Abstract
Adjudin, an analogue of lonidamine, affects adhesion between Sertoli and most germ cells, resulting in reversible infertility in rats, rabbits and dogs. Previous studies have described the apical ectoplasmic specialization, a hybrid-type of Sertoli cell–elongating/elongated spermatid adhesive junction, as a key target of adjudin. In this study, we ask if the function of the blood-testis barrier which is constituted by co-existing tight junctions, desmosome-gap junctions and basal ectoplasmic specializations can be maintained when the seminiferous epithelium is under assault by adjudin. We report herein that administration of a single oral dose of adjudin to adult rats increased the levels of several tight junction and basal ectoplasmic specialization proteins during germ cell loss from the seminiferous epithelium. These findings were corroborated by a functional in vitro experiment when Sertoli cells were cultured on Matrigel™-coated bicameral units in the presence of adjudin and transepithelial electrical resistance was quantified across the epithelium. Indeed, the Sertoli cell permeability barrier was shown to become tighter after adjudin treatment as evidenced by an increase in transepithelial electrical resistance. Equally important, the blood-testis barrier in adjudin-treated rats was shown to be intact 2 weeks post-treatment when its integrity was monitored following vascular administration of inulinfluorescein isothiocyanate which failed to permeate past the barrier and enter into the adluminal compartment. These results illustrate that a unique mechanism exists to maintain blood-testis barrier integrity at all costs, irrespective of the presence of germ cells in the seminiferous epithelium of the testis.
Keywords: testis, blood-testis barrier, cell junctions, male contraceptive, adjudin
1. Introduction
Spermatogenesis is a complex process in which elongated spermatids develop from spermatogonia under the regulation of follicle stimulating hormone (FSH), luteinizing hormone (LH), testosterone and estradiol 17β (Kerr et al., 2006, O'Donnell et al., 2006). As germ cells differentiate throughout spermatogenesis, they also traverse the seminiferous epithelium, and this requires disassembly of Sertoli–germ cell junctions, namely apical ectoplasmic specializations (apical ES, present between Sertoli cells and elongating/elongated spermatids; ultrastructurally, the apical ES has not been identified in spermatids) and desmosome-gap junctions [D-GJs, present between Sertoli cells and all germ cells up to, but not including, step 8 spermatids and beyond; it is also present between adjacent Sertoli cells] (Russell, 1993, Russell, 1977a, Russell, 1977c, Vogl et al., 2008, Mruk and Cheng, 2004, Mruk et al., 2008, Cheng and Mruk, 2002). While this aspect of spermatogenesis is difficult to study because it requires a sound in vivo system, several testis models of junction restructuring [e.g., intraperitoneal administration of CdCl2, intratesticular injection of an occludin peptide and subdermal insertion of testosterone-estradiol implants (Chiquone, 1964, Setchell and Waites, 1970, Wong et al., 2005, O'Donnell et al., 1996, Wong et al., 2004, Wong et al., 2007)] are currently available for investigation. Unfortunately, our understanding of the cellular events that underlie Sertoli–germ cell junction disassembly leading to germ cell movement during spermatogenesis is still incomplete.
Previous in vivo studies have shown that a single oral dose of adjudin [1-(2,4)-dichlorobenzyl-1H-indazole-3-carbohydrazide, 50 mg/kg b.w.] affects Sertoli–germ cell adhesion, thereby resulting in germ cell loss from the seminiferous epithelium (Cheng et al., 2001, Grima et al., 2001, Mruk et al., 2006). In this study, we use this in vivo model of Sertoli—germ cell junction restructuring to address a simple but important question: can the loss of germ cells from the seminiferous epithelium trigger restructuring of the blood-testis barrier (BTB)? This question is critical to address for the following reasons. First, the BTB sequesters germ cells residing in the adluminal compartment from the systemic circulation, thereby protecting these cells from being attacked by the host’s immune system (Hedger and Hales, 2006, Dym and Fawcett, 1970, Setchell and Waites, 1975, Setchell, 1980). If adjudin depletes germ cells from the adluminal compartment, then the function of the BTB may be compromised since the immunological barrier would no longer be needed to sustain spermatogenesis. Alternatively, the BTB forms at 15–18 days post-partum in the rat before differentiated germ cells appear in the adluminal compartment. As such, the BTB may function as a separate entity, independent of germ cell development. Finally, the BTB is constituted by co-existing tight junctions (TJs), basal ES and D-GJs, and these junctions function collectively to maintain barrier integrity (Lie et al., 2009b, Dym and Fawcett, 1970, Setchell and Waites, 1975, Setchell, 1980, Mruk and Cheng, 2004, Li et al., 2009). If the primary cellular target of adjudin is the apical ES (Cheng et al., 2001, Chen et al., 2003, Lui et al., 2003, Siu et al., 2003), which is the functional and structural counterpart of the basal ES at the BTB, then barrier integrity may also be compromised. In this study, we test these hypotheses to determine if the events that underlie Sertoli–germ cell junction disassembly are distinct from the events that underlie BTB integrity and function. Herein, we report that adjudin-mediated restructuring of anchoring junctions (AJs) strengthened BTB function in vivo and in vitro, illustrating that barrier integrity can be maintained at all costs, irrespective of the loss of germ cells from the seminiferous epithelium.
2. Materials and methods
2.1 Animals
The use of 20 day old and adult (~300 g b.w.) Sprague-Dawley outbred male rats (Charles River Laboratories, Kingston, NY) was approved by The Rockefeller University Laboratory Animal Care and Use Committee (protocol numbers 06018 and 09016). Testes from 20 day old rats were used for the isolation of Sertoli cells, whereas testes from adult rats were used for protein lysate preparation and for cryo-sectioning. Rats were sacrificed by CO2 asphyxiation as directed (Beaver et al., 2001).
2.2 Antibodies
Primary antibodies against TJ and ES proteins were obtained commercially as purified IgGs (Table 1). Each antibody used in this study was pre-screened by immunoblotting using testis (n = 3) and Sertoli cell lysates (n = 3) to ensure that it cross-reacted with a protein band of expected molecular weight.
Table 1.
Primary antibodies used in this study.
| Antibody | Catalog # | Lot # | Host | Vendor | Working dilution | |
|---|---|---|---|---|---|---|
| IB | IF | |||||
| Claudin-11 | 36-4500 | 387613A | Rabbit | Zymed/Invitrogen | 1:125 | |
| Occludin | 71-1500 | 00250207 | Rabbit | Zymed/Invitrogen | 1:400 | 1:50 |
| JAM-A | 36-1700 | 370923A | Rabbit | Zymed/Invitrogen | 1:250 | |
| CAR | sc-15405 | B2808 | Rabbit | Santa Cruz Biotechnology | 1:200 | 1:50 |
| ZO-1 | 61-7300 | 389452A | Rabbit | Zymed/Invitrogen | 1:250 | 1:50 |
| ZO-1-FITC | 33-9111 | 30879018 | Mouse | Zymed/Invitrogen | 1:50 | |
| N-Cadherin | sc-7939 | H0907 | Rabbit | Santa Cruz Biotechnology | 1:200 | 1:50 |
| 33-3900 | 1410973 | Mouse | Zymed/Invitrogen | 1:50 | ||
| γ-Catenin | 610254 | 0000074819 | Mouse | BD Transduction Laboratories | 1:1000 | 1:50 |
| Actin | sc-1616 | F2007 | Goat | Santa Cruz Biotechnology | 1:200 | |
Abbreviations: IB, immunoblotting, IF, immunofluorescent microscopy.
2.3 Treatment of animals with adjudin
To induce germ cell loss from the seminiferous epithelium, a single oral dose of adjudin (50 mg/kg b.w., suspended in 0.5% methyl-cellulose [wt/vol]) was administered to adult rats (~300 g b.w., n = 4/time point) as described previously (Cheng et al., 2001, Grima et al., 2001, Chen et al., 2003). Rats were sacrificed at 0, 1, 2, 4, 7 and 14 days after treatment. We chose these time points because they spanned the period of extensive germ cell loss from the seminiferous epithelium, as well as the period in which the adluminal compartment was largely devoid of spermatocytes and spermatids. Both testes were removed, frozen in liquid nitrogen and stored at −80 °C until used for lysate preparation or for cryo-sectioning. Control rats (n = 3) received 0.5% methyl-cellulose [wt/vol] only. The right testis from each animal was used for lysate preparation, whereas the left testis was used for cryo-sectioning.
2.4 Isolation of Sertoli cells and treatment of cells with adjudin
Sertoli cells were isolated from 20 day old rat testes as described previously (Cheng et al., 1986, Mruk et al., 2003) and seeded on Matrigel™- (BD Biosciences, Bedford, MA) [diluted 1:7 in F12/DMEM, Sigma-Aldrich, St. Louis, MO] coated 6-well plates at high density (0.5 × 106 cells/cm2). Two days after plating, cultures were treated with 20 mM Tris, pH 7.4 at 22 °C to lyse residual germ cells and to yield Sertoli cells with a purity of ~98% (Galdieri et al., 1981). Sertoli cell purity was assessed in random cultures throughout the course of this study by terminating cells in TRIzol® reagent (Invitrogen, Carlsbad, CA) and extracting RNA for RT-PCR using primers that were designed against genes previously reported to be expressed specifically by germ [e.g., proacrosin (Polakoski et al., 1973, Polakoski and McRorie, 1973)], Leydig [e.g., 3β-hydroxysteroid dehydrogenase (Baillie and Griffiths, 1964)] and peritubular myoid [e.g., alkaline phosphatase (Palombi and Di Carlo, 1988)] cells. By day 3 in vitro, functional TJs, ES, and D-GJs were established between adjacent Sertoli cells as observed by electron microscopy (Wong et al., 2000, Lee and Cheng, 2003, Siu et al., 2005, Lie et al., 2009b), thereby resembling the BTB in vivo (Byers et al., 1986, Steinberger and Jakubowiak, 1993, Chung et al., 1999, Chung et al., 2001, Siu et al., 2005, Yan et al., 2008b). On the following day (i.e., day 4 in vitro), adjudin (~1 mg/ml, dissolved in ethanol) was added to Sertoli cell cultures at either 500 ng or 1 µg/ml, and this time point was designated as 0 hour. The control consisted of adding vehicle (0.1% ethanol [vol/vol]) alone. Media supplemented with growth factors (i.e., 10 µg/ml insulin, 5 µg/ml human transferrin, 5 µg/ml bacitracin and 2.5 ng/ml epidermal growth factor) and with the appropriate concentration of adjudin was replaced daily, and the maximal volume of media added to each well was 5 ml. Cells were terminated at different time points from 0 hour to 3 days after the addition of adjudin by scraping cells with a rubber policeman in lysis buffer (10 mM Tris, pH 7.4 at 22 °C containing 0.15 M NaCl, 1% NP-40 [vol/vol] and 10% glycerol [vol/vol]), followed by a brief sonication step and centrifugation at 4 °C to clear lysates. Prior to terminating cells, lysis buffer was supplemented with protease and phosphatase inhibitor cocktails each at an inhibitor:buffer ratio of 1:100 (Sigma-Aldrich). These concentrations of adjudin were used based on preliminary results obtained from an in vitro assay which demonstrated these doses to be effective in detaching fluorescently-labeled germ cells from the Sertoli cell epithelium (Mruk and Cheng, unpublished observations). This experiment was repeated at least three times using different batches of isolated Sertoli cells treated with adjudin. Within a single experiment, each treatment group consisted of duplicate culture wells.
2.5 Assessing Sertoli cell barrier function in vitro
Sertoli cell barrier function in vitro was monitored by measuring transepithelial electrical resistance (TER) with a Millicell ERS system (i.e., meter with Ag/AgCl electrodes). In brief, Sertoli cells were seeded on Matrigel™-coated Millicell® HA bicameral units (12 mm diameter, 0.45 µm pore size) at high density (1.2 × 106 cells/cm2), and a ~2 sec pulse of current (20 µAmps) was passed through the Sertoli cell epithelium. The first TER reading was taken 24 hours after plating cells (i.e., day 1), and a total of four readings were obtained for each bicameral unit (i.e., at 12, 3, 6 and 9 o’clock positions) which were averaged into a single TER reading. An averaged reading obtained from triplicate ‘blank’ bicameral units, which contained no Sertoli cells, was subtracted from bicameral units containing cells. Each resulting value was multiplied by the surface area of the bicameral unit (i.e., 0.6 cm), and data was presented as Ω·cm2. By day 3, a functional barrier was assembled. On the following day, Sertoli cells were incubated with increasing concentrations of adjudin (100 ng, 500 ng or 1 µg/ml) in one of three ways: adjudin was added to (i) both the apical and basal compartment of bicameral units, (ii) the apical or (iii) the basal compartment only. For the control, Sertoli cells were incubated with vehicle which was added to both compartments, the apical or the basal compartment alone, and the maximal volume of media added to either compartment was 500 µl. Growth factor-supplemented media containing the appropriate concentration of adjudin was replaced daily, and TER readings were recorded every 12 hr. This experiment was repeated at least four times using different batches of isolated Sertoli cells as described previously (Chung and Cheng, 2001, Cheng and Mruk, 2006). Within a single TER experiment, each treatment group consisted of triplicate bicameral units.
2.6 Immunofluorescent microscopy
Immunofluorescent staining using either cross-sections from adult testes or cultured Sertoli cells was performed as described previously (Lie et al., 2009b, Su et al., 2009, Kopera et al., 2009). Sertoli cells were cultured on Matrigel™-coated 22 mm glass coverslips (No. 1, each coverslip was placed into one of six wells of a culture plate) at a significantly lower density (0.05 × 106 cells/cm2) when compared to cells that were used for immunoblotting and TER measurement, but at a density which still permitted the assembly of functional TJs, ES and D-GJs (Li et al., 2009, Lie et al., 2009b, Xia et al., 2007, Yan et al., 2008b). On day 2, cultures were treated with a hypotonic buffer to lyse residual germ cells, and on day 4, Sertoli cells were treated with adjudin (1 µg/ml). This time point was designated as 0 hour. For the control, Sertoli cells were incubated with vehicle alone. Growth factor-supplemented media containing the appropriate concentration of adjudin was replaced daily. Three days after the addition of adjudin, cells were fixed in 4% paraformaldehyde [wt/vol], permeabilized with 0.1% Triton X-100 [vol/vol] and non-specific binding sites blocked with 10% normal goat serum (NGS) [vol/vol] in PBS (10 mM NaH2PO4, pH 7.4 at 22 °C containing 0.15 M NaCl). Primary antibodies were used at dilutions as listed in Table 1. Secondary antibodies were goat anti-mouse or goat anti-rabbit IgG conjugated to either Alexa Fluor 488 or 555 (Invitrogen) and diluted 1:100 in PBS containing 10% NGS [vol/vol] before use. Cells were mounted with ProLong anti-fade reagent containing DAPI (Molecular Probes, Eugene, OR), and images were acquired with MicroSuite FIVE software (Version 1.224, Olympus Soft Imaging Solutions Corp., Lakewood, CO) and an Olympus DP71 12.5 MPa digital camera attached to an Olympus BX61 motorized microscope (Olympus America, Inc., Center Valley, PA). All images were adjusted for brightness and contrast using Adobe Photoshop (Version 10.0, Adobe Systems, San Jose, CA), and all images acquired within a single time-course experiment were adjusted using identical parameters. Immunofluorescent staining using testes cross-sections was performed as described previously with minor modifications (Kopera et al., 2009). Immunofluorescent experiments were repeated at least three times using different batches of isolated Sertoli cells, as well as testes from three different rats treated with adjudin. Controls included incubating testis cross-sections or cultured Sertoli cells with PBS, rabbit or mouse IgG, or a mixture of rabbit and mouse IgGs in place of the primary antibody/antibodies at a similar dilution(s).
2.7 Assessing Sertoli cell barrier function in vivo
The BTB integrity assay is a technique used to monitor BTB function, and it can effectively discriminate between an assembled and a disassembled barrier (Morrow et al., 2009, Li et al., 2006, Kopera et al., 2009, Sarkar et al., 2008). Adjudin (50 mg/kg b.w.) was administered to adult rats (~300 g b.w., n = 3/time point) as described previously. Also, CdCl2 (3 mg/kg b.w., suspended in PBS, pH 7.4 at 22 °C), an environmental toxicant, was administered to adult rats (~300 g b.w., n = 3) by intraperitoneal injection because this compound was previously shown to permanently disrupt barrier function (Chiquone, 1964, Setchell and Waites, 1970, Wong et al., 2004). As such, this group of animals served as a positive control in this experiment. Control rats (n = 3) in the adjudin group received 0.5% methyl-cellulose [wt/vol], whereas control rats (n = 3) in the CdCl2 group received PBS. To eliminate inter-experimental variations, all BTB integrity assays were performed on the same day (i.e., the time-course administration of adjudin was carried out in the reverse; animals in the last experimental group, day 14, received adjudin first), and all tissue samples were processed immediately thereafter. In brief, each rat received an intramuscular injection of ketamine HCl–xylazine HCl (300 µl, Sigma-Aldrich). The surgical site was cleansed with betadine solution, and a ~1 cm incision was made to expose the jugular vein. Thereafter, inulin-fluorescein isothiocyanate (FITC; 1 mg, suspended in 200 µl PBS, pH 7.4 at 22 °C, Mr ~5 kDa; Sigma-Aldrich) was carefully injected into the jugular vein with a 28 gauge syringe. The surgical site was sutured and cleansed, and animals were allowed to recover. Approximately 90 min thereafter, rats were sacrificed by CO2 asphyxiation, both testes removed and frozen in liquid nitrogen. On the same day, testes were cut at a thickness of 10 µm, cross-sections collected onto slides and the diffusion of inulin-FITC into the adluminal compartment of the seminiferous epithelium monitored by fluorescent optics. Approximately 50 tubules were examined from each rat testis from both control and treatment groups.
2.8 Cytotoxicity assay
Sertoli cells were plated on Matrigel™-coated 96-well plates at high density (0.5 × 106 cells/cm2). On day 2, cultures were treated with a hypotonic buffer. Two days thereafter (i.e., day 4 in vitro), Sertoli cells were incubated with increasing concentrations of adjudin from 0.001 to 500 µg/ml for 2 and 3 days. Growth factor-supplemented media containing the appropriate concentration of adjudin was replaced daily, and the maximal volume of media added to each well was 200 µl. Two and 3 days after the addition of adjudin, the Cell Proliferation II kit (Roche Diagnostics, Mannheim, Germany) was used as instructed by the manufacturer. In brief, 100 µl XTT reagent was added to each well, and cells were incubated at 35 °C for 2, 4, 6, 8 and 12 hours at which time absorbance was measured at 450 nm (650 nm, reference wavelength) using a Model 680 microplate reader (Bio-Rad Laboratories, Hercules, CA). This experiment was repeated three times using different batches of isolated Sertoli cells treated with increasing doses of adjudin.
2.9 General methods
Testes lysates were prepared for immunoblotting by using a tissue:buffer ratio of 1:5 as described previously (Lau and Mruk, 2003). Protein estimation was performed by using the Bio-Rad Dc Protein Assay using BSA as a standard. F-actin was visualized in the testis by staining 7 µm thick sections with rhodamine phalloidin (Molecular Probes) as instructed by the manufacturer. Immunoblots were scanned using Scion Image (Version 1.1, NIH, Bethesda, MD). Statistical analysis was performed with GB-STAT (Version 7.0, Dynamic Microsystems, Silver Spring, MD). Student’s t-test was used for paired comparisons against the control, whereas one-way ANOVA followed by Dunnett’s post-test was used for multiple comparisons. P<0.05 was taken as statistically significant. Table 1 lists the antibodies and experimental conditions that were used for immunoblotting and immunofluorescent microscopy.
3. Results
3.1 Adjudin-mediated Sertoli–germ cell junction disassembly strengthens barrier function in vivo
In this experiment, we investigated by immunoblotting and immunofluorescent microscopy the levels of several proteins that have important roles in BTB dynamics to determine if there were any changes after adjudin treatment. By immunoblotting, the steady-state levels of TJ [i.e., claudin-11, occludin, junctional adhesion molecule-A (JAM-A), coxsackie and adenovirus receptor (CAR) and zonula occludens-1 (ZO-1)] and basal ES (i.e., N-cadherin and γ-catenin) proteins were shown to increase several-fold after adjudin treatment (Fig. 1A, B). Of these proteins, claudin-11 was up-regulated the most, i.e., ~11-fold at 7 days post-treatment (Fig. 1A, Ba). These results were corroborated by immunofluorescent microscopy when an obvious thickening of claudin-11 (Fig. 2B, C versus A), occludin (Fig. 2E, F versus G), JAM-A (Fig. 2H, I versus G), CAR (Fig. 2K, L versus J), N-cadherin (Fig. 2N, O versus M) and γ-catenin (Fig. 2Q, R versus P) was observed at the BTB following adjudin treatment when compared to control rats. Consistent with previously published reports in the literature, all proteins localized to the BTB in the control testis (Morita et al., 1999, Gow et al., 1999, Moroi et al., 1998, Saitou et al., 2000, Tarulli et al., 2008, Yan and Cheng, 2005). CAR was also found at the site of the apical ES (Wang et al., 2007, Mirza et al., 2006). Shrinkage of seminiferous tubules was evident following adjudin administration as a result of germ cell loss (Fig. 2B, C, E, F, H, I, K, L, N, O, Q and R).
Fig. 1. Adjudin-mediated Sertoli–germ cell junction disassembly up-regulates BTB-constituent proteins in vivo.
Adjudin (50 mg/kg b.w.) was administered to rats (~300 g b.w., n = 4/time point) as described in Materials and methods. Control rats (n = 3) received 0.5% methyl-cellulose [wt/vol] only. (A) Immunoblots investigating changes in TJ and basal ES proteins in the testis following oral administration of adjudin. Actin served as a loading control. (Ba, b) Histograms summarizing results shown in (A). Each data point was normalized against its corresponding actin data point and then compared against its control [0 day (D)] which was arbitrarily set at 1. Error bars represent mean ± SD of data using testis lysates from 4 different animals. *, P<0.05; **, P<0.01 (Student’s t-test).
Fig. 2. Adjudin affects protein localization at the BTB.
Adjudin (50 mg/kg b.w.) was administered to rats (~300 g b.w., n = 4/time point) as described in Materials and methods. Control rats (n = 3) received 0.5% methyl-cellulose [wt/vol] only. Cross-sections of testes from control and adjudin-treated rats at 7 and 14 days post-treatment immunostained for claudin-11 (A–C), occludin (D–F), JAM-A (G–I), CAR (J–L), N-cadherin (M–O) and γ-catenin (P–R). Arrowheads (J) indicate the presence of CAR at the apical ES. Brackets show a thickening in claudin-11 (B, C versus A), occludin (E, F versus D), JAM-A (H, I versus G), CAR (K, L versus J), N-cadherin (N, O versus M) and γ-catenin (Q, R, versus P) immunostaining at the BTB following adjudin treatment when compared to control rats. This experiment was repeated three times using testes from three different rats treated with adjudin. Bar (A, also corresponds to B–R) = 115 µm.
3.2 Sertoli cell barrier function is strengthened by adjudin in vitro
Culturing Sertoli cells at high density on Matrigel™-coated dishes or bicameral units results in the assembly of a polarized epithelium with functional TJs, ES and D-GJs (Wong et al., 2000, Lee and Cheng, 2003, Siu et al., 2005, Lie et al., 2009b, Li et al., 2009), making this in vitro system ideal to study Sertoli cell barrier function. Equally important, this model allows the investigator to monitor with relative ease the different states (i.e., leaky, tight and tighter) of barrier function. In this experiment, increasing concentrations of adjudin (100 ng, 500 ng and 1 µg/ml) were added to both compartments of bicameral units onwards of day 4, and this strengthened the Sertoli cell permeability barrier dose-dependently when compared to control cells cultured in the absence of adjudin (Fig. 3A). Adding adjudin (500 ng/ml) to either the apical (Fig. 3B) or the basal (Fig. 3C) compartment also strengthened barrier function, consistent with results shown in Fig. 3A. The only difference between the two compartments was that adjudin’s effect on increasing TER was more pronounced when this compound was added to the apical compartment (Fig. 3B versus C). It is also worth noting that the increase in TER after adding adjudin to the apical compartment only was indistinguishable from the treatment group in which adjudin was added to both compartments (Fig. 3B). Adjudin did not affect Sertoli cell viability at doses of up to 10 µg/ml when cytotoxicity was assessed by an in vitro assay (Fig. 3D). As such, the moderate decrease in TER observed on days 6 and 7 in the adjudin treatment group (500 ng and 1 µg/ml, Fig. 3A–C) is not likely the result of cytotoxicity.
Fig. 3. Adjudin strengthens Sertoli cell barrier function in vitro and displays no cytotoxicity.
(A–C) TER was measured as a means to assess barrier function when Sertoli cells were cultured in the presence of adjudin. (A) Sertoli cells were cultured at high density on Matrigel™-coated bicameral units. Thereafter, increasing concentrations of adjudin (100 ng, 500 ng and 1 µg/ml) were added to both the apical and basal compartment of bicameral units onwards of day 4. (B, C) Adjudin (500 ng/ml) was added to the apical (B) and/or the basal (C, see schematic drawing in B and C) compartment of bicameral units onwards of day 4. Media containing the appropriate concentration of adjudin was replaced daily, and TER readings were recorded every 12 hr. Results in (A–C) are representative of at least four independent experiments using different batches of isolated Sertoli cells, and one set of results is shown in (A–C). Each adjudin treatment data point in (A–C) was compared to its corresponding control (i.e., Sertoli cells cultured in the presence of vehicle which was either added to both compartments, the apical or basal compartment alone). The addition of adjudin to both the apical and basal compartment of bicameral units (B, C) served as an internal control. Results in (B, C) represent data from a single experiment, but these results were presented as two separate plots. Error bars represent mean ± SD of triplicate bicameral units per treatment group in a single experiment. *, P<0.05; **, P<0.01 (ANOVA followed by Dunnett’s test). (D) Cytotoxicity assay to assess if adjudin displays overt toxicity in Sertoli cells. Sertoli cells, cultured at high density on Matrigel™-coated plates for 4 days, were treated with increasing concentrations of adjudin (0.001 to 500 µg/ml) for 3 days, followed by addition of using XTT reagent for 6 hours. The dotted-line indicates the maximum dose of adjudin that was used for all in vitro experiments. This cytotoxicity assay was repeated three times using different batches of isolated Sertoli cells treated with increasing doses of adjudin.
3.3 Sertoli cell TJ and basal ES proteins are up-regulated by adjudin in vitro
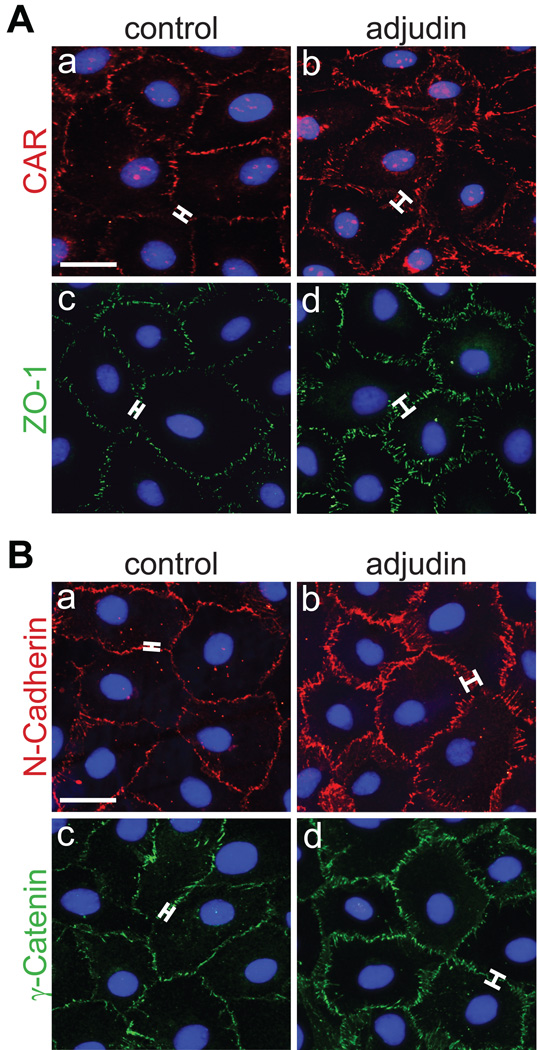
Next, we investigated by immunoblotting and immunofluorescent microscopy if adjudin up-regulates TJ and basal ES proteins in Sertoli cells in vitro. When protein levels were compared between the two experimental groups (i.e., control versus adjudin-treated) in a time-course study, claudin-11, JAM-A, CAR, ZO-1, N-cadherin and γ-catenin increased significantly after adjudin treatment (500 ng and/or 1 µg/ml; Fig. 4A, B). Moreover, when Sertoli cells were treated with adjudin (1 µg/ml) for 3 days and used for immunofluorescent staining, CAR (Fig. 5Ab versus a), ZO-1 (Fig. 5Ad versus c), N-cadherin (Fig. 5Bb versus a) and γ-catenin (Fig. 5Bd versus c) thickened at sites of cell contact when compared to their corresponding controls. This is consistent with in vivo results shown in Fig. 2. Sertoli cell nuclei were visualized by using mounting medium containing DAPI (Fig. 3Aa–d, Ba–d).
Fig. 4. Adjudin up-regulates Sertoli cell barrier constituent proteins in vitro.
Sertoli cells were cultured at high density on Matrigel™-coated dishes as described in Materials and methods. On day 4, adjudin was added (this time point was designated as 0 hour), and cells were used at different time points thereafter for lysate preparation. Control Sertoli cells consisted of adding vehicle alone. (A) Immunoblots investigating changes in TJ and basal ES proteins following treatment of Sertoli cells with adjudin (500 ng and/or 1 µg/ml). Actin served as a loading control. (Ba–f) Histograms summarizing results shown in (A). Each data point was normalized against its corresponding actin data point. Error bars represent mean ± SD of data from at least three different batches of isolated Sertoli cells treated with adjudin. *, P<0.05; **, P<0.01 (ANOVA followed by Dunnett’s test).
Fig. 5. Adjudin affects protein localization at the Sertoli cell surface.
Sertoli cells were cultured at high density on Matrigel™-coated glass coverslips as described in Materials and methods. On day 4, adjudin was added. Three days thereafter, cells were used for immunofluorescent microscopy. (A, B) Control and adjudin-treated Sertoli cells were immunostained for CAR (Aa, b), ZO-1 (Ac, d), N-cadherin (Ba, b) and γ-catenin (Bc, d). Brackets show a thickening in CAR (Ab versus a), ZO-1 (Ad versus c), N-cadherin (Bb versus a) and γ-catenin (Bd versus c) immunostaining at the Sertoli cell interface following adjudin treatment. This immunofluorescent microscopy experiment was repeated at least three times using different batches of isolated Sertoli cells treated with adjudin. Bar (Aa, also corresponds to b–d; Ba, also corresponds to b–d) = 15 µm.
3.4 Adjudin-mediated changes in F-actin at the BTB in vivo
Actin is a key structural protein to which TJ and basal ES proteins at the BTB attach either directly or indirectly. In this in vivo experiment, we investigated if there were changes in F-actin localization at the BTB following administration of adjudin to adult rats. In the control testis, F-actin was discrete and thread-like, lying close and running parallel to the basement membrane (Fig. 6A–C). F-actin staining at the BTB was weakest at stage VIII of the seminiferous epithelial cycle (Fig. 6B), coinciding with restructuring of the BTB. However, 7 days after the administration of adjudin, F-actin at the BTB thickened (Fig. 6E–G). Moreover, in the majority of seminiferous tubules, F-actin no longer ran parallel to the basement membrane; instead, it ran perpendicular to and appeared somewhat removed from the vicinity of the basement membrane (Fig. 6G). By 14 days post-treatment, F-actin at the BTB thickened even further (Fig. 6I–K). Changes in F-actin localization at the apical ES have been reported previously in a separate, unrelated study which investigated the apical ES as the cellular target of adjudin (Mruk and Lau, 2009). While these findings are not discussed in detail herein, it is worth noting that F-actin at the apical ES disintegrated completely. These observations are in contrast to what was observed at the BTB (Fig. 6). Nuclei were visualized by using mounting medium containing DAPI (Fig. 6D, H, L).
Fig. 6. Changes in F-actin at the BTB after administration of adjudin in vivo.
Adjudin (50 mg/kg b.w.) was administered to rats (~300 g b.w., n = 4/time point) as described in Materials and methods, and the integrity of F-actin at the BTB was assessed by using rhodamine phalloidin and fluorescent optics. Control rats (n = 3) received 0.5% methyl-cellulose [wt/vol] only. (A–D) Representative testis cross-section from a control rat. (E–L) Representative testis cross-sections from rats 7 (E–H) and 14 (I–L) days after administration of adjudin. Dotted-line boxes in (A, B, E, F, I, K) represent magnified views. Arrowheads (C, G, K) indicate changes in F-actin orientation at the BTB. Brackets (K versus C) indicate a thickening in F-actin after administration of adjudin when compared to the control. Nuclei were visualized by using mounting medium containing DAPI (D, H, L) which served as an indicator of germ cell loss from seminiferous tubules. Dotted-line circles (D, H, L) outline the periphery of seminiferous tubules. bv, blood vessel. Bar (A, also corresponds to E, I) = 175 µm; bar (B, also corresponds to D, F, H, J, L) = 110 µm; bar (C, also corresponds to G, K) = 80 µm.
3.5 Adjudin-mediated Sertoli–germ cell junction disassembly does not compromise barrier function in vivo
The BTB integrity assay is a technique used to monitor BTB function (Morrow et al., 2009, Li et al., 2006, Kopera et al., 2009, Sarkar et al., 2008). In the control testis, inulin-FITC failed to penetrate the BTB and was restricted to the basal compartment of the seminiferous epithelium (Fig. 7A), illustrating that the Sertoli cell barrier was intact. Since CdCl2 is known to permanently disrupt BTB function (Chiquone, 1964, Setchell and Waites, 1970, Wong et al., 2004), it was used as a positive control in this experiment. Indeed, 5 days after CdCl2 treatment inulin-FITC diffused into the adluminal compartment in all tubules examined (Fig. 7B), consistent with previously published reports in the literature (Li et al., 2006, Xia et al., 2007). On the contrary, the integrity of the BTB was not affected by adjudin 4 (Fig. 7C), 7 (Fig. 7D) and 14 (Fig. 7E) days after administration of this compound because inulin-FITC was restricted to the basal compartment, similar to the control testis (Fig. 7A).
Fig. 7. Adjudin-mediated Sertoli-germ cell junction disassembly does not compromise barrier function in vivo.
Inulin-FITC was administered to control and treated rats (~300 g b.w., n = 3/time point) as described in Materials and methods, and testis cryo-sections were examined microscopically by fluorescent optics. (A) Control rats (n = 3) in the adjudin group received 0.5% methyl-cellulose [wt/vol], whereas control rats (n = 3) in the CdCl2 group received PBS (data not shown). (B) Representative testis cross-section from a rat 5 days (D) after administration of CdCl2 (3 mg/kg b.w.). (C–E) Representative testis cross-sections from rats 4 (C), 7 (D) and 14 (E) days after administration of adjudin (50 mg/kg b.w.). Dotted-line circles (A–E) outline the periphery of seminiferous tubules. Brackets (A–E) show the relative distance that inulin-FITC had traveled from the periphery of seminiferous tubules. bv, blood vessel. Bar (A, also corresponds to B–E) = 115 µm.
4. Discussion
There are several reports in the literature illustrating that disassembly of adherens junctions (AJs) in epithelial and endothelial cells under physiological or pathophysiological conditions such as tumorigenesis also results in the concomitant disassembly of TJs (Martin and Jiang, 2008, Balda and Matter, 2009, Oliveira and Morgado-Diaz, 2007, Ben-Ze'ev and Geiger, 1998). One of the main reasons for this is that AJs are known to associate intimately with TJs within the junctional complex, and crosstalk between co-existing junction types which contributes to tissue integrity has been reported (Dejana et al., 2008). This is also true for cell junctions in the seminiferous epithelium of the testis, especially those found at the BTB (i.e., TJs, basal ES and D-GJs) since ultrastructural studies have shown these junctions to be intermingled with one another (Vogl et al., 2008). Throughout spermatogenesis, differentiating germ cells traverse the seminiferous epithelium, a cellular event that requires restructuring of Sertoli–Sertoli (i.e., BTB) and Sertoli–germ cell junctions [i.e., ES and D-GJs] (Cheng and Mruk, 2002, Mruk and Cheng, 2004, Vogl et al., 2008, Russell, 1993, Russell and Peterson, 1985). Indeed, there is coordination between disassembly of the apical ES, which is followed immediately by the release of elongated spermatids at late stage VIII of the seminiferous epithelial cycle (i.e., spermiation), and restructuring of the BTB at stages VIII–XI to permit entry of leptotene spermatocytes into the adluminal compartment for continued development (Yan et al., 2008a, Lie et al., 2009a). As such, this study’s rationale is simple but important: to investigate if the integrity of the BTB can be maintained during adjudin-mediated AJ disassembly which results in extensive germ cell loss from the seminiferous epithelium since this is somewhat analogous to AJ restructuring during spermatogenesis. We opted to use administration of adjudin as our working model because the effects of this compound on junction disassembly have been well documented in several in vitro and in vivo studies (Cheng et al., 2001, Chen et al., 2003, Mruk et al., 2006, Lui et al., 2003, Siu et al., 2003, Grima et al., 2001, Su et al., 2009, Kopera et al., 2009, Lau and Mruk, 2003, Lee et al., 2003, Cheng et al., 2005, Xia and Cheng, 2005, Wolski et al., 2006, Sarkar and Mathur, 2009). For example, more than half of all tubules examined microscopically showed signs of damage, i.e., the appearance of detached elongating/elongated spermatids in the tubule lumen by ~6.5 hours after administration of a single oral dose of adjudin (50 mg/kg b.w.) when the kinetics of germ cell loss were investigated (Chen et al., 2003). Moreover, virtually all tubules showed signs of spermatid loss by ~1–2 days post-treatment. This was followed immediately by the detachment of round spermatids and secondary spermatocytes by 3–6 days (Chen et al., 2003). Interestingly, adjudin did not appear to affect adhesion between Sertoli cells and primary spermatocytes, as well as between Sertoli cells and spermatogonia residing in the basal compartment of the seminiferous epithelium (Cheng et al., 2001, Grima et al., 2001). At this point, it is important to emphasize that junction restructuring and germ cell loss (i.e., spermiation) also occur throughout spermatogenesis in the control testis. However, administration of adjudin intensifies or exaggerates these critical cellular events in the seminiferous epithelium, thereby providing a suitable model for our investigation.
At the onset of this study, we anticipated to see a compromise in BTB integrity following administration of adjudin. However, the results of this study support a different hypothesis, and we demonstrate herein that barrier function was instead strengthened by adjudin. Following administration of a single oral dose of adjudin to adult rats, several putative TJ (e.g., claudin-11, occludin, JAM-A, CAR and ZO-1) and basal ES (e.g., N-cadherin and γ-catenin) proteins known to be present at the BTB increased significantly when their levels in the testis were assessed by immunoblotting and immunofluorescent microscopy, illustrating barrier function to be enhanced. These results were corroborated by a functional in vitro assay when Sertoli cell barrier function was assessed by TER, as well as in part by a functional in vivo assay which monitored the integrity of the BTB. These findings are important because they reveal that the seminiferous epithelium is equipped with a unique mechanism, one that can protect the BTB during Sertoli–germ cell junction restructuring which occurs during spermatogenesis. Indeed, this is the case in the control testis: the BTB begins to restructure shortly after spermiation at late stage VIII/early stage IX of the seminiferous epithelial cycle, and it ceases to restructure extensively at stage XI (and remains as such through early stage VIII) once spermatocytes enter the adluminal compartment (Russell, 1977b). While it can be argued that the increase in BTB function in vivo is simply the result of a pharmacological effect on Sertoli cells since an increase in barrier function was also observed in vitro in the absence of germ cells, we do not believe this to be the case. Sertoli cells cultured at high density in vitro, even in the absence of germ cells, are known to assemble functional (basal) ES, as well as TJs and D-GJs (Wong et al., 2000, Lee and Cheng, 2003, Siu et al., 2005), and this system, albeit simple, is known to mimic the BTB in vivo (Byers et al., 1986, Steinberger and Jakubowiak, 1993, Chung et al., 1999, Chung et al., 2001, Siu et al., 2005, Yan et al., 2008b). Furthermore, if adjudin targets the apical ES in vivo, it is also likely to target the (basal) ES in vitro due to structural and functional similarities and to elicit similar stimulatory effects on barrier function because there is crosstalk between ES and TJs, and this is exactly what we observed. Moreover, the effects of adjudin on peritubular myoid cells have not yet been investigated. Since myoid cells in the rodent testis are known to restrict the passage of electron-opaque markers into the seminiferous tubule (Dym and Fawcett, 1970, Fawcett et al., 1970), thereby contributing in part to BTB function, it would be worthwhile to examine if myoid cell function is affected by adjudin.
Striking changes were also noted with F-actin in testes from adjudin-treated rats. In testes from control rats, F-actin at the BTB was discrete and thread-like in appearance, and it ran parallel to the basement membrane, consistent with previously published reports in the literature (Vogl et al., 2008, Kopera et al., 2009, Pelletier et al., 1997). However, in testes from adjudin-treated rats, the staining pattern and orientation of F-actin at the BTB were clearly different; F-actin ran somewhat perpendicular to the basement membrane and was more diffuse (i.e., thicker) when compared to the control. Interestingly, these changes in the orientation of F-actin failed to compromise the integrity of the BTB. Also, these changes in F-actin orientation at the BTB in adjudin-treated rats are in line with immunohistochemistry results which showed several TJ and basal ES proteins to be up-regulated drastically, revealing that F-actin structurally supports the increase in barrier function. At this point, it is not known if a higher or consecutive dose(s) of adjudin can affect BTB integrity because this compound can cause permanent infertility depending on the treatment regimen used (Cheng et al., 2005). Nevertheless, our findings support the notion that AJ disassembly can trigger a cascade of events leading to strengthened barrier function in the testis. As such, these results illustrate that a unique mechanism is in place in the seminiferous epithelium, one that maintains BTB integrity during AJ restructuring throughout spermatogenesis. These findings also support the notion that barrier function conferred by TJs can be ‘disengaged’ from AJs as earlier postulated (Yan and Cheng, 2005) so that Sertoli–germ cell AJs can be restructured without compromising the Sertoli cell BTB. Additional functional studies will be needed in the future to better understand this mechanism.
Acknowledgements
This work was supported in part by NICHD, NIH (R03 HD061401 to DDM, and R01 HD056034 and U54 HD029990 Project 5 to CYC).
Abbreviations
- AJ
anchoring junction
- BTB
blood-testis barrier
- CAR
coxsackie and adenovirus receptor
- D-GJ
desmosome-gap junction
- ES
ectoplasmic specialization
- JAM-A
junctional adhesion molecule-A
- NGS
normal goat serum
- TER
transepithelial electrical resistance
- TJ
tight junction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baillie AH, Griffiths K. 3β-Hydroxysteroid dehydrogenase activity in the mouse Leydig cell. J Endocrinol. 1964;29:9–17. doi: 10.1677/joe.0.0290009. [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Beaver BV, Reed W, Leary S, McKiernan B, Bain F, Schultz R, et al. 2000 Report of the American Veterinary Medical Association Panel in Euthanasia. J Am Vet Med Assoc. 2001;218:669–696. [Google Scholar]
- Ben-Ze'ev A, Geiger B. Differential molecular interactions of β-catenin and plakoglobin in adhesion, signaling, and cancer. Curr Opin Cell Biol. 1998;10:629–639. doi: 10.1016/s0955-0674(98)80039-2. [DOI] [PubMed] [Google Scholar]
- Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Chen YM, Lee NPY, Mruk DD, Lee WM, Cheng CY. Fer kinase/Fer T and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod. 2003;69:656–672. doi: 10.1095/biolreprod.103.016881. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mather JP, Byer AL, Bardin CW. Identification of hormonally responsive proteins in primary Sertoli cell culture medium by anion-exchange high performance liquid chromatography. Endocrinology. 1986;118:480–488. doi: 10.1210/endo-118-2-480. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Tight junction assembly. In: Harris R, Graham J, Rickwood D, editors. Cell Biology Protocols. Hoboken: John Wiley & Sons; 2006. pp. 296–299. [Google Scholar]
- Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- Chiquone AD. Observations on the early events of cadmium necrosis in the testis. Anat Rec. 1964;149:23–35. doi: 10.1002/ar.1091490104. [DOI] [PubMed] [Google Scholar]
- Chung NPY, Mruk DD, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol Reprod. 2001;65:1340–1351. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- Chung NPY, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- Chung SS, Lee WM, Cheng CY. Study on the formation of specialized inter-Sertoli cell junctions in vitro. J Cell Physiol. 1999;181:258–272. doi: 10.1002/(SICI)1097-4652(199911)181:2<258::AID-JCP8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Leak LV, Heidger PM. Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil. 1970;10 Suppl 10:105–122. [PubMed] [Google Scholar]
- Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl. 1981;5:249–259. [Google Scholar]
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, et al. CNS myelin and Sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001;64:1500–1508. doi: 10.1095/biolreprod64.5.1500. [DOI] [PubMed] [Google Scholar]
- Hedger MP, Hales DB. Immunophysiology of the male reproductive tract. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. New York: Elsevier; 2006. pp. 1195–1286. [Google Scholar]
- Kerr JB, Loveland KL, O'Bryan MK, de Kretser D. Cytology of the testis and intrinsic control mechanisms. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. New York: Elsevier; 2006. pp. 827–947. [Google Scholar]
- Kopera I, Su L, Bilinska B, Cheng CY, Mruk DD. An in vivo study on adjudin and blood-testis barrier dynamics. Endocrinology. 2009;150:4724–4733. doi: 10.1210/en.2008-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau ASN, Mruk DD. Rab8B GTPase and junction dynamics in the testis. Endocrinology. 2003;144:1549–1563. doi: 10.1210/en.2002-220893. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3',5'-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Mruk DD, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell-actin-based adherens junctions (AJ) in the rat testis? Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- Li MWM, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, et al. Tumor necrosis factor-α reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Cheng CY, Mruk DD. Coordinating cellular events during spermatogenesis: a biochemical model. Trends Biochem Sci. 2009a;34:366–373. doi: 10.1016/j.tibs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Cheng CY, Mruk DD. The desmoglein-2/desmocollin-2/Src kinase protein complex regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2009b;42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol Reprod. 2003;68:2189–2206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2008;1788:872–891. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Mirza M, Hreinsson J, Strand ML, Hovatta O, Soder O, Philipson L, et al. Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp Cell Res. 2006;312:817–830. doi: 10.1016/j.yexcr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in the testis. J Cell Biol. 1999;145:579–588. doi: 10.1083/jcb.145.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yoshida O, et al. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cell testes. Am J Physiol. 1998;274:C1708–C1717. doi: 10.1152/ajpcell.1998.274.6.C1708. [DOI] [PubMed] [Google Scholar]
- Morrow CMK, Tyagi G, Simon L, Carnes K, Murphy KM, Cooke PS, et al. Claudin 5 expression in mouse seminiferous epithelium is dependent upon the transcription factor Ets-variant 5 and contributes to blood-testis barrier function. Biol Reprod. 2009;81:871–879. doi: 10.1095/biolreprod.109.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Lau ASN. RAB13 participates in ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2009;80:590–601. doi: 10.1095/biolreprod.108.071647. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Siu MKY, Conway AM, Lee NPY, Lau ASN, Cheng CY. Role of tissue inhibitor of metalloproteases-1 in junction dynamics in the testis. J Androl. 2003;24:510–523. doi: 10.1002/j.1939-4640.2003.tb02703.x. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–1328. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- O'Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996;55:895–901. doi: 10.1095/biolreprod55.4.895. [DOI] [PubMed] [Google Scholar]
- O'Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Endocrine regulation of spermatogenesis. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. New York: Elsevier; 2006. pp. 1017–1069. [Google Scholar]
- Oliveira SS, Morgado-Diaz JA. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci. 2007;64:17–28. doi: 10.1007/s00018-006-6314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombi F, Di Carlo C. Alkaline phosphatase is a marker for myoid cells in cultures of rat peritubular and tubular tissue. Biol Reprod. 1988;39:1101–1109. doi: 10.1095/biolreprod39.5.1101. [DOI] [PubMed] [Google Scholar]
- Pelletier RM, Okawara Y, Vitale ML, Anderson JM. Differential distribution of the tight-junction-associated protein ZO-1 isoforms α+ and α− in guinea pig Sertoli cells: a possible association with F-actin and G-actin. Biol Reprod. 1997;57:367–376. doi: 10.1095/biolreprod57.2.367. [DOI] [PubMed] [Google Scholar]
- Polakoski KL, McRorie RA. Boar acrosin - II: classification, inhibition, and specificity studies of a proteinase from boar acrosomes. J Biol Chem. 1973;248:8183–8188. [PubMed] [Google Scholar]
- Polakoski KL, McRorie RA, Williams WL. Boar acrosin - I: purification and preliminary characterization of a proteinase from boar sperm acrosomes. J Biol Chem. 1973;248:8178–8182. [PubMed] [Google Scholar]
- Russell LD. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977a;148:301–312. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977b;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Russell LD. Observations on rat Sertoli ectoplasmic ('junctional') specializations in their association with germ cells of the rat testis. Tissue Cell. 1977c;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- Russell LD. Morphological and functional evidence for Sertoli-germ cell relationships. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 365–390. [Google Scholar]
- Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int J Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar O, Mathur PP. Adjudin-mediated germ cell depletion alters the anti-oxidant status of adult rat testis. Mol Reprod Dev. 2009;76:31–37. doi: 10.1002/mrd.20928. [DOI] [PubMed] [Google Scholar]
- Sarkar O, Mathur PP, Cheng CY, Mruk DD. Interleukin-1α (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol Reprod. 2008;78:445–454. doi: 10.1095/biolreprod.107.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell BP. The functional significance of the blood-testis barrier. J Androl. 1980;1:3–10. [Google Scholar]
- Setchell BP, Waites GMB. The blood-testis barrier. In: Hamilton DW, Greep RO, editors. The Handbook of Physiology. Baltimore: Williams and Wilkens; 1975. pp. 143–172. [Google Scholar]
- Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the "blood-testis barrier" after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1 integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- Steinberger A, Jakubowiak A. Sertoli cell culture: historical perspective and review of methods. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 155–180. [Google Scholar]
- Su L, Cheng CY, Mruk DD. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol. 2009;41:2578–2587. doi: 10.1016/j.biocel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarulli GA, Meachem SJ, Schlatt S, Stanton PG. Regulation of testicular tight junctions by gonadotrophins in the adult Djungarian hamster in vivo. Reproduction. 2008;135:867–877. doi: 10.1530/REP-07-0572. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin: Landes Bioscience and Springer Science+Business Media; 2008. pp. 186–211. [Google Scholar]
- Wang CQF, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res. 2007;313:1373–1392. doi: 10.1016/j.yexcr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolski KM, Mruk DD, Cameron DF. The Sertoli-spermatid junctional complex adhesion strength is affected in vitro by Adjudin. J Androl. 2006;27:790–794. doi: 10.2164/jandrol.106.000422. [DOI] [PubMed] [Google Scholar]
- Wong CCS, Chung SSW, Grima J, Zhu LJ, Mruk DD, Lee WM, et al. Changes in the expression of junctional and nonjunctional complex component genes when inter-Sertoli tight junctions are formed in vitro. J Androl. 2000;21:227–237. [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lee WM, Cheng CY. Targeted and reversible disruption of the blood-testis barrier by an FSH mutant-occludin peptide conjugate. FASEB J. 2007;21:438–448. doi: 10.1096/fj.05-4144com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Wong CH, Xia W, Lee NPY, Mruk DD, Lee WM, Cheng CY. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology. 2005;146:1192–1204. doi: 10.1210/en.2004-1275. [DOI] [PubMed] [Google Scholar]
- Xia W, Cheng CY. TGF-β3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: an in vivo study. Dev Biol. 2005;280:321–343. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Cheng CY. C-type natriuretic peptide regulates blood-testis barrier dynamics in adult rat testes. Proc Natl Acad Sci USA. 2007;104:3841–3846. doi: 10.1073/pnas.0610100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci USA. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Wong EW, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008a;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008b;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]