Hepcidin, a 25 amino acid liver-derived peptide, has been recognized as a regulator of iron homeostasis. Hepcidin levels are negatively correlated with erythropoietic expansion, consistent with erythrocytes representing the major compartment that utilizes iron and hepcidin the major regulator for limiting iron availability (Nemeth, 2008). However, the molecular pathway transmitting the signal from proliferating erythrocytes to the regulation of hepcidin expression in hepatocytes has not been clearly defined.
Erythropoietin (EPO), which increases erythropoiesis, has been shown to be a potent repressor of hepcidin expression in vivo (Sun et al, 2006). The mechanism of in vivo EPO-mediated repression of hepcidin appears to be predominantly indirect, because after disruption of erythropoiesis by bone marrow irradiation, EPO-treated mice were unable to repress hepcidin (Vokurka et al, 2006). Furthermore, EPO is unable to completely repress hepcidin under inflammatory conditions, as evidenced by resistance to EPO treatment in dialysis patients with functional iron deficiency associated with high levels of serum C-Reactive Protein and interleukin-6 (Martone et al, 2003;de Francisco et al, 2009).
Tmprss6 (also called matriptase-2) was shown by Du et al.(2008) to be an important negative regulator of hepcidin expression. This raised the question whether EPO mediated it’s effect on hepcidin through tmprss6. Hypothetically, EPO could increase tmprss6 levels resulting in increased degradation of hemojuvelin leading to a decrease in hepcidin expression. If this were the case, then mutations in TMPRSS6 would impair responsiveness to EPO. In contrast, if EPO did not mediate its response through tmprss6, then EPO should be efficacious in correcting the microcytic iron refractory iron deficiency anemia (IRIDA) in patients carrying mutations in TMPRSS6. Alternatively, EPO might repress hepcidin expression through down regulation of hemojuvelin directly. This study investigated the role of tmprss6 and hemojuvelin in EPO-induced repression of hepcidin expression.
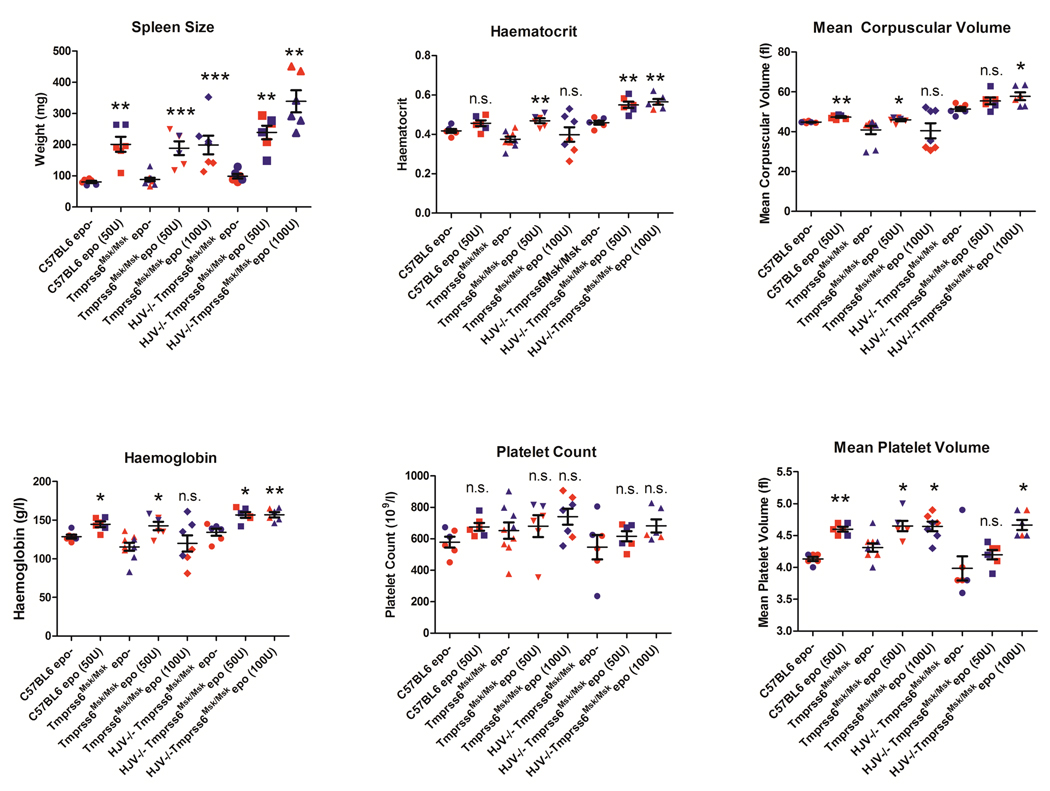
We administered 50 or 100 u/day of human recombinant EPO for four days to wildtype C57BL/6 and two types of Tmprss6 defective mice, Tmprss6msk/msk mutant mice, which exhibit iron deficient microcytic anemia, and Hfe2tm1Nca/tm1Nca Tmprss6msk/msk double mutant mice, which exhibit iron overload. Blood and tissues were harvested on day 5. Splenomegaly was significantly induced in the C57BL/6 wildtype mice and both types of Tmprss6 mutant mice (Figure 1). Furthermore, 50 u/day EPO for 4 days was effective in increasing the haemoglobin levels, mean corpuscular volume and hematocrit in wildtype and both types of Tmprss6 mutant. Tmprss6msk/msk mutant mice, which are iron deficient and express high levels of endogenous Hamp were less responsive to high levels (100 u per day for 4 days) of EPO treatment. It is unclear why high levels of EPO were ineffective in Tmprss6msk/msk mutant mice when low levels of EPO were effective but it might be related to the baseline level of EPO in these mice, which has not been determined. In contrast, mice lacking functional hemojuvelin and tmprss6, which are iron overloaded and express low levels of endogenous Hamp, demonstrated an erythropoietic response even at high concentrations of EPO. Platelet counts and platelet volume were similarly elevated demonstrating a normal physiological response of Tmprss6 mutant mice to EPO. These data clearly demonstrate that the lack of functional tmprss6 does not impair erythropoiesis stimulated by EPO, and in fact, the microcytic iron deficiency anaemia caused by mutations in Tmprss6 can be corrected with EPO administration.
Figure 1. Effect of EPO on mice spleen mass and hematological parameters.
C57BL6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The Tmprss6 mask mice (Tmprss6 msk/msk) and mice lacking both functional hemojuvelin (HFE2) and TMPRSS6 (Hfe2tm1Nca/tm1Nca;Tmprss6msk/msk) have been previously described (Du et al, 2008;Truksa et al, 2009). C57BL6, Tmprss6 msk/msk, or Hfe2tm1Nca/tm1Nca (HJV−/−)Tmprss6msk/msk mice (n>=6 each) received 50 or 100 u of EPO (Cell Sciences, Canton, MA) per day for 4 days. Mice were sacrificed on Day 5. Spleens were harvested and weighed. Blood was drawn and haematological parameters were measured as described in Methods. Each point represents one mouse. Female mice are indicated in red, male mice in blue. *, P≤0.05; **,P≤0.005; ***, P≤0.0005, as compared to respective EPO- control.
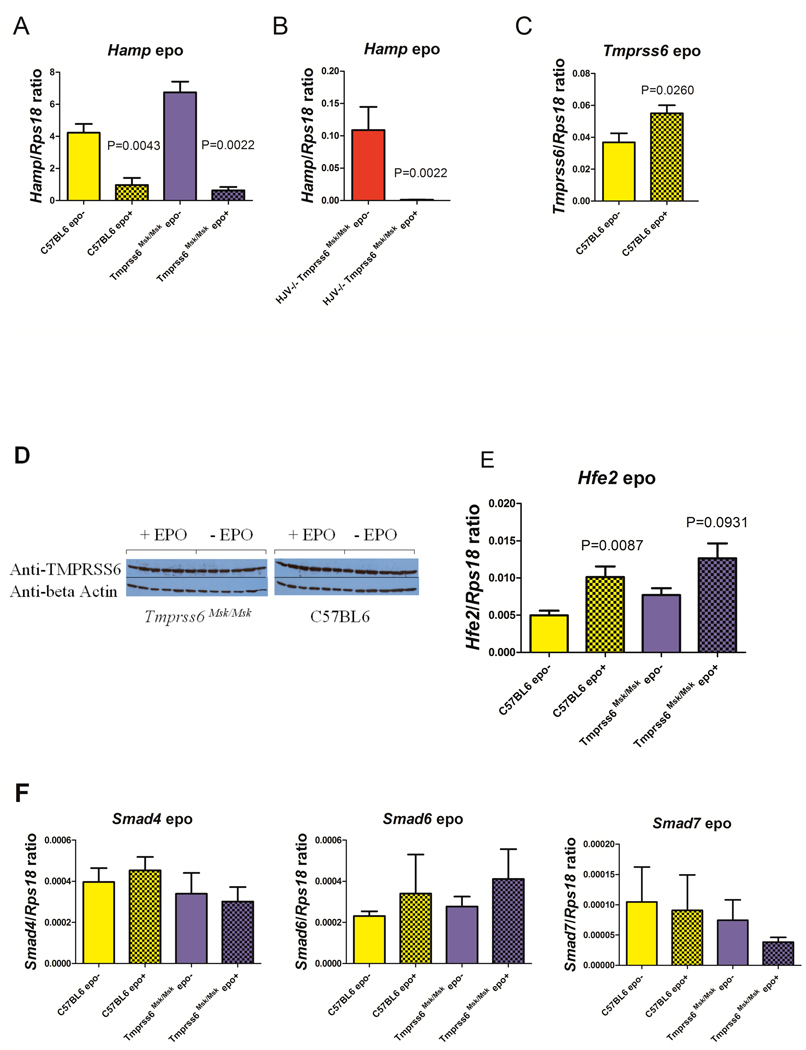
In vivo administration of rhEPO resulted in a >10-fold reduction in Hamp mRNA levels compared to the untreated wildtype mice, as assessed by quantitative reverse trasnscription polymerase chain reaction (Figure 2A). The high endogenous level of Hamp expression in Tmprss6msk/msk (iron deficient phenotype) mice was likewise suppressed in response to EPO (Figure 2A). In mice lacking functional hemojuvelin and tmprss6 (iron overloaded phenotype), the low endogenous Hamp expression was also robustly repressed by EPO (Figure 2B). These data clearly demonstrate that tmprss6 is not required for the repression of Hamp expression in response to EPO.
Figure 2. Effect of in vivo EPO treatment on Hamp, Tmprss6, and Hfe2 expression.
C57BL6 or Tmprss6 msk/msk mice or Hfe2tm1Nca/tm1Nca (HJV−/−)Tmprss6msk/msk mice (n=6 each) received 50 u/day for 4 days, were sacrificed on Day 5, and total liver RNA and protein were isolated. A,B,C,E,F Quantitative RT-PCR was performed using Hamp, Tmprss6, Hfe2, Smad4, Smad6 and Smad7 specific TaqMan probes and primers (Truksa et al, 2009). The gene encoding the 18S ribosomal protein Rps18 was used as a normalization gene. Means and standard errors are shown. Statistical analyses were performed with GraphPad Prism software using Mann Whitney non-parametric two tailed t-test. D. Western blot analysis was performed using pooled polyclonal antibodies against tmprss6/matriptase-2 protease domain, CUB domain and cytoplasmic domain (1:10,000) (Santa Cruz) or beta actin HRP (Sigma). Each lane represents the liver extract from one mouse.
The effect of EPO on mRNA and protein expression of Tmprss6 was also examined. We found that hepatic Tmprss6 mRNA expression in C57BL/6 mice was slightly increased by EPO treatment (Figure 2C). Nevertheless, Western blot analyses using antibodies against tmprss6/matriptase-2 demonstrated that levels of tmprss6/matriptase-2 in EPO-treated liver lysates were unchanged (Figure 2D). These data demonstrate that EPO does not repress hepcidin expression through increased expression of tmprss6 protein.
Given that EPO represses Hamp expression independently of tmprss6, we considered whether EPO might mediate Hamp repression through down regulation of hemojuvelin. Surprisingly, EPO increased, rather than decreased, expression of Hfe2 mRNA (Figure 2E). EPO dependent changes in expression of hemojuvelin protein could not be determined because commercially available antibodies are not specific for hemojuvelin since they recognize an identical size protein in Hfe2tm1Nca/tm1Nca mice that lack hemojuvelin (data not shown). Thus, to determine if EPO requires hemojuvelin for repression of Hamp expression, mice defective in both hemojuvelin and tmprss6 were tested for responsiveness to EPO. Mice defective in both hemojuvelin and tmprss6 were able to effectively repress hepcidin expression in response to EPO (Figure 2B). While this manuscript was in preparation, Krijt et al (2010) reported that hemojuvelin mutant (Hfe2 [Hjv]−/−) mice were responsive to EPO, in agreement with our data.
Hemojuvelin, a co-receptor for bone morphogenic proteins BMP-2, -4, and -6, signals through SMAD receptors 1/5/8 and the co-activator SMAD4 to regulate Hamp expression. We examined if EPO might mediate the repression of hepcidin expression through downregulation of SMAD4 (activating SMAD), or upregulation of SMAD6 or SMAD7 (inhibitory SMADs). Mice lacking functional tmprss6 did not express endogenously higher mRNA levels of SMAD4 or lower levels of SMAD6 or SMAD7 that might account for their abnormally high Hamp expression. Furthermore, we found that EPO treatment had no effect on the mRNA expression of SMAD4, SMAD6 or SMAD7 (Figure 2F) or protein expression (data not shown). Huang et al (2009) demonstrated that EPO treatment was associated with a decrease in phosphorylation of SMAD1/5/8. It remains to be determined if phosphorylation changes of the SMAD4,SMAD6 and SMAD7 play a role in EPO-mediated repression of hepcidin.
In conclusion, these data demonstrate that tmprss6 and hemojuvelin are not required for EPO-induced repression of hepcidin expression. Furthermore, these data suggest that EPO would be effective in restoring naormal hematological parameters to IRIDA patients carrying TMPRSS6 mutations by repressing HAMP expression thereby allowing increased iron uptake. In addition, activators of TMPRSS6 might be effective in repressing hepcidin and restoring EPO sensitivity, particularly in anaemia ofchronic inflammation.
Acknowledgements
This is manuscript 20709-MEM from the Scripps Research Institute. This work was supported by grants from the National Institutes of Health grant DK053505-11, The Skaggs Scholars in Clinical Science Program from The Scripps Research Institute, the Stein Endowment Fund, and the Beutler Foundation.
Footnotes
Disclosure
The authors have no conflicts of interest to declare.
References
- de Francisco AL, Stenvinkel P, Vaulont S. Inflammation and its impact on anaemia in chronic kidney disease: from haemoglobin variability to hyporesponsiveness. Nephrology Dialysis Transplantation Plus. 2009;2:i18–i26. doi: 10.1093/ndtplus/sfn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, Khovananth K, Mudd S, Mann N, Moresco EM, Beutler E, Beutler B. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Constante M, Layoun A, Santos MM. Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood. 2009;113:3593–3599. doi: 10.1182/blood-2008-08-173641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijt J, Jonasova A, Neuwirtova R, Necas E. Effect of erythropoietin on hepcidin expression in hemojuvelin-mutant mice. Blood Cells, Molecules, and Diseases. 2010;44:257–261. doi: 10.1016/j.bcmd.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Martone M, Zanchi R, Panzetta G. Role of iron deficiency in erythropoietin sensitivity in dialysis patients with elevated C-reactive protein. Giornale Italiano Nefrologia. 2003;20:31–37. [PubMed] [Google Scholar]
- Nemeth E. Iron regulation and erythropoiesis. Current Opinion in Hematology. 2008;15:169–175. doi: 10.1097/MOH.0b013e3282f73335. [DOI] [PubMed] [Google Scholar]
- Sun XF, Zhou DB, Zhao YQ. An iron regulator hepcidin is affected by EPO. Zhongguo Shi Yan.Xue.Ye.Xue.Za Zhi. 2006;14:778–782. [PubMed] [Google Scholar]
- Truksa J, Gelbart T, Peng H, Beutler E, Beutler B, Lee P. Suppression of the hepcidin-encoding gene Hamp permits iron overload in mice lacking both hemojuvelin and matriptase-2/TMPRSS6. British Journal of Haematology. 2009;147:571–581. doi: 10.1111/j.1365-2141.2009.07873.x. [DOI] [PubMed] [Google Scholar]
- Vokurka M, Krijt J, Sulc K, Necas E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiological Research. 2006;55:667–674. doi: 10.33549/physiolres.930841. [DOI] [PubMed] [Google Scholar]