Abstract
A convenient competitive enzyme-linked immunosorbent assay (ELISA) for ciprofloxacin (CPFX) was developed by using rabbit monoclonal antibodies (RabMAbs) against a hapten-protein conjugate of CPFX-bovine serum albumin (BSA). The indirect competitive ELISA of CPFX had a concentration at 50% inhibition (IC50) of 1.47 ng/ml and a limit of detection (LOD) of 0.095 ng/ml. The mAb exhibited some cross-reactivity, however, not so high with enrofloxacin (28.8%), ofloxacin (13.1%), norfloxacin (11.0%), fleroxacin (22.6%), and pefloxacin (20.4%). And it showed almost no cross-reactivity with other antibiotics or sulfonamides evaluated in this study. The competitive ELISA kit developed here could be used as a screening tool to detect and control illegal addition of CPFX in food products. This kit had been applied to milk detection and the recovery rates from samples spiked by CPFX were in a range of 63.02%–84.60%, with coefficients of variation of less than 12.2%.
Keywords: Ciprofloxacin, Competitive enzyme-linked immunosorbent assay (ELISA), Rabbit monoclonal antibody, Residues, Cross-reactivity
1. Introduction
Fluoroquinolone antibiotics (FQs) are a group of broad-spectrum antibiotics used in veterinary and aquatic medicine (Zhu et al., 2008). The FQs have a broad range of activity against a variety of microorganisms such as Chlamydia, Mycoplasma, and Mycobacterium tuberculosis. Furthermore, FQs are comparatively more effective against Gram-negative bacteria and some Gram-positive bacteria than quinolone antibiotics (Wang et al., 2007). With increased use, the residue of FQs entering the food chain may speed the antibiotic resistance of human pathogens (Holtzapple et al., 1997) and cause latent carcinogenicity. Therefore, more and more organizations have issued maximum residue levels (MRLs) and withdrawal criterion for FQs. According to different food resources and drugs, the MRLs of FQs are set in a range of 30–1500 μg/kg (Lu et al., 2006; Zhao et al., 2007).
Ciprofloxacin (CPFX) (Fig. 1) is one of the most widely used FQs for the treatment of urinary tract infections, lower respiratory tract infections, nosocomial pneumonia, skin infections, intra-abdominal infections, joint infections, and chronic bacterial prostatitis (Ahmad et al., 2006). On the other hand, the CPFX residues in livestock products may cause serious public health problems. An EU Regulation has set an MRL of 30 μg/kg for the sum of CPFX and enrofloxacin in edible animal tissues (Duan and Yuan, 2001).
Fig. 1.
Configuration of ciprofloxacin
Microbiological assays for CPFX in food products are usually easy to perform and inexpensive. However, these methods are complicated, time-consuming, and specificity-lacking (Kurittu et al., 2000). Instrumental methods, including liquid chromatography-mass spectrometry (LC-MS) and high-performance liquid chromatography (HPLC) (Johnston et al., 2002; van Vyncht et al., 2002; Espinosa-Mansilla et al., 2005; 2006; Toussaint et al., 2005a; 2005b; Zeng et al., 2005), are the most current widely used methods for CPFX residues detection. These methods are sensitive and highly specific, but they require expensive equipment, large volumes of solvents, and highly trained individuals for operating complicated instruments, with time-consuming sample cleanup processes. Therefore, rapid and simple detection techniques are required to determine the CPFX residue level in food products. Enzyme-linked immunosorbent assay (ELISA) is very common as a biochemical and clinical analytical method, and is available for the detection of pesticides and veterinary drugs (Watanabe et al., 2001; Zhang et al., 2007; Peng et al., 2008; Stanker et al., 2008).
Numerous studies have been reported on developing ELISA methods for the detection of FQs, including CPFX (Mellgren and Sternesjö, 1998), enrofloxacin (Mellgren and Sternesjö, 1998; Watanabe et al., 2002), norfloxacin (Watanabe et al., 2002; Huet et al., 2006), pefloxacin (Mellgren and Sternesjö, 1998; Huet et al., 2006), and sarafloxacin (Lu et al., 2006). Moreover, Bucknall et al. (2003) reported results concerning a polyclonal antibody against CPFX with a limit of detection (LOD) of 4 ng/ml (Bucknall et al., 2003). Duan and Yuan (2001) reported that the LOD of polyclonal CPFX antibody was 0.32 ng/ml.
However, there have not yet been any reports of preparation from a rabbit monoclonal antibody (RabMAb) for CPFX and development of an ELISA method based on this. The objective of this study was first to develop an indirect competitive ELISA based on high-affinity RabMAbs for the determination of CPFX in milk. Secondary purposes included the measurement of the LOD and cross-reactivity with other antibiotics of RabMAb and the development of a kit based on the RabMAb for CPFX residue detection in milk samples.
2. Materials and methods
2.1. Reagents and instrumentation
Bovine serum albumin (BSA), ovalbumin (OVA), Freund’s complete and incomplete adjuvants (cFA and iFA), 3,3′,5,5′-tetramethylbenzidine (TMB), and 1-ethyl-3-(dimethylaminopropyl)carbodiimide hydrochloride (EDC) were supplied by Sigma-Aldrich (St. Louis, MO, USA). N-hydroxysuccinimide (NHS) was purchased from Yanchang Biochemistry Ltd. (Shanghai, China). Ciprofloxacin (CPFX), enrofloxacin, ofloxacin, norfloxacin, fleroxacin, pefloxacin, and sulfonamides were provided by the Institute of Veterinary Medicine of Zhejiang (Hangzhou, China). Goat anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase (HRP) conjugate was obtained from the Pierce Inc. (Rockford, IL, USA). Dimethylformamide (DMF) was purchased from Jinling Ltd. (Shanghai, China). Hydrogen peroxide (30%), and other reagents were supplied by Zhejiang Chemical Inc. (Hangzhou, China). Milk samples were purchased from a local food store.
Fast protein liquid chromatography (FPLC) equipment AKTA FPLC with XK 16/70 was obtained from GE Inc. (Shanghai, China). Polystyrene 96-well microtiter plates were purchased from Corning Inc. (Hangzhou, China). The ELISA plate reader was purchased from TECAN Inc. (Durham, NC, USA). The ultraviolet/visible (UV/VIS) spectrometer was supplied by Shanghai Analytical Instrument, China.
The buffers used in the experiments include: (1) phosphate-buffered saline (PBS, pH 7.4) composed of 138 mmol/L NaCl, 1.5 mmol/L KH2PO4, 7 mmol/L Na2HPO4, and 2.7 mmol/L KCl; (2) washing buffer: PBS including 0.05% Tween 20 (PBST, pH 7.4); (3) coating buffer: 0.05 mol/L carbonate buffer (pH 9.6); (4) blocking buffer: 0.02 mol/L PBS (pH 7.4) including 2% (w/w) glycine; (5) substrate buffer: 0.1 mol/L sodium acetate/citrate buffer (pH 5.0); (6) substrate: 0.1% TMB and H2O2 in 0.05 mol/L citrate buffer (pH 4.5); (7) stopping reagent: 2 mol/L H2SO4.
2.2. Preparation of immunogen CPFX-BSA and coating antigen CPFX-OVA
The immunogen CPFX-BSA and the coating antigen CPFX-OVA were both prepared by carbodiimide active ester method as per a previous paper (Lu et al., 2006). A total of 3.86 mg of CPFX, 20.64 mg of EDC, and 5.76 mg of NHS were added to 0.23 ml DMF in order. The mixture solution was incubated for 24 h at room temperature in dark. Then 2.5 ml of PBS (0.01 mol/L, pH 7.4) with 10% (w/w) BSA was added slowly to the mixture solution with stirring, followed by 3-h incubation at room temperature. Finally, the reaction mixture was dialyzed (molecular weight cut-off (MWCO): 12 000–14 000 Da) under stirring against PBS (0.1 mol/L, pH 7.4) for 4 d with repeated change of the PBS solution to remove the unconjugated free hapten. The solution was lyophilized, and the obtained CPFX-BSA conjugate was stored at −20 °C. A CPFX-OVA conjugate was prepared in the same procedure. The UV absorbance method and the FPLC method were used to determine whether or not the conjugate had a success.
2.3. Production of RabMAbs
2.3.1. Immunization
Three male New Zealand white rabbits were subcutaneously immunized at a couple of sites in the back with CPFX-BSA conjugate. The first immunization was subcutaneously injected with 1 mg of conjugate in 0.5 ml of NaCl (0.85%, w/v) and 0.5 ml of cFA. Subsequent boost injections [0.5 mg of conjugate in 0.5 ml of NaCl (0.85%) plus 0.5 ml of iFA] were carried on 15 d later and then at 12-d intervals. Serum titers were detected by ELISA method after each booster. Meanwhile, the antiserum was prepared by keeping the blood to clot at 4 °C overnight, followed by centrifugation to remove unnecessary parts. Ten days after the final boost, all rabbits were exsanguinated by heart puncture under general euthanasia or anesthetic by lethal injections. The serum was separated from blood cells by storing at 4 °C overnight and centrifugation with 3000 r/min for 15 min. This crude serum was purified from saturated ammonium sulfate (SAS) precipitation method. Sodium azide was added to the purified serum as a preservative at a final concentration of 0.02% (w/w). The serum was then stored at −70 °C.
2.3.2. Cell fusion
The rabbit with the highest polyclonal antibody titer was sacrificed, and splenocytes were fused with 240E rabbit myeloma cells using polyethylene glycol (PEG 6000). The fused cells were propagated in hypoxanthine aminopterin thymidine (HAT) medium, plated in forty 96-well microculture plates, and then screened for specific MAbs using the indirect competitive ELISA (Spieker-Polet et al., 1995).
2.3.3. Hybridoma selection and subcloning
At 12 to 15 d after cell fusion, culture supernatants were screened for the presence of antibodies that recognized the analytes. Then these positive clones were transferred to 24-well plates. Seven days later, these multiclones were screened again for confirming. Supernatants showing an inhibition of >80% in the presence of 1000 ng/ml hapten compared to the absence of analyte were considered to be derived from high-affinity antibody-secreting hybridomas. The cultures showing the highest inhibition by CPFX were subcloned by limiting dilution. The clones with the highest inhibition were subcloned prior to ascite production. The antibodies were purified using saturated ammonium sulfate and stored at −20 °C in 50 μl aliquots.
2.4. Development of indirect competitive ELISA
The coating antigen and RabMAb concentrations were optimized. To each well of a 96-well plate was added coating antigen solution (CPFX-BSA, 1:1600, 100 μl/well) in bicarbonate buffer (0.05 mol/L, pH 9.6), and the mixture was incubated at 4 °C overnight. The plate was washed with washing buffer three times and added with 200 μl/well of blocking buffer, followed by the incubation at 37 °C for 2 h. After the blocking solution was removed and the plate was washed three times, 100 μl of RabMAb was added to each well followed by the addition of buffer or competitor in buffer, and the plate was incubated at 37 °C for 1 h. The plate was washed another three times, and goat anti-rabbit IgG-HRP (1:5000, 100 μl/well) was added, followed by the incubation for 1 h at 37 °C. The plate was washed three times again, the substrate solution was added (50 μl/well), and then the plate was incubated for another 15 min at 37 °C. The color development was halted by adding stopping solution (50 μl/well). The absorbance was measured at 450 nm wavelength and corrected by a blank reading. Preimmune withdrawn serum was carried as a negative control. The result was expressed in percent inhibition as follows:
| percent inhibition=(1−A/A0)×100%, |
where A is the absorbance of the well containing competitor and A 0 is the absorbance of the well without competitor. The indirect competitive ELISA was used to detect the MAb affinity and cross-reactivity.
2.5. Sensitivity and specificity of assay
The LOD, also called the least detectable dose, was evaluated as the concentration of CPFX giving a 10% inhibition of the maximum absorbance.
Five different FQs and other antimicrobials such as antibiotics and sulfonamides were assessed for cross-reactivity with anti-CPFX monoclonal antibodies. Cross-reactivity was defined as the following: (nanomoles of CPFX for 50% binding/nanomoles of other competitors for 50% binding)×100% (Duan and Yuan, 2001).
2.6. Milk sample analysis
2.6.1. Standard curve generation and standard solution preparation
The indirect competitive ELISA was performed as described above. The standard calibration curve with final CPFX concentrations between 0.05 and 10 ng/ml was estimated in PBST. CPFX solutions used for milk detection were prepared in PBS at following concentrations: 0.4, 1.0 and 2.0 ng/ml.
2.6.2. Milk sample pretreatment
Milk samples were centrifuged at 4 °C with a speed of 10 000 r/min for 30 min, and the floated fat was discarded. A total of 200 μl of the rest milk was added to tube with 200 μl PBS and 400 μl methanol. The mixture was then centrifuged at 4 °C with a speed of 12 000 r/min for 30 min. The supernatant was ready for detection procedures.
3. Results and discussion
3.1. Hapten conjugation
With a molecular mass of 331.4, CPFX is not able to stimulate the immune response in an animal for anti-CPFX antibody production and is, therefore, non-immunogenic. To make it immunogenic, it must be conjugated to a carrier protein before immunization. BSA and OVA are two of the mostly applied carrier proteins, and usually, they provide satisfying results.
From the structure (Fig. 1), it can be seen that CPFX contains a carboxylic acid group and a secondary amino group. Thus, the immunogen and coating antigen can be prepared by the conjugation of the carboxylic acid group and an amino group of a carrier protein or by the conjugation of the secondary amino group of CPFX and a carboxylic acid group of a carrier protein. In this study, the former linkage method was chosen in order to expose the structural part representing the feature of CPFX outward to increase the specificity of the antibody.
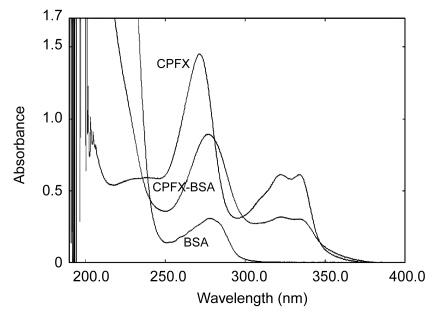
The carbodiimide active ester method was used to prepare immunogen and coating antigen. UV spectrometry and FPLC method were used to determine the efficiency of the conjugation reaction. UV absorbances for CPFX-BSA, CPFX, and BSA are presented in Fig. 2. The absorbance for CPFX-BSA (276.4, 322, 335.6 nm) gave a shifted peak at 276.4 nm compared with the 271.3 nm peak for CPFX (271.3, 321.8, 333.7 nm), which indicated the CPFX was successfully conjugated with BSA. The coating antigen CPFX-OVA gave a UV pattern similar to that of CPFX-BSA.
Fig. 2.
UV absorbances for CPFX-BSA, CPFX, and BSA
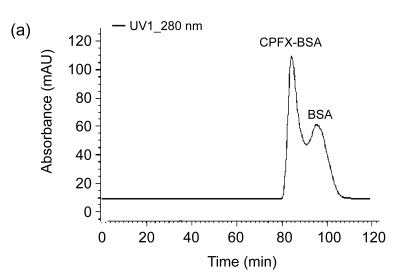
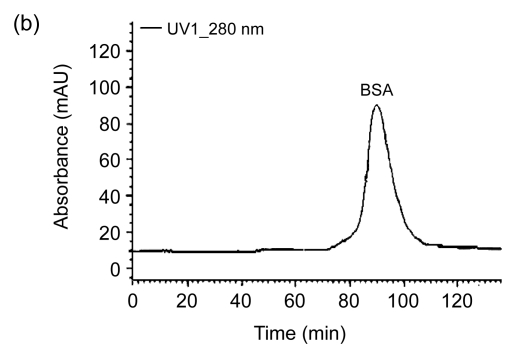
The FPLC results as shown in Fig. 3 also support the successful conjugation from different spectrogram peaks. The time for CPFX-BSA (84 min) is shorter than that for BSA (92 min). The coating antigen CPFX-OVA gave an FPLC result similar to that of CPFX-BSA.
Fig. 3.
FPLC spectrograms of CPFX-BSA (a) and BSA (b)
3.2. Characterization of RabMAb
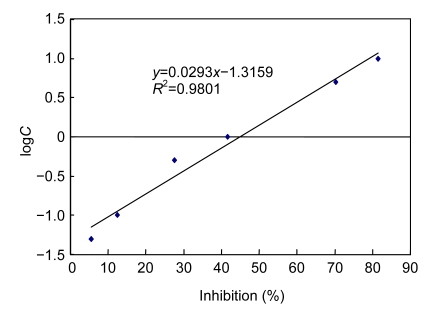
The proper RabMAb dilution method as primary antibody here was defined as the reciprocal of the dilution multiple, which results in an absorbance value that is twice of that of the background. The titer of RabMAb was then determined by indirect ELISA as >128 000 for three rabbits used in the immunization procedure. Fig. 4 shows a CPFX inhibition curve obtained by the competitive competitive ELISA with RabMAb. The inhibition curve was obtained from four replicates. The LOD estimated and the working range for ELISA calculated as the concentration of CPFX providing a 20%–80% inhibition (I 20–I 80 values) of the maximum signal, were 0.095 ng/ml and 0.19–10.00 ng/ml, respectively. The concentration at 50% inhibition (IC50) (Table 1) from RabMAb was 1.47 ng/ml, which was lower than other results obtained by polyclonal antibodies. Bucknall et al. (2003) reported a research concerning a polyclonal antibody against CPFX with an LOD of 4 ng/ml. Duan and Yuan (2001) reported the LOD of polyclonal CPFX antibody was 0.32 ng/ml. These results all confirmed that our RabMAb has higher affinity of CPFX than former polyclonal antibodies.
Fig. 4.
Standard curve of indirect competitive ELISA of CPFX monoclonal antibody
C: CPFX standard concentration (ng/ml)
Table 1.
IC50 values and cross-reactivity of anti-CPFX toward selected FQs and other antibiotics and sulfonamides
| Competitor | IC50 (ng/ml)a | Cross-reactivity (%)b |
| FQs | ||
| Ciprofloxacin | 1.47 | 100.0 |
| Enrofloxacin | 5.10 | 28.8 |
| Ofloxacin | 11.20 | 13.1 |
| Norfloxacin | 13.40 | 11.0 |
| Fleroxacin | 6.50 | 22.6 |
| Pefloxacin | 7.20 | 20.4 |
| Other antibiotics and sulfonamides | ||
| Chloramphenicol | >106 | <0.0005 |
| Oxytetracycline | >105 | <0.005 |
| Neomycin | >105 | <0.005 |
| Tetracycline | >106 | <0.0005 |
| Sulphadiazine | >105 | <0.005 |
| Sulfadimidine | >105 | <0.005 |
| Sulfamethoxazole | >105 | <0.005 |
IC50 value was the competitor concentration at which the absorbance value was decreased by half as compared to the absorbance value of no competitor. Data represent three separate experiments run on three different days
Cross-reactivity was determined by comparing the concentration of analyte required to produce A/A 0 of 50%. Results are expressed as a percentage relative to the figure for ciprofloxacin
3.3. Specificity of the antibody
The specificity of the RabMAb was estimated by determination of the IC50 and the cross-reactivity towards various FQs, some other antibiotics and sulfonamides. The IC50 values towards enrofloxacin, ofloxacin, norfloxacin, fleroxacin, and pefloxacin are summarized in Table 1. Ciprofloxacin (CPFX) and enrofloxacin had IC50 values of 1.47 and 5.10 ng/ml, respectively. Other FQs, including ofloxacin, norfloxacin, fleroxacin, and pefloxacin, showed relative affinity with IC50 values of 11.20, 13.40, 6.50, and 7.20 ng/ml, respectively. All antibiotics and sulfonamides listed in Table 1 have IC50 values of more than 105 ng/ml. The cross-reactivity evaluations were carried out by an indirect competitive ELISA, adding various standard CPFX solutions at different concentrations (from 0.001 to 1 000 000 ng/ml) to compete with the coating antigen to bind on the antibody. Cross-reactivity was measured by comparison of the competitor IC50 with that of CPFX. However, RabMAb did not show high cross-reactivity with commonly used FQs including enrofloxacin (28.8%), ofloxacin (13.1%), norfloxacin (11.0%), fleroxacin (22.6%), and pefloxacin (20.4%) (Table 1). Furthermore, in Table 1 there were almost no cross-reactivities between RabMAb and other antibiotics and sulfonamides. The results show that the RabMAb had excellent specificity.
3.4. ELISA performance in milk
The applicability of the developed assay was further verified by testing for the CPFX residue in milk. The results are shown in Table 2. In this research, samples for the recovery study were prepared by the extract after PBS dilution only. Known amounts of CPFX were spiked into milk and were analyzed using a calibration curve made in a buffer. The assay has an LOD of 0.095 ng/ml in buffer solution. The linear range of the percentage A/A 0 vs. log CPFX concentration competitive curve generated in PBS was 0.19–10.00 ng/ml (Fig. 4). Thus, the milk samples have an LOD of 0.38 ng/ml in buffer solution (four-fold dilution), while the detection range varies from 0.76 to 40.00 ng/ml. Meanwhile, the milk sample spiked at 0.4, 2.0, and 4.0 ng/ml gave an average recovery rate of 77.12%, 84.60%, and 63.02%, respectively, for intra-assay. The coefficient of variation (CV) ranged from 6.26% to 12.2%.
Table 2.
Intra-assay variations of raw milk with CPFX
| CPFX added (ng/ml) | Replicates | Measured (ng/ml)a | RR (%) | CV (%) |
| 0.4 | 8 | 0.31±0.05 | 77.12 | 12.20 |
| 2.0 | 8 | 1.69±0.16 | 84.60 | 6.26 |
| 4.0 | 8 | 2.52±0.52 | 63.02 | 11.60 |
Mean±SD
RR: recovery rate; CV: coefficient of variation
4. Conclusions
In summary, for the first time, a high-affinity rabbit monoclonal anti-CPFX antibody was prepared. Furthermore, an indirect competitive ELISA kit to detect CPFX residues in milk was developed. The RabMAb showed high sensitivity with an IC50 value of 1.47 ng/ml and excellent specificity towards other antibiotics and sulfonamides. The competitive ELISA method had an LOD of 0.095 ng/ml and was suitable for future use as a rapid screening method for liquid samples. As a concrete example, this competitive ELISA kit developed was applied to detect CPFX in milk samples with satisfying results.
Acknowledgments
We thank the Yikang Biotechnology Company and Hua’an Biotechnology Company of Hangzhou (Zhejiang, China) for their collaboration on the immunization and preparation of RabMAb.
Footnotes
Project supported by the National High-Tech R&D Program (863) of China (No. 2007AA10Z436) and the Important National Science and Technology Specific Projects of China (No. 2009ZX03012-010B)
References
- 1.Ahmad B, Parveen S, Khan RH. Effect of albumin conformation on the binding of ciprofloxacin to human serum albumin: a novel approach directly assigning binding site. Biomacromolecules. 2006;7(4):1350–1356. doi: 10.1021/bm050996b. [DOI] [PubMed] [Google Scholar]
- 2.Bucknall S, Silverlight J, Coldham N, Thorne L, Jackman R. Antibodies to the quinolones and fluoroquinolones for the development of generic and specific immunoassays for detection of these residues in animal products. Food Addit Contam. 2003;20(3):221–228. doi: 10.1080/0265203021000055388. [DOI] [PubMed] [Google Scholar]
- 3.Duan J, Yuan Z. Development of an indirect competitive ELISA for ciprofloxacin residues in food animal edible tissues. J Agric Food Chem. 2001;49(3):1087–1089. doi: 10.1021/jf000091j. [DOI] [PubMed] [Google Scholar]
- 4.Espinosa-Mansilla A, Peńa AM, Cańada-Cańada F, Gómez DG. Determinations of fluoroquinolones and nonsteroidal anti-inflammatory drugs in urine by extractive spectrophotometry and photoinduced spectrofluorimetry using multivariate calibration. Anal Biochem. 2005;347(2):275–286. doi: 10.1016/j.ab.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Espinosa-Mansilla A, Peńa AM, Gómez DG, López FS. Determination of fluoroquinolones in urine and serum by using high performance liquid chromatography and multiemission scan fluorimetric detection. Talanta. 2006;68(4):1215–1221. doi: 10.1016/j.talanta.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Holtzapple CK, Buckley SA, Stanker LH. Development of antibodies against the fluoroquinolone sarafloxacin and molecular modeling studies of cross-reactive compounds. Food Agric Immunol. 1997;9(1):13–26. doi: 10.1080/09540109709354930. [DOI] [Google Scholar]
- 7.Huet AC, Charlier C, Tittlemier SA, Singh G, Benrejeb S, Delahaut P. Simultaneous determination of (fluoro)quinolone antibiotics in kidney, marine products, eggs, and muscle by enzyme-linked immunosorbent assay (ELISA) J Agric Food Chem. 2006;54(8):2822–2827. doi: 10.1021/jf052445i. [DOI] [PubMed] [Google Scholar]
- 8.Johnston L, Mackay L, Croft M. Determination of quinolones and fluoroquinolones in fish tissue and seafood by highperformance liquid chromatography with electrospray ionization tandem mass spectrometric detection. J Chromatogr A. 2002;982(1):97–109. doi: 10.1016/S0021-9673(02)01407-3. [DOI] [PubMed] [Google Scholar]
- 9.Kurittu J, Lonnberg S, Virta M, Karp M. A group-specific microbiological test for the detection of tetracycline residues in raw milk. J Agric Food Chem. 2000;48(8):3372–3377. doi: 10.1021/jf9911794. [DOI] [PubMed] [Google Scholar]
- 10.Lu S, Zhang Y, Liu J, Zhao C, Liu W, Xi R. Preparation of anti-pefloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of pefloxacin residue in chicken liver. J Agric Food Chem. 2006;54(19):6995–7000. doi: 10.1021/jf061309q. [DOI] [PubMed] [Google Scholar]
- 11.Mellgren C, Sternesjö A. Optical immunobiosensor assay for determining enrofloxacin and ciprofloxacin in bovine milk. J AOAC Int. 1998;81(2):394–397. [PubMed] [Google Scholar]
- 12.Peng CF, Chen YW, Chen W, Xu CL, Kim JM, Jin ZY. Development of a sensitive heterologous ELISA method for analysis of acetylgestagen residues in animal fat. Food Chem. 2008;109(3):647–653. doi: 10.1016/j.foodchem.2007.12.072. [DOI] [Google Scholar]
- 13.Spieker-Polet H, Sethupath P, Yam PC, Knight KL. Rabbit monoclonal antibodies generating a fusion partner to produce rabbit-rabbit hybridomas. PNAS. 1995;92(20):9348–9352. doi: 10.1073/pnas.92.20.9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanker LH, Merrill P, Scotcher MC, Cheng LW. Development and partial characterization of high-affinity monoclonal antibodies for botulinum toxin type A and their use in analysis of milk by sandwich ELISA. J Immunol Methods. 2008;336(1):1–8. doi: 10.1016/j.jim.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Toussaint B, Chedin M, Bordin G, Rodriguez AR. Determination of (fluoro)quinolone antibiotic residues in pig kidney using liquid chromatography-tandem mass spectrometry. I. Laboratory-validated method. J Chromatogr A. 2005;1088(1-2):32–39. doi: 10.1016/j.chroma.2005.02.057. [DOI] [PubMed] [Google Scholar]
- 16.Toussaint B, Chedin M, Vincent U, Bordin G, Rodriguez AR. Determination of (fluoro)quinolone antibiotic residues in pig kidney using liquid chromatography-tandem mass spectrometry. Part II. Intercomparison exercise. J Chromatogr A. 2005;1088(1-2):40–48. doi: 10.1016/j.chroma.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 17.van Vyncht G, Jànosi A, Bordin G, Toussaint B, Maghuin-Rogister G, Pauw ED, Rodriguez AR. Multiresidue determination of (fluoro)quinolone antibiotics in swine kidney using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2002;952(1-2):121–129. doi: 10.1016/S0021-9673(02)00092-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Zhu Y, Ding S, He F, Beier RC, Li J, Jiang H, Feng C, Wan Y, Zhang S, et al. Development of a monoclonal antibody-based broad-specificity ELISA for fluoroquinolone antibiotics in foods and molecular modeling studies of cross-reactive compounds. Anal Chem. 2007;79(12):4471–4483. doi: 10.1021/ac070064t. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Satake A, Kido Y, Tsuji A. Monoclonal-based enzyme-linked immunosorbent assay and immunochromatographic rapid assay for salinomycin. Anal Chim Acta. 2001;437(1):31–38. doi: 10.1016/S0003-2670(01)00925-4. [DOI] [Google Scholar]
- 20.Watanabe H, Satake A, Kido Y, Tsuji A. Monoclonal-based enzyme-linked immunosorbent assays and immunochromatographic assay for enrofloxacin in biological matrices. Analyst. 2002;127(1):98–103. doi: 10.1039/b109427k. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Z, Dong A, Yang G, Chen Z, Huang X. Simultaneous determination of nine fluoroquinolones in egg white and egg yolk by liquid chromatography with fluorescence detection. J Chromatogr B. 2005;821(2):202–209. doi: 10.1016/j.jchromb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Lu S, Liu W, Zhao C, Xi R. Preparation of anti-tetracycline antibodies and development of an indirect heterologous competitive enzyme-linked immunosorbent assay to detect residues of tetracycline in milk. J Agric Food Chem. 2007;55(2):211–218. doi: 10.1021/jf062627s. [DOI] [PubMed] [Google Scholar]
- 23.Zhao C, Liu W, Ling H, Lu S, Zhang Y, Liu J, Xi R. Preparation of anti-gatifloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for the detection of gatifloxacin residue in milk. J Agric Food Chem. 2007;55(17):6879–6884. doi: 10.1021/jf070978g. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y, Li L, Wang Z, Chen Y, Zhao Z, Zhu L, Wu X, Wan Y, He F, Shen J. Development of an immunochromatography strip for the rapid detection of 12 fluoroquinolones in chicken muscle and liver. J Agric Food Chem. 2008;56(14):5469–5474. doi: 10.1021/jf800274f. [DOI] [PubMed] [Google Scholar]