Abstract
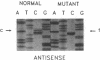
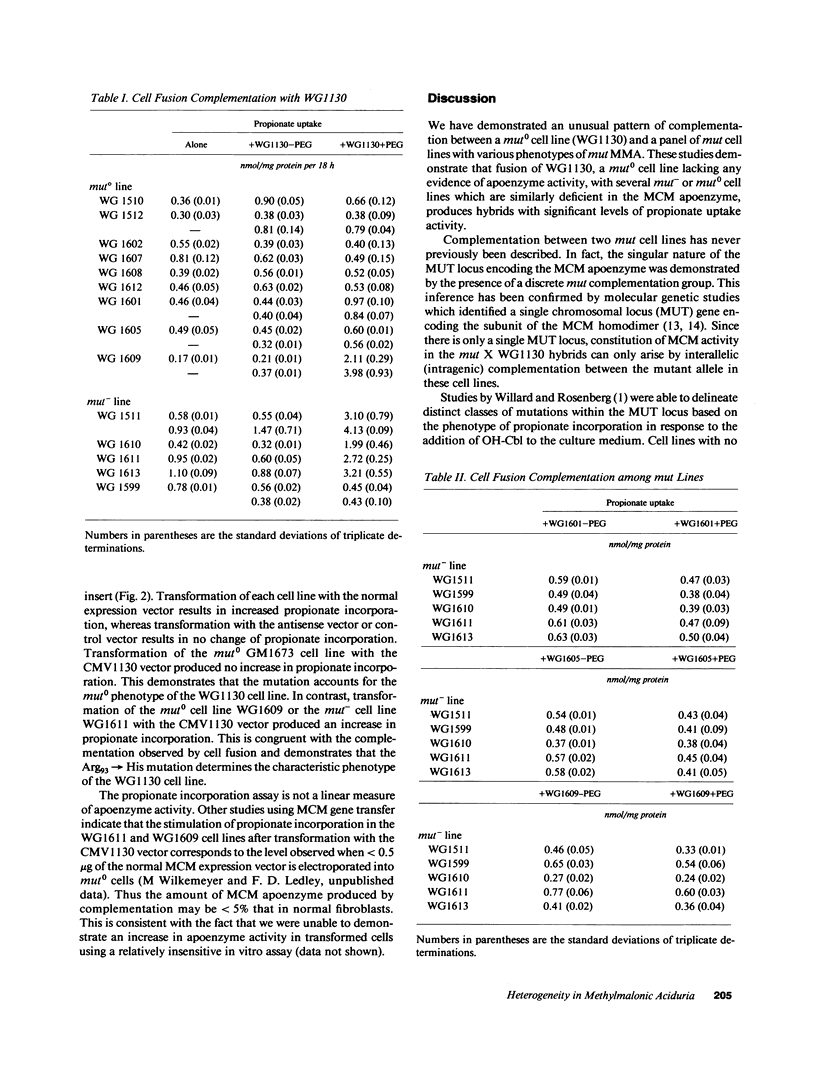
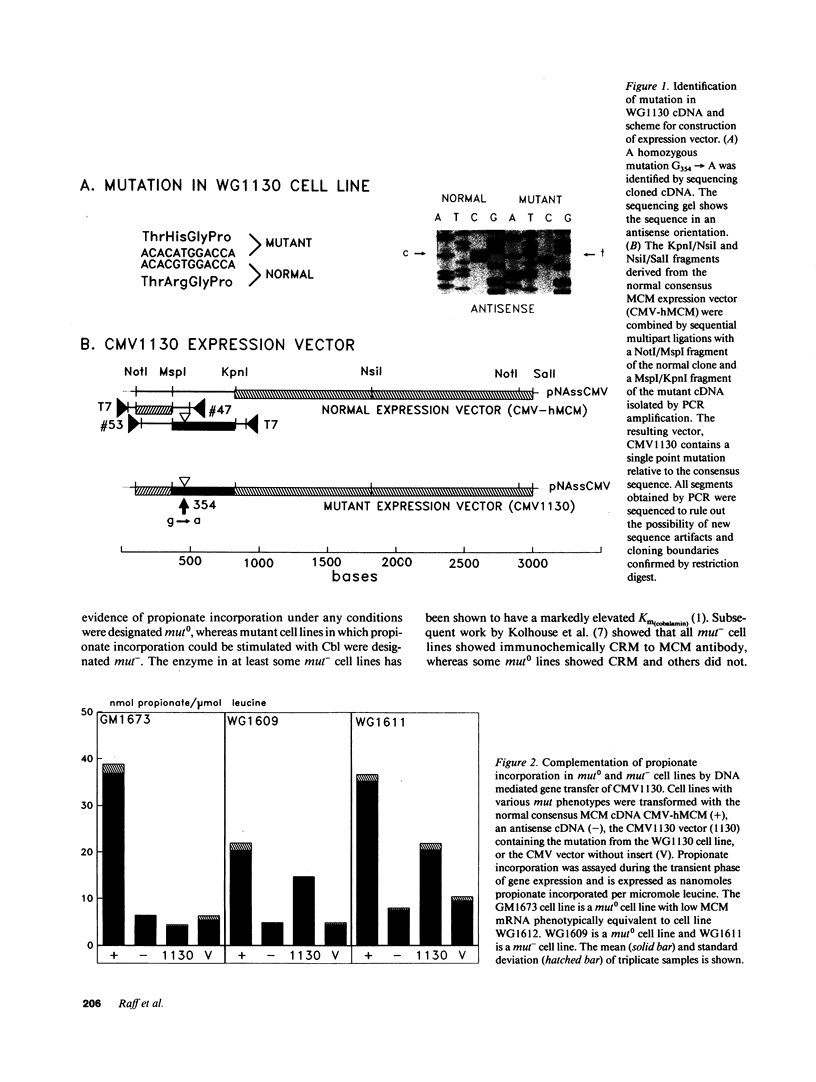
Genetic complementation of fibroblasts from patients with methylmalonic aciduria (MMA) defines a unique class of allelic mutations arising from mutations at the locus encoding the methylmalonyl coenzyme A (CoA) mutase apoenzyme. Various phenotypes of MMA have been delineated including complete absence of enzyme activity (mut0) and abnormal enzyme activity with an elevated Km for adenosylcobalamin (mut-). We describe genetic studies on a cell line (WG1130) from a patient with mut0 MMA which exhibited an unusual complementation phenotype, complementing with three of nine mut0 cell lines and four of five mut- cell lines. This suggests that interallelic complementation occurs between mutant alleles in WG1130 and subsets of alleles associated with both mut0 and mut- phenotypes. The methylmalonyl CoA mutase cDNA was cloned from WG1130 and found to contain a G354----A (Arg93----His) mutation. Gene transfer of this mutant clone into primary fibroblasts from patients with MMA confirms that this mutation expresses a mut0 phenotype when transferred into a mut0 cell line with low levels of mRNA but can contribute to apoenzyme function when transferred into mut cell lines which show correction with WG1130 by somatic cell complementation. These results point to further heterogeneity within both mut0 and mut- and may enable identification of mutations affecting discrete components of apoenzyme function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fenton W. A., Hack A. M., Kraus J. P., Rosenberg L. E. Immunochemical studies of fibroblasts from patients with methylmalonyl-CoA mutase apoenzyme deficiency: detection of a mutation interfering with mitochondrial import. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1421–1424. doi: 10.1073/pnas.84.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R., Kalousek F., Fenton W. A., Rosenberg L. E., Ledley F. D. Cloning of full-length methylmalonyl-CoA mutase from a cDNA library using the polymerase chain reaction. Genomics. 1989 Feb;4(2):198–205. doi: 10.1016/0888-7543(89)90300-5. [DOI] [PubMed] [Google Scholar]
- Jansen R., Ledley F. D. Heterozygous mutations at the mut locus in fibroblasts with mut0 methylmalonic acidemia identified by polymerase-chain-reaction cDNA cloning. Am J Hum Genet. 1990 Nov;47(5):808–814. [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Utley C., Fenton W. A., Rosenberg L. E. Immunochemical studies on cultured fibroblasts from patients with inherited methylmalonic acidemia. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7737–7741. doi: 10.1073/pnas.78.12.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Crane A. M., Lumetta M. Heterogeneous alleles and expression of methylmalonyl CoA mutase in mut methylmalonic acidemia. Am J Hum Genet. 1990 Mar;46(3):539–547. [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Jansen R., Nham S. U., Fenton W. A., Rosenberg L. E. Mutation eliminating mitochondrial leader sequence of methylmalonyl-CoA mutase causes muto methylmalonic acidemia. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3147–3150. doi: 10.1073/pnas.87.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Lumetta M. R., Zoghbi H. Y., VanTuinen P., Ledbetter S. A., Ledbetter D. H. Mapping of human methylmalonyl CoA mutase (MUT) locus on chromosome 6. Am J Hum Genet. 1988 Jun;42(6):839–846. [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Lumetta M., Nguyen P. N., Kolhouse J. F., Allen R. H. Molecular cloning of L-methylmalonyl-CoA mutase: gene transfer and analysis of mut cell lines. Proc Natl Acad Sci U S A. 1988 May;85(10):3518–3521. doi: 10.1073/pnas.85.10.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nham S. U., Wilkemeyer M. F., Ledley F. D. Structure of the human methylmalonyl-CoA mutase (MUT) locus. Genomics. 1990 Dec;8(4):710–716. doi: 10.1016/0888-7543(90)90259-w. [DOI] [PubMed] [Google Scholar]
- Rosenblatt D. S., Cooper B. A., Pottier A., Lue-Shing H., Matiaszuk N., Grauer K. Altered vitamin B12 metabolism in fibroblasts from a patient with megaloblastic anemia and homocystinuria due to a new defect in methionine biosynthesis. J Clin Invest. 1984 Dec;74(6):2149–2156. doi: 10.1172/JCI111641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigekawa K., Dower W. J. Electroporation of eukaryotes and prokaryotes: a general approach to the introduction of macromolecules into cells. Biotechniques. 1988 Sep;6(8):742–751. [PubMed] [Google Scholar]
- Willard H. F., Rosenberg L. E. Inherited methylmalonyl CoA mutase apoenzyme deficiency in human fibroblasts: evidence for allelic heterogeneity, genetic compounds, and codominant expression. J Clin Invest. 1980 Mar;65(3):690–698. doi: 10.1172/JCI109715. [DOI] [PMC free article] [PubMed] [Google Scholar]