Abstract
Emotion regulation is a critical aspect of children's social development, yet few studies have examined the brain mechanisms involved in the development of emotion regulation. Theoretical accounts have conceptualized emotion regulation as relying upon prefrontal control of limbic regions, specifying the anterior cingulate cortex (ACC) as a key brain region for the regulation of emotion. Functional magnetic resonance imaging (fMRI) in 5- to 11-year-olds during emotion regulation and processing of emotionally expressive faces revealed that older children preferentially recruited the more dorsal “cognitive” areas of the ACC, while younger children preferentially engaged the more ventral “emotional” areas. Additionally, children with more fearful temperaments exhibited more ventral ACC activity while less fearful children exhibited increased activity in the dorsal ACC. These findings provide insight into a potential neurobiological mechanism underlying well-documented behavioral and cognitive changes from more emotional to more cognitive regulatory strategies with increasing age, as well as individual differences in this developmental process as a function of temperament. Our results hold important implications for our understanding of normal development and should also help to inform our understanding and management of emotional disorders.
Keywords: Emotion Regulation, Development, fMRI, Anterior Cingulate, Temperament
Proficiency in the management of affective arousal is a critical aspect of early development. Infants move from a necessary dependence upon the support of their caregivers to the development of more self-sufficient mechanisms for emotion regulation at school age (Denham, 1998). Grolnick, Bridges, & Connell (1996), for example, discuss emotion regulation in early childhood as developing along a continuum in which young children and infants are initially unable to remove focus from emotional distress, without adult assistance, but later develop self-soothing strategies and the ability to reorient attention towards a more pleasant stimulus. Behavioral studies in preschool and school-age children have examined both children's modulation of overt emotional behavior (Cole, 1986; Saarni, 1984) and subjective emotional state (Harris, Olthof, & Terwogt, 1981). To illustrate, Cole (1986) found that preschool children are able to suppress overt negative emotional facial and vocal expression in response to social demands, while Harris and colleagues (1981) documented that by later school years (age 11-15) children become adept at modifying internal emotional states.
Since this early research, the characterization of individual differences in the typical and atypical development of affective regulation has become a well-established area of psychological science (Cole et al., 2004). However, little is known about the biological mechanisms supporting the developmental transitions in emotion regulation during early childhood or about the biological bases of individual differences in developmental pathways. This is unfortunate because information about the brain mechanisms underlying the typical and atypical development of emotion regulation could greatly inform our understanding of early social development as well as the disorders of emotion regulation (Lewis & Stieben, 2004; Lewis & Todd, 2007). Thus far, investigation of brain mechanisms for the development of emotion regulation has employed event related potential (ERP) measures to implicate the prefrontal cortex in cognitive control of emotion. Lewis, Lamm, Segalowitz, Stieben, & Zelazo (2006), for example, discovered that a temporary loss of points towards a desired prize triggered an increase in the frontal P3 response, and this response changed with age to a more midline localization. This developmental change coincided with a decrease in self-reported negative emotions in children (ages 5-16 years). Here, we report the results of one of the first functional magnetic resonance imaging (fMRI) studies of emotion regulation process in school-age children.
The anterior cingulate cortex (ACC) is a specialized prefrontal region that has been consistently implicated in affective regulation in the adult neuroimaging literature (Allman et al., 2001; Bush, Luu, & Posner, 2000). Located between the neocortex and the limbic system, the ACC is well positioned to serve as an interface between cognition and emotion. This region contains spindle-shaped neurons allowing for widespread connections to other brain areas. Found only in humans and great apes (Nimchinsky et al., 1999), these cells become functionally mature at four to six months of age, coinciding with an infant's developing ability to divert attention and self-soothe (Grolnick et al., 2006). Posner and Rothbart (1998, 2000) have noted an increase in cognitive control of emotion at the age of 4, which they relate to the child's emerging capacity to employ the ACC to manage impulsive emotional behavior through conscious regulatory strategy use. These theorists have hypothesized that variation in functional ACC maturation, and, consequently emotion regulation, is related to developmental and temperamental differences in the modulation of attention. Models of ACC function hypothesize divisions of a “cognitive” dorsal region, in proximity to dorsolateral prefrontal cortex (PFC), and an “emotional” ventral region close to orbitofrontal cortex and the amygdala (Bush et al., 2000). The dorsal division has been most often implicated in “cool” (Zelazo & Müller, 2002) executive function, activating during error monitoring and cognitive stroop tasks, while ventral activity is most often linked to “hot” executive function (Zelazo & Müller, 2002), including emotional stroop tasks and recall of emotional events (Bush et al., 2000). Both divisions project to the amygdala, and via these connections, are thought to support affective down-regulation. However, the dorsal pathway is hypothesized to be preferentially involved more in deliberate and consciously controlled regulatory processes (Lewis & Todd, 2007).
In a typically-developing sample of 5- to 11-year-old children, we used functional magnetic resonance imaging (fMRI) to investigate the development of the dorsal and ventral divisions of the ACC and their relationships to developmental and temperamental differences in the modulation of emotion. We hypothesized that when contrasting periods of high and low regulatory demand, the change in blood oxygenation level dependent (BOLD) activity in the dorsal (cognitive) area of the ACC would increase as a function of increasing age, while change in the activity of the ventral (emotional) portion of this region would decrease with age. This pattern should reflect a developmental shift to cognitively-oriented regulation strategies (Harris et al., 1981). We further hypothesized that decreased activation in the dorsal ACC would be related to fearful temperament, while increased ventral ACC activation would be correlated with fearfulness. This hypothesis was informed by previous electrophysiological studies reporting a negative influence of inhibited temperament on the brain's ability to control arousal in infancy and childhood (Fox et al., 1995).
Method
Subjects
Subjects were 20 (9 male) English-speaking, healthy, and typically developing children ages 5-11 years (average age = 8 years, 2 months) with normal or corrected-to-normal vision. An additional 6 children were scanned, but not included in data analyses due to excessive head movement (4 children), inability to complete the scan (1 child), or drowsiness (1 child). These children did not differ from children with usable data in age [t(24) = -.19, p = .85] or on any dimension of temperament [t(24) = -.50-1.83, p = .08-.97; see below]. Children were recruited from local community postings and internet advertisements and were paid for their participation. In addition, they earned an age-appropriate prize as part of their task participation (see below). A parent or legal guardian of each child provided written informed consent for their child's participation. Each child provided written assent.
Materials and Procedure
Before data collection and practice of the task, children were told that they would be playing to win a prize: their choice of a toy from a large collection. After choosing their desired prize, children were given a few minutes to play with the toy and show it to their parent and/or siblings. They were then told that they would need to earn a large amount of points during the game in order to keep their prize, and that they would earn points based on speed and accuracy. Children were not told the specific number of points which they would need to win, but if questioned, the experimenter replied, “You need a lot of points. Over a thousand, at least!” They were reminded that they would not be told whether they won or lost until the end of the game. Subjects then practiced the emotional go/no-go task outside of the scanner to ensure understanding during fMRI data collection.
Before scanning began, children participated in a “mock” scanning session to help ensure compliance with the requirement to remain motionless during data collection, and to help the children to feel as comfortable as possible while participating in the actual experiment. Children were trained to remain still while watching a children's video inside a replica of our MRI scanner. During practice, custom-written software received input from a head motion sensor worn by the child and used that input to play a sound when the child moved outside of a set threshold (3 mm). In addition to viewing approximately 10 minutes of the video, children again practiced the go/no-go task inside the mock scanner. With the addition of realistic scanner sounds played during the children's practice session, we were able to closely reproduce the scanning environment in order to familiarize our young subjects with fMRI scanning procedures, thereby improving compliance and increasing the likelihood of obtaining high-quality data from the young children.
Subjects participated in a novel, event-related within a blocked design, emotion regulation task partially adapted from previous studies (Garavan et al., 1999; Lewis et al., 2006). The emotional go/no-go task was presented using E-prime software (Psychological Software Tools, Pittsburgh, PA) and is depicted in Figure 1. Throughout the paradigm, a stream of pictures of common objects (e.g. balls, shoes, umbrellas) was presented at a rapid rate. Subjects were instructed to press a button when the object was presented in a green frame, but to inhibit their response when the object was presented in a red frame. Incorrect responses to both go and no-go trials were followed by a large “X” on the center of the screen and the sound of a buzzer. Periodically, fearful faces from the NimStim set of facial expressions (Tottenham et al., 2009), appeared for a one second duration, accompanied by a short chime. Children were told that the presentation of the fearful face was unrelated to the game and did not reflect their task performance. Subjects were also notified of their total number of accumulated points approximately every 30 seconds throughout the task. As point totals were presented, subjects heard a “slide whistle” sound indicative of point loss or point gain.
Figure 1.
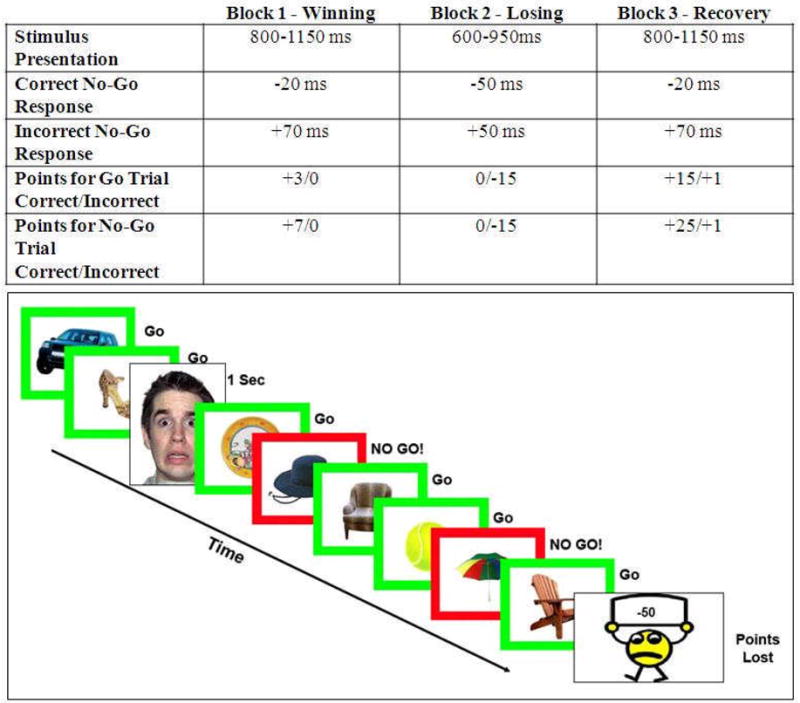
Top Panel: Stimulus presentation time and point adjustment algorithm for each of three emotion induction blocks. Subjects won points during Block 1, but then lost all earnings in Block 2. During Block 3, which had the same task difficulty level as Block 1, subjects regained their points to win their desired prize. Bottom Panel: A graphic representation of our go/no-go task. The paradigm was 12 minutes in length with 3 mood induction blocks and sporadic fearful faces presented as 1 second events.
This task was designed to maintain the same level of difficulty for all subjects. To this end, an algorithm was used to keep all children functioning at approximately the same error rate (50 ± 10%; Lewis et al., 2006). The algorithm was piloted with 25 5-year-olds (outside of the scanner) to ensure that children this young could successfully complete our task. The error rate for the task was maintained by adjusting the stimulus duration dynamically (see the top panel of Figure 1). Stimulus duration increased with each error made on a no-go trial and decreased with each correct response. Unbeknownst to subjects, the task contained three blocks designed to induce different types of emotion (Lewis et al., 2006). Each block lasted approximately four minutes. During Block 1 (Winning), subjects saw their points steadily increase to well over 1000 with a stimulus interval set between 800 – 1150 ms. This block was designed to induce positive emotions related to success in our subjects, even though the task was designed to be challenging and engaging throughout. Changes in the point-adjustment algorithm and stimulus presentation speed (600 – 950 ms) caused increased task difficultly and point loss during Block 2 (Losing), which was intended to induce negative emotions of frustration. Children lost all of their previously earned points during this block. With a return to the more generous algorithm in Block 3 (Recovery), subjects regained their points and ultimately won their desired prize. Although there was notable point gain, we designed this block to represent a recovery period after an emotionally negative event. The experiment was designed with three long blocks in order to allow subjects the necessary time to feel the induced positive or negative emotions due to relatively lengthy periods of gaining or losing points, respectively. In order to ensure that subjects were unaware of our manipulation, there were no pauses between emotion induction blocks.
In our analysis, the one second presentations of the fearful faces were modeled as events. Each block contained 20 fearful face events (60 total trials) that were presented 6-15 seconds apart regardless of emotion induction block or changes to the speed of the point adjustment algorithm. We compared the fearful face events between the Winning and Recovery blocks because they were essentially identical in speed and difficulty of the point adjustment algorithm, but offered very different regulatory contexts. In the Winning block, subjects were expected to be in a positive mood while rapidly gaining points compared to the Recovery block which followed a significant point loss and the induction of negative emotions. We note that the go/no-go task in this study was employed simply as a mechanism to challenge children within an emotional context and was presented at speeds varying by participant success rate. We, therefore, did not model specific go and no-go trials in our analysis.
Manipulation Check
Our child subjects were interviewed regarding their emotional experiences during the task. One hundred percent of children recalled losing all of their points during the game and 95% (all but one child) reported feeling negative emotions during that episode. Ninety percent reported feeling positive emotions when they discovered that they would, indeed, win their prize. Finally, 74% of children reported an overall enjoyment of the game.
fMRI data acquisition
Scanning was performed on a Siemens 3 Tesla Allegra head-only scanner (Siemens, Erlangen, Germany). High-resolution, T1-weighted anatomical images were acquired using an MPRAGE sequence (TR = 1630 ms; TE = 2.48 ms; FOV = 20.4 cm; α = 8°; image matrix = 2562; voxel size = 0.8 × 0.8 × 0.8 mm; 224 slices). Whole-brain functional images were acquired using a single-shot, gradient-recalled echoplanar pulse sequence (TR = 2000 ms; TE = 30 ms; α = 73°; FOV = 20.4 cm; image matrix = 642; voxel size = 3.2 × 3.2 × 3.2 mm; 35 slices) sensitive to blood oxygenation level-dependent (BOLD) contrast. Runs consisted of the acquisition of 224 successive brain volumes beginning with 2 discarded RF excitations to allow for steady-state equilibrium.
The BrainVoyager QX software package (Brain Innovations, Maastricht, The Netherlands) was used for all analyses. The following preprocessing procedures were performed on the raw images: slice scan time correction (using cubic spline interpolation), high-pass temporal filtering to remove nonlinear drifts of three or fewer cycles per time course, three-dimensional motion correction to detect and correct for small head movements by spatial alignment of all volumes to the first volume by rigid body transformation, and spatial data smoothing using a Gaussian kernel with a 4 mm full width at half-maximum. Head movements never exceeded 3 mm for all participants included in this analysis. Functional data were coregistered to the anatomical volume by alignment of corresponding points to obtain optimal fit and were then transformed into Talairach space (Talairach & Tournoux, 1988). We employed the adult anatomical Talairach template. The literature suggests that after transformation into a common stereotactic space, anatomical differences between children and adults are small relative to the resolution of fMRI data (Burgund et al., 2002). Another study revealed minimal differences in the time courses and locations of functional activation foci between children and adults (Kang, Burgund, Lugar, Petersen, & Schlaggar, 2003).
A multiparticipant statistical analysis was performed by multiple linear regression of the time course of the BOLD response in each voxel. The general linear model of the experiment was computed for each participant's z-normalized volume time courses. Model predictors were defined by convolving an ideal boxcar response with a gamma-function model of the hemodynamic response (Friston et al., 1995). Boxcar values were equal to 1.0 during the face presentation and were 0.0 during presentation of objects. Activation maps were visualized on a Talairach-transformed brain of an 8 year-old male subject (see Figure 2), with only clusters of more than 8 contiguous voxels displayed.
Figure 2.
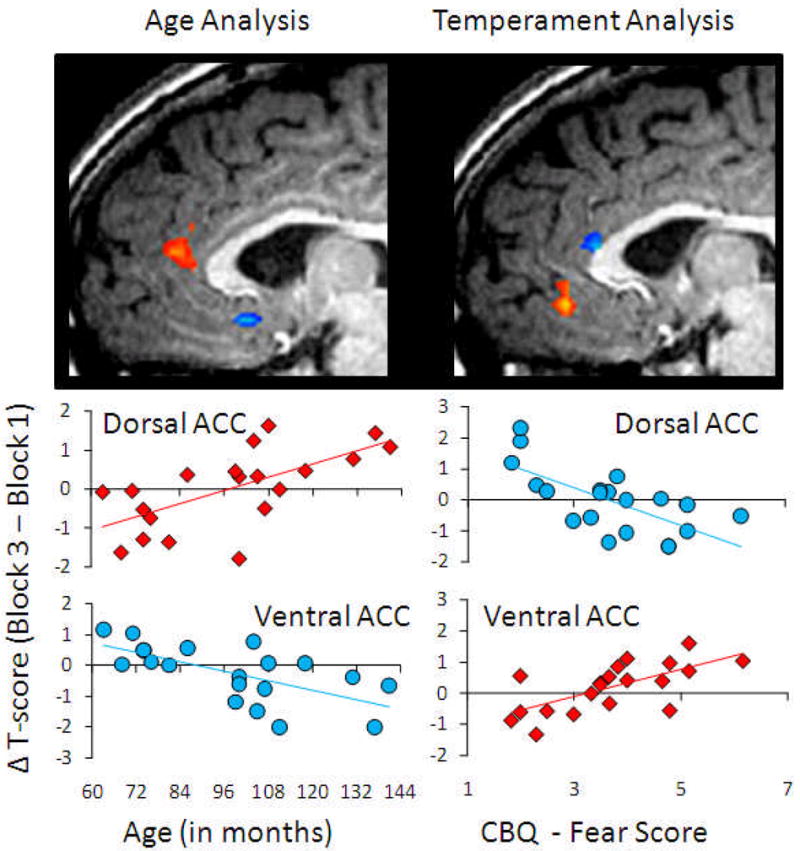
Correlations between age and fearful temperament and the difference in activation between pre-induction and post-induction blocks during 1 s presentation of fearful faces. Red represents positive correlation and blue represents negative correlation within the anatomically defined anterior cingulate cortex.
Temperament data collection
Caregivers were asked to provide information on their children's temperament. They completed the Child Behavior Questionnaire (CBQ)-Long Form (Rothbart et al., 2001), an experimentally-validated and commonly used caregiver assessment of 15 dimensions of children's temperament. Our hypotheses focused on the fearful dimension of temperament due to its relationship to behavioral inhibition and anxiety, which have been implicated in regulatory deficits (Fox et al., 1995).
Results
An examination of the main effect of condition (Winning, Losing, and Recovery) revealed several neural areas that were significantly active at the False Discovery Rate (FDR) q < .05 criterion (Genovese, Lazar, & Nichols, 2002). The ACC (BA 32) was the largest, and most significantly active, amongst them. All regions are presented in Table 1.
Table 1. Differences in BOLD signal between Winning, Losing, and Recovery.
| Talairach coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | Side | X | Y | Z | # voxels | F* |
| Main effect of condition | ||||||
| Anterior Cingulate (BA32) | R | 9 | 35 | 22 | 11445 | 18.2 |
| Middle Temporal Gyrus (BA20) | R | 57 | -43 | -8 | 3769 | 15.6 |
| Inferior Frontal Gyrus (BA9) | R | 42 | 8 | 25 | 1765 | 12.5 |
| Inferior Parietal Lobule (BA40) | R | 42 | -55 | 49 | 1057 | 8.8 |
| Medial Frontal Gyrus (BA8) | R | 6 | 20 | 43 | 703 | 9.4 |
| Cuneus (BA18) | R | 15 | -79 | 19 | 623 | 10.5 |
| Middle Occipital Gyrus (BA19) | L | -30 | -82 | 10 | 617 | 11.5 |
| Cuneus (BA19) | R | 24 | -82 | 37 | 599 | 10.2 |
| Middle Frontal Gyrus (BA10) | R | 39 | 38 | 16 | 570 | 12.1 |
| Paracentral Lobule (BA5) | R | 15 | -31 | 52 | 367 | 12.3 |
| Fusiform Gyrus (BA18) | L | -27 | -91 | -11 | 250 | 9.1 |
| Inferior Temporal Gyrus (BA20) | R | 54 | -28 | -14 | 168 | 9.9 |
| Middle Occipital Gyrus (BA37) | R | 48 | -67 | -5 | 88 | 7.04 |
| Inferior Temporal Gyrus (BA20) | L | -42 | -1 | -46 | 51 | 13.2 |
R, right; L, left; BA, Brodmann area;
= q<0.05 FDR corrected for multiple comparison
Next, brain responses to the presentation of fearful faces during the Winning period in which children gained points towards a desired prize were contrasted with activity in response to fearful faces presented during a Recovery period, which followed point loss. To focus our analysis on the ACC as an a priori region of interest, we used the Analysis of Functional Neuroimages Anterior Cingulate Gyrus region of interest (ROI) (AFNI; National Institutes of Mental Health; Bethesda, MD) to define the area structurally. Across the ACC, the Winning condition was contrasted with the recovery condition and then correlated with our auxiliary variables, at the significance level of of p < .05 [r(18) ≥ .50]. For these image analyses, we used a statistical threshold criterion of p < 0.001 corrected for multiple comparisons using a cluster-size threshold adjustment, based on a Monte Carlo simulation approach extended to 3D data sets using the cluster threshold size estimator plug-in BrainVoyager QX (Forman, Cohen, Fitzgerald, Eddy, Mintun, & Noll, 1995). This procedure takes input regarding the functional voxel size (3 mm3 native resolution), the total number of significant voxels within a map, and the estimated smoothness of a map and performs Monte Carlo simulations (1,000 iterations) to estimate the probability of clusters of a given size arising purely by chance. By applying a spatial cluster threshold of 828 mm3, or 28 contiguous voxels, the family-wise (corrected) false probability rate was p < 0.001.
This analysis identified a section of the dorsal ACC in which greater positive change in the level of activity between Winning and Recovery correlated positively with a child's age, as well as a section of the ventral ACC in which greater positive change in activity levels between Winning and Recovery correlated negatively with age (see Figure 2). A regression analysis involving the change in activation from Winning to Recovery in both the dorsal and ventral areas simultaneously regressed onto child age, revealed a significant overall model [F(2,19) = 20.375, p < .001], with both the dorsal [β = .545, p < .001] and ventral [β = -.543, p < .001] regions of the ACC being significantly different from zero.
In addition, we examined the fearful dimension of temperament in relation to activity evoked by the presentation of faces during mood induction. Our analysis revealed a portion of the dorsal ACC in which greater positive change in activity levels between Winning and Recovery correlated negatively with fearfulness and a portion of the ventral ACC in which greater positive change in activity from Winning to Recovery correlated positively with this dimension of temperament. In both our age and temperament correlations, clusters of activation appeared to be localized to the cognitive and emotional divisions of the ACC (Bush et al., 2000). Notably, child age and fearful temperament were uncorrelated in our sample [r(18) = -.04, p = .87], suggesting that age and temperament were contributing independently to the double dissociation that we observed. A regression analysis was completed with the change in activation from Winning to Recovery in both the dorsal and ventral areas simultaneously regressed onto child fearful temperament. This analysis revealed a significant overall model [F(2,19) = 25.293, p < .001] with both the dorsal [β = -.555, p < .001] and ventral [β = .550, p < .001] regions of the ACC being significantly different from zero.
Discussion
Our results provide novel insights into the biological basis of regulatory mechanisms across child development. Extrapolating from functional models of the dorsal and ventral divisions of the ACC (Bush et al., 2000), older children in our experiment appeared to recruit the more dorsal “cognitive” areas of the ACC when required to emotionally regulate in the face of a frustrating task, while younger children engaged the more ventral “emotional” areas. We propose that this shift in prefrontal activation underlies the well-documented change to more cognitive regulatory strategy use (e.g. reappraisal) as children develop (Harris et al., 1981).
Our findings concerning age-related changes in ACC function are consistent with and serve to advance a broader literature concerning the development of the PFC and its relationship to the emergence of more effective executive functioning with age (Casey et al., 2000). The PFC is among the last brain areas to fully develop, with continued synaptogenesis (Rakic et al., 1994) and increasing white matter volume (Pfefferbaum et al., 1994; Shaw et al., 2006) until late adolescence or early adulthood. This protracted developmental course has been linked to improvements in various complex cognitive skills (Caviness et al, 1996; Diamond, 1988; Shaw et al., 2006), which are known to be important for the modulation of affective arousal (Gross, 1998). FMRI studies investigating the development of self-regulation have demonstrated that differential recruitment of regions of the PFC may underlie decreased cognitive control of emotion in children relative to adults (Bunge et al, 2002; Casey et al, 1997, Luna et al, 2001). Casey and colleagues (Casey et al., 1997), for example, found that children recruited the same PFC regions as adults during a go/no-go task. Activation was greater in children than in adults, however, suggesting inefficient recruitment of the PFC in children. This finding was echoed by Lewis et al. (2006), who related their electrophysiological results to inefficient ACC functioning during their go/no-go task.
Independent of the age-related effects, the more fearful children preferentially recruited the less effective “emotional” areas of the ventral ACC in response to emotionally expressive faces, while less fearful children engaged the more “cognitive” dorsal regions of the ACC. These results suggest a neural mechanism for the influence of fearful temperament on the development of emotion regulation abilities and add to a growing understanding of the neurobiological basis of individual differences in emotion regulation. Prior research has probed the relationship between child temperament and the brain's ability to control emotional arousal in infancy and childhood (Rothbart & Sheese, 2007). Fox and his colleagues (Fox, 1994; Fox & Calkins, 2003) have implicated the construct of electroencephalograph (EEG) asymmetry in individual differences in children's temperament, emotional reactivity, and regulation. Specifically, right-lateralized frontal EEG asymmetry has been related to increased fearful temperament and impaired regulation of fear during social interaction (Fox et al., 1995). In a related line of fMRI research, adolescents who had been temperamentally classified as behaviorally inhibited displayed heightened amygdala activation while rating the emotional content of face stimuli (Pérez-Edgar et al., 2007). This amygdala hyperactivity might underlie difficulties in effective regulation during social engagement. Relatedly, Drevets & Raichle (1998) have reported competition between dorsal and ventral prefrontal activation in adult subjects using positron emission tomography (PET). Notably, these authors have found that increased anxiety is related to heightened ventral, at the expense of dorsal, ACC activity.
We found evidence that changes in the balance of dorsal and ventral anterior cingulate cortical activation during regulatory episodes may be related to differences in child age and temperament. However, our conclusions are tempered by some limitations to the current study. First, we did not include online, physiological measures of anxiety and/or arousal such as heart rate, pupil diameter, or skin conductance. Indeed, these measures would have helped to pinpoint moments of increase in task-related stress and thus provided additional precision for correlation with our brain and temperament data. Second, due to the need to induce negative emotions with point loss following a point gain, we presented the blocks in a fixed order. We note that this method could have resulted in subject “drift”. For example, young children may have been losing track of the task in the recovery block, while older children may have remained engaged longer. We do not believe this to be the case because our correlational analysis of fearful temperament and ACC activity indicated that the dorsal ACC was most active in non-fearful children, who were represented across the observed age range. Nonetheless, this is an important limitation which should be considered.
Finally, the construct of emotion regulation is a widely debated topic in the field of child development. There is general disagreement on the definition of this construct, the nature of its development, and how to separate regulation from aspects of general affective reactivity (Cole et al., 2004). Our approach was designed to put children in a real-life situation in which they are typically required to regulate emotion: the loss of a desirable prize. Consequently, there was no condition in which participants were explicitly instructed to regulate emotion, which is a commonly employed technique in the adult literature (e.g. Ochsner, Bunge, Gross, & Gabrieli, 2002). While commendable in that it is difficult to mimic ecologically valid emotional situations within the confines of the scanning environment, this method also has some drawbacks. Specifically, it is difficult to pinpoint exactly when and how children were regulating emotion during this task. Although the brain and self-report data is suggestive of the engagement of regulatory mechanisms during our go/no-go task, without the explicit instruction to regulate emotion, we cannot be sure that changes in activation between Winning and Recovery are due to increased regulation or persistent reactivity of negative emotions from point loss. We are, therefore, somewhat limited in the interpretation of our results. However, we believe that such a complicated theoretical debate (Campos, Frankel, & Camras, 2004; Cole et al., 2004) requires a variety of approaches to better characterize the nature of the neural mechanisms involved in affective self regulation. In the future, experiments will be needed to better assess the specific regulatory strategies employed during implicit emotional tasks.
Acknowledgments
We thank S. Dewhurst, M. Donovan, S. Haas, A.J. Hawthorne, C. Hudac, K-J. Jung, S. Kurdilla, S. Shade, and D. Vizlay for their assistance with programming, subject recruitment, data collection, and analysis. We are grateful to Dr. Marc Lewis and his colleagues for allowing us the use and adaptation of their emotion regulation task and to the children and families who devoted their time to participation in our study. Funding was provided by a R21 Network Grant (MH0704780), a Career Development Award from the National Institute of Mental Health (MH071284), and a John Merck Scholars award to Kevin Pelphrey. Susan Perlman is now at the University of Pittsburgh Medical Center.
References
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. Annals of the New York Academy of Sciences. The anterior cingulate: The evolution of an interface between cognition and emotion. In: Damasio AR, Harrington A, editors. Unity of knowledge: The convergence of natural and human science. New York: New York Academy of Sciences; 2001. pp. 107–117. [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, et al. The feasibility of a common stereotactic space for children and adults in fmri studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Frankel CB, Camras LA. On the nature of emotion regulation. Child Development. 2004;75(2):377–394. doi: 10.1111/j.1467-8624.2004.00681.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Geidd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Nystrom LE, Geidd JN, Castellanos X, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7-11 years: A volumetric analysis based on magnetic resonance images. Cerebral Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Cole PM. Children's spontaneous control of facial expression. Child Development. 1986;67:1686–1706. [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75(2):317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Denham SA. Emotional development in young children. New York: Guilford Press; 1998. [Google Scholar]
- Diamond A. Abilities and neural mechanisms underlying AB performance. Child Development. 1988;59:523–527. [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between cognition and emotion. Cognition & Emotion. 1998;12(3):353–385. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a clustersize threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processing underlying emotion regulation. Monographs of the Society for Research in Child Development. 1994;59(2/3):152–166. [PubMed] [Google Scholar]
- Fox NA, Calkins SD. The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;27(1):7–26. [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, et al. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1770–1784. [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TN. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Grolnick WS, Bridges LJ, Connell JP. Emotion regulation in two-year-olds: Strategies and emotional expression in four contexts. Child Development. 1996;67:928–941. [PubMed] [Google Scholar]
- Grolnick WS, McMenamy JM, Kurowski CO. Emotional self-regulation in infancy and toddlerhood. In: Balter L, Tamis-LeMonda CS, editors. Child psychology: A handbook of contemporary issues. Philadelphia: Psychology Press; 2006. pp. 3–26. [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2(3):271–299. [Google Scholar]
- Harris PL, Olthof T, Meerum Terwogt M. Children's knowledge of emotion. Journal of Child Psychology and Psychiatry. 1981;22:247–261. doi: 10.1111/j.1469-7610.1981.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18(3):430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Stieben J. Emotion regulation in the brain: Conceptual issues and directions for developmental research. Child Development. 2004;75(2):371–376. doi: 10.1111/j.1467-8624.2004.00680.x. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Todd RM. The self-regulating brain: Cortical-subcortical feedback and the development of intelligent action. Cognitive Development. 2007;22:406–430. [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Nimchinsky E, Gilissen E, Allman JM, Perl DP, Erwin JM, et al. A neuronal morphologic type unique to humans and great apes. Proceedings of the National Academy of Sciences. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky E, Vogt BA, Morrison JH, Hof PR. Spindle neurons of the human anterior cingulate cortex. Journal of Computational Neurology. 1995;335:27–37. doi: 10.1002/cne.903550106. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Kim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self regulation, and consciousness. Philosophical Transactions of the Royal Society of London, B. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: Implications for learning, memory, and mental illness. Progress in Brain Research. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at 3-7 years: The Children's Behavior Questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Sheese BE. Temperament and emotion regulation. In: Gross JJ, editor. The Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 331–350. [Google Scholar]
- Saarni C. Observing children's use of display rules: Age and sex differences. Child Development. 1984;55:1504–1513. [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3 dimensional proportional system: An approach to cerebral imaging. New York: Thieme Medical; 1988. [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Müller U. Executive function in typical and atypical development. In: Goswami U, editor. Handbook of childhood cognitive development. Oxford: Blackwell; 2002. pp. 445–469. [Google Scholar]


