Abstract
To investigate the influence of microwave radiation on the human fibroblast nuclei, the effects of three variants of electromagnetic wave polarization, linear and left-handed and right-handed elliptically polarized, were examined. Experimental conditions were: frequency (f) 36.65 GHz, power density (P) at the surface of exposed object 1, 10, 30, and 100 µW/cm2, exposure time 10 s. Human fibroblasts growing in a monolayer on a cover slide were exposed to microwave electromagnetic radiation. The layer of medium that covered cells during microwave exposure was about 1 mm thick. Cells were stained immediately after irradiation by 2% (w/v) orcein solution in 45% (w/v) acetic acid. Experiments were made at room temperature (25 °C), and control cell samples were processed in the same conditions. We assessed heterochromatin granule quantity (HGQ) at 600× magnification. Microwave irradiation at the intensity of 1 µW/cm2 produced no effect, and irradiation at the intensities of 10 and 100 µW/cm2 induced an increase in HGQ. More intense irradiation induced more chromatin condensation. The right-handed elliptically polarized radiation revealed more biological activity than the left-handed polarized one.
Keywords: Human cells, Chromatin, Heterochromatin, Electromagnetic field, Elliptical polarization
1. Introduction
The effect of microwave radiation on cell nucleus morphology was first demonstrated in the early 1970’s. A microwave-induced increase in cell nucleus volume of human cells was revealed (Kiselev and Zalyubovskaya, 1973). In human cells, 1 h after cell exposure to radiation at the intensity of 20 mW/cm2, the nuclear volume decreased, but after 24 and 48 h following irradiation, there was an increase in the volume of the nucleus and a small decrease in the volume of the nucleolus (Szmigielski et al., 1975).
Many researchers have analyzed the chromosome aberrations and DNA damage induced by microwaves. Microwave irradiation (frequency (f)=1.8 GHz, specific absorption rate (SAR)=2–4 W/kg) induces DNA damage in human lens epithelial cells, but electromagnetic noise blocks DNA damage (Yao et al., 2008). In cultured hamster cells, a dose-dependent increase of chromosome aberrations was induced by microwaves (f=7.7 GHz, power density (P)=30 µW/cm2) (Garaj-Vrhovac et al., 1990). Cell exposure to microwaves increases the quantity of micronuclei (Garaj-Vrhovac et al., 1991). Irradiation by microwaves of less intensity (P=0.5 µW/cm2) also induces chromosome aberrations and micronuclei increase in human lymphocytes (Garaj-Vrhovac et al., 1992). In lymphocytes of persons previously subjected to intensive microwave irradiation by accident, the number of micronuclei is elevated (Garaj-Vrhovac, 1999). The micronuclei induction by microwaves has been shown by some researches (Trosic et al., 2002; Zotti-Martelli et al., 2005), but not in others (Zeni et al., 2003; Görlitz et al., 2005).
In our previous work we showed that microwaves induce an increase of heterochromatinization in the nuclei of human buccal epithelium cells (Shckorbatov et al., 1998). This phenomenon is related to the state of polarization of the electromagnetic wave (Shckorbatov et al., 2009). These investigations induce interest because the frequency of approximately 35 GHz is commonly used in many scenarios and, in particular, in police radars (Tillemans, 2008). In the present work, we investigate the biological effect of microwaves of close frequency (36.64 GHz). The intensity of irradiation in our experiments (10 µW/cm2) is far below the level (10 mW/cm2) that induces a considerable thermal effect, and is believed to be non-hazardous (Michaelson, 1980). The low intensity of irradiation and short exposure time (10 s) in our experiments were chosen in order to determine the minimal intensity of electromagnetic irradiation that induces chromatin condensation. The process of heterochromatinization is connected with DNA damage (Luijsterburg et al., 2009), so, in principle, microwave-induced heterochromatinization may be connected with microwave-induced mutations.
In our previous work, we analyzed the effects of microwaves on human buccal epithelium cells (Shckorbatov, 1999; Shckorbatov et al., 2009), but the effect of microwaves on the process of heterochromatinization in other types of cells remained unexplored. In the present work, we studied the effect of microwaves of different elliptical polarization on quantity of heterochromatin granules in cultured human fibroblasts.
2. Materials and methods
2.1. Cell line and cell culture
The primary diploid fibroblast cell line was derived from a skin specimen of a 35-year-old donor. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) plus 10% (w/v) fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 mg/ml streptomycin in 5% CO2 at 37 °C.
2.2. Irradiation procedure
To obtain microwave radiation of frequency (f) 36.65 GHz, we used a semi-conductor device developed at the Department of Theoretical Radiophysics of Kharkiv National University, Ukraine. Exposure of a cell sample was achieved in a free space. Cells growing in a monolayer on a cover slide were exposed to radiation. The layer of cultured medium covering cells during exposure to microwaves was about 1 mm thick. Immediately after exposure, cells were stained by orcein. All experiments were conducted at room temperature (25 °C), the control cell samples (not exposed to radiation) being processed under the same conditions. The temperature was chosen for convenience. In our previous experiments, cells retained viability at this temperature for 8 h, as revealed by modified Nassonov’s test for cellular ability to concentrate vital stain indigo carmine in granules after 30 min of staining (Shckorbatov et al., 1995).
In our experiments, the radiation power density (P) on the surface of exposed object was 1, 10, 30, and 100 µW/cm2. We applied a linearly or elliptically polarized radiation. Irradiation time in all experiments was 10 s. At the low energy of irradiation applied in our experiments, the calculated increase of the cell sample temperature is very small. Calculation of temperature changes of cell sample carried out by precise numerical simulation of the experimental setup has shown that, even in the case of maximal electromagnetic power applied, i.e., 100 µW/cm2, the increase of the cell sample temperature during 10-s irradiation is less than 0.0076 °C.
In order to obtain an elliptically polarized radiation, we applied a grating polarizer, the surface of which was perpendicular to the vector K (direction of electromagnetic field propagation) and was oriented at angle φ=45° to the vector E (electric field component of electromagnetic field). The elliptically polarized electromagnetic field can be interpreted as a result of superposition of two linearly polarized mutually orthogonal waves,
| Ex=E1cos2πf0t |
and
| Ey=E2cos(2πf0t+ψ) |
, of an identical frequency (f 0), but different amplitudes (E 1 and E 2) and shifted phase (ψ) spreading along the axis of z. Here t denotes time. If ψ=0 or ψ=±π, the field has linear polarization; if
ψ=±π/2 and E 1=E 2, circular polarization. In the case when ψ≠nπ and E 1≠E 2, the field is elliptically polarized. Thus, the circularly polarized field is the special case of the elliptically polarized field. In this study, the source of microwave radiation generated the linearly polarized wave, which, by the grating polarizer, was transformed into elliptically polarized wave with the coefficient of elliptisity of 0.65. This is the same experimental setup used in our previous work (Shckorbatov et al., 2009).
2.3. Chromatin state evaluation
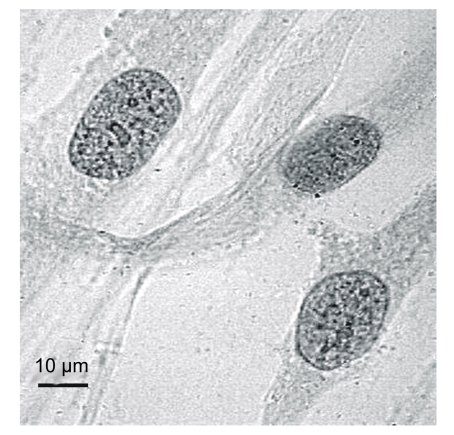
We estimated the heterochromatin granule quantity (HGQ) in human cells by the method described previously (Shckorbatov, 1999). After cell exposure to microwaves, cells were stained with 2% (w/v) orcein in 45% (w/v) acetic acid (Reachem, Moscow, Russia) (Sanderson and Stewart, 1961). Cells stained with orcein are presented in Fig. 1.
Fig. 1.
Human fibroblasts stained by orcein
Cells were investigated at 600× magnification. In each variation of the experiment, we assessed HGQ in 30 nuclei. The mean HGQ value and the standard error of mean (SEM) were calculated for the 30 nuclei (Figs. 2–3). The optimal number for analysis was determined in our previous experiments. The SEM was less than 5% of the mean. Each experiment was repeated three times.
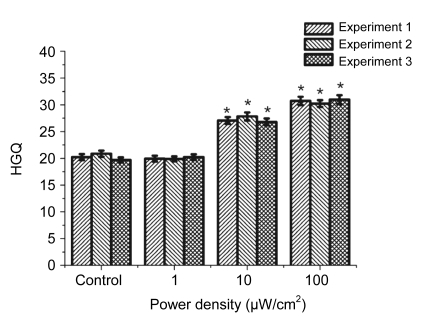
Fig. 2.
Changes of HGQ in human fibroblasts after exposure to microwaves of different intensities
* Statistically significant difference at P<0.05, compared with the control
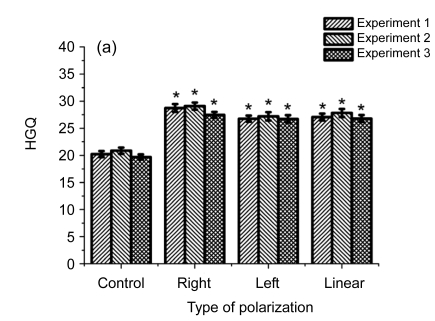
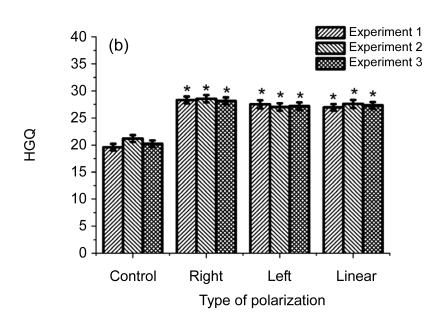
Fig. 3.
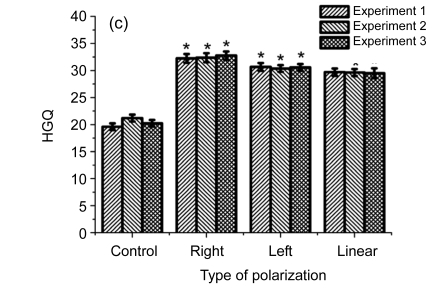
Influence of differently elliptically polarized microwave irradiation at the intensities of 10 (a), 30 (b), and 100 (c) µW/cm2 on HGQ in human fibroblasts
* Statistically significant difference at P<0.05, compared with the control
2.4. Cell viability evaluation
For evaluation of cell membrane damage, we used the trypan blue test. This test is also used for the assessment of cell viability (Hodgson, 2004). We assessed the percentage of unstained cells after 5 min of cell staining with 1% (w/v) trypan blue in 3.03 mmol/L phosphate buffer (pH=7.0) with 2.89 mmol/L CaCl2.
2.5. Statistical analysis
The data were processed by Student t-test and analysis of variance (ANOVA) test. The difference was considered statistically significant when P<0.05.
3. Results and discussion
The level of cell viability (percentage of cells unstained with trypan blue) was 86%. In the first set of experiments (Fig. 2), we studied changes in HGQ after cell sample exposure to linearly polarized radiation at different intensities of 1, 10, and 100 µW/cm2.
As shown in Fig. 2, the HGQ increased after microwave irradiation at the intensities of 10 and 100 µW/cm2, but the irradiation at the intensity of 1 µW/cm2 did not produce any effect.
The biological meaning of the process of heterochromatin granule formation, so-called heterochromatinization, is a decrease of general gene activity or transcriptional activity. As is known, heterochromatin is mainly the inactive part of chromatin with low transcriptional activity (Lewin, 2004). Our data on microwave-induced heterochromatin granule formation in human fibroblasts were in a good agreement with previous data in human buccal epithelium cells (Shckorbatov et al., 1998; 2009). The biological consequences of electromagnetic irradiation in connection to heterochromatin granule formation may be the following. As a contributor to cell stress, microwave radiation may evoke a reversible reaction of cell nucleus that leads to cardinal change in cell metabolic activity. Of course, if it is evoked once or relatively rarely, it may be reversed without consequence, but the continuous provocation may be an impediment to normal functioning of cells.
In Fig. 3 are presented data on the influence on cells of differently polarized microwaves at the intensities of 10, 30, and 100 µW/cm2.
As shown in Fig. 3, the right-handed polarized microwaves induced more heterochromatin granules than the left-handed polarized microwaves. Our data indicate that the action of polarized microwave radiation on cell nucleus depends on the state of polarization of electromagnetic wave (Table 1), and that the influence of polarization factor is statistically significant in variants after cell exposure to 10 and 100 μW/cm2. Table 2 showed that cell exposure to microwaves with right-handed elliptical polarization is more effective than that with left-handed polarization (P<0.05), and that cell exposure to microwaves at the intensity of 100 µW/cm2 has a stronger effect than cell exposure at 10 µW/cm2 (P<0.05).
Table 1.
ANOVA test for assessment of the influence of differently polarized microwave irradiation on the state of chromatin in cell nucleus
| Polarization factor | F | P |
| 10 μW/cm2 | ||
| A | 4.93* | 0.01 |
| B | 1.73 | 0.18 |
| B×A | 0.29 | 0.88 |
| 30 μW/cm2 | ||
| A | 2.64 | 0.08 |
| B | 0.05 | 0.95 |
| B×A | 0.20 | 0.94 |
| 100 μW/cm2 | ||
| A | 13.22* | 0.00 |
| B | 0.03 | 0.97 |
| B×A | 0.08 | 0.99 |
A: independent factor of state of polarization (right-handed elliptical, left-handed elliptical, and linear polarization); B: dependent factor (1–3 experiments)
Significance at 0.05 level
Table 2.
Influence of differently polarized microwaves on heterochromatin granule quantity (HGQ) in human fibroblasts
| Polarization type | HGQ |
| 10 μW/cm2 | |
| Control | 20.27±0.31 |
| Right | 28.43±0.38 |
| Left | 26.90±0.39 |
| Linear | 27.23±0.39 |
| 100 μW/cm2 | |
| Control | 20.34±0.36 |
| Right | 32.46±0.46 |
| Left | 30.53±0.37 |
| Linear | 29.61±0.43 |
Data are expressed as mean±SEM (n=90)
We propose that difference in biological effect of differently elliptically polarized microwaves is related to the differential absorption of the energy of electromagnetic waves by asymmetric macromolecules and macromolecular complexes (including DNA and DNA-protein complexes) in the cell nuclei.
Comparing the present data concerning the differences in the activity of differently polarized microwaves with results obtained previously (Shckorbatov et al., 2009), we can conclude that the reaction of chromatin to right-polarized and left-polarized irradiation in human buccal cells is individual. In human buccal epithelium cells, the greater effectiveness of linearly polarized irradiation compared to left-polarized irradiation is clear, but this effect was not demonstrated in human fibroblasts.
4. Conclusions
Microwave irradiation at the frequency of 36.65 GHz and the intensities of 10 and 100 µW/cm2 induces chromatin condensation in human fibroblasts. The right-handed elliptically polarized radiation demonstrated more biological activity than the left-handed elliptically polarized one. As the left-handed elliptically polarized microwaves induce less effect of heterochromatinization, it is possible that left-handed polarized microwaves may be preferred for use in various systems of radio communications.
References
- 1.Garaj-Vrhovac V. Micronucleus assay and lymphocyte mitotic activity in risk assessment of occupational exposure to microwave radiation. Chemosphere. 1999;39(13):2301–2312. doi: 10.1016/S0045-6535(99)00139-3. [DOI] [PubMed] [Google Scholar]
- 2.Garaj-Vrhovac V, Horvat D, Koren Z. The effect of microwave radiation on the cell genome. Mutat Res Lett. 1990;243(2):87–93. doi: 10.1016/0165-7992(90)90028-I. [DOI] [PubMed] [Google Scholar]
- 3.Garaj-Vrhovac V, Horvat D, Koren Z. The relationship between colony-forming ability, chromosome aberrations and incidence of micronuclei in V79 Chinese hamster cells exposed to microwave radiation. Mutat Res Lett. 1991;263(3):143–149. doi: 10.1016/0165-7992(91)90054-8. [DOI] [PubMed] [Google Scholar]
- 4.Garaj-Vrhovac V, Fucic A, Horvat D. The correlation between the frequency of micronuclei and specific chromosome aberrations in human lymphocytes exposed to microwaves. Mutat Res Lett. 1992;281(3):181–186. doi: 10.1016/0165-7992(92)90006-4. [DOI] [PubMed] [Google Scholar]
- 5.Görlitz BD, Müller M, Ebert S, Hecker H, Kuster N, Dasenbrock C. Effects of 1-week and 6-week exposure to GSM/DCS radiofrequency radiation on micronucleus formation in B6C3F1 mice. Radiat Res. 2005;164(4):431–439. doi: 10.1667/RR3440.1. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson E. A Textbook of Modern Toxicology. 3rd Ed. New Jersey: John Wiley & Sons; 2004. pp. 14–16. [DOI] [Google Scholar]
- 7.Kiselev RI, Zalyubovskaya NP. Influence of electromagnetic waves of millimeter range on cell and some structural elements of cell. Uspekhi Fizicheskih Nauk (Success in Physical Sciences) 1973;110(3):464–466. (in Russian) [Google Scholar]
- 8.Lewin B. Genes VIII. Upper Saddle River, New Jersey: Pearson Education Inc; 2004. p. 1002. [Google Scholar]
- 9.Luijsterburg MS, Dinant C, Lans H, Stap J, Wiernasz E, Lagerwerf S, Warmerdam DO, Lindh M, Brink MC, Jurek WJW, et al. Heterochromatin protein 1 is recruited to various types of DNA damage. J Cell Biol. 2009;185(4):577–586. doi: 10.1083/jcb.200810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michaelson SM. Microwave biological effects: an overview. Proc. IEEE. 1980;68(1):40–49. doi: 10.1109/PROC.1980.11579. [DOI] [Google Scholar]
- 11.Sanderson A, Stewart J. Nuclear sexing with acetoorcein. BMJ. 1961;2(5259):1065–1067. doi: 10.1136/bmj.2.5259.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shckorbatov YG. He-Ne laser light induced changes in the state of chromatin in human cells. Naturwissenschaften. 1999;86(9):452–453. doi: 10.1007/s001140050653. [DOI] [PubMed] [Google Scholar]
- 13.Shckorbatov YG, Shakhbazov VG, Bogoslavsky AM, Rudenko AO. On age-related changes of cell membrane permeability in human buccal epithelium cells. Mech Ageing Dev. 1995;83(2):87–90. doi: 10.1016/0047-6374(95)93574-M. [DOI] [PubMed] [Google Scholar]
- 14.Shckorbatov YG, Shakhbazov VG, Grigoryeva NN, Grabina VA. Microwave irradiation influences on the state of human cell nuclei. Bioelectromagnetics. 1998;19(7):414–419. doi: 10.1002/(SICI)1521-186X(1998)19:7<414::AID-BEM2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Shckorbatov YG, Pasiuga VN, Kolchigin NN, Grabina VA, Batrakov DO, Kalashnikov VV, Ivanchenko DD, Bykov VN. The influence of differently polarized microwave radiation on chromatin in human cells. Int J Radiat Biol. 2009;85(4):322–329. doi: 10.1080/09553000902781113. [DOI] [PubMed] [Google Scholar]
- 16.Szmigielski S, Luszak M, Wiranowska M. Kariometric observations of WISH cell cultures irradiated with 36 GHz microwaves. Folia Histochem Cytochem. 1975;13(3-4):151–159. [PubMed] [Google Scholar]
- 17.Tillemans A. Kontroverse um Radarstrahlung. 2008. wissenschaft.de Available from: http://www.wissenschaft.de/wissenschaft/hintergrund/297264.html [Accessed on Nov. 15, 2008] (in German)
- 18.Trosic I, Busljeta I, Kasuba V, Rozgaj R. Micronucleus induction after whole-body microwave irradiation of rats. Mutat Res. 2002;521(1-2):73–79. doi: 10.1016/s1383-5718(02)00214-0. [DOI] [PubMed] [Google Scholar]
- 19.Yao K, Wu W, Wang K, Ni S, Ye P, Ye Y, Ye J, Sun L. Electromagnetic noise inhibits radiofrequency radiation-induced DNA damage and reactive oxygen species increase in human lens epithelial cells. Mol Vis. 2008;19:964–969. [PMC free article] [PubMed] [Google Scholar]
- 20.Zeni O, Schiavoni AS, Sannino A, Antolini A, Forigo D, Bersani F, Scarfi MR. Lack of genotoxic effects (micronucleus induction) in human lymphocytes exposed in vitro to 900 MHz electromagnetic fields. Radiat Res. 2003;160(2):152–158. doi: 10.1667/RR3014. [DOI] [PubMed] [Google Scholar]
- 21.Zotti-Martelli L, Peccatori M, Maggini V, Ballardin M, Barale R. Individual responsiveness to induction of micronuclei in human lymphocytes after exposure in vitro to 1800-MHz microwave radiation. Mutat Res. 2005;582(1-2):42–52. doi: 10.1016/j.mrgentox.2004.12.014. [DOI] [PubMed] [Google Scholar]