Abstract
Complete deficiency of a member of the type II transmembrane serine protease family, tmprss1 (also known as hepsin), is associated with severe to profound hearing loss in mice and a gross enlargement of the tectorial membrane in the cochlea. Levels of thyroxine in these mice have been shown to be significantly lower compared to wild type controls. Since thyroxine is critical for inner ear development, we delivered thyroxine to these mice during the prenatal or postnatal stage of development. Both treatments could not ameliorate hearing loss or correct deformities in the tectorial membrane of these mutant mice, suggesting that a deficiency in tmprss1 affects thyroxine responsiveness in the inner ear in vivo.
Keywords: transmembrane serine protease, hepsin, tectorial membrane, hypothyroidism, cochlea, thyroid hormones, resistance to thyroid hormones
INTRODUCTION
Distinct members of the type II transmembrane serine protease (TMPRSS), tmprss1 and tmprss3, are important for hearing. It is unclear how these proteases contribute to hearing but emerging data have shown that mutations in the tmprss3 cause non-syndromic congenital and childhood onset autosomal recessive deafness [1,2] whereas a complete deficiency in tmprss1 in mice leads to hearing loss and inner ear defects [3]. These defects include a grossly enlarged tectorial membrane - an extracellular sheet overlying the organ of Corti with an important role in amplifying low-level signals from outer hair cells; as well as a reduced myelination of the auditory nerve [3,4]. These pathologies are also present in hypothyroidism [5,6]. Congenital or drug-induced hypothyroidism is known to affect the proper development and function of the auditory system [4-7]. Because free serum thyroxine (T4) levels in tmprss1−/− mice were found to be significantly depressed [3], we administered thyroxine to these mice to determine if it could potentially rescue their auditory deficits.
MATERIALS AND METHODS
Mouse colony
We crossed a male tmprss1−/− mouse to a female tmprss1+/+ mouse to obtain heterozygote offsprings. Matings between these offsprings generate tmprss1−/− and tmprss1+/+ breeders which were used to stock the colony. Mice were genotyped using polymerase chain reaction (PCR) as described [8]. Mice used in this study were approved by the animal ethics committees of the Royal Victorian Eye and Ear Hospital, the Walter Eliza Hall Institute of Medical Research, and the University of Melbourne Gene Technology and Biosafety Committee.
Thyroxine supplementation
For postnatal (P) treatment, newborn tmprss1+/+ and −/− mice were subcutaneously injected with 50ng T4 (Sigma Aldrich)/g body weight (BW) in 0.9% saline every 2nd day from P2 – P28. In prenatal treatments, 60-day release pellets containing different doses of T4 (5, 15 or 25 mg) or placebo (5, 15 or 25 mg) were purchased from Innovative Research America and implanted subcutaneously in tmprss1−/− dams. Implanted dams were mated with tmprss1−/− studs 2 weeks later.
Hearing measurements
At 5-6 weeks old, mice were anaesthetised intra-peritoneally with ketamine (75 mg/kg BW; Parnell Laboratories) and xylazil (7.5 mg/kg BW; Troy Laboratories). Computer-generated rarefaction clicks were channelled to a loud speaker positioned 10 cm from the pinna of the measured ear. The non-measured ear was covered with synthetic foam (Otoform, Dreve Otoplastik). Stimuli were presented at a rate of 33 per second and responses were amplified 105 fold and bandpass filtered (150 Hz – 3 kHz). Recording typically starts at 100 decibels peak equivalent sound pressure level (dB SPL) and nearer to the threshold; intensity was varied by 5 dB SPL to determine as accurately as possible the auditory brainstem response (ABR) threshold. ABR threshold was determined as the smallest stimulus level required to give peak-trough response amplitude of > 0.3 μV for wave II of the ABR. At each stimulus level, 500 responses were averaged and each recording was repeated to ensure consistency.
Histology
After ABR recordings, mice were sacrificed by CO2 euthanasia. Cochleae were rapidly dissected, fixed for 2 hrs in 4% paraformaldehyde, decalcified in 10% ethylene diamine tetra-acetic acid (pH7.4), and incubated overnight in 30% sucrose before embedding along the modiolar axis in OCT compound (Sakura). Cochleae were sectioned in 10 μm thickness and stained with haematoxylin and eosin. Cochlear sections separated by a distance of 20-50 μm were examined with a Zeiss Axioplan 2 microscope and a Zeiss Axio Cam camera. Control and treated cochleae in each experiment were treated equally.
Reverse transcription-PCR (RT-PCR)
Age-matched tmprss1+/+ and −/− cochleae were dissected, snap frozen in liquid nitrogen. RNA isolation and reverse transcription were performed as described [9]. Primers used for glyceraldehyde-3-phosphate dehydrogenase, gapdh, and type II iodothyronine deiodinase, dio2, were identical to those previously tested and characterised [9,10]. We used HotStarTaq Plus DNA polymerase (Qiagen) and PCR conditions were: denaturation 94°C 3mins; amplification 94°C 1min/54°C 1min/72°C 1min (cycle varied); final extension 72°C 10mins.
RESULTS
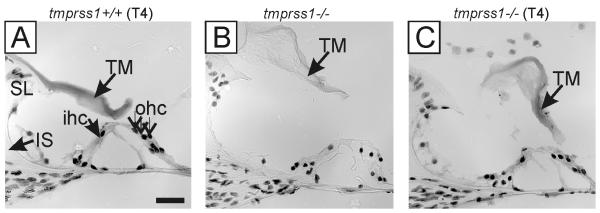
It was previously reported that athyroid pax8−/− mice have hearing loss and structural abnormalities in the cochlea including a grossly enlarged tectorial membrane; features manifested in tmprss1−/− mice [5]. Because early postnatal treatment of T4 could correct the size of the tectorial membrane in pax8−/− mice [5], we administered T4 to 11 tmprss1−/− and 3 tmprss1+/+ pups from postnatal day 2 to 28 to determine if postnatal treatment would be sufficient to correct otopathological defects in tmprss1−/− mice (Fig. 1). Analysis of at least 5 sections/cochlea from these mice, with section interval of 20-50 μm, showed that tmprss1−/− mice receiving T4 continued to show a tectorial membrane similar in size to untreated tmprss1−/− mice (Fig. 1C vs B); but the membrane was notably enlarged compared to wild type mice that also received T4 (Fig. 1C vs A). This initial finding prompted us to investigate the use of prenatal T4 treatment in tmprss1−/− mice.
Fig. 1.
Postnatal T4 treatment in tmprss1+/+ and tmprss1−/− mice. Tectorial membrane (TM) in tmprss1−/− mice remained enlarged after T4 treatment (C), similar to the untreated tmprss1−/− mice (B), but larger than that of tmprss1+/+ mice receiving identical T4 (A). Abbreviations: SL, spiral limbus; IS, inner sulcus; ihc, inner hair cell; ohc, outer hair cell. Scale bar: 20 μm.
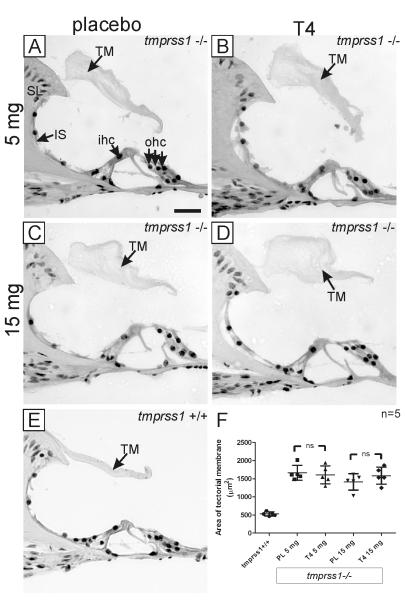
Sprenkle et al. (2001) showed that pellets containing T4, purchased from Innovative Research of America, released quantifiable T4 which significantly improved auditory thresholds in congenitally hypothyroid and deaf hyt/hyt mice [11]. Therefore, we implanted 3 different doses of T4 (5, 15, 25mg) and their corresponding placebo pellets in tmprss1−/− dams before mating to tmprss1−/− studs. No pregnancy was achieved in 5 tmprss1−/− dams when 25mg T4 pellets were used. For each implanted group, 5-10 pups were randomly selected from at least 2 litters for cochlear histology and ABR measurements. We also included 4 age-matched tmprss1+/+ mice for comparison. When sections were examined from 5 cochleae in each group, we noticed that the tectorial membranes of all tmprss1−/− mice - regardless of treatment - were clearly thicker than tmprss1+/+ mice (Fig. 2A-D vs 2E). Mean areas and standard deviations (SD) of the tectorial membrane of tmprss1−/− mice receiving 5mg T4 or 5mg placebo were 1606±250 μm2 and 1663±206 μm2, respectively; and Student’s t-test revealed no statistical significance between both groups (Fig. 2F, p=0.7). Although the mean area of the tectorial membrane of tmprss1−/− mice receiving 15mg T4 was 1582±235 μm2, higher than that of the corresponding placebo group (1412±226 μm2), no statistical significance could be established with Student’s t-test (Fig. 2F, p=0.3).
Fig. 2.
Prenatal T4 treatment in tmprss1−/− mice and cochlear morphology. Representative cochlear images of tmprss1−/− mice receiving 5 mg placebo (A), 5 mg T4 (B), 15 mg placebo (C), 15 mg T4 (D), and a tmprss1+/+ mouse (E). Scale bar: 20 μm. A graph displaying data point of each area measurement of the tectorial membrane per group, including means and standard deviations, is shown in (F). Abbreviation: ns, not significant.
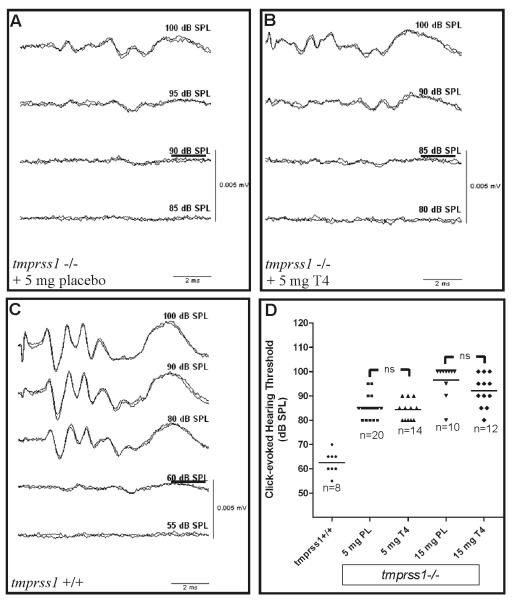
Because hyt/hyt mice exposed to prenatal thyroxine treatment showed a significant improvement of hearing thresholds [11], we determined ABR thresholds of tmprss1−/− mice receiving T4 or placebo, as well as age-matched tmprss1+/+ mice. Representative ABR recordings are shown in Fig. 3A-C. Mean ABR threshold and SD of tmprss1+/+ mice is 63±5 dB SPL (n=8) whereas those of tmprss1−/− mice receiving 5mg placebo or T4 were 85±4 dB SPL (n=20) and 84±4 dB SPL (n=14), respectively (Fig. 3D). Increasing T4 dosage failed to improve ABR thresholds since the mean ABR threshold of tmprss1−/− mice treated with 15mg T4 remained high at 92±7 dB SPL (n=12), slightly lower than that of the corresponding placebo group, 97±7 dB SPL (n=10). Statistical analysis of ABRs using Student’s t-test did not indicate any statistical significance between cohorts receiving 5mg T4 or placebo (Fig. 3D, p=0.6), nor cohorts receiving 15 mg T4 or placebo (Fig. 3D, p=0.09).
Fig. 3.
Prenatal T4 treatment in tmprss1−/− mice and hearing thresholds. Representative ABR recordings of tmprss1−/− mice receiving 5 mg placebo (A), 5 mg T4 (B), and a tmprss1+/+ mouse (C). Threshold of each recording is underlined. A graph displaying data point for each ABR measurement, including means (solid line), is shown in (D).
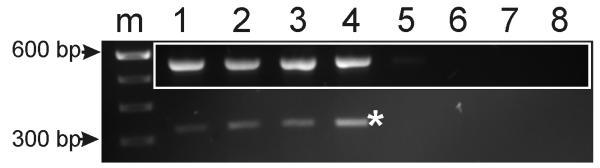
Because T4 has to be converted to 3′,3,5-tri-iodothyronine (T3) - the biologically active form - by the cochlear deiodinase gene, dio2, we used RT-PCR technique to examine dio2 gene expression in tmprss1−/− and tmprss1+/+ mice. We first optimised the number of amplification cycles for dio2 and gapdh, a reference gene, to establish conditions for non-saturating amplification. Next, in 2 experiments using the same number of PCR cycles (30), we fail to observe a diminished level of dio2 in tmprss1−/− mice, relative to tmprss1+/+ mice (Fig. 4). By amplifying gapdh from the same cDNA sample, we noticed that changes of dio2 are independent of those of gapdh (Fig. 4, inset). Therefore, prenatal T4 treatment in tmprss1−/− mice does not appear to be compromised by inadequate dio2 levels in these mice.
Fig. 4.
RT-PCR of dio2 and gapdh genes in tmprss1+/+ and −/− mice. Expression of dio2 gene (*) in tmprss1+/+ (lanes 1, 3) compared to tmprss1−/− (lanes 2, 4) mice. Inset shows gapdh expression from the same cDNA samples. Negative RT-PCR reactions corresponding to lanes 1-4 are shown in lanes 5-8, respectively. Abbreviation: m, 100 base pair (bp) DNA markers.
Discussion
The discovery that mutations in tmprss3 cause non-syndromic recessive hearing loss in humans underpins the importance of proteases for hearing [1,2]. However, the mechanism which tmprss3 influences hearing remains unclear although cell culture studies indicated that it activates epithelial sodium channels which putatively regulate Na+ levels in the perilymph [12]. Because human temporal bones of deaf individuals affected by tmprss3 mutations are unavailable, we could not establish if tmprss3 can be linked to any cochlear pathology. With the availability of tmprss1 mutant mice, we provided evidence that a deficiency in tmprss1 induces both abnormalities in tectorial membrane and hearing thresholds [3]. More importantly, the discovery of hypothyroidism in tmprss1−/− mice and the indispensable role of thyroid hormones in inner ear development raise a possibility of correcting hearing impairment in these mice with thyroxine treatments [3,13].
Such treatments have been successfully used in mice with mutations in genes that have clearly-defined roles in hypothyroidism [5,11]; for examples, pax8−/− athyroid mice which lack thyroid follicles and hyt/hyt mice which lack the receptor for thyroid stimulating hormones. In this study, prenatal thyroxine treatment on tmprss1−/− mice apparently has no effects in improving hearing thresholds or correcting tectorial membrane abnormality, suggesting that T4 dosage maybe inadequate. This likelihood is ruled out because (i) the dosage of T4 pellets used in this study is either equal or higher than those tested in hyt/hyt mice; and (ii) the less severe hypothyroidism in tmprss1−/− mice - ~40% less T4 compared to 80% less T4 in hyt/hyt mice – would make it more likely for T4 treatment to be effective [11,14].
Because a deficiency in dio2 expression in mice also causes a grossly enlarged tectorial membrane and raised hearing thresholds [15], it is plausible that a reduction of dio2 gene expression in tmprss1−/− mice can explain our findings. Our RT-PCR experiments show that dio2 gene expression was not diminished in tmprss1−/− mice, indicating that other mechanisms might account for T4 resistance in these mice. Resistance to thyroid hormones (RTH) in humans is commonly attributed to mutations in the thyroid hormone receptor β (TR β) gene [16] but several patients which display clinical symptoms of RTH do not bear mutations in the TR β gene [17], suggesting that other pathways maybe involved. Thus, it is plausible that tmprss1 may have a role in RTH in vivo but further investigation would be necessary to establish the link between tmprss1 and T4 responsiveness.
Conclusion
Deficiency in tmprss1 causes a reduction in thyroxine and auditory deficits in mice similar to those in congenital hypothyroidism. Thyroxine treatments fail to correct these deficits suggesting a novel role of tmprss1 in T4 responsiveness.
Acknowledgements
We would like to thank Maria Clarke, Lauren Donley, Ping ZF Cannon, Michelle Fornito, Wu Q, Bayer Healthcare and the Howard Hughes Medical Institute. The Bionic Ear Institute acknowledges the support it receives from the Victorian Government through its Operational Infrastructure Support Program.
Grant disclosure: Supported by the Garnett Passe and Rodney Williams Memorial Foundation, the Royal National Institute for Deaf People (UK), the Royal Victorian Eye and Ear Hospital (all awarded to JT); the National Institute on Deafness and Other Communication Disorders (grant HHS-N-263-2007-00053-C); the National Health & Medical Research Council, Australia (grants 257501, 215305, 71601, 461204 to HSS); Swiss National Science Foundation grant 3100A0-114077-1 (to MG).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masmoudi S, Antonarakis SE, Schwede T, Ghorbel AM, Gratri M, Pappasavas MP, Drira M, Elgaied-Boutila A, Wattenhofer M, Rossier C, Scott HS, Ayadi H, Guipponi M. Novel missense mutations of TMPRSS3 in two consanguineous Tunisian families with non-syndromic autosomal recessive deafness. Human Mutation. 2001;18:101–108. doi: 10.1002/humu.1159. [DOI] [PubMed] [Google Scholar]
- 2.Scott HS, Kudoh J, Wattenhofer M, Shibuya K, Berry A, Chrast R, Guipponi M, Wang J, Kawasaki K, Asakawa S, Minoshima S, Younus F, Mehdi SQ, Radhakrishna U, Papasavvas MP, Gehrig C, Rossier C, Korostishevsky M, Gal A, Shimizu N, Bonne-Tamir B, Antonarakis SE. Insertion of beta-satellite repeats identifies a transmembrane protease causing both congenital and childhood onset autosomal recessive deafness. Nature Genetics. 2001;27:59–63. doi: 10.1038/83768. [DOI] [PubMed] [Google Scholar]
- 3.Guipponi M, Tan J, Cannon PZF, Donley L, Crewther P, Clarke M, Wu QY, Shepherd RK, Scott HS. Mice deficient for the type II transmembrane serine protease, TMPRSS1/hepsin, exhibit profound hearing loss. American Journal of Pathology. 2007;171:608–616. doi: 10.2353/ajpath.2007.070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legan PK, Lukashkina VA, Goodyear RJ, Kossl M, Russell IJ, Richardson GP. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28:273–285. doi: 10.1016/s0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 5.Christ S, Biebel UW, Hoidis S, Friedrichsen S, Bauer K, Smolders JWT. Hearing loss in athyroid Pax8 knockout mice and effects of thyroxine substitution. Audiology and Neuro-Otology. 2004;9:88–106. doi: 10.1159/000076000. [DOI] [PubMed] [Google Scholar]
- 6.Knipper M, Bandtlow C, Gestwa L, Kopschall I, Rohbock K, Wiechers B, Zenner HP, Zimmermann U. Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development. 1998;125:3709–3718. doi: 10.1242/dev.125.18.3709. [DOI] [PubMed] [Google Scholar]
- 7.Sprenkle PM, McGee J, Bertoni JM, Walsh EJ. Consequences of hypothyroidism on auditory system function in Tshr mutant (hyt) mice. Jaro. 2001;2:312–329. doi: 10.1007/s101620010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu QY, Yu DY, Post J, Halks-Miller M, Sadler JE, Morser J. Generation and characterization of mice deficient in hepsin, a hepatic transmembrane serine protease. Journal of Clinical Investigation. 1998;101:321–326. doi: 10.1172/JCI1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan J, Ruttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Kopschall I, Rohbock K, Knipper M. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007;145:715–726. doi: 10.1016/j.neuroscience.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 10.Torkos A, Wissel K, Warnecke A, Lenarz T, Stover T. Technical report: Laser microdissection and pressure catapulting is superior to conventional manual dissection for isolating pure spiral ganglion fractions from the cochlea. Hearing Research. 2008;235:8–14. doi: 10.1016/j.heares.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Sprenkle PM, McGee J, Bertoni JM, Walsh EJ. Prevention of auditory dysfunction in hypothyroid Tshr mutant mice by thyroxin treatment during development. Jaro. 2001;2:348–361. doi: 10.1007/s101620010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guipponi M, Vuagniaux G, Wattenhofer M, Shibuya K, Vazquez M, Dougherty L, Scamuffa N, Guida E, Okui M, Rossier C, Hancock M, Buchet K, Reymond A, Hummler E, Marzella PL, Kudoh J, Shimizu N, Scott HS, Antonarakis SE, Rossier BC. The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Human Molecular Genetics. 2002;11:2829–2836. doi: 10.1093/hmg/11.23.2829. [DOI] [PubMed] [Google Scholar]
- 13.Sohmer HFS. The importance of thyroid hormone for auditory development in the fetus and neonate. Audiology and Neuro-Otology. 1996;1:137–147. doi: 10.1159/000259194. [DOI] [PubMed] [Google Scholar]
- 14.Omalley BW, Li DQ, Turner DS. Hearing-Loss and cochlear abnormalities in the congenital hypothyroid (hyt/hyt) mouse. Hearing Research. 1995;88:181–189. doi: 10.1016/0378-5955(95)00111-g. [DOI] [PubMed] [Google Scholar]
- 15.Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, St Germain DL, Galton VA, Forrest D. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3474–3479. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usala SJ, Bale AE, Gesundheit N, Weinberger C, Lash RW, Wondisford FE, McBride OW, Weintraub BD. Tight linkage between the syndrome of generalized thyroid-hormone resistance and the human c-erba-beta gene. Molecular Endocrinology. 1988;2:1217–1220. doi: 10.1210/mend-2-12-1217. [DOI] [PubMed] [Google Scholar]
- 17.Pohlenz J, Weiss RE, Macchia PE, Pannain S, Lau IT, Ho H, Refetoff S. Five new families with resistance to thyroid hormone not caused by mutations in the thyroid hormone receptor beta gene. Journal of Clinical Endocrinology & Metabolism. 1999;84:3919–3928. doi: 10.1210/jcem.84.11.6080. [DOI] [PubMed] [Google Scholar]