Abstract
The ciliary band is a distinct region of embryonic ectoderm that is specified between oral and aboral ectoderm. Flask-shaped ciliary cells and neurons differentiate in this region and they are patterned to form an integrated tissue that functions as the principal swimming and feeding organ of the larva. TGFβ signaling, which is known to mediate oral and aboral patterning of the ectoderm, has been implicated in ciliary band formation. We have used morpholino knockdown and ectopic expression of RNA to alter TGFβ signaling at the level of ligands, receptors, and signal transduction components and assessed the differentiation and patterning of the ciliary band cells and associated neurons. We propose that the primary effects of these signals are to position the ciliary cells, which in turn support neural differentiation. We show that Nodal signaling, which is known to be localized by Lefty, positions the oral margin of the ciliary band. Signaling from BMP through Alk3/6, affects the position of the oral and aboral margins of the ciliary band. Since both Nodal and BMP signaling produce ectoderm that does not support neurogenesis, we propose that formation of a ciliary band requires protection from these signals. Expression of BMP2/4 and Nodal suppress neural differentiation. However, the response to receptor knockdown or dominant negative forms of signal transduction components indicate signaling is not acting directly on unspecified ectoderm cells to prevent their differentiation as neurons. Instead, it produces a restricted field of ciliary band cells that supports neurogenesis. We propose a model that incorporates spatially regulated control of Nodal and BMP signaling to determine the position and differentiation of the ciliary band, and subsequent neural patterning.
INTRODUCTION
Members of the transforming growth factor-β (TGFβ) superfamily play central roles in cell fate specification in development. TGFβ ligands are secreted proteins that diffuse from their source and activate complex signaling networks that regulate differentiation. TGF-β signaling patterns are complicated because a range of factors modify ligand availability and receptor and signal transduction functions, creating complex developmental patterns from seemingly simple arrangements of localized signaling sources and widespread receptors. Well-known examples are Nodal and BMP4. In vertebrates, nodal is expressed on the left side of the embryo and its localized effects are controlled by Lefty-1 and Lefty-2 (Meno et al., 1996). Leftys bind to the EGF-CFC proteins that are required for Nodal to bind to the activin-like kinase (Alk) receptor at the midline of the body, thereby blocking Nodal binding, and preventing Nodal signals from spreading to right side (Chen and Shen, 2004). BMP4, which is expressed on the future ventral side of vertebrate ectoderm, diffuses throughout the embryo, but is antagonized by the direct binding of Chordin, which is expressed in the dorsal organizer. The consequence is that dorsal tissues form where BMP4’s ventralizing effects are blocked (De Robertis and Kuroda 2004). It is a hallmark of TGFβ signaling that molecular antagonists pattern the effects of the secreting ligands with surprising precision.
In sea urchin embryos four regions of ectoderm – the animal plate, oral ectoderm, aboral ectoderm and ciliary band – are produced by animal hemisphere blastomeres (Davidson et al., 1998; Yaguchi et al., 2006). Incompletely characterized events, dependent on vegetal canonical Wnt, restrict the animal plate to the animal pole (Yaguchi et al., 2006) and eliminate a repressor of nodal expression (Yaguchi et al., 2008). As a consequence, the TGFβ signals, Nodal and subsequently BMP2/4, begin to pattern the remaining ectoderm in the animal hemisphere, producing oral, aboral and ciliary band ectoderm (Duboc et al., 2004; Yaguchi et al., 2006). Models of ectodermal specification suggest that Nodal signaling is limited to the oral ectoderm by Lefty, which depends on Nodal and has long-range inhibitory functions (Duboc et al., 2008). A reaction-diffusion model in which Lefty acts as a feedback inhibitor has been proposed to explain how it restricts Nodal signaling to oral ectoderm (Duboc et al., 2008; Bolouri and Davidson, 2009). BMP2/4, which also acts downstream of Nodal, is transcribed in the oral ectoderm (Angerer et al., 2000; Duboc et al., 2004), yet acts outside of oral ectoderm to induce aboral ectoderm (Lapraz et al., 2009). Bradham et al. (2009) and Lapraz et al. (2009) showed that Chordin, expressed in the oral ectoderm under the control of Nodal, blocks BMP2/4 activity. In its absence, or in the absence of Nodal, differentiation of ciliary band neurons is altered as well as the normal expression pattern of a ciliary band marker. Although TGFβ signaling accounts for many aspects of oral and aboral ectoderm specification, we understand very little of the mechanisms involved in ciliary band formation, and the differentiation of ciliary band neurons.
The ciliary band is the principal swimming and feeding organ of the larva. It is a tightly packed strip of flask-shaped, ciliary cells that beat away from the mouth, producing a force that moves the larva forward and captures food particles deflected by ciliary reversals (Strathmann, 2007). In addition to the ciliary cells, there is a series of neurons, mostly on the oral side of the ciliary band, that have short, microvillar dendritic processes on their surface (Burke, 1978). A tract of axons that lies at the base of the ciliary cells interconnects the nerve cells. The nervous system is thought to regulate the direction of ciliary beat, as depolarization of the ciliary cells accompanies reversals of ciliary beat (Mackie et al., 1969; Satterlie and Cameron, 1985). Thus, the ciliary band is an integrated tissue innervated by neurons arranged in a precise pattern.
Our objective was to determine how components of the oral-aboral signaling network specify and pattern the ciliary cells and neurons of the ciliary band. We manipulated the signaling network by knocking down ligands and receptors with morpholinos and expressing RNAs encoding antagonists and dominant negative, or constitutively active signal transduction components. We anticipated that by assessing the distribution of different types of ectoderm and neurons, we would be able to deduce how oral/aboral ectoderm patterning mechanisms regulate formation of the ciliary band. Our results indicate that the ciliary band is positioned by TGFβ signaling, yet it is a region in which TGFβ signaling is suppressed. In addition, we identify novel roles for known components of the oral-aboral signaling network in patterning the ectoderm of sea urchin embryos.
MATERIALS AND METHODS
Animals and embryo culture
Strongylocentrotus purpuratus were collected near Victoria, BC or purchased from The Cultured Abalone, Goleta, CA. Gametes were obtained by intra-coelomic injection of 0.5M KCl and embryos were cultured by standard methods with filtered seawater (FSW) or artificial seawater at 15°C.
Microinjection of morpholino anti-sense oligonucleotides (MO) and mRNAs
Eggs were prepared as described previously (Yaguchi et al., 2006). Morpholinos (Gene Tools, Eugene, OR) were microinjected in 22.5% glycerol with the following concentration in the injection needles: nodal-MO (600µM), lefty-MO (200µM), BMP2/4-MO (150µM), and Alk3/6-MO (200µM). The morpholino sequences are:
nodal-MO: 5’-GATGTCTCAGCTCTCTGAAATGTAG-3’
lefty-MO: 5’-AGCACCGAGTGATAATTCCATATTG-3’
BMP2/4-MO: 5’-GTGGTAACCATGATGGGTCTGAAAG-3’
Alk3/6-MO: 5'-TAGTGTTACATCTGTCGCCATATTC-3'
The preparation and concentration for nodal, lefty, modified smad2/3 and BMP2/4 mRNAs are described previously (Yaguchi et al., 2006; Yaguchi et al., 2007). To misexpress modified smad1/5, the C-terminal of Sp-Smad1/5 was substituted or deleted in a manner similar to that described for Smad2/3 modification (Yaguchi et al., 2007). The concentration of act-smad1/5 and dn-smad1/5 mRNAs were 3.0µg/µl in injection needles.
Immunohistochemistry
Immunohistochemistry was done as described previously (Yaguchi et al., 2006). Primary antibodies were incubated overnight at 4°C using the following dilutions: Synaptotagmin (1E11, 1:800; Nakajima et al., 2004), Goosecoid (Gsc, 1:600; Kenny et al., 2003), Hnf6 (1:500), serotonin (1:1000, Sigma), and Nk2.1 (1:800; Takacs et al., 2004). The specimens were observed using Leica (DM6000) and Zeiss (Axiovert 200M and LSM410) microscopes. Counts of immunoreactive cells and DAPI stained nuclei were done manually from Z projections of optical sections of individual embryos. The images were analyzed with ImageJ (NIH) and Adobe Photoshop and the figures were prepared with Canvas8.
To prepare an antiserum against Sp-Hnf6, a full-length cDNA (1–1479) was amplified by RT-PCR using synthesized primers (SpHnf6_hisF1 GGGATCCATGCTTTCAAGTGAGTTAGTTGGC, SpHnf6_hisR1 CTCGAGCGCCTTGTACTCTGACTTTGGT). Products were cloned using pGEM-T vector (Promega), sequenced, and subcloned into the bacterial expression plasmid pET28a (EMD Biosciences). Clones containing insert were transformed into E. coli (BL21) screened for IPTG inducible expression of a 6XHis-containing protein of the expected size (54 kDa). A 1 L liquid culture was induced, and after 4h cells were pelleted, and extracted in 6M urea. The extract was passed over a 5 ml column of chelating sepharose fast flow (GE Healthcare), and bound protein eluted with 15 mM imidazole. Eluted fractions were dialyzed into PBS and the purity of the protein was determined with PAGE. Two Sprague-Dawley rats were immunized and boosted 4 times following Canadian Council of Animal Care standard procedures. Antisera were screened with immunoblots of expressed protein or native protein and with whole mount immunofluoresence using pre-immune serum as a control.
RESULTS
Ciliary Band Neurons
The ciliary band in a pluteus larva is composed of 3–4 rows of columnar cells that surround the oral ectoderm (Fig. 1A, green line). To identify differentiating ciliary band cells, we prepared an antibody to Hnf6, a transcription factor of the ONECUT family, which is the earliest marker of the ciliary band (Poustka et al., 2004) and reported to be required for its formation (Otim et al., 2004). In prism and pluteus stages, Hnf6 protein is detected in the nuclei of the tightly packed columnar cells of the ciliary band. Double staining for Goosecoid (Gsc), a transcription factor expressed in the oral ectoderm (Angerer et al., 2001) and Hnf6 shows that these do not co-localize and ciliary band is a distinct region of ectoderm (Fig 1C–E; oral view).
Figure 1.
Ciliary band and ciliary band neurons in the sea urchin pluteus larvae. (A) Schematic diagrams of ciliary band in the larva. Green lines show ciliary band between oral ectoderm and aboral ectoderm, and magenta and orange dots show ciliary band neurons and lateral ganglia. m, mouth; a, anus. (B) The entire nervous systems of the pluteus larva revealed with a pan-neural marker, anti-synaptotagmin (1E11). Arrowheads show the two axonal tracts underlying the oral ectoderm at the base of the postoral arms. (C–E) Double staining using anti-goosecoid (C; Gsc) for oral ectoderm and anti-Hnf6 for ciliary band (D, E). Green dots outside of the band are non-specific signal produced by the Hnf6 antibody. The ciliary band lies adjacent to the oral ectoderm, but never overlaps. (F–G) Double staining with anti-synaptotagmin and anti-Hnf6. Magnified image for the rectangle in (G) shows the detail of this co-localization (H–K). The neurons have nuclear Hnf6 (K). (L–N) Serotonergic neurons of the animal plate are a subset of ciliary band-associated neurons in the early pluteus larva. Bars = 20 µm.
In the early pluteus larva some neurons are in the thickened animal plate and around the mouth, but most reside in the ciliary band (Fig. 1A, B, H–K). The cell bodies of ciliary band neurons are predominantly on the oral side of the ciliary band and bundled axon tracts connect the neurons and encircle the oral ectoderm (Fig. 1F–G). Synaptotagmin B-containing projections from the ciliary band neurons underlie the aboral ectoderm, are oriented toward the posterior end of the larva, and are not bundled. On the left and right sides of the pluteus, lateral ganglia each include a cluster of 2–4 neural cell bodies beneath the aboral ectoderm that extend projections posteriorly and into the axonal tracts of the ciliary band. The only neural projections under the oral ectoderm are two bundles of axons that cross the oral ectoderm at the base of the postoral arms (Fig 1B, arrow). The serotonergic neurons are restricted to the animal plate at this stage of development (Fig 1L–N). Thus, the types and organization of neurons and neural projections are distinctive in the oral and aboral ectoderm as well as in different parts of the ciliary band. The key features of the ciliary band neurons are: 1. Neuronal cell bodies are restricted to the oral side of the ciliary band; 2. Axons in the ciliary band form bundles; 3. Unbundled axons project posteriorly under the aboral ectoderm; 4. Only two axonal tracks at the base of the postoral arms project under the oral ectoderm.
Suppressing Nodal signaling
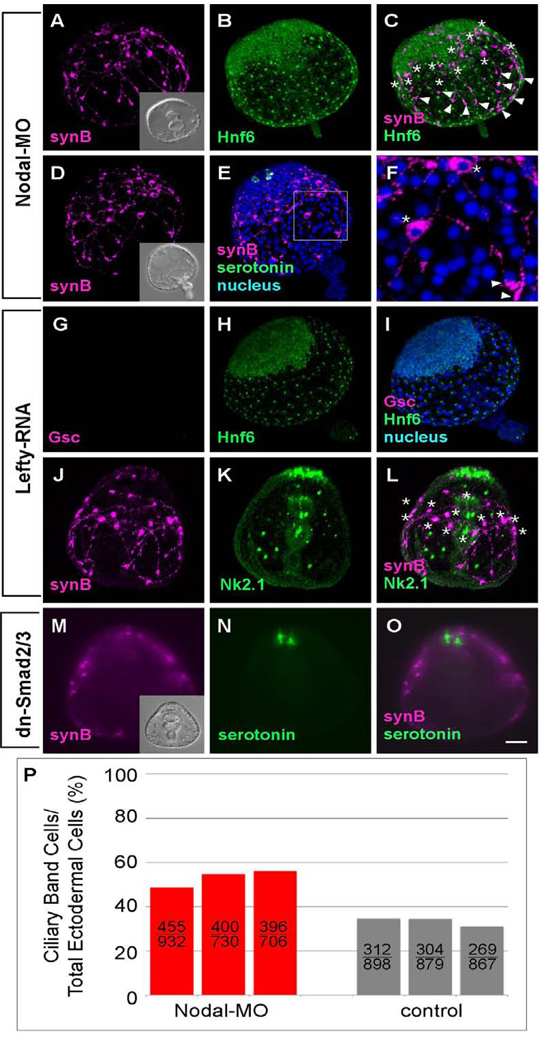
Injecting eggs with an Sp-nodal morpholino oligonucleotide (nodal-MO) results in embryos that are radialized with a gut that elongates toward the animal plate, yet no mouth forms. This phenotype is identical to that previously reported for Paracentrotus lividis (Duboc et al., 2004; Fig. 2A inset). A more severe phenotype with an everted archenteron, which has not been reported in P. lividus (Duboc et al., 2004) or Hemicentrotus pulcherrimus (data not shown), is common in S. purpuratus. At the end of gastrulation, there is a large disk of tightly packed, columnar cells, 10–14 cells wide, that contain nuclear Hnf6 (Fig. 2B, C). This disk includes the animal plate, as it expresses Nk2.1 (Fig. 2J–L; Takacs et al., 2004; Yaguchi et al., 2006) and serotonergic neurons (Fig. 2E, F), and additional ectoderm derived from animal blastomeres. The number and percentage of Hnf6-positive ciliary band cells in nodal-MO injected embryos are greater than those in normal embryos (Fig. 2P). The ciliary band cells comprise approximately 50% of the ectoderm in nodal-MO-injected embryos, whereas in normal embryos they are about one-third of the total ectoderm cells. There is another region lacking nuclear Hnf6, but marked by small, non-nuclear spots of non-specific staining in the basal bodies. Cells in this region are less densely packed and squamous. Thus in nodal-MO embryos, the ectoderm is specified as animal plate, surrounded by an expanded region of ciliary band cells, and a squamous epithelium that has features of aboral ectoderm.
Figure 2.
The ciliary band is expanded and shifted to the animal pole in embryos in which Nodal-MO blocks the oral pathway. Ciliary band-associated neurons differentiate, most of the cell bodies are near the Hnf6-expressing ciliary band cells and axons with strongly immunoreactive growth cones project toward the vegetal pole. The position of cell bodies(*) and growth cones (arrowheads) are marked (A–C) Ciliary band cells, identified with anti-Hnf6, cover the lateral ectoderm initially, and become restricted to a thickened epithelium, 10–14 cells wide, surrounding the animal plate. Ectoderm surrounding blastopore thins and expands to eventually cover the vegetal half of the embryo. (B). Serotonergic neurons are present exclusively at the animal plate (D, E). (F) The magnified image of rectangle in (E) shows the neural cell body (*) and growth cones (arrowheads). (G–L) Lefty RNA-injected embryos have the same morphology as embryos injected with Nodal-MO. (G–I) Goosecoid (Gsc) is not expressed (G), indicating that the oral ectoderm has not differentiated. The ectoderm is covered with ciliary band and thin, vegetal ectoderm (H and I). (J–L) Ciliary band-associated neurons are present throughout the entire lateral ectoderm (* mark the position of cell bodies) and the animal plate marker, Nk2.1, is expressed exclusively in the animal plate. (M–O) Embryos injected with RNA encoding a dominant-negative Smad2/3 have the same morphology as embryos injected with Nodal-MO. Ciliary band neurons are spread throughout the entire lateral ectoderm. Serotonergic neurons are present at the animal plate region (N and O). Inset in (M) shows the DIC image of dn-Smad2/3 injected embryo. (P) The number and ratio of ciliary band cells are increased in Nodal morpholino injected embryos. The upper and lower numbers on the column show the counted number of ciliary band cells and total ectodermal cells, respectively. Those cells are counted in three individual embryos of each treatment. Bar = 20µm.
Synaptotagmin-expressing neurons differentiate throughout much of the ectoderm in nodal-MO embryos, although most of the cell bodies are associated with the ciliary band. Much of the Synaptotagmin signal is in growth cones on neurites projecting toward the blastopore beneath the aboral ectoderm (Fig. 2A–F). The neurons in the ciliary band region do not interconnect to form bundled axon tracts (Fig 2A, D). An alternative, although not equivalent, means of suppressing Nodal signaling is over-expression of lefty, an endogenous antagonist of Nodal (Duboc et al., 2008). As expected, the Nodal-dependent gene, gsc, is not expressed in these embryos (Fig. 2G, I; Gsc protein), which are similar in form to nodal-MO embryos, with a radialized ectoderm, a straight archenteron and no mouth. The Hnf6 expression pattern, the distribution of neurons and axon projections are also the same in lefty RNA-injected embryos as described for nodal-MO containing embryos (Fig. 2G–L). Similarly, embryos injected with a dominant negative version of smad2/3 (Yaguchi et al., 2007), a downstream effector of Nodal signals, have a similar phenotype to nodal-MO- or lefty RNA-injected embryos: Synaptotagmin neurons differentiate along the margin of the thickened ectoderm and extend neurites posteriorly (Fig. 2M–O). Taken together, these results show that embryos lacking Nodal function have 3 types of ectoderm: animal plate, a region with some ciliary band features, and a more vegetal region with aboral ectoderm features. Most of nerve cell bodies are near the ciliary band, but they lack the neural patterning characteristic of ciliary band.
Enhancing Nodal signaling
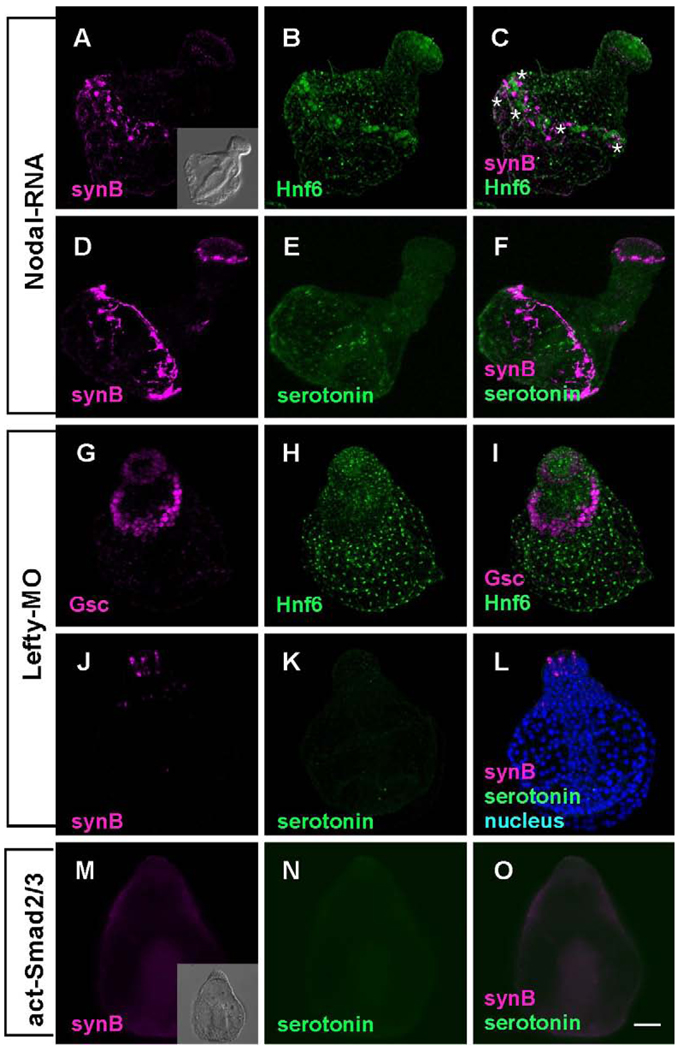
Injecting eggs with nodal RNA also produces a radialized embryo, but in this case, four regions of ectoderm are present and arranged along the animal-vegetal axis: the animal plate, and successive rings of oral ectoderm, ciliary band, and aboral-like ectoderm (Duboc et al., 2004; Yaguchi et al., 2006). Hnf6 protein is detected in cells of the animal plate and in a thin, interrupted strip of ciliary band, 1–2 cells wide (Fig. 3B), confirming previously reported in situ hybridization data (Yaguchi et al., 2006). In nodal RNA-injected embryos, the cell bodies of Synaptotagmin-expressing neurons are predominantly in the ciliary band, axons form a single tract that joins the neurons and short neurites project posteriorly toward the blastopore. (Fig. 3A, C, D, F). In 4-day plutei, neurons expressing Synaptotagmin appear in the animal plate, but there are no cells expressing serotonin (Fig. 3D–F).
Figure 3.
Embryos are radialized and the ciliary band is reduced to a band 1–2 cells wide that is shifted toward the vegetal pole when Nodal signaling is enhanced. (A–F) A thin Hnf6-positive band contains ciliary band neurons forming tracts of axons that interconnect cells. (C). Serotonergic neurons do not differentiate, because the animal plate is surrounded by oral ectoderm, but non-serotonergic neurons are present in the animal plate (E and F) (* mark the position of cell bodies). (G–L) Lefty-MO-injected embryos are superficially similar to Nodal mRNA-injected embryos, but there is no ciliary band (Hnf6 is restricted to the animal plate) and no ciliary band neurons. (G) Gsc is expressed radially and neither Hnf6-expressing ciliary cells nor synaptotagmin-positive neurons are detected in the lateral ectoderm (H–L). (M–O) Constitutively activated Smad2/3 mRNA-injected embryos have a similar form to other embryos with enhanced Nodal signaling, but they are like the Lefty-MO- injected embryos in lacking ciliary band neurons. Inset in (M) shows the DIC image of act-Smad2/3 injected embryo. Bar = 20 µm.
Lefty-MO-injected embryos are similar to nodal mRNA-injected embryos and identical to those previously reported for P. lividus (Duboc et al., 2008). The expression of the oral ectoderm marker, Gsc, is radialized in both cases (Fig. 3G–I; Duboc et al., 2008) and serotonergic neurons do not differentiate in the animal plate (Fig. 3J–L; Yaguchi et al., 2007). However, there is no ciliary band, as Hnf6 protein (Fig. 3H) and synaptotagmin neurons are found only in the animal plate (Fig. 3J–L) in lefty-MO injected embryos. Embryos injected with act-smad2/3 mRNA are similar in form to lefty-MO injected embryos, being radialized, covered with thin ectoderm and lacking serotonergic neurons (Fig. 3N and O), but the constriction of mouth region is slightly delayed (Yaguchi et al., 2007). However, unlike lefty-MO embryos, there are no Synaptotagmin expressing animal plate neurons (Fig. 3M–O).
Injection of nodal RNA results in ectopic Nodal signaling and also ectopic expression of Nodal-dependent genes like lefty, BMP2/4 and chordin. The neural patterning is normal in the ciliary band that forms in these embryos as cells are interconnected with bundled axons and extend aboral projections. The fact that misexpressed nodal can still direct the formation of a set of fully integrated ectodermal tissues supporting the differentiation and patterning of neural components reinforces the idea that it functions near the top of the oral/aboral ectoderm specification pathway.
Suppressing BMP2/4 signaling
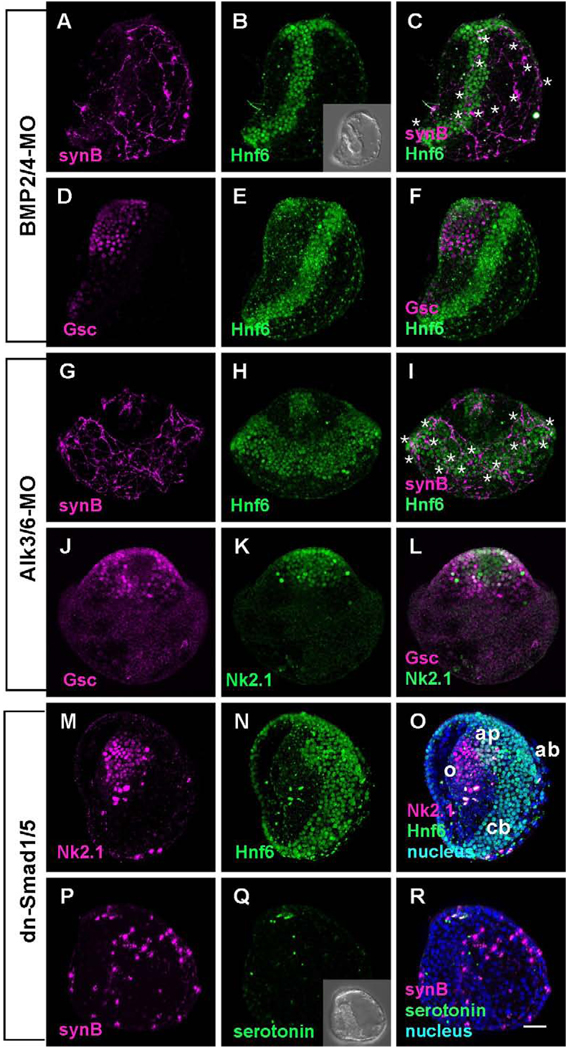
BMP2/4-MO-injected embryos developed as previously described (Duboc et al., 2004). They are not radialized, as the gut bends to the oral side and fuses to form a mouth (Fig. 4B, inset). The overall form of the embryo is distorted, but they contain four regions of ectoderm: animal plate, oral ectoderm, ciliary band, and aboral ectoderm. However, the oral ectoderm marker, Gsc, is not restricted to the oral side but surrounds the animal plate (Fig. 4D–F). A band 5–6 cells wide of Hnf6-expressing ciliary cells, slightly wider than ciliary bands in control embryos, is present but is displaced to the aboral side and does not intersect the ciliated cells in the animal plate, as in normal embryos (Fig. 4A–F). The squamous ectoderm opposite to the oral ectoderm has a low cell density. Synaptotagmin-expressing neurons differentiate in embryos injected with BMP2/4-MO; however, they are not restricted to the ciliary band and some are found in the ectoderm on the aboral side of the embryo (Fig. 4A, C). The cell bodies are multipolar and neurites project randomly without forming bundled tracts. Few neurites associate with the oral ectoderm and no serotonergic neurons develop in the animal plate.
Figure 4.
The embryos in which BMP2/4 signaling is blocked have wider than normal Hnf6-expressing ciliary bands that are shifted vegetally and unpatterned neurons. (A) Ciliary band neurons differentiate in association with the ciliary band and the aboral ectoderm. (B) The ciliary band is wider (5–6 cells in width) and shifted toward the aboral ectoderm. (C) Merged image of (A) and (B) (* mark the position of cell bodies). (D) Oral ectoderm identified with anti-Gsc surrounds the animal plate. (E) Hnf6 marks the animal plate and shifted ciliary band away from animal plate. (F) Merged image of (D) and (E). (G–L) Alk3/6 is the only BMP receptor in the sea urchin genome, yet morpholinos produce embryos with a more severe phenotype with a greatly expanded, radialized ciliary band (* mark the position of cell bodies). (G) Neurons differentiate in association with the ciliary band and the aboral ectoderm. (H) Hnf6 reveals that the ciliary band is shifted vegetally, does not connect with the animal plate, and is 10–12 cells wide. (J–L) An oral ectoderm, expressing Gsc surrounds the animal plate (Nk2.1). (M–R) Embryos in which a dominant-negative form of Smad1/5 is injected are similar to embryos in which Alk3/6 receptor expression is suppressed. (M–O) The animal plate, identified with an Nk2.1 antibody, is surrounded by oral ectoderm and there is a thickened ciliary band (10–12 cells wide; animal pole view). (P–R) Ciliary band neurons differentiate in association with the ciliary band and project neurites with strongly immunoreactive growth cones toward the vegetal end of the embryo. Inset in (Q) shows the DIC image of dn-Smad1/5 injected embryo. Bar = 20µm; o; oral ectoderm; ap, animal plate; cb, ciliary band; ab, aboral ectoderm.
Similarly, knockdown of Alk3/6, the only BMP receptor in the sea urchin genome, produced embryos with some features in common with embryos in which BMP2/4 expression is blocked (Lapraz et al., 2009; Fig. 4G–I). Again, the ciliary band shifts toward the aboral side around the animal plate (Fig. 4H and I), oral ectoderm, marked by Gsc expression, surrounds the animal plate (Fig. 4J and L), and the animal plate marker Nk2.1 is expressed in animal pole cells, although this domain is larger than normal (Fig. 4L). Alk3/6-MO injected embryos are more radial than BMP2/4-MO injected embryos as the ciliary band is in a plane almost orthogonal to the animal-vegetal axis. The major difference between the BMP2/4-MO and the Alk3/6-MO embryos is that the band of Hnf6-expressing cells is much wider, 10–12 cells, than it is in BMP2/4-MO embryos. Synaptotagmin-expressing neurons differentiate throughout this broad band of cells expressing Hnf6 as well as ectopically throughout the aboral region. The neurons project neurites randomly throughout the non-oral half of the embryo and the neurons interconnect, but axon tracts fail to form. Thus, embryos in which expression of the Alk3/6 receptor is blocked are not identical to embryos in which one of the ligands, BMP2/4, is suppressed in this study.
When RNA encoding a dominant-negative form of smad1/5 (dn-smad1/5) is injected into eggs, the embryos are similar in form to embryos injected with Alk3/6-MO. The timing of ingression of primary mesenchyme cells and archenteron invagination are the same as control or Alk3/6-MO-injected embryos, and the oral-aboral polarity is also maintained as in Alk3/6-MO embryos (Fig. 4Q, inset). The oral territory is expanded to surround the animal plate and the Hnf6-expressing ciliary band is shifted aborally and is as wide as that in Alk3/6-MO-injected embryos (Fig. 4M–O). Similarly, embryos expressing dn-smad1/5 have Synaptotagmin-expressing neurons throughout the aboral ectoderm. Only short randomly oriented projections form, the cells are not interconnected and axons do not bundle into tracts (Fig. 4P–R). Thus, suppressing signaling that specifies aboral ectoderm with either Alk3/6-MO or dn-smad1/5 results in a large fraction of the ectoderm that supports the differentiation but not the patterning of neurons. These neurons are not associated with the band of Hnf6 cells, indicating BMP ligands are involved in the process that patterns neurons within the ciliary band.
Enhancing BMP2/4 signaling
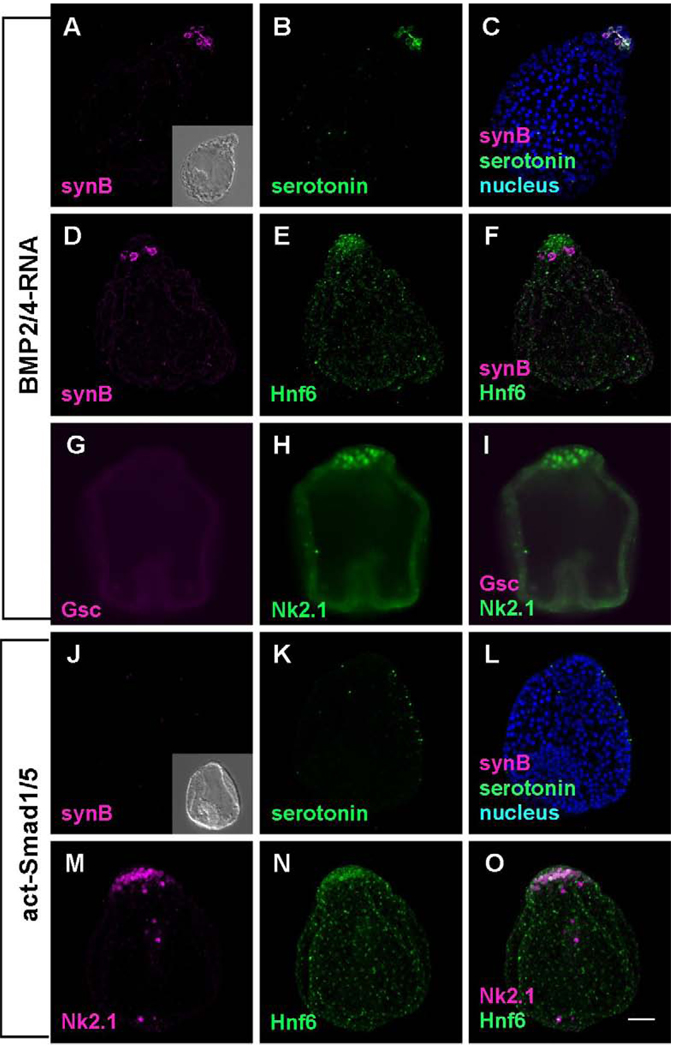
Embryos injected with BMP2/4 mRNA develop as previously described (Angerer et al., 2000; Yaguchi et al., 2006). Most of the ectoderm is squamous and does not express an oral marker, Gsc (Fig. 5G–I). As well, neither ciliary band cells nor Synaptotagmin-expressing ciliary band neurons differentiate (Fig. 5A–F). The animal plate is pronounced, expresses Hnf6 and Nk2.1 and contains serotonergic neurons that express Synaptotagmin (Fig. 5C, F, and I). The embryos expressing act-smad1/5 have shortened archenterons and are phenotypically similar to those of BMP2/4 expressing embryos (Fig. 5A vs J). This phenotype also has neither ciliary band ectoderm nor ciliary band neurons (Fig. 5J and N). However, unlike BMP2/4 misexpressing embryos, these embryos lack serotonin-containing neurons in the animal plate. Taken together, these experiments indicate that BMP2/4 can inhibit formation of ciliary band and suppress differentiation of ciliary band neurons.
Figure 5.
The embryos in which BMP2/4 signaling is enhanced are radialized and lack ciliary bands and ciliary band neurons. (A–I) BMP2/4- misexpressing embryos. (A–F) The embryos lack synaptotagminB-expressing neurons and Hnf6-expressing ciliary band cells throughout the lateral ectoderm. However, serotonergic neurons are invariably present in the animal plate (B and C), indicating the animal plate is not influenced by the misexpression of BMP2/4. (G–I) Embryos lack oral ectoderm expressing Gsc, but express NK2.1 in the animal plate. (J–O) The embryo in which constitutively activated Smad1/5 mRNA has been injected is similar to BMP2/4-misexpressing embryos. They have an animal plate identified with Hnf6 and Nk2.1 (M–O), but no serotonergic neurons differentiate because of the intracellular activation of Smad1/5 (K–L). Inset in (J) shows the DIC image of dn-Smad1/5 injected embryo. Bar = 20µm.
DISCUSSION
TGFβ signaling and differentiation of neurons
Treatments that enhance BMP2/4 or Nodal signaling appear to inhibit neural differentiation. Misexpressed ligands could be acting on neural progenitors directly, or they could be acting indirectly on the non-neural ectoderm, which in turn either supports or suppresses neural differentiation. If signaling acts directly on ectodermal cells to prevent their differentiation as neurons, then blocking of that signaling with either a receptor morpholino or introduction of dn-smads should result in a cell autonomous increase in the number of neurons. However, if this happens it must affect only a small fraction of the precursors to the ciliary band neurons, indicating that most of them respond indirectly to TGFβ signals. Our model proposes that the indirect effect of TGFβ signaling is to provide the appropriate environment for neural development and the Hnf6-expressing ciliary cells provide this environment. Our experiments cannot eliminate the possibility that there is a direct effect of TGFβ signaling on committed neural progenitors. Indeed, Yaguchi et al. (2006, 2007) have demonstrated that Nodal suppresses the differentiation of serotonergic cells on the oral margin of the animal plate. Understanding how TGFβ signaling affects the specification and differentiation of neurons is hampered by not knowing the precise origin of neural progenitors.
Much of the behavior of neurons reported in untreated embryos and in embryos resulting from the perturbations described here is consistent with a model in which a region free of TGFβ is required for the differentiation of neurons and the bundling of axons. Neurons do not differentiate in treatments that result in the loss of the ciliary band and when the ciliary band is displaced, neurons differentiate at the new site. The exception is the appearance of synaptotagmin-expressing neurons in the aboral ectoderm of BMP2/4 morpholino injected embryos, which is, nevertheless, a TGFβ-deficient region. Whether they develop in this region or their precursors migrate there is not clear because synaptotagmin is a late differentiation marker. There are numerous situations in neural development of other metazoans in which neural progenitors must receive appropriate neurotrophic support to differentiate and neurite outgrowth is directed by axon guidance cues that determine the direction of neurite growth and regulate axon bundling by regulating adhesion (Chilton, 2006). We propose that the ciliary band provides an environment conducive to neural development and organization.
A model for patterning the ciliary band
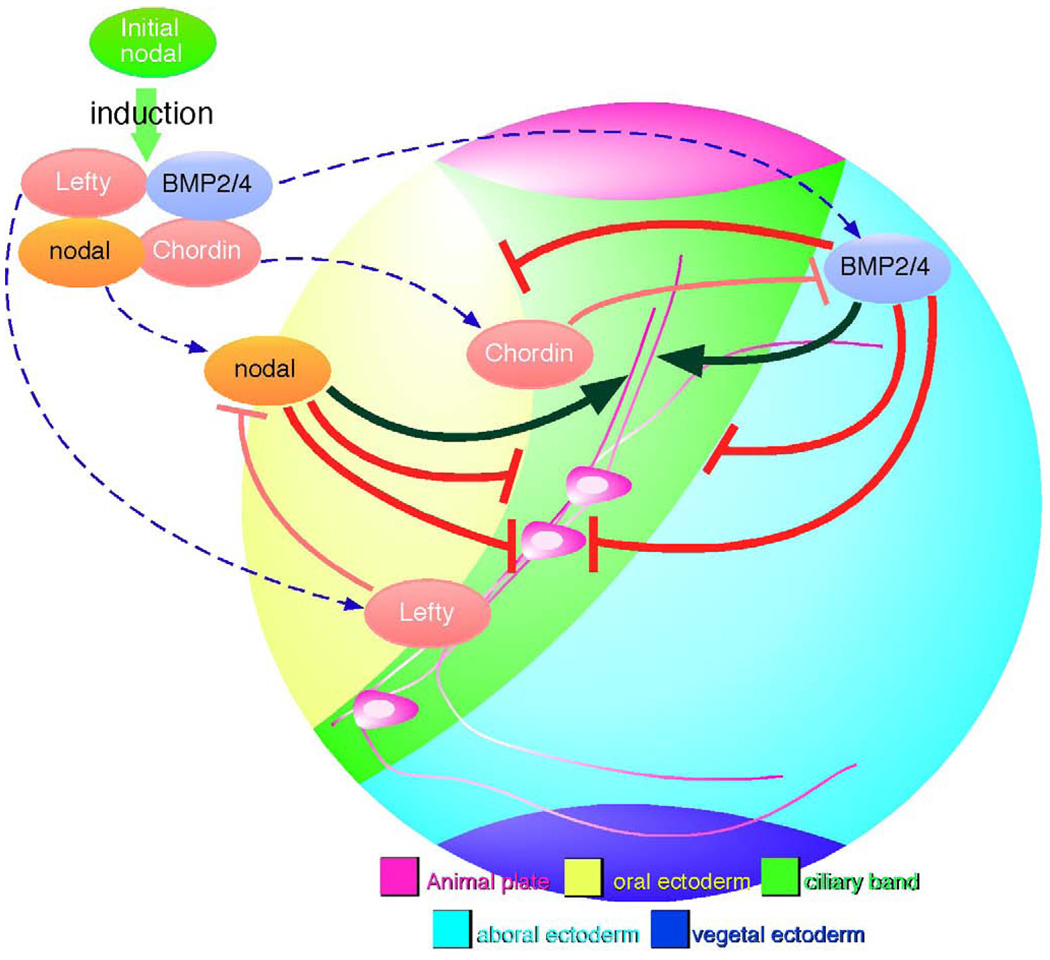
When Nodal is expressed in the oral ectoderm, it initiates a sequence of signaling and differentiation events that includes the expression of factors that antagonize or modify Nodal and BMP2/4 signals (Fig. 6). So far, Lefty and Chordin have this role (Duboc et al., 2004, 2008; Bradham et al., 2009; Lapraz et al., 2009), and we propose that they exclude TGFβ signals from the ciliary band. Here we show that Nodal signaling, localized by Lefty, positions the oral margin of the ciliary band. Signaling through the BMP receptor, Alk3/6, also has an effect on the position of the oral margin and determines the aboral margin of the ciliary band. This narrows the potential width of the band from about 12 cells to 4. Signaling by BMP2/4 also affects the position of both margins and blocks the development of cilary band neurons. Although Nodal strongly reduces and shifts the position of the ciliary band and BMP2/4 signaling blocks it, each with corresponding changes in the development of ciliary band neurons, both signals are required for the correct patterning of these cells within the ciliary band. In the normal embryo, the ectoderm that is subject to these TGFβ signals includes all of the ectoderm except the animal plate and the ectoderm surrounding the blastopore. Little is known about specification of the vegetal ectoderm, but it likely involves canonical Wnt signaling, which is active in precursors during cleavage stages. However, the specification mechanisms of the ectoderm at the animal pole (Wei et al., 2009) and that of oral and aboral ectoderm, which require TGFb signals, are beginning to emerge (Duboc et al., 2004; Range et al., 2007; Nam et al., 2007; Bradham et al., 2009; Su et al., 2009; Lapraz et al., 2009). Here we have examined how these signals position cells in the intervening region that expresses Hnf6 and form the ciliary band.
Figure 6.
Schematic diagram for the signaling pathways regulating ciliary band formation and the differentiation of ciliary band-associated neurons. Nodal expression in the oral ectoderm initiates expression of factors that antagonize or modify Nodal and BMP2/4 signals. Lefty and Chordin exclude TGFβ signals from the presumptive ciliary band (thin red barheads). Localized Nodal signaling positions the oral margin of the ciliary band and BMP signaling through Alk3/6 narrows the width of the ciliary band (barheads to ciliary band margins). This model proposes that an indirect effect of TGFβ signaling is to provide the appropriate environment for neural differentiation. Exclusion of BMP and Nodal from the ciliary band ectoderm is required for the differentiation of neurons and the outgrowth and bundling of axons. Oral ectoderm cannot support these events and aboral ectoderm only promotes growth of unbundled neurites. Thus, Nodal and BMP2/4 block development of most, if not all, ciliary band neurons (barheads to neural cell body), yet the correct patterning of these cells within the ciliary band depends on the presence of both of these signals (black arrowheads to axons).
The Nodal pathway is sufficient for patterning of the ciliary band
All the data presented here suggest that Nodal initiates a series of events, including expression of another TGFβ, BMP2/4, that are required for the differentiation and patterning of the neural components of the ciliary band in the TGFβ-responsive ectoderm. We found that differentiated Synaptotagmin-expressing neurons form in embryos mis-expressing act-smad2/3, which transduces the effects of Nodal signaling throughout the embryo, and bypasses negative feedback regulation by Lefty, a Nodal antagonist. In contrast, an innervated ciliary band can form in embryos mis-expressing nodal, and its downstream target lefty, even though nodal is initially expressed uniformly throughout the embryo.
Our data support the proposition that Lefty is a critical regulator of ciliary band formation. When Lefty expression is blocked, the domain of endogenous Nodal signaling expands and no ciliary band is detectable by Hnf6 staining. In addition, the ectoderm that results does not support differentiation of Synaptotagmin-expressing neurons. The loss of ciliary band and neurons in embryos with suppressed expression of Lefty argues that in the normal embryo prevention of ectopic Nodal signaling by Lefty is an essential feature of patterning of the TGFβ-responsive ectoderm, as proposed by Duboc and Lepage (2008).
When Nodal signaling is blocked, all but the most vegetal ectoderm continues to express Hnf6. When BMP signaling is blocked, the number of ciliary band cells expressing Hnf6 also increases and appear ectopically on both the oral and aboral sides of the embryo. In contrast, in embryos mis-expressing BMP2/4, they are absent (this paper; Angerer et al., 2000; Duboc et al., 2004; Bradham et al., 2009; Lapraz et al., 2009). In the normal embryo, Hnf6 is expressed broadly throughout the embryo before Nodal or BMP2/4 signaling begins, and continues to be widespread until the hatching blastula stage (Otim et al., 2004). Subsequently it restricts to a narrow strip of cells during mesenchyme blastula stages in a process shown here to depend on these signals. Control of BMP signaling appears to be mediated, at least in part, by Chordin, which has been shown to antagonize BMP2/4 in sea urchin embryos (L. variegateus) and is necessary for correct formation of the ciliary band and development of ciliary band neurons (Bradham et al., 2009), although the responses to Chordin perturbations in another species (Paracentrotus lividus) are somewhat different (Lapraz et al., 2009). Taken together, these observations suggest that TGFβ signaling transforms most of the early ectoderm into an epidermal regulatory state except in cells where these signals are excluded; the site of ciliary band formation. It follows that the ciliary band, and subsequently the development and patterning of neurons within it, require protection from Nodal and BMP.
Restriction of the ciliary band to a narrow strip of cells expressing Hnf6 follows shortly after the activation of the oral signaling network. The levels of nodal and lefty mRNAs increase significantly during early blastula stages and chordin and BMP2/4 transcription is up-regulated a few hours later during mesenchyme blastula stage (Angerer et al., 2000; Bradham et al., 2009). This precedes by only a few hours the emergence of the ciliary band at late mesenchyme blastula stage (Otim et al., 2004; Poustka et al., 2004). Exactly how spatially regulated TGFβ signaling restricts the expression of hnf6 is not yet clear, however, it is likely that mechanisms that control the levels and distribution of Lefty, Chordin and other TGFβ antagonists are involved.
Nodal and BMP signaling position the ciliary band
The position and size of the band of cells expressing Hnf6 is dramatically altered when the domain of Nodal expression is altered. When it is blocked, the band is 10–14 cells wide and shifts toward the animal pole of the embryo, whereas, when it is mis- expressed, the band is reduced to a width of only 1 cell and shifts toward the vegetal end of the embryo. As there is a small difference in the proportion of cells expressing Hnf6 in embryos injected with nodal-MO, it appears the predominant effect is to respecify a significant fraction of ectoderm cells, which alters the position of the cells expressing Hnf6. These observations suggest that Nodal signaling regulates the position of the oral margin of Hnf6 expressing ciliary band cells.
When embryos are injected with BMP2/4 RNA or act-smad1/5 no band of Hnf6 cells forms. As well, when BMP2/4 signaling is suppressed (BMP2/4-MO, dn-smad1/5 RNA, Alk3/6-MO), the band of Hnf6 cells that forms is shifted away from the animal plate toward the vegetal pole and is wider. These data indicate that BMP signaling also has a role in positioning the ciliary band. BMP also appears to have a clear effect on the position of the aboral margin of the ciliary band. Its posterior margin is restricted by factors that signal through ALK3/6. BMP2/4 appears to play a relatively small role in this process. Loss of BMP2/4 increases the band from 4 to 5 or 6 cells in width, even at concentrations of morpholino approaching toxicity, while loss of Alk3/6 further increases it to 10–12 cells. This raises the possibility that more than one BMP ligand may be involved in patterning of this region of the aboral ectoderm. However, Lapraz et al. (2009) do not report a large difference between the effects of BMP2/4-MO and Alk3/6-MO on embryos of Paracentrotus lividus. This suggests that there is either an incomplete suppression of BMP2/4 in S. purpuratus or species differences in regulation of aboral ectoderm specification by BMP pathways.
Vegetal ectoderm is resistant to TGFβ signals
The specification and differentiation of the most vegetal region of ectoderm is poorly understood. Perturbations of Nodal or BMP signaling make it clear that this ectoderm responds differently than more animal ectoderm. Although loss of BMP signaling results in expansion of the ciliary band, it does not extend into the vegetal ectoderm. Loss of Nodal signaling, and consequently BMP2/4, reveals that the vegetal ectoderm continues to express aboral markers rather than Hnf6 (Duboc et al., 2004). Although misexpression of nodal generates a ciliary band near the vegetal pole, there remains a vegetal strip of aboral-like ectoderm (this work, Duboc et al., 2004). At least some of this ectoderm is probably derived from veg1 blastomeres, which surround the blastopore (Davidson et al., 1998), and as such its regulatory state is likely to be different from animal blastomere-derived ectoderm as a result of vegetal Wnt signaling (Davidson et al., 2002).
Patterning of the ciliary band nervous system
Nodal and BMP2/4 specify oral and aboral ectoderm, and suppression of these signals in a narrow region of ectoderm between them produces the ciliary band. These tissues appear to control how the neurons develop within this band. Oral ectoderm inhibits differentiation of neurons and outgrowth of neurites, but aboral ectoderm supports outgrowth of unbundled neurites. The ciliary band cells are under the influence of a gene regulatory network that includes hnf6, but the presence of Hnf6 is not sufficient to ensure correct patterning of ciliary band neurons. The Hnf6-expressing cells are capable of forming a thickened, ciliated epithelium but, in the absence of TGFβ signals, they do not support correct formation of bundled axonal tracts that interconnect. The mechanisms by which TGFβ signaling affects the direction of neural projections and the interactions among them are not understood. Rigorous testing will be required to understand the intricate mechanisms by which TGFβ signaling patterns the elegant, yet relatively simple, tissues that serve the critical functions of swimming and feeding in the larva.
Acknowledgements
Supported in part by a Discovery grant from the Natural Sciences and Engineering Research Council (Canada) to RDB, in part by the Intramural Program of the National Institutes of Health, NIDCR, and in part by Special Coordination Funds for Promoting Science and Technology of the Japanese Government to SY. We thank Thierry Lepage and Francois Lapraz for sharing reagents and unpublished data with us.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angerer LM, Oleksyn DW, Levine AM, Li X, Klein WH, Angerer RC. Sea urchin goosecoid function links fate specification along the animal-vegetal and oral-aboral embryonic axes. Development. 2001;128:4393–4404. doi: 10.1242/dev.128.22.4393. [DOI] [PubMed] [Google Scholar]
- Angerer LM, Oleksyn DW, Logan CY, McClay DR, Dale L, Angerer RC. A BMP pathway regulates cell fate allocation along the sea urchin animal-vegetal embryonic axis. Development. 2000;127:1105–1114. doi: 10.1242/dev.127.5.1105. [DOI] [PubMed] [Google Scholar]
- Bradham CA, Oikonomou C, Kuhn A, Core AB, Modell JW, McClay DR, Poustka AJ. Chordin is required for neural but not axial development in sea urchin embryos. Developmental Biology. 2009;328:221–233. doi: 10.1016/j.ydbio.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri H, Davidson EH. The gene regulatory network basis of the "community effect," and analysis of a sea urchin embryo example. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.06.007. http://dx.doi.org/10.1016/j.ydbio.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RD. The structure of the nervous system of the pluteus larva of Strongylocentrotus purpuratus. Cell and Tissue Research. 1978;191:233–247. doi: 10.1007/BF00222422. [DOI] [PubMed] [Google Scholar]
- Chen CH, Shen MM. Two modes by which lefty proteins inhibit Nodal signaling. Current Biology. 2004;14:618–624. doi: 10.1016/j.cub.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Chilton JK. Molecular mechanisms of axon guidance. Developmental Biology. 2006;292:13–24. doi: 10.1016/j.ydbio.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Cameron RA, Ransick A. Specification of cell fate in the sea urchin embryo: summary and some proposed mechanisms. Development. 1998;125:3269–3290. doi: 10.1242/dev.125.17.3269. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Rast JP, Oliveri P, Ransick A, Calestani C, Yuh CH, Minokawa T, Amore G, Hinman V, Arenas-Mena C, et al. A provisional regulatory gene network for specification of endomesoderm in the sea urchin embryo. Developmental Biology. 2002;246:162–190. doi: 10.1006/dbio.2002.0635. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annual Review of Cell and Developmental Biology. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboc V, Lapraz F, Besnardeau L, Lepage T. Lefty acts as an essential modulator of Nodal activity during sea urchin oral-aboral axis formation. Developmental Biology. 2008;320:49–59. doi: 10.1016/j.ydbio.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Duboc V, Lepage T. A conserved role for the nodal signaling pathway in the establishment of dorso-ventral and left-right axes in deuterostomes. Journal of Experimental Zoology Part B-Molecular and Developmental Evolution. 2008;310B:41–53. doi: 10.1002/jez.b.21121. [DOI] [PubMed] [Google Scholar]
- Duboc V, Rottinger E, Besnardeau L, Lepage T. Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Developmental Cell. 2004;6:397–410. doi: 10.1016/s1534-5807(04)00056-5. [DOI] [PubMed] [Google Scholar]
- Kenny AP, Oleksyn DW, Newman LA, Angerer RC, Angerer LM. Tight regulation of SpSoxB factors is required for patterning and morphogenesis in sea urchin embryos. Developmental Biology. 2003;261:412–425. doi: 10.1016/s0012-1606(03)00331-2. [DOI] [PubMed] [Google Scholar]
- Lapraz F, Besnardeau L, Lepage T. Patterning of the Dorsal-Ventral Axis in Echinoderms: Insights into the Evolution of the BMP-Chordin Signaling Network. Plos Biology. 2009;7(11) doi: 10.1371/journal.pbio.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie GO, Spencer AN, Strathmann R. Electrical Activity Associated with Ciliary Reversal in an Echinoderm Larva. Nature. 1969;223:1384–1385. [Google Scholar]
- Meno C, Saijoh Y, Fujii H, Ikeda M, Yokoyama T, Yokoyama M, Toyoda Y, Hamada H. Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature. 1996;381:151–155. doi: 10.1038/381151a0. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Kaneko H, Murray G, Burke RD. Divergent patterns of neural development in larval echinoids and asteroids. Evolution & Development. 2004;6:95–104. doi: 10.1111/j.1525-142x.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Nam J, Su YH, Lee PY, Robertson AJ, Coffman JA, Davidson EH. Cis-regulatory control of the nodal gene, initiator of the sea urchin oral ectodenn gene network. Developmental Biology. 2007;306:860–869. doi: 10.1016/j.ydbio.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otim O, Amore G, Minokawa T, McClay DR, Davidson EH. SpHnf6, a transcription factor that executes multiple functions in sea urchin embryogenesis. Developmental Biology. 2004;273:226–243. doi: 10.1016/j.ydbio.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Poustka AJ, Kuhn A, Radosavljevic V, Wellenreuther R, Lehrach H, Panopoulou G. On the origin of the chordate central nervous system: expression of onecut in the sea urchin embryo. Evolution & Development. 2004;6:227–236. doi: 10.1111/j.1525-142X.2004.04028.x. [DOI] [PubMed] [Google Scholar]
- Range R, Lapraz F, Quirin M, Marro S, Besnardeau L, Lepage T. Cis-regulatory analysis of nodal and maternal control of dorsal-ventral axis formation by Univin, a TGF-beta related to Vg1. Development. 2007;134:3649–3664. doi: 10.1242/dev.007799. [DOI] [PubMed] [Google Scholar]
- Satterlie RA, Cameron RA. Electrical-Activity at Metamorphosis in Larvae of the Sea-Urchin Lytechinus-Pictus (Echinoidea, Echinodermata) Journal of Experimental Zoology. 1985;235:197–204. [Google Scholar]
- Strathmann RR. Time and extent of ciliary response to particles in a non-filtering feeding mechanism. Biological Bulletin. 2007;212:93–103. doi: 10.2307/25066587. [DOI] [PubMed] [Google Scholar]
- Su YH, Li E, Geiss GK, Longabaugh WJR, Kramer A, Davidson EH. A perturbation model of the gene regulatory network for oral and aboral ectoderm specification in the sea urchin embryo. Developmental Biology. 2009;329:410–421. doi: 10.1016/j.ydbio.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs CM, Amore G, Oliveri P, Poustka AJ, Wang D, Burke RD, Peterson KJ. Expression of an NK2 homeodomain gene in the apical ectoderm defines a new territory in the early sea urchin embryo. Developmental Biology. 2004;269:152–164. doi: 10.1016/j.ydbio.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Wei Z, Yaguchi J, Yaguchi S, Angerer RC, Angerer LM. The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development. 2009;136:1179–1189. doi: 10.1242/dev.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi S, Yaguchi J, Angerer RC, Angerer LM. A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Developmental Cell. 2008;14:97–107. doi: 10.1016/j.devcel.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Yaguchi S, Yaguchi J, Burke RD. Specification of ectoderm restricts the size of the animal plate and patterns neurogenesis in sea urchin embryos. Development. 2006;133:2337–2346. doi: 10.1242/dev.02396. [DOI] [PubMed] [Google Scholar]
- Yaguchi S, Yaguchi J, Burke RD. Sp-Smad2/3 mediates patterning of neurogenic ectoderm by nodal in the sea urchin embryo. Developmental Biology. 2007;302:494–503. doi: 10.1016/j.ydbio.2006.10.010. [DOI] [PubMed] [Google Scholar]