mitochondrial activities to drugs and drug candidate compounds in both “wild type” and transgenic parasites.
Keywords: cell fractionation, mitochondria, malaria, hemozoin, magnetic separation, spectrophotometric enzyme assay
The highly divergent mitochondrion of the malaria parasite [1,2] is a proven drug target [3,4]. The ubiquinone antagonist atovaquone is an inhibitor of Complex III (ubiquinol-cytochrome c oxidoreductase) of the mitochondrial electron transport chain. Several additional classes of compounds have been found to inhibit this complex in P. falciparum, leading to additional potential drugs in early stages of development (reviewed in [2]). Inhibitors of another essential mitochondrial enzyme, dihydroorotate dehydrogenase (DHODH), are also advancing toward drug development [5,6]. Studies of Plasmodium mitochondrial physiology, however, have been hampered by the difficulty of consistently obtaining a clean preparation of sufficient yield. Intraerythrocytic parasites are physically tough, requiring prolonged homogenization [7] or high pressures [8] to release their contents, presumably due to the several membranous systems and associated cytoskeleton surrounding parasites isolated from infected erythrocytes as well as their relatively small size. The parasites also contain numerous internal membrane compartments and hemozoin particles [9], adding to the difficulty of separating mitochondria from other components.
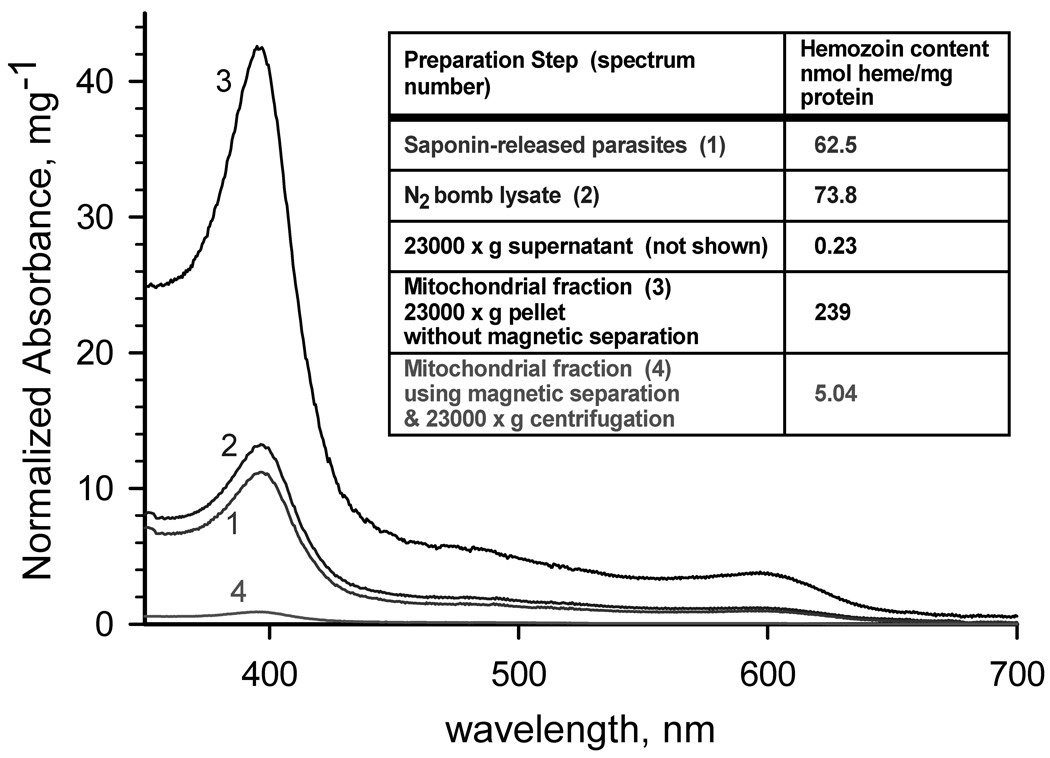
Differential centrifugation is perhaps the most common procedure for the rapid isolation of cellular fractions containing mitochondria (see e.g., Part I, Isolation and Subfractionation of Mitochondria, in [10]). The presence of hemozoin particles in Plasmodium spp., however, presents a challenge to the adaptation of this technique in members of this genus. Hemozoin has significant absorbance throughout the 300 – 625 nm region (Fig. 1), and in extracts exists as opaque, dark brown to black micro-crystalline particles. During differential centrifugation, the densest hemozoin particles sediment with the nuclei and cellular debris in the first low speed centrifugation step, but the particles that remain in the low speed supernatant are concentrated in the crude organellar fraction subsequently obtained by high speed centrifugation, resulting in an even higher final concentration of hemozoin (e.g., sample 3 in Fig. 1). As the activities of many mitochondrial enzymes are relatively low in blood-stage parasites [7] (and see below), a relatively large amount of mitochondrial preparation must be used in each individual measurement of activity, underscoring the importance of removing hemozoin to minimize interference due to light scattering and high background absorbance for traditional spectrophotometric enzyme assays.
Fig. 1. Hemozoin content of fractions of a mitochondrial preparation.
Spectra of hemozoin extracted from (1) saponin-released parasites, (2) parasite lysate produced by N2 cavitation, (3) 23000g pellet without a magnetic separation step, and (4) 23000g pellet obtained after magnetic separation using MACS. Inset: Calculated hemozoin contents. Parasite mitochondria were isolated from P. falciparum cultures that were synchronized at least twice by treatment with alanine [11], expanded and harvested at 8–15% parasitemia in the mid to late trophozoite stage. Parasitized erythrocytes were harvested by centrifugation, and lysed at 37°C with 0.05% (w/v) saponin in lysis buffer (120 mM KCl, 20 mM NaCl, 20 mM glucose; 6 mM (HEPES), 6 mM MOPS, 1 mM MgCl2, 0.1 mM EGTA, pH 7.0). After washing 3 times with lysis buffer and once with mitochondrial isolation buffer (225 mM mannitol, 75 mM sucrose, 4.3 mM MgCl2, 0.25 mM EGTA, 10 mM HEPES [Tris], 5 mM HEPES [KOH]; pH 7.4) containing 5 mM glucose, the parasites were pressurized with compressed nitrogen in a 4639 Cell Disruption Bomb (N2 bomb) (Parr, USA) at 1000 psi for 20 min at 4°C in reduced-oxygen mitochondrial isolation buffer containing 5 mM glucose and mitochondrial substrates (2.5 mM succinate, 5 mM D,L-malate, 2 mM α-glycerophosphate, and 1 mM dihydroorotate) in the presence of 1 mM phenylmethylsulfonyl fluoride and 1 µl per ml fungal protease inhibitor cocktail (Sigma-Aldrich, Inc., St. Louis, MO, USA). Cavitation was performed by slow drop-wise release of the parasite suspension through the valve of the N2 bomb. The 1000 psi pressure in the N2 bomb was reestablished whenever the release caused a drop of more than 100 psi. Upon completion of the disruption, another aliquot of protease inhibitors was added, and the unbroken cells and cell debris were removed by centrifugation at 900×g for 6 min at 4°C. The low speed supernatant was passed slowly (~0.2 ml/min) through a MACS CS column prewashed with mitochondrial isolation buffer in a Vario MACS magnetic separation apparatus (Miltenyi Biotec, Auburn, CA, USA) to remove hemozoin. The mitochondria were recovered as a pellet by centrifugation at 23000×g for 20 min at 4°C. The supernatant was removed as completely as possible, and the pellet was suspended in a minimal volume of mitochondrial isolation buffer containing 0.75 mM succinate (and/or 0.5 mM dihydroorotate, if DHODH activity is to be measured) and used for enzymatic assay or stored at −80°C. To determine hemozoin content, protein and lipids were removed from each sample aliquot using 2 washes with 2 % sodium dodecyl sulfate, and the hemozoin collected by centrifugation. The hemozoin pellet was depolymerized as described by Sullivan et al [12]. The solution was neutralized with HEPES, and the heme concentration in the sample was determined spectroscopically (ε400nm = 100,000 M−1cm−1).
Extending the method of Takashima et al. [8], we developed a modified differential centrifugation procedure incorporating a magnetic separation step that yields a mitochondrial preparation with about a 50-fold reduced level of hemozoin (compare samples 3 and 4 in Fig. 1) in a few straightforward steps (see Fig. 1 caption for details), notably, without requiring extensive mechanical breakage with a tissue homogenizer, or gradient centrifugation steps. There may be significant co-purification of the apicoplast in this preparation [13], however, the enzyme sets of these two organelles appear to be largely distinct and in any event the volume of the mitochondrion is much larger in the trophozoite stage. The residual hemozoin level varied with the stage of the parasite culture and the care with which the procedure is carried out, particularly, maintaining a uniform slow release from the N2 bomb and slow passage through the MACS column. In order to achieve better control of the release valve on the N2 bomb, we attached a round plastic disk to the control knob to increase its effective diameter to 10.4 cm (see supplemental Fig. S1). The overall yield has been in the range of 0.1–0.3 pg protein/parasitized erythrocyte, i.e., ~3–8 mg protein per 500 mL of 5% hematocrit culture with 10% parasitemia at mid to late trophozoite stage.
As noted above, the activities of many P. falciparum mitochondrial enzymes are low in blood-stage parasites [7], and a relatively large amount of mitochondrial preparation is required for each individual measurement of activity. In traditional spectrophotometric enzyme assays, even after the virtual elimination of hemozoin, there will typically be significant light scattering due to the mitochondria in suspension, which can obscure the signal (absorbance change due to enzymatic activity). Therefore, the use of a spectrophotometer that can minimize or compensate for particulate light scattering is recommended. Here we demonstrate the use of our mitochondrial preparations in assays of mitochondrial quinone-linked dehydrogenases and electron transfer complexes using a dual wavelength spectrophotomer.
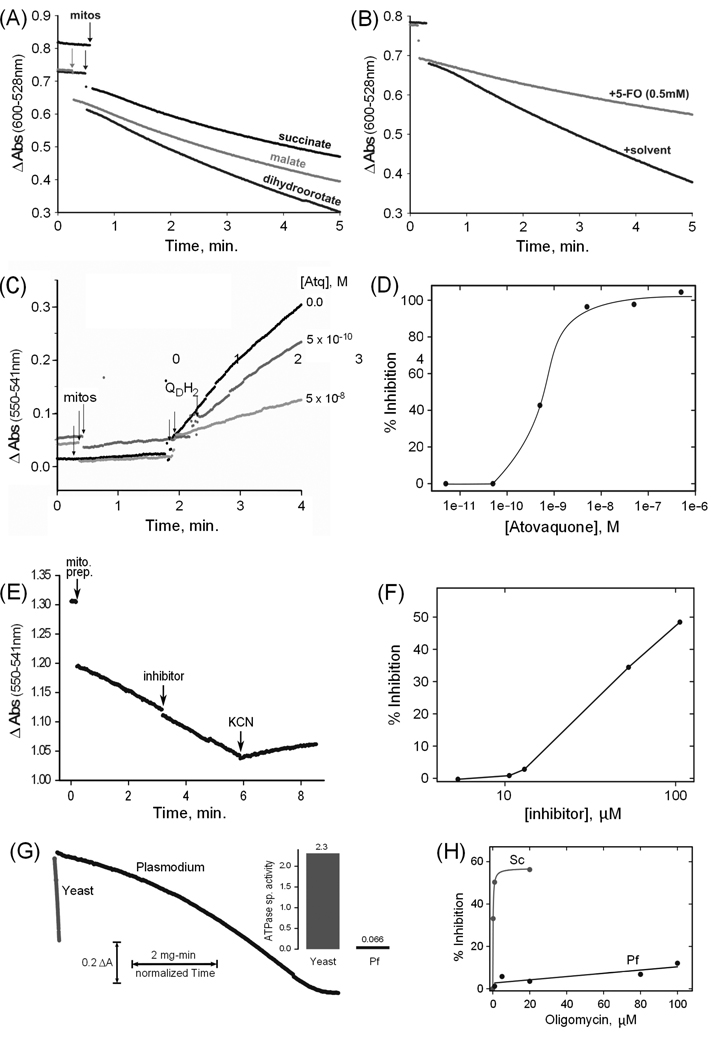
The emerging drug target DHODH is an example of a mitochondrial flavin-dependent, ubiquinone-linked dehydrogenase, which are often assayed by linking the substrate oxidation reaction to the reduction of a soluble dye, the absorbance of which changes upon reduction. Here we assayed DHODH, succinate dehydrogenase and malate-quinone oxidoreductase by observing the reduction of QD coupled to 2,6-dichloroindophenol (Fig. 2A). The specificity of a reaction can be supported by demonstrating inhibition with a specific inhibitor, such as 5-fluoroorotate for the DHODH reaction (Fig. 2B). The enzyme activities of the three enzymes measured in this experiment (Table 1) were all of the same order of magnitude, i.e. ~10 nmol substrate oxidized per min per mg of mitochondrial protein. For DHODH this is similar to the activity level found in mammalian mitochondria [18]. However, in the case of succinate dehydrogenase the level in the parasite preparation is about 2 orders of magnitude lower [19] (mammalian mitochondria do not possess a malate-quinone oxidoreductase).
Fig. 2. Spectrophotometric assays of mitochondrial enzyme activities.
(A) Example assays of dihydroorotate, malate and succinate QD/2,6-Dichloroindophenol reductase activities of a P. falciparum mitochondrial preparation (B) Example dihydroorotate reductase assays plus and minus the inhibitor 5-fluoroorotate (5-FO). (C) Time courses of ubiquinone cytochrome c reductase assays in the absence and in the presence of two concentrations of atovaquone. The points of addition of aliquots of mitochondrial preparation (mitos) and of substrate (QDH2) are indicated. (D) Profile of the inhibition by atovaquone of the ubiquinone cytochrome c reductase activity of a mitochondrial preparation. (E) Time course of a cytochrome c oxidase assay. The points of addition of an aliquot of mitochondrial preparation (mito. prep.), of the potential inhibitor 2,5-bis(4-amidinophenyl)furan (inhibitor; 13 µM final), and of potassium cyanide (KCN; 2 mM final) are indicated. Cytochrome c is present from the beginning of the assay. (F) Profile of the inhibition by 2,5-bis(4-amidinophenyl)furan of the cytochrome c oxidase activity of a mitochondrial preparation. (G) Example ATPase assay time course curves for an aliquot of P. falciparum mitochondrial preparation compared to one of yeast mitochondria, normalized to mitochondrial protein to allow visualization of the rate difference. The inset to the right of the curves displays the corresponding ATPase specific activities in µmol ATP/min/mg protein (Pf = P. falciparum). (H) Profile of oligomycin inhibition of the ATPase activities of a P. falciparum preparation (Pf) and a yeast (Sc) mitochondrial preparation. Succinate, malate, and dihydroorotate quinone reductase activities were measured essentially as described [14], using 10 mM succinate, 10 mM D,L-malate, or 2 mM dihydroorotate. Cytochrome c reductase activity was assayed by a modification of the method of Trumpower and Edwards [15], using 100 µM QDH2 and 100 µM horse heart cytochrome c (Sigma-Aldrich). Cytochrome c oxidase activity was determined by measuring the oxidation of 100 µM reduced horse heart cytochrome c. The diamidine compound 2,5-bis(4-amidinophenyl)furan was provided by Steven Meshnick, University of North Carolina. ATPase activity was determined by a coupled assay modified from Pullman et al [16], using 3 mM phosphoenolpyruvate, 0.3 mM NADH, 4 units lactate dehydrogenase (Sigma), 4 units pyruvate kinase (Sigma) and 1 mM ATP, and including inhibitors of possible contaminating activities. The assays described above were all recorded with a modified SLM-AMINCO DW2C dual wavelength spectrophotometer (On-Line Instrument Systems, Inc., Bogart, GA, USA) in dual mode (600 nm – 528 nm for 2,6-Dichloroindophenol reduction, 550 nm – 541 nm for cytochrome c oxidation/reduction, and 341 nm – 401 nm for NADH oxidation). Yeast mitochondria were prepared as described [17] and stored in aliquots at −80 °C.
Table 1.
Activities of three mitochondrial ubiquinone oxidoreductases
| substrate | inhibitor | specific activitya nmol/min/mg prot |
|---|---|---|
| DHOb | 13.8 | |
| DHO | (50 µl DMSO) | 12.1 |
| DHO | 5FOc, 0.5 mM (DMSO) | 5.71 |
| malate | 11.1 | |
| succinate | 9.73 | |
| succinate | malonate, 10 mM | 5.79 |
acceptor cosubstrate = 100 µM QD + 60 µM 2,6-dichloroindophenol
DHO = L-dihydroorotate
5FO = 5-fluoroorotate
As in the case of succinate-ubiquinone oxidoreductase, the enzyme complexes of the mitochondrial electron transfer chain are present at relatively low levels in blood stage parasites. Nevertheless, complex III (cytochrome bc1 complex) is the site of action of several classes of antimalarial compounds under development. Complex IV (cytochrome c oxidase) is inhibited by various agents such as cyanide and could be considered as a potential target. These complexes are often assayed by following the reduction or oxidation, respectively, of cytochrome c. Fig. 2 illustrates such assays of complexes III (2C) and IV (2E) with and without inhibitors, and inhibition curves (2D,2F) resulting from such assays.
Mitochondrial ATP synthase is an essential enzyme in many organisms. It has not been characterized in malaria parasites, but genomic data suggest it likely has unique features and could therefore be a potential target [2,3]. ATP synthase is routinely assayed using the reverse reaction; an enzyme-coupled spectrophotometric ATP hydrolysis assay is illustrated in Fig. 2G. The parasite mitochondria evince a very low ATPase level, and that activity is relatively insensitive to the well-known ATP synthase/hydrolase inhibitors oligomycin (Fig. 2H) and azide (not shown).
In summary, we have developed a straightforward procedure for the preparation of a parasite extract enriched in mitochondrial enzymes and largely depleted of hemozoin. The removal of hemozoin particles reduces the background light scattering and absorbance associated with mitochondrial sample aliquots, greatly facilitating spectrophotometric measurements and allowing enzyme activities present at relatively low levels to be readily measured.
Supplementary Material
Fig. S1. Parr Cell Disruption Bomb No. 4639 before (A) and after (B) modification to increase diameter of the control valve knob from 1.8 cm to 10.4 cm. A hole is cut in the center of a plastic disk such that it fits snugly over the knurled knob.
Acknowledgements
We thank Dr. Karl Henry for assistance in the preparation of yeast mitochondria. Supported by NIH grant AI028398 to A.B.V., as well as by Drexel University College of Medicine.
Abbreviations
- DHODH
dihydroorotate dehydrogenase
- QD
2,3-dimethoxy-5-methyl-6-decyl-1,4-benzoquinone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael W. Mather, Email: mmather@drexelmed.edu.
Akhil B. Vaidya, Email: avaidya@drexelmed.edu.
References
- 1.Mather MW, Vaidya AB. Mitochondria in malaria and related parasites: ancient, diverse and streamlined. J Bioenerg Biomembr. 2008;40:425–433. doi: 10.1007/s10863-008-9176-4. [DOI] [PubMed] [Google Scholar]
- 2.Vaidya AB, Mather MW. Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol. 2009;63:249–267. doi: 10.1146/annurev.micro.091208.073424. [DOI] [PubMed] [Google Scholar]
- 3.Mather MW, Henry KW, Vaidya AB. Mitochondrial drug targets in apicomplexan parasites. Curr Drug Targets. 2007;8:49–60. doi: 10.2174/138945007779315632. [DOI] [PubMed] [Google Scholar]
- 4.Vaidya AB. Mitochondrial physiology as a target for atovaquone and other antimalarials. In: Sherman IW, editor. Malaria: Parasite biology, pathogenesis, and protection. Washington, DC: ASM Press; 1998. pp. 355–368. [Google Scholar]
- 5.Gujjar R, Marwaha A, El Mazouni F, White J, White KL, Creason S, Shackleford DM, Baldwin J, Charman WN, Buckner FS, Charman S, Rathod PK, Phillips MA. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J Med Chem. 2009;52:1864–1872. doi: 10.1021/jm801343r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng X, Gujjar R, El Mazouni F, Kaminsky W, Malmquist NA, Goldsmith EJ, Rathod PK, Phillips MA. Structural plasticity of malaria dihydroorotate dehydrogenase allows selective binding of diverse chemical scaffolds. J Biol Chem. 2009;284:26999–27009. doi: 10.1074/jbc.M109.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry M, Beesley JE. Mitochondria of mammalian Plasmodium spp. Parasitology. 1991;102(Pt 1):17–26. doi: 10.1017/s0031182000060297. [DOI] [PubMed] [Google Scholar]
- 8.Takashima E, Takamiya S, Takeo S, Mi-ichi F, Amino H, Kita K. Isolation of mitochondria from Plasmodium falciparum showing dihydroorotate dependent respiration. Parasitol Int. 2001;50:273–278. doi: 10.1016/s1383-5769(01)00085-x. [DOI] [PubMed] [Google Scholar]
- 9.Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol Today. 2000;16:427–433. doi: 10.1016/s0169-4758(00)01755-5. [DOI] [PubMed] [Google Scholar]
- 10.Pon LA, Schon EA, editors. Mitochondria. London & San Diego: Academic Press; 2007. [Google Scholar]
- 11.Haynes JD, Moch JK. Automated synchronization of Plasmodium falciparum parasites by culture in a temperature-cycling incubator. Methods Mol Med. 2002;72:489–497. doi: 10.1385/1-59259-271-6:489. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan DJ, Jr, Gluzman IY, Russell DG, Goldberg DE. On the molecular mechanism of chloroquine's antimalarial action. Proc Natl Acad Sci U S A. 1996;93:11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T, Sato S, Takamiya S, Komaki-Yasuda K, Yano K, Hirata A, Onitsuka I, Hata M, Mi-ichi F, Tanaka T, Hase T, Miyajima A, Kawazu S, Watanabe Y, Kita K. Mitochondria and apicoplast of Plasmodium falciparum: behaviour on subcellular fractionation and the implication. Mitochondrion. 2007;7:125–132. doi: 10.1016/j.mito.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin J, Farajallah AM, Malmquist NA, Rathod PK, Phillips MA. Malarial dihydroorotate dehydrogenase. Substrate and inhibitor specificity. J Biol Chem. 2002;277:41827–41834. doi: 10.1074/jbc.M206854200. [DOI] [PubMed] [Google Scholar]
- 15.Trumpower BL, Edwards CA. Purification of a reconstitutively active iron-sulfur protein (oxidation factor) from succinate . cytochrome c reductase complex of bovine heart mitochondria. J Biol Chem. 1979;254:8697–8706. [PubMed] [Google Scholar]
- 16.Pullman ME, Penefsky HS, Datta A, Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960;235:3322–3329. [PubMed] [Google Scholar]
- 17.Meisinger C, Sommer T, Pfanner N. Purification of Saccharomcyes cerevisiae mitochondria devoid of microsomal and cytosolic contaminations. Anal Biochem. 2000;287:339–342. doi: 10.1006/abio.2000.4868. [DOI] [PubMed] [Google Scholar]
- 18.Hines V, Keys LD, 3rd, Johnston M. Purification and properties of the bovine liver mitochondrial dihydroorotate dehydrogenase. J Biol Chem. 1986;261:11386–11392. [PubMed] [Google Scholar]
- 19.Blair PV, Oda T, Green DE. Studies on the Electron Transfer System. LIV. Isolation of the Unit of Electron Transfer. Biochemistry. 1963;2:756–764. doi: 10.1021/bi00904a023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Parr Cell Disruption Bomb No. 4639 before (A) and after (B) modification to increase diameter of the control valve knob from 1.8 cm to 10.4 cm. A hole is cut in the center of a plastic disk such that it fits snugly over the knurled knob.