Abstract
Background
Cutaneous PGD2 levels increase following scratching. CRTH2 mediates chemotaxis to PGD2 and is expressed on Th2 cells and eosinophils, which infiltrate skin lesions in atopic atopic dermatitis (AD).
Objective
Examine the role of CRTH2 in a murine model of AD.
Methods
CRTH2−/− mice and WT controls were epicutaneously (EC) sensitized by repeated application of ovalbumin (OVA) to tape-stripped skin for 7 weeks, then challenged by OVA application to tape-stripped previously unsensitized skin for one week. Skin histology was assessed by H&E staining and immunohistochemistry. Cytokine mRNA expression was examined by quantitative RT-PCR. Levels of PGD2, antibody and cytokines were measured by ELISA.
Results
PGD2 levels significantly increased in skin 24 hrs after tape-stripping, but not in skin subjected to repeated sensitization with OVA. Allergic skin inflammation developed normally at sites of chronic EC sensitization with OVA in CRTH2−/− mice, but was severely impaired in previously unsensitized skin challenged with OVA, as evidenced by significantly decreased skin infiltration with eosinophils and CD4+ cells and impaired Th2 cytokine mRNA expression. Impaired skin inflammation at sites of acute OVA challenge in CRTH2−/− mice was not due to impaired systemic response to EC sensitization, as OVA specific IgG1 and IgE antibody levels and OVA driven splenocyte secretion of cytokines in these mice were comparable to those of WT controls.
Conclusions
CRTH2 promotes allergic skin inflammation in response to cutaneous exposure to antigen in previously sensitized mice.
Keywords: CRTH2, PGD2, atopic dermatitis
INTRODUCTION
Prostaglandins (PGs) are lipid-derived mediators generated by the sequential metabolism of arachidonic acid (AA) by the cyclooxygenase (COX) and prostaglandin synthase enzymes. Upon its release from the membrane by phospholipase A2, AA undergoes oxidation by COX-1, and COX-2 to PGG2 followed by reduction to the unstable endoperoxide PGH2, which serves as substrates for prostaglandin synthase enzymes that are responsible for the generation of the bioactive PGs and thromboxane. PGs include PGD2, PGE2, PGF2α and PGI2 1.
PGD2 is the predominant PG produced by activated mast cells, which initiate type I allergic responses 2. PGD2 is also released by other immune cells, that include Th2-type helper T cells, dendritic cells (DCs) and skin Langerhans cells, and by keratinocytes 3–6. PGD2 is released into the airways following antigen challenge, and PGD2 inhalation challenge elicits bronchoconstriction and airway eosinophil infiltration. PGD2 is also released in skin during an acute allergic response 2.
PGs are potent inflammatory mediators and mediate their effects through rG-protein-coupled receptors. PGD2 has two receptors: DP1, which shares 20–30% identity with receptors for other PGs, and a second receptor, DP2, also known as chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2), which is more closely related to chemoattractant receptors than to DP1 and the other PG receptors 1. Both DP1 and CRTH2 bind PGD2 with similar high affinity.
Mice deficient in DP1 exhibit decreased Th2 mediated airway inflammation and airway hypersensitivity in the OVA-induced model of asthma 7. DP1 is expressed on airway epithelial cells and likely mediates PGD2 driven mucus secretion in asthma and rhinitis. CRTH2 is expressed on Th2 lymphocytes, eosinophils, mast cells and basophils and mediates PGD2 driven chemotaxis of these cells in vitro 8–12. CRTH2 amplifies Th2 responses by preventing apoptosis of Th2 cells and enhancing their capacity to secrete cytokines 3, 13, 14. CRTH2 also amplifies eosinophil function by mobilizing them from the bone marrow, preventing their apoptosis, and promoting their chemokinesis and degranulation 15–17.
Expression of CRTH2 is increased on T cells and eosinophils in patients with allergic asthma and there is accumulation of CRTH2+ lymphocytes in human allergic nasal mucosa 18–20. Genetic variations in CRTH2 have been associated with allergic asthma in different ethnic groups 21–23. Activation of CRTH2 exacerbates allergic inflammation in an allergic inflammatory mouse model of asthma24, while CRTH2 antagonism ameliorates airway inflammation and hyperreactivity in this model 25, 26. However, in one study using the same model, CRTH2−/− mice had the same degree of allergen-induced airway eosinophilia as WT controls 27, and in another study, they showed enhanced eosinophil recruitment into the lung and produced significantly more IL-5 and IL-13 in vitro 28. Thus, the exact role of CRTH2 asthma remains unresolved.
Atopic dermatitis (AD) is characterized by skin barrier dysfunction. This results in scratching of the dry skin and sensitization to cutaneously introduced protein allergens. CRTH2 mRNA expression is high in peripheral blood mononuclear cells of patients with AD, and circulating eosinophils and T cells in AD patients have increased surface expression of CRTH2 29, 30. Since AD skin lesions are characterized by infiltration with Th2 cells and eosinophils, it is important to define the potential role of CRTH2 in AD. We have developed a mouse model of allergic skin inflammation with many similarities to AD. In this model, repeated EC sensitization of tape-stripped skin with OVA results in a Th2 dominated systemic immune response characterized by elevated total and antigen specific IgE, and in dermatitis characterized by dermal infiltration of CD4+ T cells and eosinophils and increased local expression of Th2 cytokines 31, 32. We have taken advantage of the availability of CRTH2−/− mice to examine the potential role of CRTH2 in allergic skin inflammation. The results demonstrate that CRTH2 is essential for the development of allergic inflammation in skin acutely challenged with OVA.
METHODS
Mice
CRTH2−/− mice on BALB/c background were kindly provided by Millennium Pharmaceuticals Inc. BALB/c wild-type (WT) mice were purchased from Charles River Laboratories (Wilmington, MA). J-KitW/KitW-v (WBB6F1) mice, which virtually lack tissue mast cells and possess <1.0% of the number of mast cells in the skin, and congenic WBB6F1 wild-type (WT) mice were obtained from the Jackson Laboratory (Bar Harbor, Me). All mice were kept in a pathogen-free environment and fed an OVA-free diet. All procedures performed on the mice were in accordance with the Animal Care and Use Committee of the Children’s Hospital Boston.
EC sensitization with ovalbumin and antigen challenge
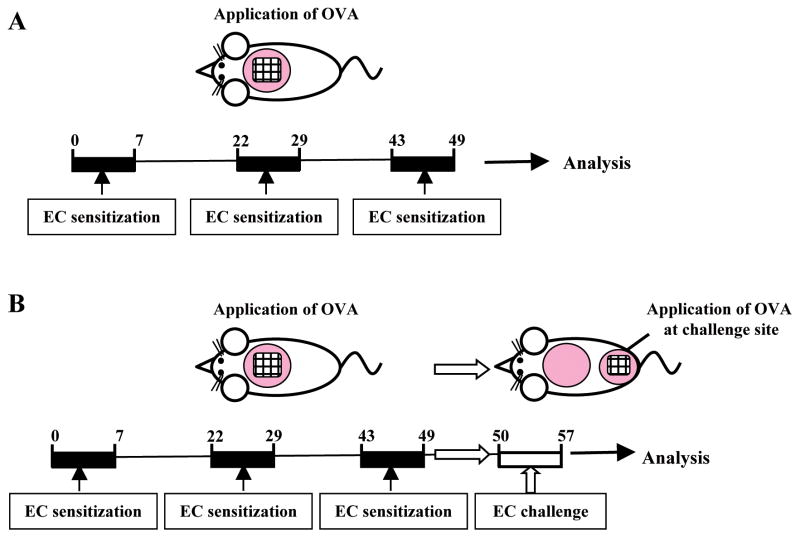
Four- to six-week-old female mice were EC sensitized as described previously 31. Briefly, the dorsal skin of anesthetized mice was shaved and tape stripped 6 times. 100 μg OVA (Grade V; Sigma Chemical Co.) in 100 μL of normal saline, or placebo (100 μL of normal saline), were placed on a patch of sterile gauze (1×1 cm), which was secured to dorsal skin with a transparent bio-occlusive dressing (Tegaderm, Westnet Inc.). Each mouse had a total of three one-week exposures to the patch separated by two-week intervals. In the first model, mice were studied at the end of the 7 week sensitization protocol (day 49). In the second model, mice were challenged on days 0 and 4 after sensitization (days 50 and 54 from the start) by application of OVA or saline to shaved and tape stripped previously unsensitized skin, and were studied on day 7 of challenge (day 57 from the start).
Histological and immunohistochemical analysis of mouse skin
Skin specimens were fixed in 10% buffered formalin and embedded in paraffin. Multiple 4 μm sections of skin were stained with hematoxylin and eosin (H&E) by HistoScientific Research Laboratories. CD4 staining of skin sections was performed as previously described 31. Eosinophils and CD4+ cells were counted blinded in 10–15 high-power fields (HPFs) at a magnification of 400X.
Quantitative RT-PCR
Skin specimens were homogenized using a Polytron RT-3000 (Kinematica, AG) in lysis buffer solution provided in the RNAqueous extraction kit (Ambion Inc.). Reverse transcription was performed using iScript cDNA synthesis kit (Bio-Rad). PCR reactions were run on an ABI Prism 7300 (Applied Biosystems) sequence detection system platform. Taqman primers and probes were obtained from Applied Biosystems. The housekeeping gene β2-microglobulin was used as a control. Relative gene expression was determined using the method described by Pfaffl 33.
Serum antibody determination
The BD-PharMingen protocol for sandwich ELISA was used to quantify OVA specific serum antibody levels.
Cell culture and in vitro cytokine production
Single cell suspensions of spleen cells were cultured in complete RPMI 1640 (JRH Biosciences Inc., Lenexa, Kansas) supplemented by 10% FCS, 1 mM sodium pyruvate, 2mM L-glutamine, 0.05 mM 2-mercapto-ethanol, 100 U/ml penicillin, and 1 mg/ml streptomycin at 2×105/ml in 96 well plates or 2×106/ml in 24 well plates in the presence of OVA (200 μg/ml). Cytokine secretion in supernatants from 24 well plates after 96 hrs of culture was determined by ELISA following the manufacture’s instructions (BD-PharMingen).
Measurement of PGD2
PGD2 levels in skin were determined using solid phase extraction followed by PGD2-EIA kit measurement or PGD2-MOX ELISA according to the manufacturer’s instructions (Cayman Chemical Company). Results obtained with these two assays we found to be similar.
Statistical analysis
Student’s t-test for unpaired data was used. A p value smaller than 0.05 was considered statistically significant.
RESULTS
Mouse models of allergic skin inflammation used
We examined the effect of lack of the PGD2 receptor CRTH2 on allergic skin inflammation in two models. In one model, mouse skin was chronically sensitized with OVA over a total of 7 weeks and the site of repeated sensitization was examined on day 49 (Fig. 1A). In a second model, the mice were sensitized similarly, then previously unsensitized skin was tape stripped and challenged by application of OVA twice at days 50 and 54, and examined at day 57 (Fig. 1B).
Figure 1. Mouse models of allergic skin inflammation.
(A) Skin site subjected to repeated EC sensitization. (B) Acute skin challenge by application of OVA to tape stripped previously unsensitized skin of EC sensitized mice.
PGD2 levels are modestly elevated in tape stripped skin, but not in skin chronically sensitized with OVA
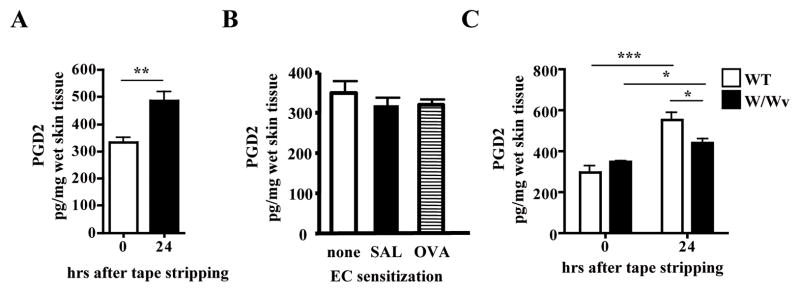
Scratching is a hallmark of AD and triggers skin flares. Fig. 2A shows that PGD2 is readily detectable in mouse skin and its levels modestly, but significantly increased 24 hrs after tape stripping. In contrast, we found no significant differences in PGD2 levels between unsensitized skin and skin EC sensitized for three cycles by repeated tape stripping and application of either saline or OVA (Fig. 2B).
Figure 2. Levels of PGD2 levels in tape stripped skin and in chronically EC sensitized skin.
(A) PGD2 levels in tape stripped skin of WT BALB/c mice (n=4 mice per group). (B) PGD2 levels in unsensitized skin of BALB/c mice taken immediately after shaving, and in skin taken at the end of 7 week sensitization with saline or OVA (n=4–5 mice per group). Experiments in A and B were performed at different times. (C) PGD2 levels in tape stripped skin of WBB6F1/J-KitW/KitW-v mice (n=3 mice per group). *p<0.05, **p<0.01, ***p<0.001.
Since mast cells are a rich source of PGD2 2, we examined the effect of acute mechanical skin injury on PGD2 levels in mast cell deficient WBB6F1/J-KitW/KitW-v mice. There was significantly lower PGD2 levels in injured skin of WBB6F1/J-KitW/KitW-v mice compared to genetically matched WBB6F1 controls (Fig. 2C), suggesting that mast cells are an important source of increased PGD2 skin levels in skin injured by tape stripping.
Allergic skin inflammation in skin sites repeatedly sensitized with OVA is intact in CRTH2−/− mice
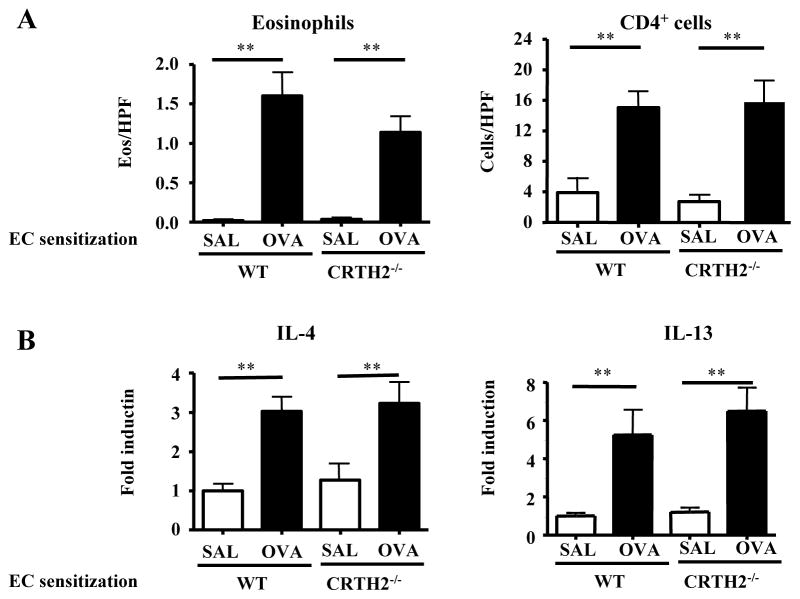
As we previously reported 31, 32, skin sites of WT BALB/c mice repeatedly sensitized with OVA, developed Th2-dominated inflammation characterized by epidermal thickening, dermal infiltration with mononuclear cells, CD4+ cells and eosinophils (Fig. 3A) and upregulation of IL-4 and IL-13, but not IFN-γ, mRNA expression (Fig. 3B). Dermal infiltration with CD4+ cells and eosinophils and upregulation of IL-4 and IL-13 were comparable in CRTH2−/− mice and WT controls (Fig. 3). These results suggest that CRTH2 does not play an important role in chronic skin inflammation elicited by repeated sensitization with antigen application to the same site.
Figure 3. Allergic skin inflammation in skin sites repeatedly sensitized with OVA is intact in CRTH2−/− mice.
(A) Dermal infiltration with eosinophils and CD4+ cells. (B) Q-PCR analysis of mRNA levels of IL-4, IL-5 and IL-13 and IFN-γ Results are expressed as fold induction relative to saline sensitized skin of WT mice (n=5–6 mice per group). **p<0.01.
Impaired skin inflammation in OVA challenged skin of CRTH2−/− mice EC sensitized with OVA
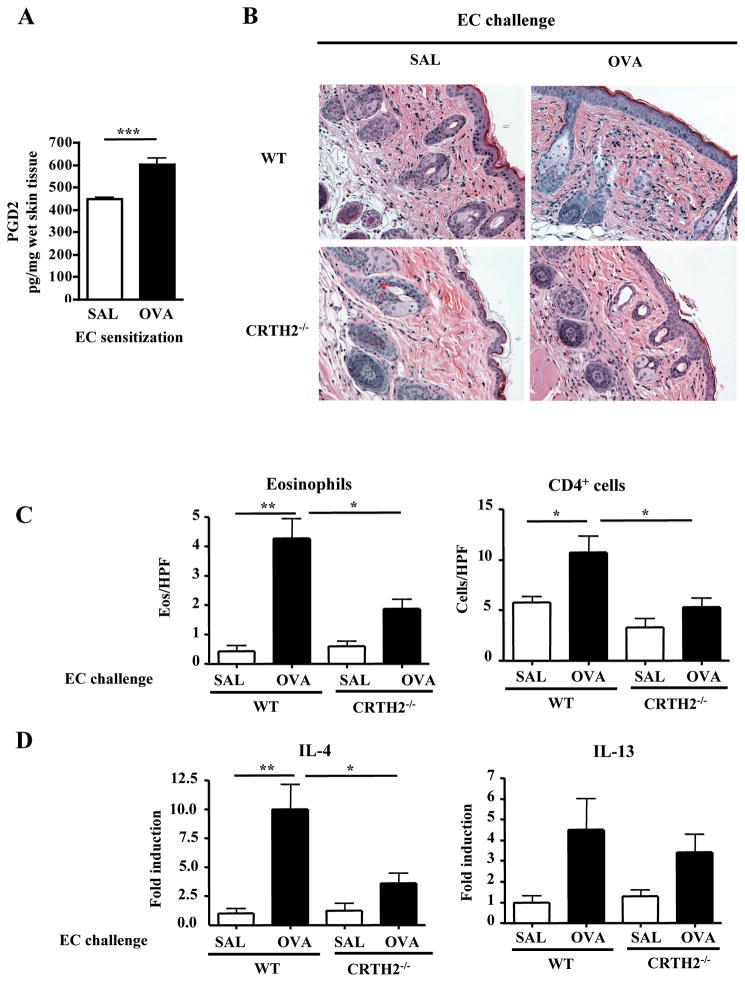
We next examined the potential role of CRTH2 in the development of skin inflammation in mice EC sensitized with OVA in response to antigen application to acutely tape-stripped previously unsensitized skin. Twenty four hours after challenge, there was a significant increase in PGD2 levels in OVA challenged previously unsensitized skin compared to saline challenged previously unsensitized skin (Fig. 4A). Skin challenge of EC sensitized WT mice with OVA at a previously unsensitized skin site caused increased dermal infiltration with mononuclear cells (Fig. 4B), a significant increase in dermal infiltration with CD4+ cells and eosinophils (Fig. 4C), and significant upregulation of IL-4 and IL-13 mRNA expression compared to skin challenge with saline (Fig. 4D). There were no significant differences in the numbers of CD4+ and eosinophils between tape-stripped saline challenged skin and unmanipulated skin of mice EC sensitized with OVA (data not shown). Skin challenge of EC sensitized CRTH2−/− mice with OVA caused less dermal infiltration with mononuclear cells (Fig. 4B), significantly less dermal infiltration with CD4+ cells and eosinophils (Fig. 4C), and significantly less increase in IL-4 mRNA compared to WT mice (Fig. 4D). There was also less IL-13 mRNA expression, but the difference was not significant (Fig. 4D).
Figure 4. Impaired allergic skin inflammation in response to acute skin challenge with OVA in EC sensitized CRTH2−/− mice.
(A) PGD2 levels in challenged previously unsensitized skin from BALB/c mice previously EC sensitized with OVA. Measurement was performed 24 hrs after tape stripping and challenge with OVA or saline. (B) Representative skin histology. Magnification 200X. (C) Skin infiltrating CD4+ T cells and eosinophils. (D) Q-PCR analysis of mRNA levels of IL-4 and IL-13. Results are expressed as fold induction relative to saline challenged skin of EC sensitized WT mice (n=4–5 mice per group). *p<0.05, **p<0.01.
Intact systemic immune response to OVA in EC sensitized and OVA-challenged CRTH2−/− mice
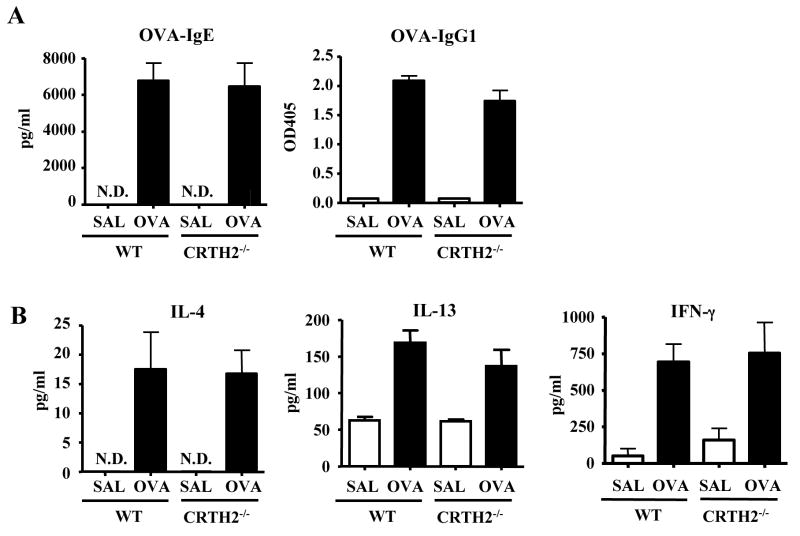
We considered the possibility that decreased skin inflammation in response to acute skin challenge with OVA in EC sensitized CRTH2−/− mice was secondary to a less vigorous systemic immune response to EC sensitization. Fig. 5A shows that serum IgG1 anti-OVA and IgE anti-OVA antibody levels were comparable in CRTH2−/− mice and WT controls that were EC sensitized with OVA. Fig 5B shows that secretion of the Th2 cytokines IL-4 and IL-13 and of the Th1 cytokine IFN-γ was also comparable in CRTH2−/− mice and WT controls.
Figure 5. Intact systemic immune response to OVA in EC sensitized CRTH2−/− mice.
(A) Serum levels of OVA-specific immunoglobulin isotypes. (B) Cytokine secretion by splenocytes in vitro in response to OVA stimulation (n=4–5 mice per group). N.D.= not detectable
DISCUSSION
This work demonstrates that CRTH2 is essential important for the development of allergic inflammation at sites of cutaneous antigen challenge.
PGD2 levels were modestly, but significantly increased in skin 24 hrs after tape stripping, a procedure that mimics mechanical injury caused by scratching (Fig. 2A). In contrast, PGD2 levels were not elevated in skin subjected to repeated sensitization with OVA, although with each cycle of sensitization the skin was tape stripped (Fig. 2B). These findings are consistent with the previous observation that PGD2 levels transiently increase in mouse skin after mechanical injury inflicted by scratching with a wire brush, but not in skin chronically subjected to scratching 34. Mast cells were shown to be an important, but not the sole, source for increased PGD2 levels after acute mechanical skin injury (Fig. 2C). Increased PGD2 levels in acutely tape stripped skin, but not in chronically sensitized skin, is consistent with our previous finding that mRNA levels of COX-2 mRNA, the enzyme that generates PGG and PGH, the substrates for PGD2 synthase, are rapidly, but transiently, upregulated in the skin after acute tape stripping, but are not elevated in skin sites repeatedly subjected to tape-stripping and exposure to OVA or saline for 7 weeks 35.
We found no detectable role for CRTH2 in the development of allergic skin inflammation in skin sites repeatedly sensitized with OVA (Fig. 3). This suggests that Th2 and eosinophil attractant chemokines other than PGD2 are important for chronic allergic inflammation. Levels of the Th2 chemoattractant CCL17/TARC and of the eosinophil chemoattractant CCL11/eotaxin are elevated both in the skin lesions of patients with AD 36, 37 and in mouse skin chronically sensitized with OVA 32, 38. Furthermore, CCL11/eotaxin was essential for eosinophil infiltration in skin chronically sensitized with OVA 38, and infiltration of CD4+ cells in these sites was significantly diminished in CCR4−/− mice (our unpublished observations). Expression of CCL17/TARC and CCL11/eotaxin mRNAs were elevated to a comparable degree in chronically EC sensitized skin of CRTH2−/− mice and ET controls (Fig. 6), suggesting that these chemokines may account for the normal infiltration of CD4+ cells and eosinophils in chronically sensitized skin CRTH2−/− mice.
Figure 6. Expression of CCL17/TARC and CCL11/eotaxin in chronically sensitized mouse skin.
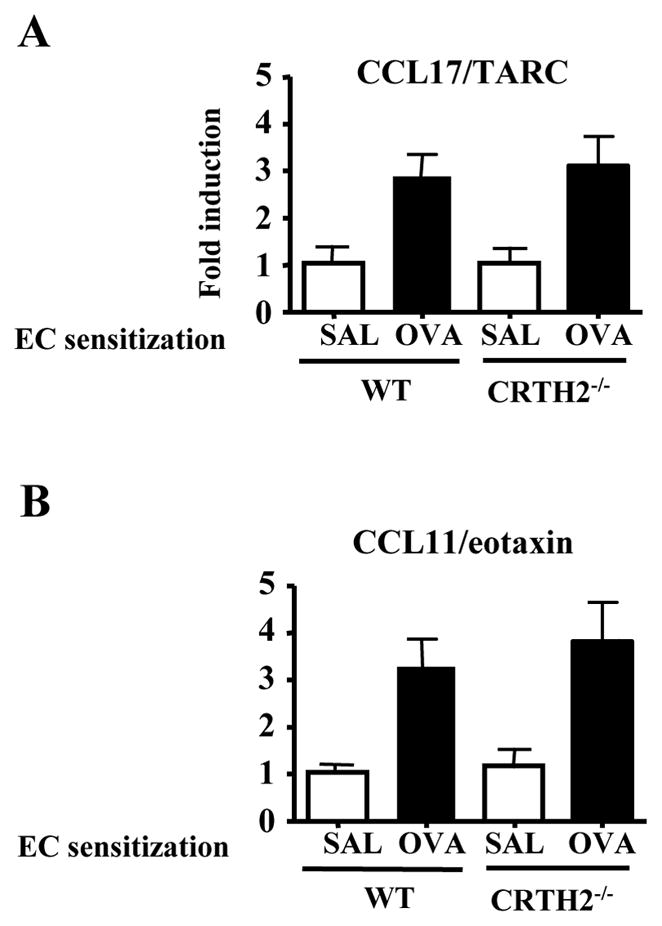
CCL17/TARC (A) and CCL11/eotaxin (B) mRNA levels in skin from CRTH2−/− mice and WT BALB/c controls, chronically sensitized with saline or OVA sensitized. Results are expressed as fold induction relative to saline sensitized skin of WT mice (n=4–5 mice per group).
Boehme et al. recently reported that a proprietary CRTH2 antagonist inhibits allergic skin inflammation in skin EC sensitized with OVA using a protocol similar to ours 39. However, mice treated with the CRTH2 antagonist had impaired immune response to EC sensitization. This was evidenced by reduced total IgE, OVA specific IgE, IgG1 and IgG2a antibody levels and cytokine secretion by OVA stimulated splenocytes. In addition, there was evidence that the antagonist interfered with the migration of antigen bearing DCs from skin to draining lymph nodes. These findings raise the possibility that the inhibitory effect of the CRTH2 antagonist used by Boehme et al. on skin inflammation could have been secondary to a CRTH2 independent inhibitory effect of the antagonist on the immune response to EC sensitization. In contrast, we found that CRTH2−/− mice had an intact systemic immune response to EC sensitization with OVA, as evidenced by comparable levels of OVA specific IgE, IgG1 and IgG2a antibody and splenocyte secretion of Th2 and Th1 cytokines in response to OVA stimulation that were comparable to those in WT controls (Fig. 5).
In contrast to normal allergic inflammation at skin sites that have been chronically sensitized with OVA, allergic inflammation in previously unsensitized skin sites that were tape stripped and acutely challenged with OVA was significantly impaired in CRTH2−/− mice. This was evidenced by significantly decreased dermal infiltration with eosinophils and CD4+ cells and impaired induction of Th2 cytokine mRNAs in antigen challenged skin (Fig. 4B–D). Furthermore, PGD2 levels were significantly higher in previously unsensitized skin sites that were tape stripped and challenged with OVA, compared to those challenged with saline (Fig. 4A). These findings suggest that PGD2 is a critical chemoattractant for Th2 cells and eosinophils following acute mechanical injury inflicted by scratching and introduction of antigen in previously uninflamed skin sites in patients with AD. Our results are in agreement with impaired skin inflammation in CRTH2−/− mice sensitized by painting FITC or 2,4-dinitrofluorobenzene on abdominal skin, then acutely challenged by painting hapten on the ears 40. This impairment was evidenced by reduced ear swelling, decreased infiltration of lymphocytes, eosinophils and basophils and decreased production of macrophage-derived chemokine and RANTES at inflammatory sites.
Although no significant increase in the numbers of CD4+ cells and eosinophils were detected in tape stripped and saline challenged skin compared to unmanipulated skin of WT mice, we cannot rule out the possibility that these cells were transiently recruited in response to a transient increase in PGD2 levels in skin acutely injured by tape stripping. In the presence of OVA, recruited CRTH2+ Th2 cells would be engaged and activated by antigen presenting dermal DCs to proliferate and secrete Th2 cytokines, which would cause accumulation of eosinophils by stimulating resident skin cells to produce eotaxins and cytokines that promote eosinophil survival 32, 38.
Scratching of dry skin, because of a defective barrier, is a hallmark of AD. To the extent that tape stripping mimics mechanical injury inflicted by scratching, our results suggest that CRTH2 blockade may be beneficial in preventing AD flares caused by scratching of non-inflamed dry skin and cutaneous introduction of antigen.
Acknowledgments
The authors thank Drs H. Oettgen and C. Matthias for discussions and advice.
Declaration of all sources of funding: This work was supported by The Atopic Dermatitis and Vaccinia Immunization Network NIH/NIAID contract NO1 (AI 40030) and a USPHS grant AR-047417 to Raif Geha.
Abbreviations used
- AA
arachidonic acid
- AD
atopic dermatitis
- COX
cyclooxygenase
- CRTH2
Chemoattractant receptor-homologous molecule expressed on receptor on Th2 cells
- DCs
dendritic cells
- DP1
prostaglandin D2 receptor 1
- EC
epicutaneous
- FITC
fluorescein isothiocyanate
- IL-4
interleukin-4
- IL-13
interleukin-13
- IFN-γ
interferon-gamma
- OVA
ovalbumin
- PG
prostaglandin
Footnotes
Clinical Implications
Blockage of PGD2 production may inhibit allergic skin inflammation elicited in AD patients by re-exposure to antigens to which they have been sensitized.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–66. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Roberts LJ, 2nd, Sweetman BJ, Lewis RA, Austen KF, Oates JA. Increased production of prostaglandin D2 in patients with systemic mastocytosis. N Engl J Med. 1980;303:1400–4. doi: 10.1056/NEJM198012113032405. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Hirai H, Takano S, Nakamura M, Nagata K. Effects of prostaglandin D2 on helper T cell functions. Biochem Biophys Res Commun. 2004;316:1009–14. doi: 10.1016/j.bbrc.2004.02.151. [DOI] [PubMed] [Google Scholar]
- 4.Vinall SL, Townsend ER, Pettipher R. A paracrine role for chemoattractant receptor-homologous molecule expressed on T helper type 2 cells (CRTH2) in mediating chemotactic activation of CRTH2+ CD4+ T helper type 2 lymphocytes. Immunology. 2007;121:577–84. doi: 10.1111/j.1365-2567.2007.02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenbach T, Czernielewski J, Hecker M, Czarnetzki B. Comparison of eicosanoid generation by highly purified human Langerhans cells and keratinocytes. J Invest Dermatol. 1990;95:104–7. doi: 10.1111/1523-1747.ep12874064. [DOI] [PubMed] [Google Scholar]
- 6.Ruzicka T, Aubock J. Arachidonic acid metabolism in guinea pig Langerhans cells: studies on cyclooxygenase and lipoxygenase pathways. J Immunol. 1987;138:539–43. [PubMed] [Google Scholar]
- 7.Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–7. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 8.Nagata K, Hirai H, Tanaka K, Ogawa K, Aso T, Sugamura K, et al. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s) FEBS Lett. 1999;459:195–9. doi: 10.1016/s0014-5793(99)01251-x. [DOI] [PubMed] [Google Scholar]
- 9.Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–86. [PubMed] [Google Scholar]
- 10.Boehme SA, Franz-Bacon K, Chen EP, Ly TW, Kawakami Y, Bacon KB. Murine bone marrow-derived mast cells express chemoattractant receptor-homologous molecule expressed on T-helper class 2 cells (CRTh2) Int Immunol. 2009;21:621–32. doi: 10.1093/intimm/dxp031. [DOI] [PubMed] [Google Scholar]
- 11.Monneret G, Gravel S, Diamond M, Rokach J, Powell WS. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood. 2001;98:1942–8. doi: 10.1182/blood.v98.6.1942. [DOI] [PubMed] [Google Scholar]
- 12.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue L, Barrow A, Pettipher R. Novel function of CRTH2 in preventing apoptosis of human Th2 cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009;182:7580–6. doi: 10.4049/jimmunol.0804090. [DOI] [PubMed] [Google Scholar]
- 14.Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 15.Schratl P, Royer JF, Kostenis E, Ulven T, Sturm EM, Waldhoer M, et al. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J Immunol. 2007;179:4792–9. doi: 10.4049/jimmunol.179.7.4792. [DOI] [PubMed] [Google Scholar]
- 16.Gervais FG, Cruz RP, Chateauneuf A, Gale S, Sawyer N, Nantel F, et al. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108:982–8. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- 17.Shiraishi Y, Asano K, Nakajima T, Oguma T, Suzuki Y, Shiomi T, et al. Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J Pharmacol Exp Ther. 2005;312:954–60. doi: 10.1124/jpet.104.078212. [DOI] [PubMed] [Google Scholar]
- 18.Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30:2972–9. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Yahara H, Satoh T, Miyagishi C, Yokozeki H. Increased expression of CRTH2 on eosinophils in allergic skin diseases. J Eur Acad Dermatol Venereol. 2009 doi: 10.1111/j.1468-3083.2009.03267.x. [DOI] [PubMed] [Google Scholar]
- 20.Shirasaki H, Kikuchi M, Kanaizumi E, Himi T. Accumulation of CRTH2-positive leukocytes in human allergic nasal mucosa. Ann Allergy Asthma Immunol. 2009;102:110–5. doi: 10.1016/S1081-1206(10)60239-6. [DOI] [PubMed] [Google Scholar]
- 21.Huang JL, Gao PS, Mathias RA, Yao TC, Chen LC, Kuo ML, et al. Sequence variants of the gene encoding chemoattractant receptor expressed on Th2 cells (CRTH2) are associated with asthma and differentially influence mRNA stability. Hum Mol Genet. 2004;13:2691–7. doi: 10.1093/hmg/ddh279. [DOI] [PubMed] [Google Scholar]
- 22.Cameron L, Depner M, Kormann M, Klopp N, Illig T, von Mutius E, et al. Genetic variation in CRTh2 influences development of allergic phenotypes. Allergy. 2009;64:1478–85. doi: 10.1111/j.1398-9995.2009.02053.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Xu Y, Zhao H, Sui H, Liang H, Jiang X. Genetic variations in chemoattractant receptor expressed on Th2 cells (CRTH2) is associated with asthma susceptibility in Chinese children. Mol Biol Rep. 2009;36:1549–53. doi: 10.1007/s11033-008-9349-6. [DOI] [PubMed] [Google Scholar]
- 24.Spik I, Brenuchon C, Angeli V, Staumont D, Fleury S, Capron M, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–8. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 25.Uller L, Mathiesen JM, Alenmyr L, Korsgren M, Ulven T, Hogberg T, et al. Antagonism of the prostaglandin D2 receptor CRTH2 attenuates asthma pathology in mouse eosinophilic airway inflammation. Respir Res. 2007;8:16. doi: 10.1186/1465-9921-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukacs NW, Berlin AA, Franz-Bacon K, Sasik R, Sprague LJ, Ly TW, et al. CRTH2 antagonism significantly ameliorates airway hyperreactivity and downregulates inflammation-induced genes in a mouse model of airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2008;295:L767–79. doi: 10.1152/ajplung.90351.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiraishi Y, Asano K, Niimi K, Fukunaga K, Wakaki M, Kagyo J, et al. Cyclooxygenase-2/prostaglandin D2/CRTH2 pathway mediates double-stranded RNA-induced enhancement of allergic airway inflammation. J Immunol. 2008;180:541–9. doi: 10.4049/jimmunol.180.1.541. [DOI] [PubMed] [Google Scholar]
- 28.Chevalier E, Stock J, Fisher T, Dupont M, Fric M, Fargeau H, et al. Cutting edge: chemoattractant receptor-homologous molecule expressed on Th2 cells plays a restricting role on IL-5 production and eosinophil recruitment. J Immunol. 2005;175:2056–60. doi: 10.4049/jimmunol.175.4.2056. [DOI] [PubMed] [Google Scholar]
- 29.Hijnen D, Nijhuis E, Bruin-Weller M, Holstege F, Koerkamp MG, Kok I, et al. Differential expression of genes involved in skin homing, proliferation, and apoptosis in CD4+ T cells of patients with atopic dermatitis. J Invest Dermatol. 2005;125:1149–55. doi: 10.1111/j.0022-202X.2005.23932.x. [DOI] [PubMed] [Google Scholar]
- 30.Iwasaki M, Nagata K, Takano S, Takahashi K, Ishii N, Ikezawa Z. Association of a new-type prostaglandin D2 receptor CRTH2 with circulating T helper 2 cells in patients with atopic dermatitis. J Invest Dermatol. 2002;119:609–16. doi: 10.1046/j.1523-1747.2002.01862.x. [DOI] [PubMed] [Google Scholar]
- 31.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–11. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto M, Arai I, Futaki N, Honma Y, Sakurai T, Hashimoto Y, et al. Putative mechanism of the itch-scratch circle: repeated scratching decreases the cutaneous level of prostaglandin D2, a mediator that inhibits itching. Prostaglandins Leukot Essent Fatty Acids. 2007;76:93–101. doi: 10.1016/j.plefa.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Laouini D, Elkhal A, Yalcindag A, Kawamoto S, Oettgen H, Geha RS. COX-2 inhibition enhances the TH2 immune response to epicutaneous sensitization. J Allergy Clin Immunol. 2005;116:390–6. doi: 10.1016/j.jaci.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 36.Zheng X, Nakamura K, Furukawa H, Nishibu A, Takahashi M, Tojo M, et al. Demonstration of TARC and CCR4 mRNA expression and distribution using in situ RT-PCR in the lesional skin of atopic dermatitis. J Dermatol. 2003;30:26–32. doi: 10.1111/j.1346-8138.2003.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 37.Yawalkar N, Uguccioni M, Scharer J, Braunwalder J, Karlen S, Dewald B, et al. Enhanced expression of eotaxin and CCR3 in atopic dermatitis. J Invest Dermatol. 1999;113:43–8. doi: 10.1046/j.1523-1747.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 38.Ma W, Bryce PJ, Humbles AA, Laouini D, Yalcindag A, Alenius H, et al. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J Clin Invest. 2002;109:621–8. doi: 10.1172/JCI14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boehme SA, Chen EP, Franz-Bacon K, Sasik R, Sprague LJ, Ly TW, et al. Antagonism of CRTH2 ameliorates chronic epicutaneous sensitization-induced inflammation by multiple mechanisms. Int Immunol. 2009;21:1–17. doi: 10.1093/intimm/dxn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satoh T, Moroi R, Aritake K, Urade Y, Kanai Y, Sumi K, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177:2621–9. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]