Summary
Interactions between the nuclear factor (NF)-κB inhibitor parthenolide and the pan-histone deacetylase inhibitors (HDACIs) vorinostat and LBH589 were investigated in human acute myeloid leukaemia (AML) cells, including primary AML blasts. Co-administration of parthenolide blocked HDACI-mediated phosphorylation/activation of IKK and RelA/p65 in association with increased JNK1 activation in various AML cell types. These events were accompanied by an increase in apoptosis in multiple AML cell lines (e.g. U937, HL-60, NB4, MV-4-11, and MOLM-13). Significantly, parthenolide also increased HDACI-mediated cell death in haematopoietic cells transduced with the MLL-MLLT1 fusion gene, which exhibit certain leukaemia-initiating cell characteristics, as well as primary AML blasts. Exposure to parthenolide/HDACI regimens clearly inhibited the growth of AML-colony-forming units but was relatively sparing toward normal haematopoietic progenitors. Notably, blockade of JNK signaling by either pharmacological inhibitors or genetic means (e.g., dominant-negative JNK1 or JNK1 shRNA) diminished parthenolide/HDACI-mediated lethality. Moreover, dominant-negative MKK7, but not dominant-negative MKK4/SEK1, blocked JNK1 activation and apoptosis induced by parthenolide/HDACI regimens. Together, these findings indicate that parthenolide potentiates HDACI lethality in human AML cells through a process involving NF-κB inhibition and subsequent MKK7-dependent activation of the SAPK/JNK pathway. They also raise the possibility that this strategy may target leukaemic progenitor cells.
Keywords: AML, NF-κB, JNK, histone deacetylase inhibitor, parthenolide
Introduction
Histone deacetylase inhibitors (HDACIs) represent a class of compounds that modify chromatin structure and gene expression. In preclinical studies, HDACIs induced apoptosis in human leukaemia cells (Grant et al, 2007), and in recent clinical trials the pan-HDACI vorinostat has shown activity in certain types of lymphoma (e.g., cutaneous T-cell lymphoma) (Grant et al, 2007) and acute leukaemia (Garcia-Manero et al, 2008a). HDACs also regulate the acetylation of diverse non-histone proteins (Marks, 2007), events which may contribute to HDACI lethality (Bolden et al, 2006). Such findings provide a rationale for exploring novel therapeutic strategies combining other signal transduction modulators with HDACIs.
Nuclear factor (NF)-κB is composed of homo- and heterodimers of five members of the Rel family including RelA (p65), RelB, c-Rel, NF-κB1 (p50 and its precursor p105), NF-κB2 (p52 and its precursor p100). The NF-κB family represents transcription factors implicated in diverse cellular activities, including inflammatory responses, survival, growth, and differentiation, among others (Baud & Karin, 2009). In human acute myelogenous leukaemia (AML) and myelodysplastic syndrome (MDS), nuclear NF-κB (RelA-p50) is generally constitutively expressed in primary leukaemic blasts as well as CD34+ leukaemia stem cells (LSCs) (Bueso-Ramos et al, 2004;Guzman et al, 2001). On the other hand, interruption of the NF-κB signaling pathway (e.g., via inhibition of IκB kinaseβ [IKKβ]) kills or chemosensitizes AML and MDS tumour cells and LSCs (Guzman et al, 2002;Guzman et al, 2005;Frelin et al, 2005). Interestingly, recent reports indicate that constitutive NF-κB activation is associated with mutations in FLT3 (Grosjean-Raillard et al, 2008) and NPM1 (nucleophosmin) (Cilloni et al, 2008) in AML and MDS, the most specific and frequent mutational events in AML. Moreover, FLT3 inhibition disrupts physical interactions between IKKβ and FLT3, leading to inhibition of NF-κB and selective killing of primary CD34+ AML and MDS cells versus normal CD34+ cells (Grosjean-Raillard et al, 2008). RelA-p50 heterodimer, the most abundant form of NF-κB, is retained in the cytoplasm by binding to IκBα. Upon stimulation by diverse stimuli, the family of IκB kinases (IKKs), particularly IKKβ, is activated, which phosphorylate IκBα, leading to ubiquitination and proteasomal degradation. Following release from IκBα, RelA-p50 translocates to the nucleus, binds to gene promoter regions, and triggers transcription of various genes (Baud & Karin, 2009). Alternatively, recent findings have highlighted post-translational modifications in the regulation of NF-κB activation (Perkins, 2006). Specifically, both RelA phosphorylation and acetylation have been associated with enhanced nuclear import, DNA binding and transactivation (Chen et al, 2001). Treatment with HDACIs leads to enhanced RelA acetylation and activation (Liu et al, 2006;Chen et al, 2001;Dai et al, 2005a), whereas blockade of these events promotes HDACI lethality in human leukaemia and other malignant cell types (Dai et al, 2005a;Duan et al, 2007). However, cell death mechanisms acting downstream of NF-κB inhibition are less clear. In this context, evidence indicates that in the classical NF-κB model of tumour necrosis factor α (TNFα)-induced apoptosis, sustained activation of the SAPK/JNK (stress-activated protein kinase/c-Jun N-terminal kinase) pathway plays an essential role (Kamata et al, 2005).
Parthenolide (PTL) is a sesquiterpene lactone extracted from the herbal medicine Feverfew (tanacetum parthenium). PTL is a natural NF-κB inhibitor that has shown activity in preclinical studies involving diverse transformed cell types, including leukaemia cells (Guzman et al, 2005;Zunino et al, 2007). It also sensitizes transformed cells to various cytotoxic agents (Patel et al, 2000;Nakshatri et al, 2004). PTL inhibits NF-κB by binding to, and blocking activation of, IKK (Hehner et al, 1999), and possibly by directly alkylating and inhibiting RelA at cysteine residues (Garcia-Pineres et al, 2001). PTL may also exert its anti-neoplastic effects through other mechanisms. For example, PTL inhibits STAT3 (Sobota et al, 2000), activates JNK (Nakshatri et al, 2004), depletes HDAC1 (Gopal et al, 2007), and triggers reactive oxygen species (ROS) generation/endoplasmic reticulum (ER) stress (Liu et al, 2009a). Notably, PTL may selectively target primitive leukaemic progenitor cells that exhibit LSC characteristics (Guzman et al, 2005). Efforts to develop PTL analogues (e.g., LC-1) as antileukaemic agents are currently underway (Hewamana et al, 2008a;Guzman et al, 2007;Jenkins et al, 2008).
In view of evidence that NF-κB activation opposes HDACI lethality, the possibility arose that PTL might increase HDACI antileukaemic activity. The purpose of this study was to determine whether PTL interacts with HDACIs to kill human AML cells and their progenitors, and to gain insights into mechanisms underlying such interactions. The present results indicated that PTL markedly increases the anti-leukaemic activity of clinically relevant HDACIs, including vorinostat and LBH589 (panobinostat) (Giles et al, 2006), through a mechanism involving attenuation of HDACI-mediated NF-κB activation and resulting MKK7- rather than MKK4/SEK1-dependent activation of JNK1. They also suggest that AML progenitor cells may be vulnerable to this strategy. Collectively, these data provide a rationale for employing PTL or other NF-κB antagonists to enhance HDACI activity in AML.
Materials and methods
Cells and reagents
U937 (AML, M4-M5 with t[10;11][p13;q14]), HL-60 (acute promyelocytic leukaemia [APL], M2 with loss and several rearrangements involving chromosome 5), NB4 (APL, M3 with t[15;17]), MV-4-11 (biphenotypic B-AML) were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA), and MOLM-13 (AML, M5a) from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ, Braunschweig, Germany). These cells were maintained in RPMI 1640 medium containing 10% or 20% (for MOLM-13) fetal bovine serum (FBS). MV-4-11 and MOLM-13 cells harbour FLT3-internal tandem duplication (ITD) mutation and MLL rearrangement. MLL-ENL cells exhibiting certain leukaemia-initiating cell (L-IC) characteristics were kindly provided by Dr. John E Dick (University Health Network, Toronto, Ontario, Canada) (Barabe et al, 2007) and cultured in α-minimum essential medium (MEMα) containing 20% FBS, 5% human plasma with 10 units/ml Na heparin, 1 × β-ME, 50 ng/ml recombinant human stem cell factor (SCF), 10 ng/ml interleukin (IL)-3, 5 ng/ml IL-7, 5 ng/ml FLT3L (R&D Systems, Minneapolis, MN).
The pan-HDAC inhibitors vorinostat (suberoylanilide hydroxamic acid, SAHA) and LBH589 (panobinostat) were obtained from Merck (Whitehouse Station, NJ, USA) and Novartis Pharmaceuticals (Basel, Switzerland), respectively. PTL and the SAPK/JNK activator anisomycin were purchased from Biomol (Plymouth Meeting, PA). JNK inhibitor VIII (Vivanco et al, 2007) (designated JNKi throughout) was obtained from Calbiochem (San Diego, CA). All reagents were formulated in dimethyl sulphoxide (DMSO), stored at −20°C, and subsequently diluted with serum-free RPMI medium prior to use. In all cases, final DMSO concentrations were less than 0.1%. Recombinant human TNFα was obtained from R&D Systems (Minneapolis, MN). The cell-permeable JNK1 peptide inhibitor D-JNK1 and its negative control D-TAT were purchased from Alexis (San Diego, CA).
All experiments were performed in cell lines utilizing logarithmically growing cells (4–6×105 cells/ml) or in primary samples at density of 1×106 cells/ml. Cells were treated with PTL for 1 h prior to addition of HDACIs (24 h).
Patient samples
Bone marrow (BM) or peripheral blood (PB) samples were obtained with informed consent, in accordance with the Declaration of Helsinki, from patients with AML or non-myeloid haematological disorder (e.g., iron deficiency) undergoing routine diagnostic procedures with approval from the institutional review board of Virginia Commonwealth University. In all AML samples, the percentage of blasts was > 70%. Umbilical cord blood (CB) cells were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA, USA) (Guzman et al, 2005). Mononuclear cells were isolated by Ficoll-Hypaque (Sigma, St Louis, MO) as previously described (Dai et al, 2005b).
Methylcellulose colony-forming assays
Previously described methylcellulose colony-forming assays were used to assess the effects of drug treatment on the clonogenic growth of normal and leukaemia cells (Guzman et al, 2005). Briefly, leukaemic blasts or normal CB mononuclear cells were incubated in serum-free medium for 1 h prior to the addition of drugs. Cells were then exposed to PTL ± vorinostat for 24 h, washed, and plated at a density of 50,000 cells/ml in Methocult GF H4534 (1% methylcelluose in Iscove’s modified Eagle medium (IMDM), 30% FBS, 1% bovine serum albumin (BSA), 10−4M 2-mercaptoethanol, 2mM L-glutamine, 50 ng/ml SCF, 10 ng/ml granulocyte-macrophage colony-stimulating factor, 10 ng/ml IL-3; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 3 units/ml of erythropoietin and 50 ng/ml granulocyte colony-stimulating factor (R&D Systems). Colonies, consisting of ≥ 50 cells (normal CB granulocyte-macrophage colony-forming units [GM-CFU]) or ≥ 20 cells (leukaemic colony-forming units [L-CFU]) were scored at the end of 10–14 days incubation. Values for each condition were expressed as a percentage of untreated, control cell colony formation.
Stable transfection
cDNAs encoding dnJNK1 (JNK1/APF), dnMKK7, or dnSEK1 were kindly provided by the laboratory of Dr. Stanley Korsmeyer (Dana-Farber Cancer Institute, Boston, MA) and Dr. Silvio Gutkind (National Institute of Dental and Craniofacial Research, National Institute of Health, Bethesda, MD, USA), respectively. SureSilencing™ plasmids (neomycin resistance) encoding shRNA targeting human MAPK8 (shJNK1, CCTGACAAGCAGTTAGATGAA) or negative control shRNA (shNC, GGAATCTCATTCGATGCATAC) were purchased from SABioscience (Frederick, MD). Transfections were performed using an Amaxa Nucleofector device and Cell Line Specific Nucleofector Kits (Amaxa GmbH, Cologne, Germany) as per the manufacturers’ instructions. Cells were continuously cultured under selection with G418 (400 μg/ml), and ectopic expression of target proteins or down-regulation of JNK1 (JNK1 shRNA) was detected by Western blot.
Flow cytometry
The extent of apoptosis was evaluated by flow cytometric analysis utilizing annexin V-fluorescein isothiocyanate (FITC; BD Pharmingen, San Diego, CA), 7-aminoactinomycin D (7-AAD; Sigma) or 3,3-dihexyloxacarbocyanine (DiOC6; Molecular Probes Inc., Eugene, OR) as described previously (Dai et al, 2005b).
Western blot analysis
Samples from either whole-cell pellets or S-100 cytosolic fractions were prepared and subjected to Western blot analysis as previously described in detail (Dai et al, 2005a). Each lane was loaded with 30μg of protein; blots were stripped and reprobed with α-tubulin or β-actin antibodies to ensure equivalent loading and transfer. The primary antibodies included: IKKβ, RelA/p65, phospho-JNK (Thr183/Tyr185, recognizing p46 JNK1 and p54 JNK2/3), phospho-c-Jun (Ser73), JNK (FL, recognizing p46 JNK1 and p54 JNK2/3), cytochrome c, and AIF (Santa Cruz Biotechnology, Santa Cruz, CA, USA); phospho-IKKα (Ser180)/IKKβ (Ser181), phospho-RelA/p65 (Ser536), p100/p52, phospho-MKK7 (Ser271/Thr275), MKK7, phospho-SEK1 (Ser257/Thr261), SEK1, and cleaved caspase-3 (Cell Signaling, Danvers, MA, USA); caspase-3 (BD PharMingen, San Diego, CA); PARP antibody (Biomol); nuclear matrix protein p84 (Abcam, Cambridge, MA). Representative experiments are shown; two additional experiments yielded equivalent results.
NF-κB activity assays
U937 cells were stably transfected with NF-κB TransLucent Reporter Vector (NF-κB/Luc; Panomics, Redwood City, CA) as previously described (Dai et al, 2005a). Luciferase assays were performed using a Luciferase Reporter Assay Kit (BD Clontech, Polo Alto, CA). Relative luciferase activities were normalized to total protein. RelA-specific DNA binding activity was measured by using Nuclear Extract Kit and TransAM™ NF-κB p65 Chemi Kit (Active Motif, Carlsbad, CA). For each cell type, wild-type and mutated consensus oligonucleotides were used as competitors for RelA-DNA binding in order to monitor assay specificity (Fig S1A). NF-κB activity was expressed as fold increase relative to untreated controls.
Statistical analysis
For NF-κB activity assays and flow cytometric analysis, values represent the means ± S.D. for at least three separate experiments performed in triplicate. The significance of differences between experimental variables was determined by using the Student’s t test. Analysis of synergism was performed according to Median Dose Effect analysis using the software program Calcusyn (Biosoft, Ferguson, MO) (Dai et al, 2005b). P values < 0.05 were considered significant.
Results
PTL prevents HDACI-induced activation of the canonical NF-κB pathway
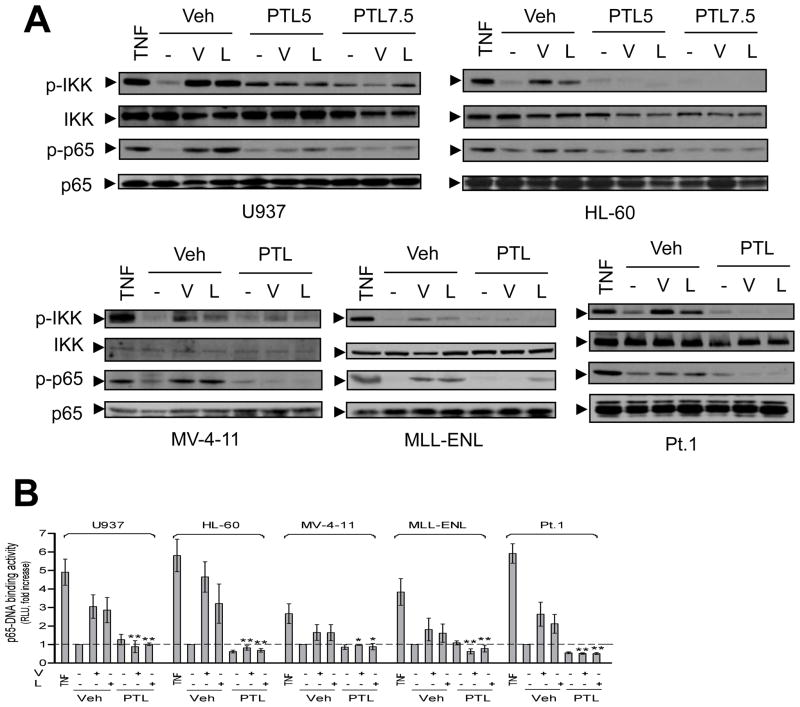
Previous studies have shown that exposure of U937 cells to HDACIs triggers cytoprotective NF-κB activation (Dai et al, 2005a). We therefore examined whether this phenomenon occurs in other human AML cell types, including those harbouring the FLT3-ITD mutation. Exposure to marginally toxic concentrations of the pan-HDACIs vorinostat (0.5–1.5 μM) or LBH589 (3–15 nM) increased IKK and RelA/p65 phosphorylation in multiple types of human AML cells that exhibited varied basal levels of NF-κB activity (Fig S1A), including U937, HL-60, NB4, MV-4-11 (FLT3-ITD), and MLL-ENL, as well as primary AML blasts (Fig 1A and Fig S1B). These events were associated with increased nuclear translocation of RelA/p65 (Fig S1C) and RelA/p65-DNA binding activity (Fig 1B). Interestingly, PTL alone modestly increased IKK phosphorylation in certain cell lines (e.g., U937, Fig 1A), possibly reflecting a compensatory response to NF-κB inhibition. Importantly, co-administration of PTL (3–7.5 μM) diminished HDACI-induced IKK and RelA/p65 phosphorylation (Fig 1A and Fig S1B), as well as nuclear translocation (Fig S1C) and RelA/p65-DNA binding (Fig 1B) in diverse AML cell types. In these experiments, treatment with TNFα, a classical activator of the canonical NF-κB pathway, was used as a positive control. These results were further confirmed by NF-κB luciferase assays using U937 cells stably transfected with an NF-κB luciferase reporter gene (Fig S1D, P < 0.05 and P < 0.01 for 5 μM and 7.5μM of PTL, respectively). No major changes were observed in total IKK or RelA protein levels. In contrast, HDACIs did not promote processing of p100 into p52 (Fig S1E), a marker reflecting activation of the non-canonical NF-κB pathway (Yao et al, 2009). However, HDACIs, similar to TNFα (Yao et al, 2009), increased expression of p100 in AML cells, an event diminished by PTL co-administration (Fig S1E). Because p100 is an NF-κB downstream target (Lu et al, 2009), these phenomena may reflect changes in NF-κB activity induced by HDACIs alone or with PTL. Collectively, these findings indicate that PTL effectively blocks HDACI-mediated activation of NF-κB via the RelA/p65-mediated canonical NF-κB pathway in various continuously cultured AML cell lines as well as primary human AML blasts, independently of their basal NF-κB activity.
Figure 1. PTL opposes HDACI-mediated NF-κB activation in various human AML cell types.
(A) Various AML cells were exposed (24 h) to PTL (U937 and HL-60, 5 μM and 7.5 μM; MV-4-11, 5 μM; primary AML, 4 μM; MLL-ENL, 3μM) ± vorinostat (V, U937 and primary AML/Patient 1, 1.5 μM; HL-60, 1 μM; MV-4-11 and MLL-ENL, 0.5μM) or LBH589 (L, U937, 15 nM; primary AML/Patient 1, 10nM; HL-60, 5 nM; MLL-ENL, 4 nM; MV-4-11, 3 nM). In parallel, cells were treated with 10 ng/ml TNFα for 30 min as positive controls. Western blot analysis was performed to monitor expression of total and phosphorylated/activated IKKα/IKKβ (Ser180/Ser181) and RelA/p65 (Ser536). (B) AML cells were treated (16 h) as described in panel 1A, nuclear extracts were prepared and subjected to a RelA/p65-specific NF-κB-DNA binding assay (* P < 0.05 and ** P < 0.01 versus vorinostat or LBH589 alone). RLU = relative light unit.
PTL synergistically potentiates HDACI-induced apoptosis in various human AML cell lines
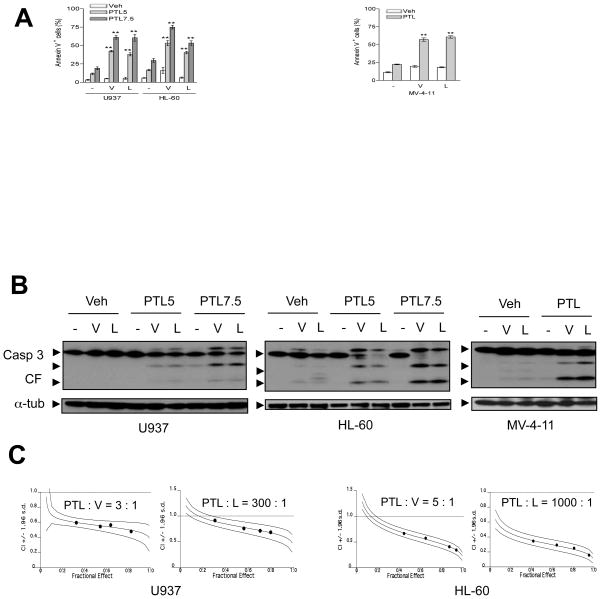
The biological consequences of PTL-mediated interruption of NF-κB activation in human AML cells exposed to HDACIs were then examined. AML cell lines were exposed (24 h) to various concentrations of vorinostat (0.5–3 μM) or LBH589 (5–30 nM for U937, HL-60, and NB4; 2.5–15 nM for MV-4-11 and MLL-ENL) in the presence or absence of PTL (3–7.5 μM). As shown in Fig S2A, cells exhibited diverse sensitivities to HDACIs. In general, cells bearing fusion (e.g., MLL-ENL) or mutant (e.g., MV-4-11 and MOLM-13, FLT3-ITD) proteins were more sensitive than cells without these genetic backgrounds (e.g., U937 and NB4, but not HL-60). Nevertheless, co-administration of PTL significantly potentiated HDACI lethality over a broad concentration range, particularly at subtoxic doses (Fig 2A; P < 0.001 versus HDACI alone, and Fig S2A and S2B). Furthermore, co-administration of PTL with either HDACI increased mitochondrial injury (manifested by loss of mitochondrial membrane potential/Δψm; data not shown), as well as cytochrome c and AIF release (Fig S2C), and caspase-3 activation (Fig 2B and Fig S2D). Median dose-effect analysis of apoptosis induction performed in multiple AML cell lines, in which PTL/HDACIs were administered at fixed concentration ratios, yielded Combination Index (C.I.) values less than 1.0, reflecting synergistic interactions (Fig 2C and Fig S2E). Together, these findings indicate that PTL-mediated inhibition of HDACI-induced NF-κB activation is accompanied by an increase in mitochondrial injury, caspase activation, and apoptosis in various human AML cell lines, including those harbouring FLT3-ITD.
Figure 2. PTL interacts synergistically with HDACIs to induce apoptosis in multiple human AML cell lines.
(A) AML cell lines (U937, HL-60, and MV-4-11) were exposed (24 h) to PTL ± vorinostat or LBH589 as described in Fig 1A, after which the percentage of apoptotic (Annexin V+) cells was determined by flow cytometry (** P < 0.001 versus each agent alone). (B) Alternatively, Western blot analysis was performed to monitor caspases-3 cleavage/activation. CF indicates cleavage fragments. (C) Median Dose Effect analyses was performed to determine status of interactions between PTL (U937 and HL-60, 5–8 μM) and vorinostat (V, U937, 1.67–2.67 μM; HL-60, 1–1.6 μM) or LBH589 (L, U937, 16.67–26.67 nM; HL-60, 5–8 nM) administered (24 h) at the fixed concentration ratios as indicated. Combination indices (C.I.) were calculated for each Fractional Effect; C.I. values < 1.0 correspond to synergism.
PTL enhances HDACI lethality in primary AML blasts
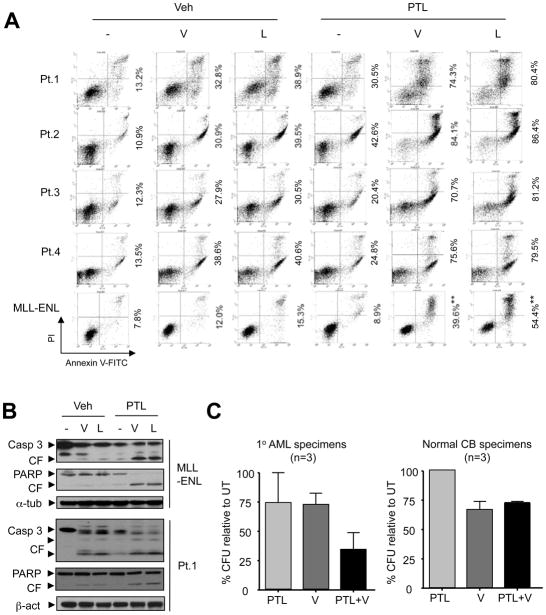
To determine whether these findings could be extended to primary AML samples, parallel studies were performed using primary AML specimens obtained from unselected patients with AML (French-American-British classification M2 phenotype). All samples consisted of > 70% blasts, as shown in a representative Giemsa-Wright stained cytospin specimen (Fig S3A). Primary AML samples were treated ex vivo with 1.5 μM vorinostat or 10 nM LBH589 ± 4 μM PTL for 24 h. Although primary samples obtained from eight AML patients displayed differential sensitivity to HDACI or PTL alone, in each case, combined treatment resulted in a clear increase in lethality compared to the effects of agents administered individually, determined by annexin V/PI (Patients 1–4, Fig S3A), 7AAD (Patients 5–8, Supplemental Fig S4A) and/or DiOC6 (Patients 5–6, Supplemental Fig S4B) staining and flow cytometric analysis. Results were confirmed by Western blot analysis to monitor increased caspase-3 activation and PARP cleavage in primary AML samples co-exposed to HDACIs and PTL (Fig 3B). In addition, Giemsa-Wright staining exhibited classical morphology of apoptosis in AML blasts following co-treatment with PTL and HDACIs (Fig 3A). Immunohistochemical staining of primary AML samples demonstrated that co-exposure to PTL and HDACIs increased the number of cells expressing activated caspase-3 (Fig S3B). Interestingly, parallel treatment of non-malignant bone marrow mononuclear cells with identical concentrations of these agents alone or in combination produced minimal toxicity (Fig S4A and S4B). Notably, 24-h exposure of three bone marrow blast specimens (Patients 9–11) to PTL (2.0 μM) or vorinostat (1.0 μM) had modest effects (~25% reduction relative to untreated controls) on the colony-forming capacity (L-CFU) of AML samples, whereas combined treatment resulted in a large decline in L-CFU (Fig 3C, ~75% reduction). In contrast, co-administration of PTL did not result in a further reduction in GM-CFU from normal cord blood (CB) samples exposed to vorinostat (Fig 3C). These findings indicate that interactions between PTL and HDACIs (e.g., vorinostat and LBH589) occur in primary AML blasts and raise the possibility that, as in the case of PTL alone (Guzman et al, 2005), normal haematopoietic cells may be less susceptible to this strategy than their leukaemic counterparts.
Figure 3. PTL potentiates HDACI lethality in primary AML blasts and MLL-ENL leukaemia cells.
(A) Primary AML samples from 4 patients with AML were exposed to vorinostat (V, 1.5 μM) or LBH589 (L, 10 nM) in the presence or absence of 4 μM PTL for 24 h. MLL-ENL were incubated (24 h) with 0.5 μM vorinostat (V) or 4 nM LBH589 (L) ± 3 μM PTL. After treatment, apoptosis was monitored by flow cytometry. Cells in the right quadrants indicate early (lower quadrants, annexin V+/PI−) and late (upper quadrants, annexin V+/PI+) apoptotic cells respectively, whereas cells in the upper left quadrants (annexin V−/PI+) reflect necrotic cells. Values indicate the percentage of annexin V+ cells (** P < 0.01 versus vorinostat or LBH589 alone). (B) Primary AML samples (Patient 1) and MLL-ENL cells were treated as described in Fig 3A, after which Western blot analysis was performed to monitor caspase-3 cleavage/activation and PARP degradation. CF indicates cleavage fragments. (C) AML blasts from bone marrow samples of 3 patients with AML (Patients 9–11) were exposed (24 h) to 1 μM vorinostat (V) ± 2 μM PTL, after which a methylcellulose colony-forming assay was performed to determine leukaemic colony formation (L-CFU) of AML samples. Parallel studies were performed using 3 normal cord blood (CB) mononuclear cell samples to monitor normal GM-CFU.
PTL interacts with HDACIs to induce apoptosis in MLL-ENL cells displaying leukaemia-initiating cell (L-IC) characteristics
Given evidence that PTL targets human LSCs (Guzman et al, 2005), interactions between PTL and HDACIs were then examined in MLL-ENL cells, generated by retrovirally transducing a lineage-depleted fraction of human umbilical CB enriched for stem/progenitor cells (Lin–CB) with the MLL-MLLT1 (previously MLL-ENL) fusion gene (the product of t[11;19]), and which have recently been shown to display L-IC characteristics in immunodeficient mice (Barabe et al, 2007). Co-exposure of MLL-ENL cells to various concentrations of either vorinostat (e.g., 0.5–3 μM) or LBH589 (e.g., 2.5–15 nM) in conjunction with PTL (3 μM) resulted in a significant increase in annexin V+ apoptotic cells (Fig 3A and Fig S2A). Western blot analysis revealed that co-treatment of MLL-ENL cells with PTL and HDACIs increased caspase-3 activation and PARP degradation (Fig 3B). Together, these results indicate that regimens that combined PTL with HDACIs effectively killed MLL-ENL cells, which exhibit certain L-IC features.
Co-treatment with PTL and HDACI induces activation of SAPK/JNK
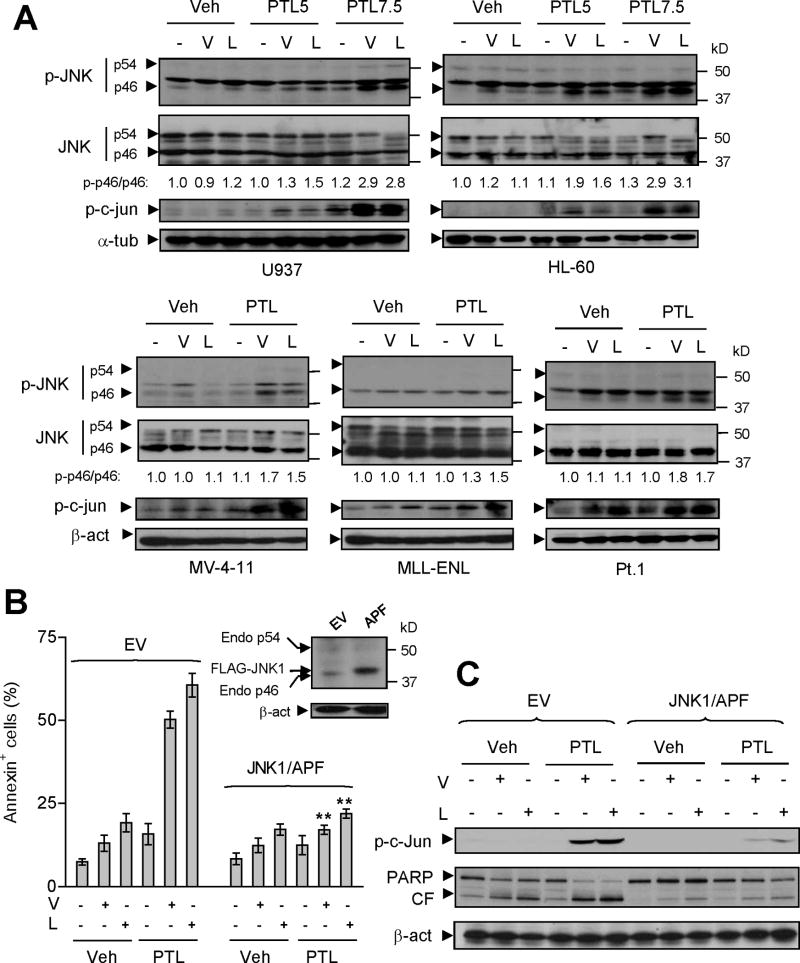
In view of evidence linking lethal actions of NF-κB inhibition to activation of the stress-related JNK pathway (Tang et al, 2001;De Smaele et al, 2001), JNK signaling was examined in various human AML cells exposed to PTL ± vorinostat or LBH589. Whereas PTL or either of the HDACIs administered individually had modest effects, co-administration of PTL with HDACIs resulted in increased phosphorylation of JNK (specifically p46 JNK1 rather than p54 JNK2/3) or its substrate c-Jun in AML cell lines (U937, HL-60, and NB4), as well as those bearing FLT3-ITD (MV-4-11 and MOLM-13; Fig 4A and Fig S5A). Notably, similar results were also observed in MLL-ENL cells and primary AML specimens (Fig 4A and Fig S5A). These findings raise the possibility that, in association with interference with NF-κB activation, PTL/HDACI-mediated lethality toward human AML cells may involve activation of the SAPK/JNK signaling pathway.
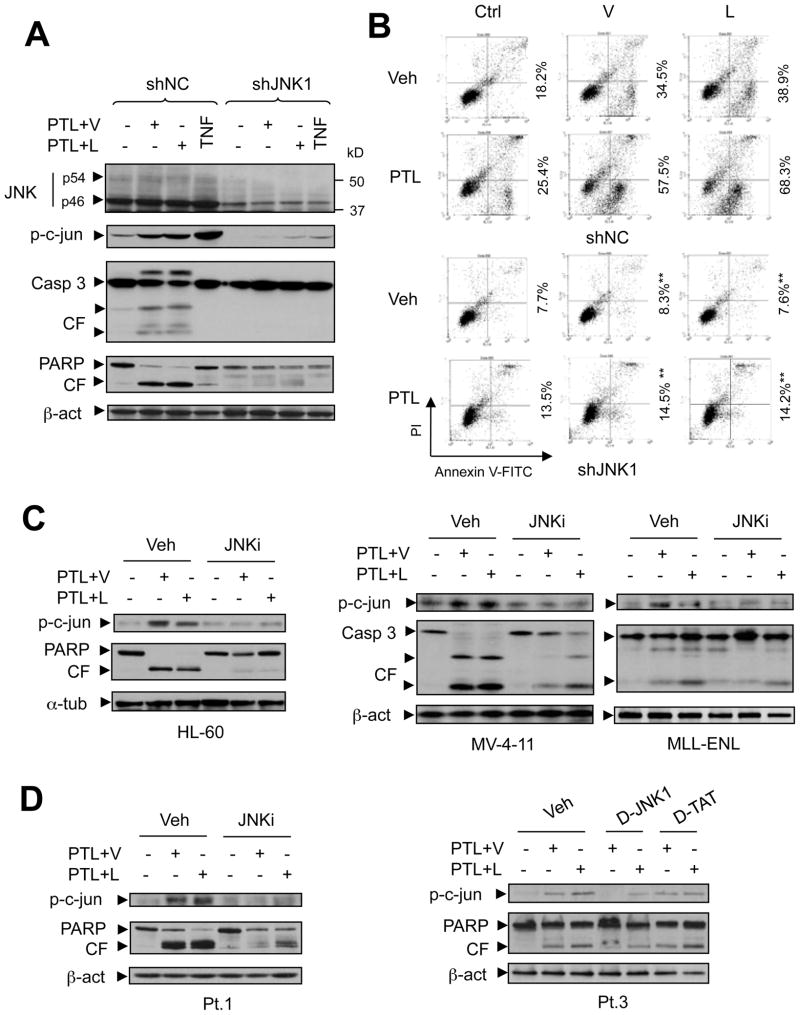
Figure 4. Attenuation of PTL/HDACI-mediated JNK activation by dominant-negative JNK1 diminishes the lethality of this regimen.
(A) Various AML cell types were exposed (24 h) to PTL ± vorinostat or LBH589 as described in Fig 1A. After drug treatment, Western blot analysis was performed to monitor phosphorylation/activation of JNK (Thr183/Tyr185, JNK1, p46; JNK2/3, p54) and its substrate c-Jun (Ser73). The density of blots for both total (p46) and phosphorylated JNK1 (p-p46) was quantified by determining integrated density. Values reflect the ratio (p-p46 versus total p46) of changes between untreated and drug-treated cells respectively. (B) Western blot analysis demonstrating ectopic expression of FLAG-JNK1/APF in U937 cells stably transfected with dominant-negative JNK1 (JNK1/APF, inset). EV = empty vector. Cells were exposed to vorinostat (V, 1.5 μM) or LBH589 (L, 20 nM) ± PTL (7.5μM), after which apoptosis was monitored by annexin V/PI staining using flow cytometry (** P < 0.01 compared with same treatment in EV cells). (C) Alternatively, c-Jun phosphorylation (Ser73) and PARP degradation were monitored by Western blot analysis.
SAPK/JNK activation plays a functional role in PTL/HDACI-mediated lethality
To investigate the functional significance of SAPK/JNK activation in potentiation of HDACI lethality by PTL, U937 cells were stably transfected with a dominant-negative JNK1 (JNK1/APF) construct (Yamamoto et al, 1999) (Fig 4B, inset). Ectopic expression of JNK1/APF blocked JNK signaling in response to the classical JNK activators TNFα and anisomycin, manifested by diminished c-Jun phosphorylation (Fig S5B). In accord with these findings, co-exposure to PTL and vorinostat or LBH589 failed to activate JNK signaling in JNK1/APF cells (Fig 4C). Notably, stable transfection with JNK1/APF significantly abrogated the lethality of PTL/vorinostat or/LBH589 regimens (P < 0.01 vs empty vector (EV) controls; Fig 4B), a phenomenon confirmed by diminished PARP cleavage in JNK1/APF cells following combination treatments (Fig 4C). To validate results obtained with the dominant-negative JNK1 approach, JNK1 (p46) was knocked down in U937 cells by stable transfection with a construct encoding JNK1 shRNA (shJNK1). As shown in Fig 5A, down-regulation of JNK1 by shRNA prevented c-Jun phosphorylation induced by either TNFα or co-treatment with PTL and vorinostat or LBH589, accompanied by diminished caspase-3 activation and PARP cleavage (Fig 5A), as well as apoptosis (Fig 5B and Fig S5C, P < 0.01). Consistent with these findings using genetic approaches, similar phenomena were also observed in other AML cell lines when a pharmacological JNK inhibitor (JNKi) (Vivanco et al, 2007) was employed. As shown in Fig 5C (HL-60, MV-4-11 and MLL-ENL) and Fig S5D (NB4 and MOLM-13), administration of JNKi clearly diminished c-Jun phosphorylation and lethality (i.e., cleavage of caspase-3 or PARP). The latter was also manifested by annexin V staining and flow cytometric analysis (Fig S6A, P < 0.05). Notably, blockade of JNK activation, reflected by diminished c-Jun phosphorylation, by either JNKi or a specific JNK peptide inhibitor D-JNK1, but not a negative control peptide D-TAT, prevented PARP cleavage (Fig 5D) and lethality (annexin V+; Fig S6A and S6B, P < 0.05) of the PTL/vorinostat or/LBH589 regimens in primary AML specimens. Together, these findings argue that activation of SAPK/JNK signaling plays a significant functional role in PTL/HDACI lethality in various human AML cell types.
Figure 5. JNK1 shRNA and pharmacological JNK inhibitors interrupt JNK signaling and attenuate apoptosis in human AML cells.
(A) U937 cells were stably transfected with a construct encoding JNK1 shRNA or negative control shRNA (shNC). Cells were then co-exposed (24 h) to vorinostat (V, 1.5 μM) or LBH589 (L, 15 nM) with PTL (7.5 μM), after which Western blot analysis was performed to examine expression of JNK (JNK1, p46; JNK2/3, p54) and phospho-c-Jun (Ser73), as well as caspase-3 cleavage/activation and PARP degradation. In parallel, cells were incubated with 10 ng/ml TNFα for 30 min as control. (B) Alternatively, flow cytometry was performed to monitor apoptosis (Annexin V+, ** P < 0.01 versus the same treatment in shNC cells). Roughly identical results were obtained in another clone of JNK1 shRNA cells (Fig S5C). (C) Various AML cell types as indicated were co-exposed (24 h) to vorinostat (V, HL-60, 1 μM; MV-4-11 and MLL-ENL, 0.5 μM;) or LBH589 (L, HL-60, 5 nM; MV-4-11, 3 nM; MLL-ENL, 4 nM) with PTL (HL-60, 7.5 μM; MV-4-11, 5 μM; MLL-ENL, 3 μM) in the absence or presence of a specific JNK inhibitor (JNKi, 5 μM). (D) Primary AML samples were incubated with 1.5 μM vorinostat or 10 nM LBH-589 + 4 μM PTL in the absence or presence of JNKi (5 umol/l), or in the presence of either a specific JNK peptide inhibitor (D-JNK1, 1 μM) or a negative control peptide (D-TAT, 1 μM). After drug treatment, Western blot analysis was performed to monitor c-Jun phosphorylation (Ser73), caspase-3 cleavage/activation, and/or PARP degradation. CF indicates cleavage fragments.
MKK7 but not SEK1 is required for PTL/HDACI-induced SAPK/JNK activation and apoptosis
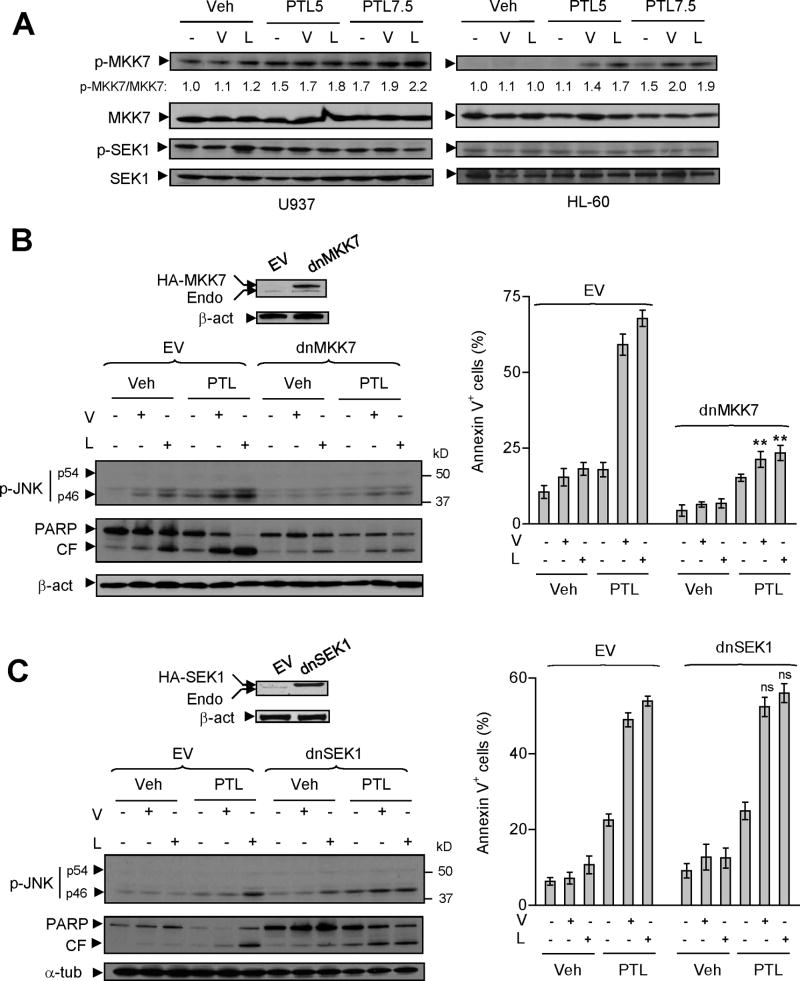
The roles of MKK7 and MKK4/SEK1, the major upstream JNK kinases, in SAPK/JNK activation and lethality mediated by PTL/HDACI regimens were then examined. Co-exposure to PTL and vorinostat or LBH589 resulted in a modest but discernible increase in MKK7 phosphorylation in various AML cells e.g., U937, HL-60, NB4, and MV-4-11, as well as MLL-ENL cells and primary AML samples (Fig 6A and Fig S6C), compared to treatment with individual agents. To examine the functional role of MKK7, U937 cells were stably transfected with dominant-negative MKK7 (dnMKK7; Fig 6B). Consistent with previous findings (Tournier et al, 2001), ectopic expression of dnMKK7 blocked phosphorylation of JNK1 (p46) and c-Jun in response to TNFα, and to a lesser extent, anisomycin (Fig S5B). These events were associated with abrogation of TNFα-induced apoptosis in dnMKK7 cells, and partial but significant protection of these cells from anisomycin lethality (data not shown). Notably, as in the case of JNK1/APF and JNK1 shRNA, stable transfection with dnMKK7 also diminished PTL/vorinostat or/LBH589-mediated PARP cleavage and lethality (annexin V+, P < 0.01 vs EV controls), associated with attenuation of JNK1 (p46) phosphorylation in response to combined treatments (Fig 6B).
Figure 6. Dominant-negative MKK7 rather than SEK1 diminishes PTL/HDACI-mediated JNK activation and lethality in human leukaemia cells.
(A) U937 and HL-60 were treated (24 h) as described in Fig 1A, after which Western blot analysis was performed to monitor expression of total and phosphorylated/activated MKK7 (Ser271/Thr275) and SEK1 (Ser257/Thr261). The density of blots for both total and phosphorylated MKK7 was quantified by determining integrated density and values reflect the ratio (phospho-MKK7 versus total MKK7) of changes between untreated and drug-treated cells respectively. (B–C) U937 cells were stably transfected with either dominant-negative MKK7 (dnMKK7) or SEK1 (dnSEK1), as well as their empty-vectors (EV), and Western blot analysis performed to demonstrate ectopic expression of HA- tagged dnMKK7 or dnSEK1 respectively. Cells were then treated (24 h) with vorinostat (1.5 μM) or LBH589 (20 nM) in the presence or absence of PTL (7.5 μM), after which JNK phosphorylation and PARP degradation were monitored by Western blot analysis. CF indicates cleavage fragments. Alternatively, flow cytometry was performed to monitor annexin V+ apoptotic cells (** P < 0.01 and ns = no significant difference, compared with same treatment in EV cells).
Parallel studies were then performed to define the role of SEK1 in SAPK/JNK activation and lethality mediated by PTL/HDACI regimens. As shown in Fig 6A, co-exposure to PTL and vorinostat or LBH589 did not increase SEK1 phosphorylation in AML cells (e.g., U937 and HL-60). As reported previously (Tournier et al, 2001), ectopic expression of dnSEK1 (Fig 6C) partially attenuated anisomycin-induced phosphorylation of JNK or c-Jun, but had no effect on TNFα responses (Supplemental Fig S5B). Consistent with these findings, dnSEK1 partially but significantly protected cells from anisomycin-mediated lethality, but failed to do so in cells treated with TNFα (data not shown). In contrast to results obtained in dnMKK7 cells, stable transfection with dnSEK1 did not block JNK1 (p46) phosphorylation triggered by PTL/vorinostat or/LBH589 (Fig 6C). Furthermore, dnSEK1 also failed to prevent PARP degradation and apoptosis (annexin V+, P > 0.05 vs EV controls) induced by PTL/HDACI regimens (Fig 6C). Collectively, these findings indicate that in human AML cells, PTL inhibits HDACI-induced NF-κB activation, and promotes SAPK/JNK activation as well as apoptosis via an MKK7- rather than SEK1-dependent mechanism.
Discussion
The dependence of malignant haematopoietic cells on NF-κB for survival provides a rationale for developing agents that interrupt this pathway. The natural NF- κB inhibitor PTL and its analogue LC-1 have been shown to be active, either singly or in combination with conventional agents, against malignant human haematopoietic cells including AML (Guzman et al, 2001;Jenkins et al, 2008), chronic lymphocytic leukaemia (Hewamana et al, 2008a;Hewamana et al, 2008b), pre-B leukaemia (Zunino et al, 2007), and myeloma (Suvannasankha et al, 2008). However, while it is uncertain whether monotherapy involving NF-κB antagonists will be effective in cancer treatment, employing such agents to enhance the anticancer activity of conventional or novel agents represents a rational strategy. In light of preclinical findings documenting HDACI-mediated anti-leukaemic activity (Vrana et al, 1999), as well as emerging evidence of a role for such agents in the treatment of AML (Garcia-Manero et al, 2008a), the finding that PTL potentiates HDACI (e.g., vorinostat and LBH589/panobinostat) lethality in various types of human AML cells including those harbouring FLT3-ITD mutations as well as primary blasts, takes on added significance.
PTL and its analog LC-1 selectively target leukaemia progenitor cells, including those exhibiting LSC characteristics, while exerting relatively modest toxicity toward their normal haematopoietic counterparts (Guzman et al, 2005;Guzman et al, 2007). Notably, combined PTL/vorinostat treatment, as observed in the case of PTL alone, also inhibited colony-forming capacity (L-CFU) of AML specimens to a greater extent than normal myeloid progenitors (GM-CFU). It is possible that the greater sensitivity of transformed versus normal cells to HDACIs (Ungerstedt et al, 2005) may also contribute to this differential effect. Significantly, PTL/HDACI regimens were effective in killing MLL-ENL cells, which display certain L-IC features, e.g., generation of leukaemias of both myeloid and lymphoid lineages in vivo (Barabe et al, 2007). Interestingly, interactions in MLL-ENL cells as well as AML cell lines bearing FLT3-ITD required relatively low concentrations of vorinostat (0.5μM) or LBH589 (3–5nM). This phenomenon may stem from the increased sensitivity of leukaemia cells bearing fusion or mutant oncoproteins to HDACIs, as previously observed in the case of cells expressing Bcr/Abl (Nimmanapalli et al, 2003) or FLT3 mutants (George et al, 2005), reflecting dependence upon chaperone protein function for maintenance and stability of these proteins (Yang et al, 2007).
Interactions between PTL and HDACIs were closely associated with interruption of IKK/RelA-mediated NF-κB activation. Previous studies have demonstrated that HDACIs activate NF-κB in various cell types, including leukaemia cells (Chen et al, 2001;Liu et al, 2006;Dai et al, 2005a). Significantly, interference with NF-κB activation by genetic means e.g., transfection of cells with an IκBα “super-repressor” or RelA siRNA lowers the threshold for HDACI-induced apoptosis (Dai et al, 2005a;Duan et al, 2007). Thus, NF-κB activation by HDACIs may serve as a cytoprotective mechanism limiting the lethality of these agents. Consistent with these findings, PTL inhibited HDACI-mediated phosphorylation of both IKK and RelA (e.g., on serine 536), an event that facilitates RelA acetylation and transcriptional activity (Chen et al, 2005), and attenuated NF-κB activation. Such findings support the notion that PTL potentiates HDACI lethality across a broad spectrum of human AML cell types, at least in part, by blocking a putatively cytoprotective NF-κB activation response to HDACIs. However, PTL lethality may stem from multiple effects (e.g., ROS generation/ER stress (Liu et al, 2009a), depletion of HDAC1 (Gopal et al, 2007), or inhibition of DNA methylation by alkylating DNMT1 (Liu et al, 2009b), among others). Consequently, the possibility that additional mechanisms may contribute to PTL/HDACI interactions cannot be excluded. Finally, although different human AML cell types exhibited varied basal (unstimulated) NF-κB activity, which may reflect disparate dependencies on this pathway for their survival, the present findings suggest that blockade of HDACI-stimulated (rather than basal) NF-κB activation by PTL triggers apoptosis across a broad spectrum of AML cell types, most likely independently of their basal NF-κB activation status.
The present findings indicate that activation of SAPK/JNK plays a functional role in PTL-mediated potentiation of HDACI lethality in human AML cells including those bearing FLT3-ITD, MLL-ENL cells and primary blasts. Previous studies have implicated JNK activation in PTL-mediated potentiation of TRAIL or TNFα lethality (Nakshatri et al, 2004;Zhang et al, 2004). PTL/HDACI-induced JNK activation appears to be closely associated with NF-κB inactivation. In this context, complex cross-talk exists between NF-κB inactivation and JNK activation through several possible mechanisms (Tang et al, 2001;Kamata et al, 2005). Notably, TNFα-induced NF-κB activation leads to upregulation of GADD45β and c-FLIPL, which directly bind to and inhibit MKK7 (Papa et al, 2004;Nakajima et al, 2006). Such findings suggest that NF-κB inactivation-mediated JNK activation may stem from kinases upstream of JNK. The kinases responsible for JNK activation appear to be stimulus-dependent (Wang et al, 2007). The major kinases upstream of JNK identified to date are SEK1 (MKK4), which is primarily activated in response to environmental stresses, and MKK7, which is required for both stress- and cytokine-related JNK signaling (Tournier et al, 2001). It has been demonstrated that SEK1 and MKK7 exhibit distinct biochemical properties in vitro and serve nonredundant functions in vivo (Weston & Davis, 2002), while cooperation between SEK1 and MKK7 is required for full JNK activation in some circumstances (Tournier et al, 2001). In the present setting, co-exposure to PTL and vorinostat or LBH589 increased phosphorylation of MKK7 rather than SEK1, and transfection of AML cells with dnMKK7, but not dnSEK1, abrogated PTL/HDACI-induced JNK activation and apoptosis. These findings argue that MKK7 is the kinase most likely responsible for PTL/HDACI-mediated JNK activation, analogous to the case of TNFα (Tournier et al, 2001). In the latter model, sustained JNK activation and lethality represents an important consequence of inhibition of TNFα-induced NF-κB activation. Analogously, the present observations support the view that PTL potentiates HDACI lethality by promoting JNK activation through an MKK7-dependent but SEK1- independent process, in all likelihood resulting from interruption of NF-κB activation.
In summary, the present findings indicate that the NF-κB inhibitor PTL markedly increases the antileukaemic activity of the clinically relevant pan-HDACIs vorinostat and LBH589 through the MKK7-dependent induction of SAPK/JNK activation and apoptosis. Moreover, such interactions occur in AML cells with or without FLT3-ITD mutations, suggesting that this strategy may act independently of or bypass signaling pathways mediated by the FLT3 receptor. Furthermore, PTL/HDACI regimens are active against primary AML specimens as well as MLL-ENL cells displaying L-IC features, and may also target leukaemic versus normal progenitors. In view of emerging interest in HDACIs (Garcia-Manero et al, 2008a, b) as well as parthenolide analogues (e.g., LC-1) (Guzman et al, 2007;Jenkins et al, 2008) in leukaemia therapy, the finding that such agents interact synergistically and in a mechanistic-based manner in human AML cells provides an attractive rationale for such a combination strategy. The ultimate clinical utility of this approach will depend upon several factors, particularly the availability of a clinically relevant PTL analog. In this regard, the recent development of LC-1, a bioavailable analog of PTL (Hewamana et al, 2008a;Guzman et al, 2007), which has recently entered Phase I testing in humans, should help to address these issues. Demonstration of similar interactions between HDACIs (e.g., vorinostat or LBH589/panobinostat) and the clinically relevant form of parthenolide (LC-1) will be important for the future translation of these findings into the clinic. Finally, the preceding findings also raise the possibility that monitoring JNK activation in leukaemic cells may represent a rational response determinant in this setting.
Supplementary Material
Acknowledgments
This work was supported by awards CA63753, CA 93738, and CA100866, and 1 P50 CA130805-01 from the National Cancer Institute, award 6045-03 from the Leukemia and Lymphoma Society of America, and award from the V Foundation. We gratefully acknowledge Drs. John Dick and Jean Wang for providing us with MLL-ENL cells.
Footnotes
Contributions: Y.D. planned and performed experiments, and wrote the manuscript. M.G., S.C., L.W., S-K.Y, and X-Y.P. performed experiments. P.D. helped plan experiments. C.T.J. helped plan experiments and wrote the manuscript. S.G. planned experiments and wrote the manuscript.
Conflict-of-interest disclosure: There is no potential conflict of interest to disclose.
References
- Barabe F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316:600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- Bueso-Ramos CE, Rocha FC, Shishodia S, Medeiros LJ, Kantarjian HM, Vadhan-Raj S, Estrov Z, Smith TL, Nguyen MH, Aggarwal BB. Expression of constitutively active nuclear-kappa B RelA transcription factor in blasts of acute myeloid leukemia. Hum Pathol. 2004;35:246–253. doi: 10.1016/j.humpath.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilloni D, Messa F, Rosso V, Arruga F, Defilippi I, Carturan S, Catalano R, Pautasso M, Panuzzo C, Nicoli P, Messa E, Morotti A, Iacobucci I, Martinelli G, Bracco E, Saglio G. Increase sensitivity to chemotherapeutical agents and cytoplasmatic interaction between NPM leukemic mutant and NF-kappaB in AML carrying NPM1 mutations. Leukemia. 2008;22:1234–1240. doi: 10.1038/leu.2008.68. [DOI] [PubMed] [Google Scholar]
- Dai Y, Rahmani M, Dent P, Grant S. Blockade of Histone Deacetylase Inhibitor-Induced RelA/p65 Acetylation and NF-{kappa}B Activation Potentiates Apoptosis in Leukemia Cells through a Process Mediated by Oxidative Damage, XIAP Downregulation, and c-Jun N-Terminal Kinase 1 Activation. Mol Cell Biol. 2005a;25:5429–5444. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Rahmani M, Pei XY, Khanna P, Han SI, Mitchell C, Dent P, Grant S. Farnesyltransferase inhibitors interact synergistically with the Chk1 inhibitor UCN-01 to induce apoptosis in human leukemia cells through interruption of both Akt and MEK/ERK pathways and activation of SEK1/JNK. Blood. 2005b;105:1706–1716. doi: 10.1182/blood-2004-07-2767. [DOI] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Duan J, Friedman J, Nottingham L, Chen Z, Ara G, Van Waes C. Nuclear factor-kappaB p65 small interfering RNA or proteasome inhibitor bortezomib sensitizes head and neck squamous cell carcinomas to classic histone deacetylase inhibitors and novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2007;6:37–50. doi: 10.1158/1535-7163.MCT-05-0285. [DOI] [PubMed] [Google Scholar]
- Frelin C, Imbert V, Griessinger E, Peyron AC, Rochet N, Philip P, Dageville C, Sirvent A, Hummelsberger M, Berard E, Dreano M, Sirvent N, Peyron JF. Targeting NF-kappaB activation via pharmacologic inhibition of IKK2-induced apoptosis of human acute myeloid leukemia cells. Blood. 2005;105:804–811. doi: 10.1182/blood-2004-04-1463. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, Faderl S, Koller C, Morris G, Rosner G, Loboda A, Fantin VR, Randolph SS, Hardwick JS, Reilly JF, Chen C, Ricker JL, Secrist JP, Richon VM, Frankel SR, Kantarjian HM. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008a;111:1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Assouline S, Cortes J, Estrov Z, Kantarjian H, Yang H, Newsome WM, Miller WH, Jr, Rousseau C, Kalita A, Bonfils C, Dubay M, Patterson TA, Li Z, Besterman JM, Reid G, Laille E, Martell RE, Minden M. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008b;112:981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pineres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, Merfort I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- George P, Bali P, Annavarapu S, Scuto A, Fiskus W, Guo F, Sigua C, Sondarva G, Moscinski L, Atadja P, Bhalla K. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood. 2005;105:1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, Masson E, Rae P, Laird G, Sharma S, Kantarjian H, Dugan M, Albitar M, Bhalla K. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- Gopal YN, Arora TS, Van Dyke MW. Parthenolide specifically depletes histone deacetylase 1 protein and induces cell death through ataxia telangiectasia mutated. Chem Biol. 2007;14:813–823. doi: 10.1016/j.chembiol.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Grant S, Easley C, Kirkpatrick P. Vorinostat. Nature Review Drug Discovery. 2007;6:21–22. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- Grosjean-Raillard J, Ades L, Boehrer S, Tailler M, Fabre C, Braun T, De Botton S, Israel A, Fenaux P, Kroemer G. Flt3 receptor inhibition reduces constitutive NFkappaB activation in high-risk myelodysplastic syndrome and acute myeloid leukemia. Apoptosis. 2008;13:1148–1161. doi: 10.1007/s10495-008-0243-4. [DOI] [PubMed] [Google Scholar]
- Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, Jordan CT. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci USA. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Becker MW, Bennett JM, Sullivan E, Lachowicz JL, Vaughan A, Sweeney CJ, Matthews W, Carroll M, Liesveld JL, Crooks PA, Jordan CT. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehner SP, Hofmann TG, Droge W, Schmitz ML. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol. 1999;163:5617–5623. [PubMed] [Google Scholar]
- Hewamana S, Alghazal S, Lin TT, Clement M, Jenkins C, Guzman ML, Jordan CT, Neelakantan S, Crooks PA, Burnett AK, Pratt G, Fegan C, Rowntree C, Brennan P, Pepper C. The NF-kappaB subunit Rel A is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukemia and represents a promising therapeutic target. Blood. 2008a;111:4681–4689. doi: 10.1182/blood-2007-11-125278. [DOI] [PubMed] [Google Scholar]
- Hewamana S, Lin TT, Jenkins C, Burnett AK, Jordan CT, Fegan C, Brennan P, Rowntree C, Pepper C. The novel nuclear factor-kappaB inhibitor LC-1 is equipotent in poor prognostic subsets of chronic lymphocytic leukemia and shows strong synergy with fludarabine. Clin Cancer Res. 2008b;14:8102–8111. doi: 10.1158/1078-0432.CCR-08-1673. [DOI] [PubMed] [Google Scholar]
- Jenkins C, Hewamana S, Gilkes A, Neelakantan S, Crooks P, Mills K, Pepper C, Burnett A. Nuclear factor-kappaB as a potential therapeutic target for the novel cytotoxic agent LC-1 in acute myeloid leukaemia. Br J Haematol. 2008;143:661–671. doi: 10.1111/j.1365-2141.2008.07392.x. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Liu Y, Adachi M, Zhao S, Hareyama M, Koong AC, Luo D, Rando TA, Imai K, Shinomura Y. Preventing oxidative stress: a new role for XBP1. Cell Death Differ. 2009a;16:847–857. doi: 10.1038/cdd.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Denlinger CE, Rundall BK, Smith PW, Jones DR. Suberoylanilide hydroxamic acid induces Akt-mediated phosphorylation of p300, which promotes acetylation and transcriptional activation of RelA/p65. J Biol Chem. 2006;281:31359–31368. doi: 10.1074/jbc.M604478200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Liu S, Xie Z, Pavlovicz RE, Wu J, Chen P, Aimiuwu J, Pang J, Bhasin D, Neviani P, Fuchs JR, Plass C, Li PK, Li C, Huang TH, Wu LC, Rush L, Wang H, Perrotti D, Marcucci G, Chan KK. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. J Pharmacol Exp Ther. 2009b;329:505–514. doi: 10.1124/jpet.108.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZG, Liu H, Yamaguchi T, Miki Y, Yoshida K. Protein kinase Cdelta activates RelA/p65 and nuclear factor-kappaB signaling in response to tumor necrosis factor-alpha. Cancer Res. 2009;69:5927–5935. doi: 10.1158/0008-5472.CAN-08-4786. [DOI] [PubMed] [Google Scholar]
- Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Komazawa-Sakon S, Takekawa M, Sasazuki T, Yeh WC, Yagita H, Okumura K, Nakano H. An antiapoptotic protein, c-FLIPL, directly binds to MKK7 and inhibits the JNK pathway. EMBO J. 2006;25:5549–5559. doi: 10.1038/sj.emboj.7601423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004;23:7330–7344. doi: 10.1038/sj.onc.1207995. [DOI] [PubMed] [Google Scholar]
- Nimmanapalli R, Fuino L, Bali P, Gasparetto M, Glozak M, Tao J, Moscinski L, Smith C, Wu J, Jove R, Atadja P, Bhalla K. Histone deacetylase inhibitor LAQ824 both lowers expression and promotes proteasomal degradation of Bcr-Abl and induces apoptosis of imatinib mesylate-sensitive or -refractory chronic myelogenous leukemia-blast crisis cells. Cancer Res. 2003;63:5126–5135. [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De Smaele E, Tang WJ, D’Adamio L, Franzoso G. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- Patel NM, Nozaki S, Shortle NH, Bhat-Nakshatri P, Newton TR, Rice S, Gelfanov V, Boswell SH, Goulet RJ, Jr, Sledge GW, Jr, Nakshatri H. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene. 2000;19:4159–4169. doi: 10.1038/sj.onc.1203768. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- Sobota R, Szwed M, Kasza A, Bugno M, Kordula T. Parthenolide inhibits activation of signal transducers and activators of transcription (STATs) induced by cytokines of the IL-6 family. Biochem Biophys Res Commun. 2000;267:329–333. doi: 10.1006/bbrc.1999.1948. [DOI] [PubMed] [Google Scholar]
- Suvannasankha A, Crean CD, Shanmugam R, Farag SS, Abonour R, Boswell HS, Nakshatri H. Antimyeloma effects of a sesquiterpene lactone parthenolide. Clin Cancer Res. 2008;14:1814–1822. doi: 10.1158/1078-0432.CCR-07-1359. [DOI] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt JS, Sowa Y, Xu WS, Shao Y, Dokmanovic M, Perez G, Ngo L, Holmgren A, Jiang X, Marks PA. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2005;102:673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ, Jiao J, Rose J, Xie W, Loda M, Golub T, Mellinghoff IK, Davis RJ, Wu H, Sawyers CL. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Vrana JA, Decker RH, Johnson CR, Wang Z, Jarvis WD, Richon VM, Ehinger M, Fisher PB, Grant S. Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP1, but independent of p53. Oncogene. 1999;18:7016–7025. doi: 10.1038/sj.onc.1203176. [DOI] [PubMed] [Google Scholar]
- Wang X, Destrument A, Tournier C. Physiological roles of MKK4 and MKK7: insights from animal models. Biochim Biophys Acta. 2007;1773:1349–1357. doi: 10.1016/j.bbamcr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Thompson MA, Brandt SJ, Hiebert SW. Histone deacetylase inhibitors induce the degradation of the t(8;21) fusion oncoprotein. Oncogene. 2007;26:91–101. doi: 10.1038/sj.onc.1209760. [DOI] [PubMed] [Google Scholar]
- Yao Z, Xing L, Boyce BF. NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest. 2009;119:3024–3034. doi: 10.1172/JCI38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Lin ZN, Yang CF, Shi X, Ong CN, Shen HM. Suppressed NF-kappaB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis. 2004;25:2191–2199. doi: 10.1093/carcin/bgh234. [DOI] [PubMed] [Google Scholar]
- Zunino SJ, Ducore JM, Storms DH. Parthenolide induces significant apoptosis and production of reactive oxygen species in high-risk pre-B leukemia cells. Cancer Lett. 2007;254:119–127. doi: 10.1016/j.canlet.2007.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.