Abstract
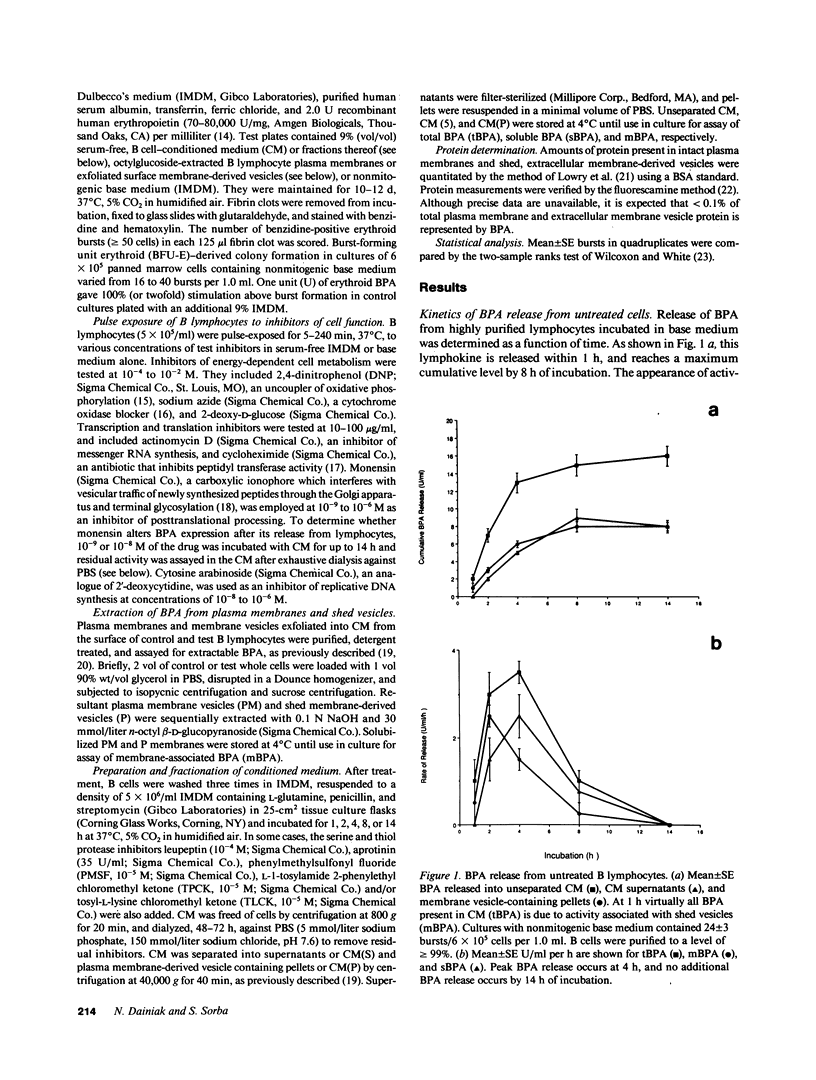
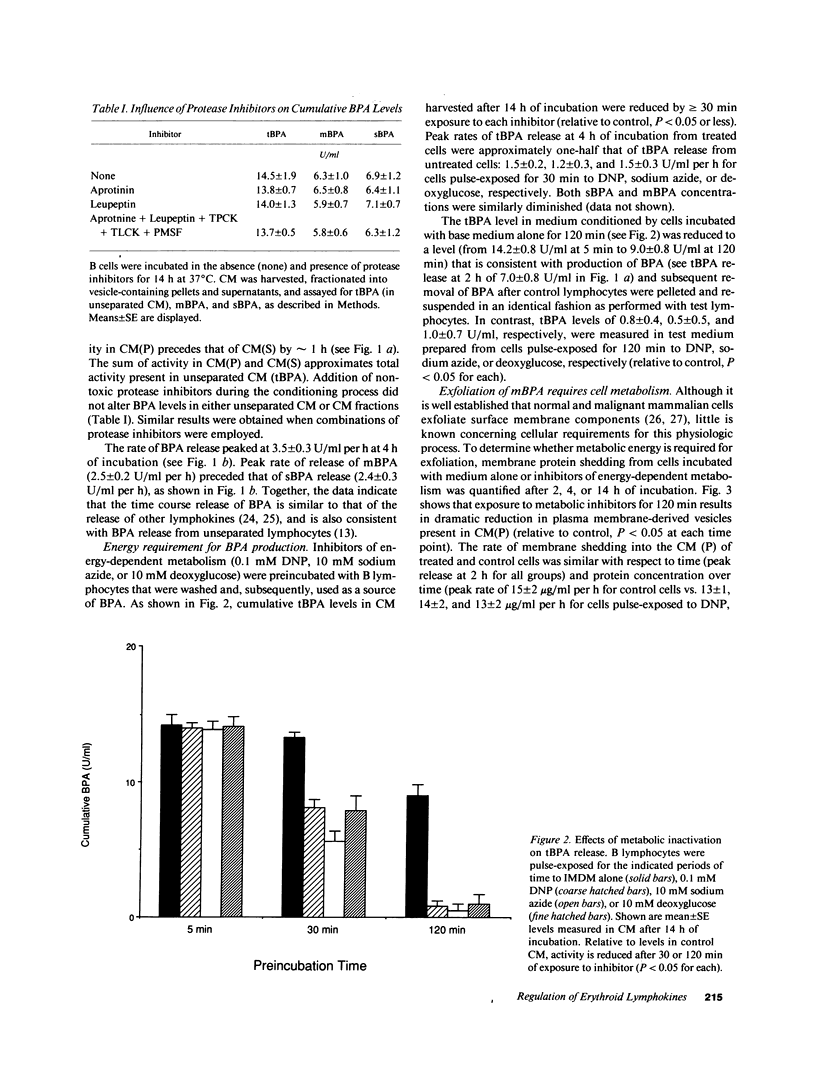
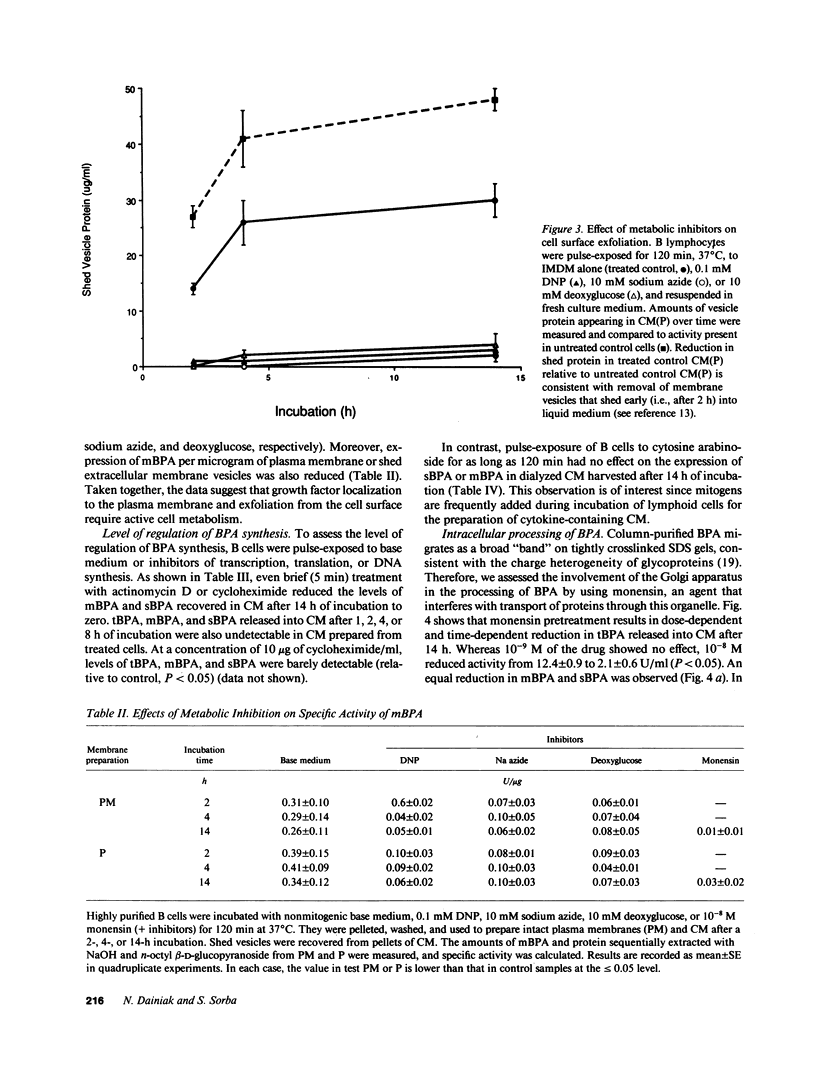
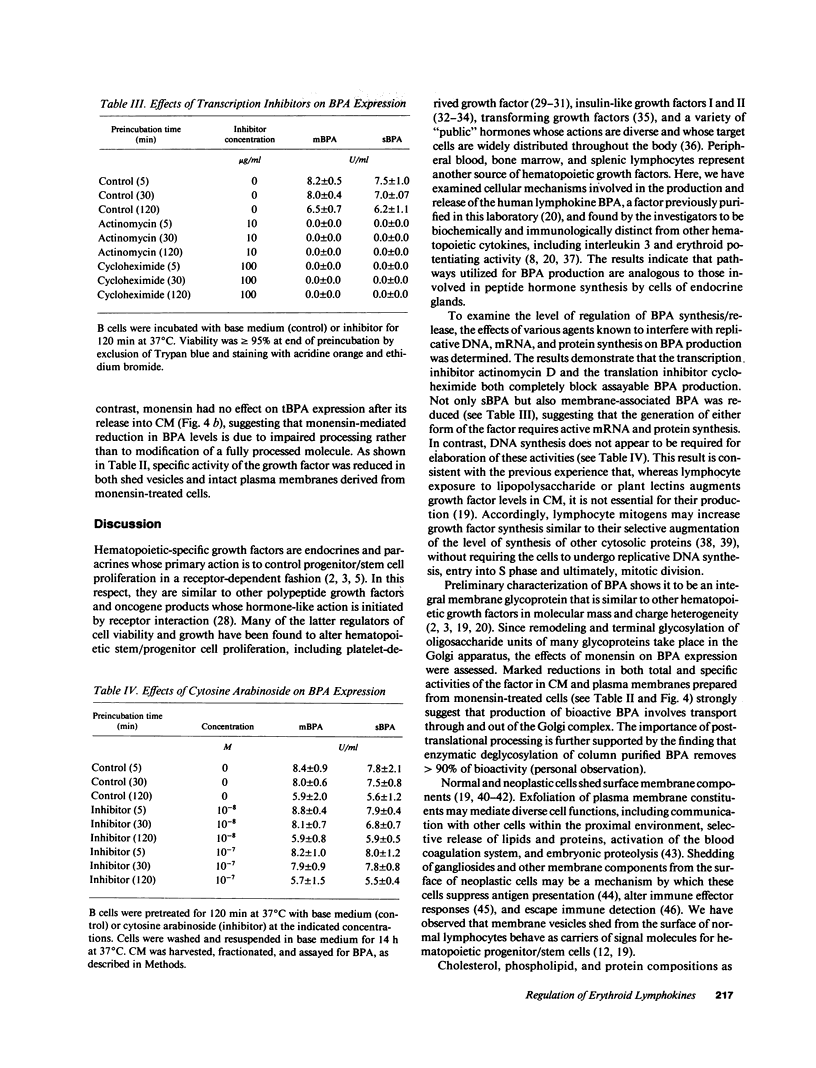
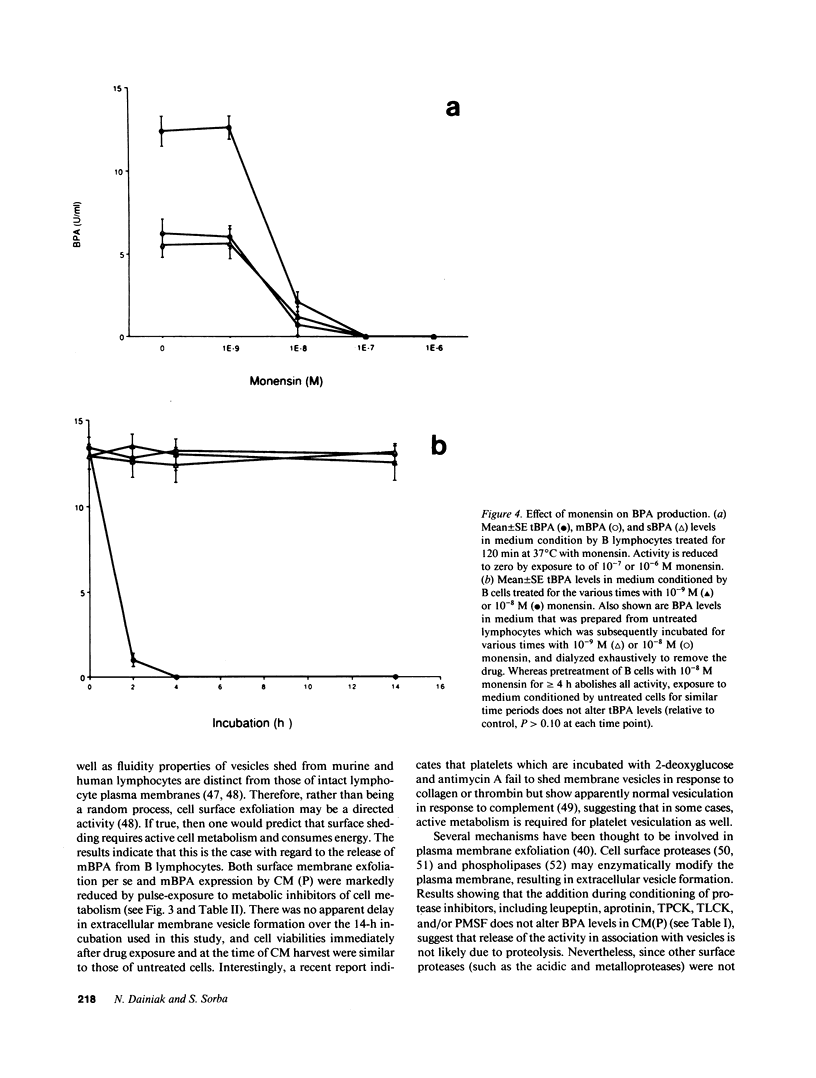
Erythroid burst-promoting activity (BPA) is released from B lymphocytes in soluble (sBPA) and membrane-bound (mBPA) forms. To study intracellular processes involved in production of these physically separable factors, we measured their time course release into serum-free medium from B cells that were pulse-exposed for 5-240 min to nonmitogenic base medium or inhibitors of energy-dependent metabolism (2,4-dinitrophenol, sodium azide, and 2-deoxy-D-glucose), transcription and translation (actinomycin D and cycloheximide), replicative DNA synthesis (cytosine arabinoside), or posttranslational processing (monensin). mBPA and sBPA were initially detectable after 1 and 2 h, respectively. Maximum cumulative levels of 8 +/- 0.6 and 9 +/- 1.0 U/ml, respectively, were reached after 8 h. In contrast, cumulative mBPA and sBPA levels in medium prepared from cells treated with metabolic inhibitors were reduced by up to 90%. Both surface exfoliation and mBPA expression by intact plasma membranes were diminished. Whereas pulse-exposure to cytosine arabinoside had no effect, treatment with actinomycin D or cycloheximide abolished BPA expression. Exposure to monensin reduced mBPA and sBPA levels to zero in a concentration-and time-dependent fashion. We conclude that production and release of BPA is an energy-dependent process, requiring mRNA synthesis and translation and posttranslational remodeling of the protein but not replicative DNA synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong M. J., Storch J., Dainiak N. Structurally distinct plasma membrane regions give rise to extracellular membrane vesicles in normal and transformed lymphocytes. Biochim Biophys Acta. 1988 Dec 8;946(1):106–112. doi: 10.1016/0005-2736(88)90462-2. [DOI] [PubMed] [Google Scholar]
- Dainiak N., Cohen C. M. Regulation of human erythroid proliferation in vitro by leukocyte surface components. Ann N Y Acad Sci. 1985;459:129–142. doi: 10.1111/j.1749-6632.1985.tb20821.x. [DOI] [PubMed] [Google Scholar]
- Dainiak N., Cohen C. M. Surface membrane vesicles from mononuclear cells stimulate erythroid stem cells to proliferate in culture. Blood. 1982 Sep;60(3):583–594. [PubMed] [Google Scholar]
- Dainiak N., Davies G., Kalmanti M., Lawler J., Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. J Clin Invest. 1983 May;71(5):1206–1214. doi: 10.1172/JCI110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Kreczko S., Cohen A., Pannell R., Lawler J. Primary human marrow cultures for erythroid bursts in a serum-substituted system. Exp Hematol. 1985 Nov;13(10):1073–1079. [PubMed] [Google Scholar]
- Dainiak N., Kreczko S. Interactions of insulin, insulinlike growth factor II, and platelet-derived growth factor in erythropoietic culture. J Clin Invest. 1985 Sep;76(3):1237–1242. doi: 10.1172/JCI112079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Najman A., Kreczko S., Baillou C., Mier J., Feldman L., Gorin N. C., Duhamel G. B-lymphocytes as a source of cell surface growth-promoting factors for hematopoietic progenitors. Exp Hematol. 1987 Nov;15(10):1086–1096. [PubMed] [Google Scholar]
- Dainiak N., Riordan M. A., Strauss P. R., Feldman L., Kreczko S. Contractile proteins participate in release of erythroid growth regulators from mononuclear cells. Blood. 1988 Jul;72(1):165–171. [PubMed] [Google Scholar]
- Dainiak N., Sutter D., Kreczko S. L-triiodothyronine augments erythropoietic growth factor release from peripheral blood and bone marrow leukocytes. Blood. 1986 Dec;68(6):1289–1297. [PubMed] [Google Scholar]
- Dainiak N., Warren G., Sutter D., Kreczko S., Howard D. A monoclonal antibody to exfoliated surface vesicles that recognizes a membrane-associated erythroid burst-promoting activity. Blood. 1988 Sep;72(3):989–994. [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Delwiche F., Raines E., Powell J., Ross R., Adamson J. Platelet-derived growth factor enhances in vitro erythropoiesis via stimulation of mesenchymal cells. J Clin Invest. 1985 Jul;76(1):137–142. doi: 10.1172/JCI111936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doljanski F., Kapeller M. Cell surface shedding--the phenomenon and its possible significance. J Theor Biol. 1976 Oct 21;62(2):253–270. doi: 10.1016/0022-5193(76)90119-3. [DOI] [PubMed] [Google Scholar]
- Emerson S. G., Cone R. E. Differential effects of colchicine and cytochalasins on the shedding of murine B cell membrane IgM and IgD. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6582–6586. doi: 10.1073/pnas.76.12.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L., Cohen C. M., Riordan M. A., Dainiak N. Purification of a membrane-derived human erythroid growth factor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6775–6779. doi: 10.1073/pnas.84.19.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L., Dainiak N. B-lymphocyte-derived erythroid burst-promoting activity is distinct from other known lymphokines. Blood. 1989 May 15;73(7):1814–1820. [PubMed] [Google Scholar]
- Hall D. J., O'Leary J. J., Rosenberg A. Early synthesis of specific cytoplasm proteins is correlated with the rate of exit of lymphocytes from the resting state. J Cell Biol. 1984 Nov;99(5):1814–1821. doi: 10.1083/jcb.99.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. J., O'Leary J. J., Rosenberg A. Early synthesis of specific cytoplasm proteins is correlated with the rate of exit of lymphocytes from the resting state. J Cell Biol. 1984 Nov;99(5):1814–1821. doi: 10.1083/jcb.99.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Härtl W., Jelkmann W., Zapf J., Bauer C. Activity in fetal bovine serum that stimulates erythroid colony formation in fetal mouse livers is insulinlike growth factor I. J Clin Invest. 1985 Oct;76(4):1643–1648. doi: 10.1172/JCI112149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ladisch S., Gillard B., Wong C., Ulsh L. Shedding and immunoregulatory activity of YAC-1 lymphoma cell gangliosides. Cancer Res. 1983 Aug;43(8):3808–3813. [PubMed] [Google Scholar]
- Ladisch S., Kitada S., Hays E. F. Gangliosides shed by tumor cells enhance tumor formation in mice. J Clin Invest. 1987 Jun;79(6):1879–1882. doi: 10.1172/JCI113031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepins A., Hillman A. J. Shedding of tumor cell surface membranes. Cell Biol Int Rep. 1981 Jan;5(1):15–26. doi: 10.1016/0309-1651(81)90153-3. [DOI] [PubMed] [Google Scholar]
- Merchav S., Tatarsky I., Hochberg Z. Enhancement of human granulopoiesis in vitro by biosynthetic insulin-like growth factor I/somatomedin C and human growth hormone. J Clin Invest. 1988 Mar;81(3):791–797. doi: 10.1172/JCI113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Najman A., Baillou C., Drouet X., Leblanc G., Douay L., Gorin N. C., Duhamel G. Regulation of human peripheral blood BFU-E growth in vitro by leukaemic B-lymphocytes. Br J Haematol. 1985 Aug;60(4):643–650. doi: 10.1111/j.1365-2141.1985.tb07468.x. [DOI] [PubMed] [Google Scholar]
- Niskanen E., Gorman J., Isakson P. C. Hematopoetic precursors respond to a unique B lymphocyte-derived factor in vivo. Blood. 1987 Dec;70(6):1784–1789. [PubMed] [Google Scholar]
- Richert N. D., Ryan R. J. Proteolytic enzyme activation of rat ovarian adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4857–4861. doi: 10.1073/pnas.74.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin R., Chou I. N., Black P. H. Proteolytic enzymes, cell surface changes, and viral transformation. Adv Cancer Res. 1975;22:203–260. doi: 10.1016/s0065-230x(08)60178-5. [DOI] [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Wiedmer T. Repolarization of the membrane potential of blood platelets after complement damage: evidence for a Ca++ -dependent exocytotic elimination of C5b-9 pores. Blood. 1986 Aug;68(2):556–561. [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Sytkowski A. J., O'Hara C., Vanasse G., Armstrong M. J., Kreczko S., Dainiak N. Characterization of biologically active, platelet-derived growth factor-like molecules produced by murine erythroid cells in vitro and in vivo. J Clin Invest. 1990 Jan;85(1):40–46. doi: 10.1172/JCI114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sármay G., István L., Gergely J. Shedding and reappearance of Fc, C3 and SRBC receptors on peripheral lymphocytes from normal donors and chronic lymphatic leukaemia (CLL) patients. Immunology. 1978 Feb;34(2):315–321. [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Taylor D. D., Black P. H. Inhibition of macrophage Ia antigen expression by shed plasma membrane vesicles from metastatic murine melanoma lines. J Natl Cancer Inst. 1985 Apr;74(4):859–867. [PubMed] [Google Scholar]
- Taylor D. D., Black P. H. Shedding of plasma membrane fragments. Neoplastic and developmental importance. Dev Biol (N Y 1985) 1986;3:33–57. doi: 10.1007/978-1-4684-5050-7_3. [DOI] [PubMed] [Google Scholar]
- Torok-Storb B. Cellular interactions. Blood. 1988 Aug;72(2):373–385. [PubMed] [Google Scholar]
- Uckun F. M., Vallera D. A., Wee S. L. B lymphocyte regulation of human hematopoiesis. J Immunol. 1985 Dec;135(6):3817–3822. [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk W. J., De Veer G., Krol J. H., Emmelot P. Comparative lipid analysis of purified plasma membranes and shed extracellular membrane vesicles from normal murine thymocytes and leukemic GRSL cells. Biochim Biophys Acta. 1982 Jun 14;688(2):495–504. doi: 10.1016/0005-2736(82)90361-3. [DOI] [PubMed] [Google Scholar]
- Wiedmer T., Shattil S. J., Cunningham M., Sims P. J. Role of calcium and calpain in complement-induced vesiculation of the platelet plasma membrane and in the exposure of the platelet factor Va receptor. Biochemistry. 1990 Jan 23;29(3):623–632. doi: 10.1021/bi00455a005. [DOI] [PubMed] [Google Scholar]



