Abstract
To establish the relevance of targeting disease-associated T cells in anti-RNP-associated glomerulonephritis, mice developing nephritis following immunization with U1-70-kd small nuclear ribonucleoprotein (snRNP) were treated with a single dose of irradiated antigen-selected T cell vaccine. T cell receptor usage in nephritic kidneys revealed oligoclonal use of T Cell Receptor V Beta (TRBV) genes as previously found in spleens and lungs of immunized mice with pulmonary disease. The CDR3 regions from T cell isolates showed sequence homology to those in humans with anti-RNP autoimmunity. Following T cell vaccination, urinalysis returned to normal in 5/7 treated mice (71% response rate) whereas all mock treated mice continued to have an active urinary sediment (Fisher’s Exact p=0.02). An oligoclonal population of T cells homologous to those identified in humans with anti-RNP autoimmunity is implicated in disease pathogenesis, and T cell vaccination is associated with a high rate of clinical improvement in established nephritis.
Introduction
Antibodies to components of the U1 small nuclear ribonucleoprotein autoantigen (U1 snRNP) are frequently encountered in patients with systemic lupus erythematosus,[1] and have been correlated with increased severity of disease and increased incidence of renal disease in lupus.[2; 3] Indeed, the strength of the correlation between renal disease and anti-RNP antibodies has been reported to be higher than the correlation between anti-double-stranded DNA antibodies and lupus nephritis.[4] However, anti-B cell therapy has been disappointing in the treatment of anti-RNP-associated lupus.[5] In contrast, a putative anti-RNP T cell tolerogen has shown promising results in this spectrum of disease,[6] suggesting that T cell activity may be central to the pathogenesis of anti-RNP-associated lupus.
Anti-RNP T cells are a plausible target for immunotherapy in lupus nephritis because they are oligoclonal and are persistent in individual patients, [7] They also show consistent Vbeta chain usage both in multiple human patients and in an induced model of murine anti-RNP nephritis.[8] Anti-RNP T cells in humans have been shown to have degenerate autoantigen reactivity towards other lupus autoantigens including Sm,[9] and in addition have been shown to be capable of increasing human B cell secretion of lupus autoantibodies.[10] In our models of induced anti-RNP autoimmunity, we have previously shown that adoptive transfer of CD4+ T cells from immunized mice to non-irradiated syngeneic naïve recipients is sufficient to induce the development of nephritis in the recipients.[11]
T cell vaccination involves the infusion of killed T cells of a restricted specificity to engender a specificity-specific reduction in T cell reactivity. This has been reported to be an effective approach to the treatment of human autoimmune disease in other models.[12] Current treatments for lupus nephritis are broadly immunosuppressive and associated with frequent and severe side effects. One attempt to use an anti-RNP T cell clone for T cell vaccination therapy in MRL/lpr mice did not lead to a clinically significant effect,[13] but cells expressing the T cell receptor used for this vaccination had not been linked to end organ damage, and expressed a different Vbeta chain than those we have identified in humans and in lesional tissues in mice.[8]
We therefore sought to characterize the therapeutic potential of targeting T cells associated with induced anti-RNP glomerulonephritis. Based on evidence that these cells were homologous to the anti-RNP T cells we have previously identified in human and murine systems, we attempted to treat established anti-RNP-associated nephritis in an induced murine model with T cell vaccination. We observed that by two months after a single course of therapy, active urinary sediment resolved in over 70% of treated mice while persisting in all of the sham-treated animals. These results highlight a role for T cells in the pathogenesis of anti-RNP associated glomerulonephritis, and suggest that targeting a limited set of T cells in anti-RNP autoimmunity can lead to clinically relevant results.
Methods
Mice
Experiments were performed using C57BL/6Ntac-[KO]Abb-[Tg]DR-4 mice (Taconic, Germantown, NY), transgenic for the expression of a chimeric human/mouse class II MHC in which the extracellular antigen presentation domains of HLA-DR4 have replaced the native murine class II regions, with the remainder of the native murine molecule intact, that we will refer to as DR4 mice.[14] All experiments were performed according to IACUC-approved protocols in AALAC-certified facilities. DR4 mice were immunized subcutaneously once in the flank with 50 micrograms of U1-70kD small nuclear ribonucleoprotein fusion protein (70k) and 50 micrograms of U1-RNA adjuvant at 8–10 weeks of age, and boosted 2 weeks later with the same preparation. The 70k antigen was a maltose binding protein fusion protein form of peptides 63–205 of the U1-70kD snRNP antigen, produced and purified as previously reported.[14] We observed that boosting the mice did not affect the rate of anti-RNP seroconversion, but did lead to an increased rate of development of renal manifestations clinically (data not shown). Mice that had anti-RNP antibodies and active urinary sediment one month after the final immunization were randomly assigned to either treatment or mock treatment groups until 7 treatment and 7 mock-treatment mice were entered into the study. Ear punch markings placed at the time of immunization were used to identify individual mice.
T Cell Line/Vaccine Preparation
Murine RNP-specific T cell lines were generated as previously described.[8]] In brief, spleen and lymph node cells were obtained sterilely at necropsy, mechanically disrupted, filtered through sterile 100 µm nylon mesh and subjected to density gradient centrifugation using Histopaque (Sigma). Cells obtained from immunized mice were used immediately for the generation of T cell lines. Cells obtained from naïve mice were irradiated with 30 Grey (Gy) and used as APC to stimulate T cells for the generation of T cell lines and in proliferation assays. Approximately 5 × 106 cells were cultured in DMEM with 2 mM L-glutamine (complete medium), supplemented with 20 µg/ml gentamicin, 15% fetal calf serum and containing 70k fusion protein at a final concentration of 50 µg/ml, as described.[8] Cells in a final volume of 5 ml were placed in 25-cm2 flasks and incubated in 5% carbon dioxide at 37°C. Cells were restimulated with 5 × 106 syngeneic APC from a naïve donor that had been irradiated with 30 Gy plus antigen in fresh medium on days 7–10. Following one cycle of stimulation, cells were harvested, washed, counted, irradiated with 30 Gy, and brought to a concentration of 1.5 × 107 cells per ml in sterile PBS. For TCR analysis, RNA was extracted from cells that had been stimulated with plate-bound anti-CD3 (applied overnight at 4 degrees C at 10 µg/ml) in the absence of APC at 48 h for TCR analyses using RNeasy (InVitrogen, Carlsbad, CA) (REF: Talken, 2001,Scan J immunol)[15]
T Cell Proliferation Assays
Spleen and lymph node cells were separated from study mice and grown for seven days with irradiated APCs from naïve syngeneic mice plus 70k antigen to generate T cell lines as above. The cells were then washed, sorted by AutoMACS for CD4 positivity, and placed into culture in the same medium with either: irradiated naïve syngeneic APCs only, irradiated naïve syngeneic APCs plus 70k antigen as above, or plate-bound anti-CD3 only. After 24 hours of incubation, cells were pulsed with tritiated thymidine, and harvested 18 hours later. Thymidine uptake was measured by scintillation counting of low energy proton events, and results were expressed as a ratio in each test condition to thymidine uptake with APCs only (specific activity). Each experiment was performed in triplicate; mean values are presented.
T Cell Vaccine Delivery
100 microliters of fresh irradiated T cell vaccine was delivered to each mouse in the treatment arm by subcutaneous injection into the flank while under isoflurane anesthesia. Mock treated mice received 100 ul of sterile PBS.
Disease assessment
The presence of active urinary sediment was determined by urinalysis on urine specimen taken at the time of T cell vaccination and again at the time of sacrifice. Specimens were obtained and results recorded by an investigator blinded to the treatment status of the mice. The presence of histologic abnormalities was determined by blinded reading of fixed, paraffin-embedded tissues stained with Hematoxylin and Eosin, as previously reported.[14] Anti-RNP antibodies were measured by ELISA using genenase-cleaved, gel-purified 70k protein (from the purified 63–205 70k fusion protein) as coating antigen, as previously described.[8,11,14,16]. In brief, 96 well flat-bottomed microtitre plates were incubated at 4°C overnight with purified Ag in PBS. The plates were washed with PBS/0.05% Tween 20 buffer, blocked with 3% powdered milk in PBS/Tween 20, incubated with mouse sera at a final dilution of 1:100 and developed with HRP-linked Fc region-specific goat anti-mouse-IgG secondary antibody followed by orthophenylenediamine. Absorbance at 450 nm was measured in a microtitre plate reader. Samples were tested in duplicate. Pre-immune sera, pre-treatment sera, and post-treatment sera for the same mice were analyzed at the same time on the same ELISA plate, blinded to the treatment status of the study mice. Sera were designated as positive for anti-70k (and hence for anti-RNP) if the mean optical density of the test serum was greater than the mean optical density of at least 5 syngeneic contemporaneously raised unimmunized mice (normal controls) measured in duplicate on the same plate, plus five standard deviations of the normal control optical densities (mean + 5S.D.).
Data Analysis
Statistics were calculated with Prism 5.0 (GraphPad Software, San Diego). Contingency tables were analyzed using Fisher’s Exact test. Continuous variables were analyzed using the Student’s T Test (data was shown to approximate Gaussian distributions for each variable analyzed; results using Mann-Whitney U Test nonparametric analysis yielded equivalent results [not shown]).
Results
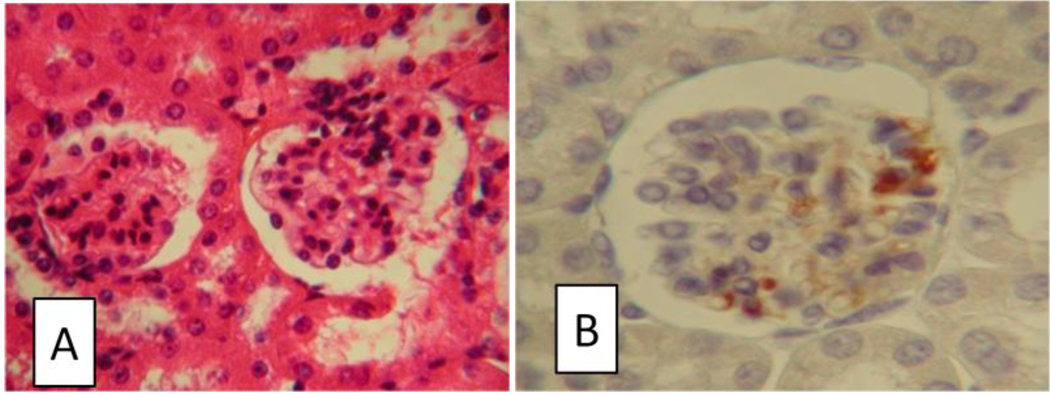
We have previously observed that B6 mice expressing the extracellular domain of HLA-DR4 (study mice) can develop a diffuse proliferative glomerulonephritis after immunization with 70k and that this nephritic presentation can be transferred to naïve syngeneic mice by 70k-specific CD4+ T cells.[11] Using immunohistochemical staining of the kidneys of mice with active urinary sediment after anti-70k immunization, we have confirmed the presence of mononuclear cells staining for CD4 in glomerular infiltrates (Figure 1).
Figure 1.
Presence of glomerular infiltrates with CD4+ cells in a model of anti-RNP autoimmunity. Mice sacrificed two months after immunization with 70k and U1-RNA, in which anti-RNP antibodies were present and proteinuria was observed, were sectioned. Images were captured at 40× magnification, cropped, and resized. A: Glomerular and periglomerular infiltrates were present in mice with active urinary sediment on haematoxylin and eosin (H&E) stained slides. B: CD4+ cells were present in glomeruli in active lesions by immunohistochemistry.
Single cell suspensions of kidneys were prepared from anti-70k-immunized study mice with nephritis, grown in short-term culture under T cell selective conditions, and assessed for T cell receptor usage. We found that the same oligoclonal use of TRBV5, TRBV13-1, and TRBV13-3 genes were present in these cells as we have previously reported in the spleens of 70k immunized mice and in the lungs of immunized mice that develop pulmonary disease (Table 1).[8] The CDR3 regions of these murine T cell isolates also are closely homologous to the CDR3 regions of anti-70k T cells cloned from human patients with anti-RNP autoimmunity (Table 2).
Table 1.
Number of T cell receptor (TCR) Vβ sequences isolated from T-cell lines and hybridomas of 70kD-immunized mice
| TRBV | Vβ | NO. OF SEQUENCES | |||
|---|---|---|---|---|---|
| Gene | Spleen | Lung | Kidney | Hybridomas | |
| (na=8) | (n=2) | (n=4) | (n=5) | ||
| TRBV5 | Vβ1 | 18b/18c | 4/4 | 1/1 | 1/1 |
| TRBV1 | Vβ2 | 14/14 | |||
| TRBV26 | Vβ3 | 12/12 | |||
| TRBV12-2 | Vβ5.1 | 6/6 | |||
| TRBV12-1 | Vβ5.2 | 5/5 | |||
| TRBV19 | Vβ6 | 20/19 | |||
| TRBV13-3 | Vβ8.1 | 21/20 | 5/5 | 3/2 | 1/1 |
| TRBV13-2 | Vβ8.2 | 16/15 | 7/7 | ||
| TRBV13-1 | Vβ8.3 | 24/23 | 9/8 | 4/1 | 3/2 |
| TRBV4 | Vβ10 | 8/7 | 2/2 | ||
| TRBV16 | Vβ11 | 5/5 | |||
| TRBV14 | Vβ13 | 2/1 | |||
| TRBV31 | Vβ14 | 9/9 | 10/8 | ||
| TRBV3 | Vβ16 | 8/8 | |||
| TRBV23 | Vβ20 | 6/6 | 2/1 | ||
Number of mice
Number of total sequences isolated
CDR3 sequences with ≥90% a.a. identity were counted as unique
Table 2.
Alignment of deduced amino acid sequences for v T cell receptor (TCR) from human and murine T cell lines, and mouse T cell hybridomas
| Origin [a] | TRBV sequences | ||
|---|---|---|---|
| CDR3 | J | J region | |
| Human | CAS SQNGQHYEQY | FGPGTRLTVT | 2S7 |
| CAI SGQGDYEQY | FGPGTRLTVT | 2S7 | |
| CAT SGQGYEQY | FGPGTRLTVT | 2S7 | |
| CAS SEGLAGVPDTQY | FGPGTRLTVL | 2S5 | |
| Murine | |||
| Spleen | CAS SPTGGRSYEQY | FGPGTRLTVL | 2S7 |
| CAS SMGQPYEQY | FGPGTRLTVL | 2S7 | |
| CAS SQEGWGGEDTQY | FGPGTRLLVL | 2S5 | |
| CAS SLGGLQDTQY | FGPGTRLLVL | 2S5 | |
| CAS SPGDSNERLF | FGHGTKLSVL | 1S4 | |
| CAS SPRDRNTGQLY | FGEGSKLTVL | 2S2 | |
| Lung | CAS SPGQGAYEQY | FGPGTRLTVL | 2S7 |
| CAS SSGTGSYEQY | FGPGTRLTVL | 2S7 | |
| CAS SLLGGQQDTQY | FGPGTRLTVL | 2S5 | |
| CAS SRENQDTQY | FGPGTRLTVL | 2S5 | |
| CAS SDASRTNERLF | FGHGTKLSVL | 1S4 | |
| Kidney | CAS SDEGVSSYEQY | FGPGTRLTVT | 2S1 |
| Hybridoma | |||
| 9 | CAS RGDISYEQY | FGPGTRLLVL | 2S7 |
| 50 | CAS SDGFNQDTQY | FGPGTRLLVL | 2S5 |
| 133 | CAS RGLGLDTQY | FGPGTRLLVL | 2S5 |
| 11 | CAS SDALFSNERLF | FGHGTKLSVL | 1S4 |
| 114 | CAS SPPGLGNTQLY | FGEGSKLTVL | 2S2 |
Human sequences are from T cell clones derived from Mixed Connective Tissue Disease patients.16 Murine sequences are from T cell lines generated using 70kD-immunised mice restimulated invitro using 70kD. T cell hybridomas were generated from spleen cells of mice immunized with 70kD that were fused with the thymoma cell line BW5147 in the presence of polyethylene glycol.
Sequences which were shared for T cell receptor Vbeta region (TRBV) are underlined.
Based on these findings, we developed T cell vaccination therapy using irradiated anti-70k T cells, under the rationale that immune responses to these oligoclonal cells could induce reductions in pathologic T cell immunity in anti-RNP autoimmunity. We chose to assess the effects of T cell vaccination on established anti-RNP nephritis 2 months after treatment, at which point test mice were sacrificed and analyzed for urinary sediment and for renal histology. Mice induced to have anti-70k autoimmunity that were found to have nephritis on the basis of active urinary sediment were randomly assigned to receive either treatment with anti-RNP T cell vaccination or mock vaccination. Thus, 7 mice with nephritis received T cell vaccination and 7 mice with nephritis (from the same litters and housed along with the vaccinated mice) received mock vaccination.
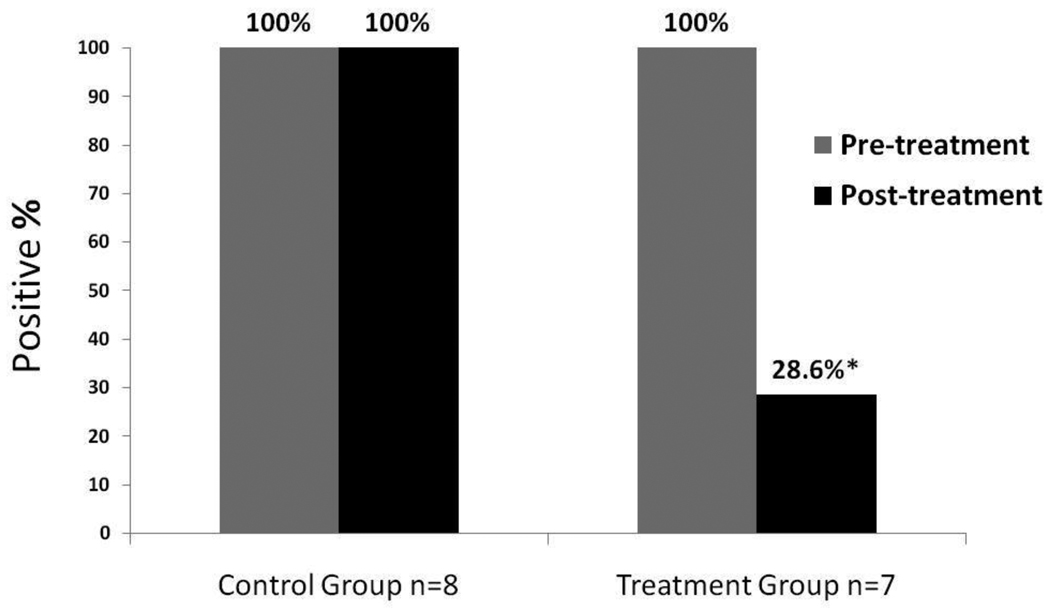
Mice receiving either mock vaccination with PBS or the T cell vaccination were observed to have no adverse effects after treatment administration. In contrast to the mock treated mice, who continued to have active urinary sediment in 7/7 cases (0% response rate), normalization of the urinalysis was observed in 5/7 mice two months after receiving the T cell vaccination (71% response rate, Fisher’s Exact p = 0.02 versus mock treated) (Figure 2). Renal histology results correlated with the urinalysis findings (Figure 3).
Figure 2.
Efficacy of T cell vaccination in nephritic mice with anti-RNP autoimmunity. Urine was assessed immediately prior to treatment with anti-70k-specific T cell vaccination (Treatment Group), or with phosphate buffered saline (PBS) mock vaccination (Control Group). Mice with active urinary sediment (including proteinuria in each case) prior to treatment were characterized as having Kidney Disease. Two months later, urine was again obtained from each study mouse and assessed for active sediment including protein. An absence of active urinary sediment was observed in 5/7 mice in the treatment group, but in none of the mice in the control group, Fisher’s Exact p = 0.02.
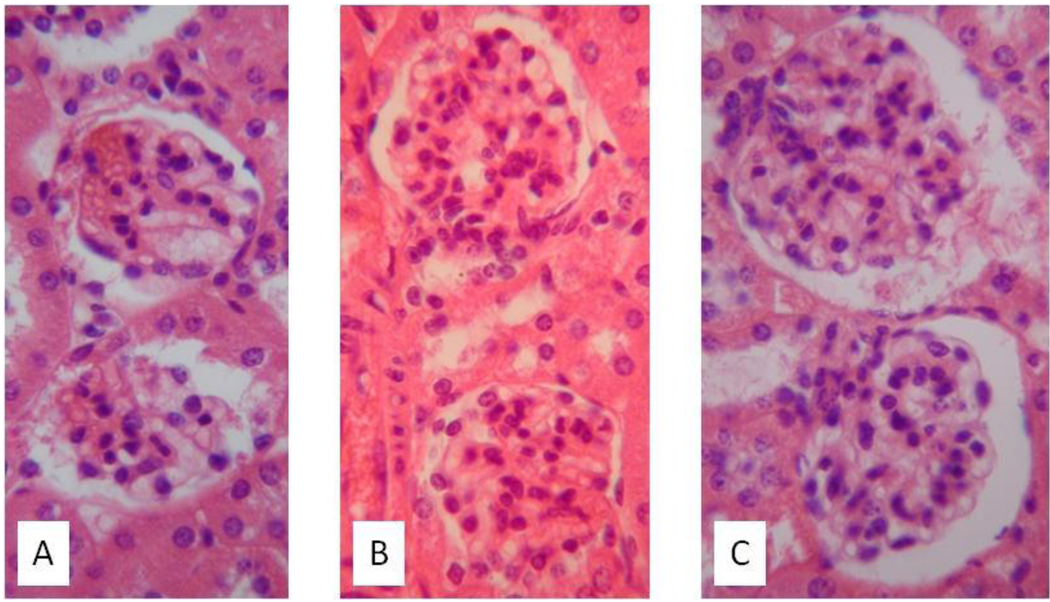
Figure 3.
Histological findings in recipients of T cell vaccination and mock treatment. At the conclusion of the study protocol, mice were sacrificed and haematoxylin and eosin (H&E) stained renal images were prepared as in Figure 1. Representative images are shown. A: T cell vaccine recipient with normalization of urinalysis (“responder”) shows normal renal histology. B: T cell vaccine recipient with persistently active urinary sediment (“nonresponder”) shows proliferative glomerulonephritis. C: Mock vaccine recipient with persistent active urinary sediment shows proliferative glomerulonephritis.
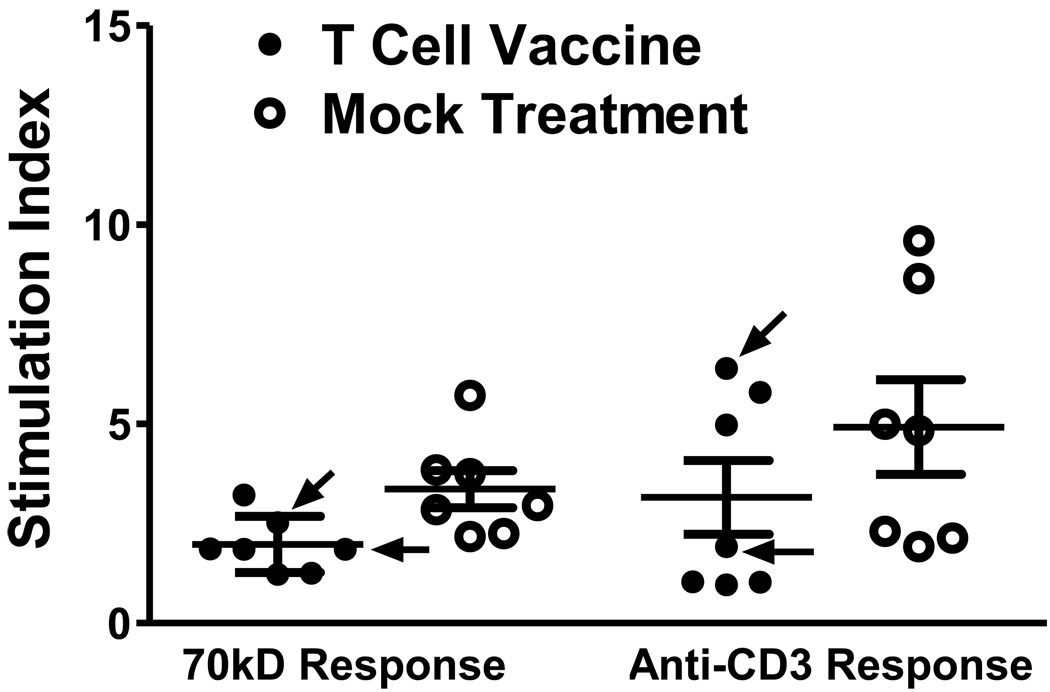
To characterize the mechanism of action of the renal response in treated mice, we tested the effects of T cell vaccination on splenic T cells from our study mice. We found that, in comparison to mock-treated mice with anti-70k autoimmunity, splenic T cells from treated mice had lower proliferative responses to 70k antigen (Figure 4, p = 0.02). In contrast to the anti-70k responses, the rate of nonspecific T cell proliferation in response to anti-CD3 could not be distinguished between the treated and mock-treated mice (Figure 4, p = 0.3). In the two nephritic mice that received T cell vaccination but continued to have active nephritis (“nonresponders”), the anti-70k-specific T cell proliferative responses were among the higher values observed among vaccinated mice, though they were both still over 1 SD below the mean for anti-70kD responses in mock-treated mice. In contrast, with nonspecific anti-CD3 proliferation, one of the two “nonresponder” mice showed proliferation over 2 standard deviations below the mock vaccinated mean, while two “responder” mice showed nonspecific T cell proliferation after T cell vaccination that was higher than the mean observed with mock-treated mice (Figure 4). These results suggest that attenuation of anti-70k-specific T cell activity but not anti-CD3 T cell activity is associated with clinical response after T cell vaccination, and provides evidence that the anti-T cell effects of the vaccination protocol are not nonspecific.
Figure 4.
Antigen-specific inhibition of T cell proliferation after T cell vaccination. Immune effects of T cell vaccination versus mock vaccination were assessed in anti-70k treated study mice. Splenic T cells were tested for proliferation to irradiated antigen presenting cells (APCs) plus 70k peptide (70kD Response), or to plate bound anti-CD3 antibody (Anti-CD3 Response); results are expressed as Stimulation Index relative to proliferation in response to irradiated APCs alone. Each data point is plotted, and mean +/− SD are shown. T cell vaccine inhibits 70k-induced T cell proliferation compared to mock vaccination (p = 0.02), but anti-CD3-induced proliferation does not differ between T cells from vaccinated and mock-vaccinated mice (p = 0.3). Arrows denote T cell vaccine-treated mice with persistent nephritis (nonresponders).
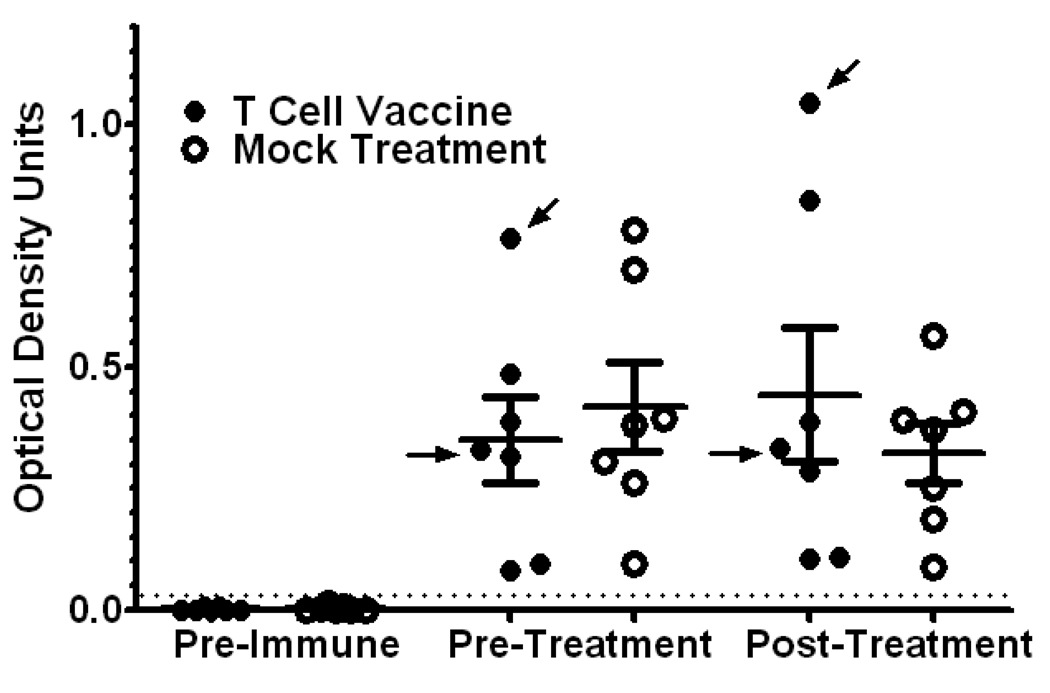
To determine whether the T cell vaccination protocol had effects on autoantibody levels, anti-70k antibodies were quantitated by ELISA (Figure 5). Sera were judged to be positive for anti-70k antibodies if their ELISA optical density results were at least 5 standard deviations higher than the results for normal control mice measured on the same ELISA plates. Levels of anti-70k IgG autoantibodies varied widely among study animals, but autoantibody levels changed little between pre-immune and post-immune sera drawn two months apart. The ordinal ranking of mice in terms of anti-70k serum titers from highest titer to lowest titer was unchanged in both the T cell vaccination group and the mock vaccination group between the pre-treatment and post-treatment ELISA values. T cell vaccination did not reduce the titers of anti-70k antibodies in test mice compared to mock treatment (Figure 5), suggesting that inhibition of B cell activity was not its mechanism of action.
Figure 5.
Anti-RNP antibody levels with T cell vaccination versus mock treatment. Anti 70k IgG antibodies were measured by ELISA from sera obtained prior to injection with 70k and U1-RNA (Pre-Immune), immediately prior to T cell vaccination (Pre-Treatment) and 2 months after T cell vaccination (Post-Treatment), all measured on the same ELISA plate at the same time. Mean optical density results are shown for each serum tested; group means +/− SD are also shown. For each set of sera, results are presented normalized to the same mean + 5 S.D. criterion for test positivity (dotted line). Arrows indicate results for the mice receiving T cell vaccination that continued to have nephritis despite T cell vaccination (nonresponders, as in Figure 3). The presence of high titer anti-70kD responses was not consistently present in mice with clinical nephritis. Despite its clinical benefit, T cell vaccination was not associated with a reduction in autoantibody levels. No differences between responder and nonresponder mice were noted.
Discussion
The same set of oligoclonal anti-RNP CD4+ T cells that we have previously identified in human anti-RNP autoimmunity and in the spleens and lungs of DR4 mice with anti-RNP autoimmunity are also present in the kidneys of mice with nephritis, where they localize to the glomerular and peri-glomerular areas. We have previously shown that adoptive transfer of anti-70k specific T cells purified in the same manner as in the current report, but not irradiated, induces nephritis in recipients. That T cell vaccination leads to antigen-specific reductions in T cell proliferation and is associated with a high rate of clinical improvement in established nephritis in this model, further establishes a role for this T cell subset in the pathogenesis of anti-RNP-associated glomerulonephritis. This result is consistent with other reports demonstrating that therapies that specifically modulate T cell responses are capable of treating established murine lupus nephritis[17]. Targeting anti-T cell therapies to oligoclonal sets of T cells thus has the potential to be clinically effective while sparing patients the risks of more general T cell immunosuppression.
Given that anti-RNP mice with interstitial pneumonitis also have T cell infiltrates with the same oligoclonal T cell subset, it is plausible that T cell vaccination could be effective for this spectrum of disease as well. We are currently working to standardize assessment of interstitial pneumonitis by noninvasive means in our animal model to allow us to assess the response to therapy in mice with established lung disease in the same manner that this study examined responses to therapy in established renal disease. Commonalities between the T cell populations in the kidneys of renal disease mice and the lungs of lung disease mice emphasizes that factors other than T cell specificity appear to dictate the tissue targeting of anti-RNP autoimmunity.
Limitations of this study include the model of nephritis itself, which though associated with diffuse glomerular proliferative changes and the development of hematoxylin bodies, is not associated with the development of crescents or glomerular deposition of immunoglobulin and complement spit products, and seldom progresses to end stage renal failure.[11] It is thus possible that the benefits of T cell vaccination in subsets of lupus nephritis with prominent humoral immunity effector mechanisms could be less profound than the effects observed here.
The mechanism of action of T cell vaccination in this study is not completely defined, but appears to be the reduction of antigen-specific T cell activation. In support of this idea, vaccination leads to reduced anti-70k-specific T cell proliferation compared to mock vaccination, in the context of an absence of detectable differences in the rates of nonspecific T cell proliferation between vaccinated and mock vaccinated mice. Likewise, no differences in anti-70k-specific antibody responses could be detected between vaccinated and mock-vaccinated mice.
An alternative explanation of the effects of our irradiated T cell preparation on our model of anti-RNP autoimmunity could propose that the infusion of irradiated cell debris induced nonspecific immunomodulatory signals. We view this as unlikely, because the time course of action of a non-specific anti-inflammatory signal leading to sustained remission of active nephritis for two months after administration despite the persistence of autoantibodies at stable titers is implausible. Such an effect, if present, would imply an anti-inflammatory activity more profound than glucocorticoid administration in sustained efficacy yet more focused than steroids in terms of the lack of effect on autoantibody levels, based on reports in other systems of murine nephritis.[18,19] Moreover, a nonspecific anti-inflammatory effect would not explain the modulation of anti-70k-specific but not anti-CD3-induced T cell activity. Future studies will explore whether the benefits of T cell vaccination are due to elimination of the baseline oligoclonal anti-RNP T cells, or due to modulation of their function and/or activity. In particular, studies assessing the effects of irradiated T cells that target alternative antigens (and that have different TCR usage) are planned.
This is the first study to demonstrate that oligoclonal anti-RNP T cells migrating to the kidney are linked to the pathogenesis of glomerulonephritis, and to demonstrate that a specific therapy targeting these T cells can induce a prominent clinical response in established disease. That these experiments were performed using an HLA-DR4 restricted model in which the oligoclonal T cells targeted are homologous to T cells isolated from human patients with anti-RNP-associated autoimmunity raises the possibility that a similar approach may be feasible and effective for the treatment of human anti-RNP-associated autoimmune syndromes.
Acknowledgements
The authors thank Dr. Mehrdad Nadji of the University of Miami Miller School of Medicine Department of Pathology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest with regard to the work presented.
Contributor Information
Sapna Trivedi, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
YunJuan Zang, University of Miami, Miller School of Medicine, Miami VA Medical Center.
Schartess Culpepper, University of Miami, Miller School of Medicine, Miami VA Medical Center.
Erica Rosenbaum, University of Miami, Miller School of Medicine, Miami VA Medical Center.
Irina Fernandez, University of Miami, Miller School of Medicine, Miami VA Medical Center.
Laisel Martinez, University of Miami, Miller School of Medicine, Miami VA Medical Center.
Robert W. Hoffman, University of Miami, Miller School of Medicine, Miami VA Medical Center
Eric L. Greidinger, University of Miami, Miller School of Medicine, Miami VA Medical Center
REFERENCES
- 1.Sharp GC, Irvin WS, May CM, Holman HR, McDuffie FC, Hess EV, Schmid FR. Association of antibodies to ribonucleoprotein and Sm antigens with mixed connective-tissue disease, systematic lupus erythematosus and other rheumatic diseases. N Engl J Med. 1976;295:1149–1154. doi: 10.1056/NEJM197611182952101. [DOI] [PubMed] [Google Scholar]
- 2.Hoet RM, Koornneef I, de Rooij DJ, van de Putte LB, van Venrooij WJ. Changes in anti-U1 RNA antibody levels correlate with disease activity in patients with systemic lupus erythematosus overlap syndrome. Arthritis Rheum. 1992;35:1202–1210. doi: 10.1002/art.1780351013. [DOI] [PubMed] [Google Scholar]
- 3.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 4.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 5.Cambridge G, Isenberg DA, Edwards JC, Leandro MJ, Migone TS, Teodorescu M, Stohl W. B cell depletion therapy in systemic lupus erythematosus: relationships among serum B lymphocyte stimulator levels, autoantibody profile and clinical response. Ann Rheum Dis. 2008;67:1011–1016. doi: 10.1136/ard.2007.079418. [DOI] [PubMed] [Google Scholar]
- 6.Muller S, Monneaux F, Schall N, Rashkov RK, Oparanov BA, Wiesel P, Geiger JM, Zimmer R. Spliceosomal peptide P140 for immunotherapy of systemic lupus erythematosus: results of an early phase II clinical trial. Arthritis Rheum. 2008;58:3873–3883. doi: 10.1002/art.24027. [DOI] [PubMed] [Google Scholar]
- 7.Greidinger EL, Foecking MF, Schäfermeyer KR, Bailey CW, Primm SL, Lee DR, Hoffman RW. T cell immunity in connective tissue disease patients targets the RNA binding domain of the U1-70kDa small nuclear ribonucleoprotein. J Immunol. 2002;169:3429–3437. doi: 10.4049/jimmunol.169.6.3429. [DOI] [PubMed] [Google Scholar]
- 8.Greidinger EL, Zang YJ, Jaimes K, Martinez L, Nassiri M, Hoffman RW. CD4+ T cells target epitopes residing within the RNA-binding domain of the U1-70-kDa small nuclear ribonucleoprotein autoantigen and have restricted TCR diversity in an HLA-DR4-transgenic murine model of mixed connective tissue disease. J Immunol. 2008;180:8444–8454. doi: 10.4049/jimmunol.180.12.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Silva-Udawatta M, Kumar SR, Greidinger EL, Hoffman RW. Cloned human TCR from patients with autoimmune disease can respond to two structurally distinct autoantigens. J Immunol. 2004;172:3940–3947. doi: 10.4049/jimmunol.172.6.3940. [DOI] [PubMed] [Google Scholar]
- 10.Greidinger EL, Gazitt T, Jaimes KF, Hoffman RW. Human T cell clones specific for heterogeneous nuclear ribonucleoprotein A2 autoantigen from connective tissue disease patients assist in autoantibody production. Arthritis Rheum. 2004;50:2216–2222. doi: 10.1002/art.20287. [DOI] [PubMed] [Google Scholar]
- 11.Greidinger EL, Zang Y, Fernandez I, Berho M, Nassiri M, Martinez L, Hoffman RW. Tissue targeting of anti-RNP autoimmunity: effects of T cells and myeloid dendritic cells in a murine model. Arthritis Rheum. 2009;60:534–542. doi: 10.1002/art.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Li N, Zang YC, Zhang D, He D, Feng G, Ni L, Xu R, Wang L, Shen B, Zhang JZ. Vaccination with selected synovial T cells in rheumatoid arthritis. Arthritis Rheum. 2007;56:453–463. doi: 10.1002/art.22316. [DOI] [PubMed] [Google Scholar]
- 13.Fujii T, Okada M, Fujita Y, Sato T, Tanaka M, Usui T, Umehara H, Mimori T. Vaccination with autoreactive CD4(+)Th1 clones in lupus-prone MRL/Mp-Fas(lpr/lpr) mice. J Autoimmun. 2009;33:125–134. doi: 10.1016/j.jaut.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Greidinger EL, Zang Y, Jaimes K, Hogenmiller S, Nassiri M, Bejarano P, Barber GN, Hoffman RW. A murine model of mixed connective tissue disease induced with U1 small nuclear RNP autoantigen. Arthritis Rheum. 2006;54:661–669. doi: 10.1002/art.21566. [DOI] [PubMed] [Google Scholar]
- 15.Talken BL, Bailey CW, Reardon SL, Caldwell CW, Hoffman RW. Structural analysis of TCRalpha and beta chains from human T-Cell clones specific for small nuclear ribonucleoprotein polypeptides Sm-D, Sm-B and U1-70 kDa: TCR complementarity determining region 3 usage appears highly conserved. Scand J Immunol. 2001;54:204–210. doi: 10.1046/j.1365-3083.2001.00930.x. [DOI] [PubMed] [Google Scholar]
- 16.Greidinger EL, Foecking MF, Ranatunga S, Hoffman RW. Apoptotic U1-70 kd is antigenically distinct from the intact form of the U1-70-kd molecule. Arthritis Rheum. 2002;46:1264–1269. doi: 10.1002/art.10211. [DOI] [PubMed] [Google Scholar]
- 17.Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171:489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 18.Borel Y, Lewis RM, André-Schwartz J, Stollar BD, Diener E. Treatment of lupus nephritis in adult (NZB + NZW)F1 mice by cortisone-facilitated tolerance to nucleic acid antigens. J Clin Invest. 1978;61:276–286. doi: 10.1172/JCI108937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appleby P, Webber DG, Bowen JG. Murine chronic graft-versus-host disease as a model of systemic lupus erythematosus: effect of immunosuppressive drugs on disease development. Clin Exp Immunol. 1989 Dec;78(3):449–453. [PMC free article] [PubMed] [Google Scholar]