Abstract
Objectives: A hallmark of cholesteatoma is hyperproliferation of keratinocytes with abundant production of keratins in the middle ear under chronic inflammatory conditions. However, little is known about the driving force of cellular proliferation and keratin production of cholesteatomal matrix. The purpose of this study was to investigate the cellular proliferation and keratin production of keratinocytes under the influence of Id1, a candidate transcription factor to cell proliferation.
Materials and methods: Keratinocytes were transfected with Id1 and the responses of keratinocytes to Id1 were studied by using cellular and molecular biologic methods.
Results: Id1 positively contributed to the cell cycle progression and negatively to the p16Ink4a downregulation via the nuclear factor‐kappa B (NF‐κB)/cyclin D1 pathway. Id1 significantly increased the promoter activity of NF‐κB which, in turn, up‐regulated the expression of cyclin D1 and keratin 10 in keratinocytes. Specific NF‐κB inhibitors (pyrrolidine dithiocarbamate, PDTC), or dominant‐negative inhibitor (I kappa B alpha mutant, IκBαM) abrogated the Id1‐induced cell proliferation and keratin 10 production whereas p65, a subunit of the NF‐κB heterodimer and an enhancer of the NF‐κB activity, strengthened the Id1‐induced cell proliferation and keratin 10 production.
Conclusions: Id1 contributed to hyperproliferation of keratinocytes via enhancement of cell cycle progression, removal of cell cycle inhibition, and simultaneously increased keratin production.
Introduction
Chronic otitis media (COM) is linked to cholesteatoma, a condition of aggressive proliferation of keratinocytes and abundant production of keratins, in the middle ear. Morphologically, cholesteatoma forms an epithelial cyst that grows aggressively and erodes the ossicular chain and bony wall of the middle ear. It represents a threat to neighbouring tissues or organs; however, little is known about the driving force behind this abnormal growth of the epithelial cyst and abundant production of keratins in cholesteatoma. Recently, we found that expression of Id1 was up‐regulated in the middle ear mucosa of rats following bacterial infection (1) and in the middle ear mucosa of humans with COM and/or cholesteatoma (2). It suggests that Id1 plays a role in pathogenesis of middle ear cholesteatoma.
The Id gene family encodes four related proteins, from Id1 to Id4, which are involved in control of cell cycle progression in a wide range of organisms, from flies to humans (3, 4). Id proteins are involved in cell population growth and proliferation (3, 5, 6) by antagonizing actions of basic helix–loop–helix (bHLH) transcription factors, which are essential for cell differentiation. According to the literature, forced expression of Id1 in primary human keratinocytes leads to cell lifespan extension and cell immortalization (7, 8). Transfection of the rat middle ear with Id1 in vivo has resulted in proliferative responses in the mucosal layer (9). These led us to examine the role of Id1 in proliferation of keratinocytes and production of keratins.
In this study, we hypothesized that Id1 induced proliferation of keratinocytes through up‐regulation of NF‐κB/cyclin D1, a signalling pathway leading to G0‐ to S‐phase progression, and down‐regulation of p16Ink4a, an inhibitor for suppression of cyclin‐dependent kinase (cdk) activity. In addition, we hypothesized that Id1 induced production of keratin 10, one of the major keratin products of keratinocytes, via NF‐κB. To test these hypotheses, cell and molecular biological experiments were performed (i) on human middle ear cholesteatoma specimens, to evaluate importance of Id1‐induced signalling in pathogenesis of cholesteatoma and (ii) on cultured human keratinocytes to verify the signalling pathway.
Materials and methods
Fourteen cholesteatoma tissue specimens were procured from Ichinomiya City Hospital in Nagoya, Japan, Cincinnati Children’s Hospital Medical Center (CCHMC) and University of Minnesota Hospitals and Clinics (UMHC). Eight normal (no history of otitis media) middle ear mucosal; head and neck skin specimens procured from UMHC served as controls. Diagnosis of these specimens was made clinically and verified pathologically. All tissues were procured, handled and maintained according to protocols approved by each Institutional Review Board (IRB).
Rhek‐1A cell line, representative epidermal keratinocytes (10), was maintained in Eagle’s minimal essential medium (MEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% foetal bovine serum (FBS), penicillin/streptomycin (50 μg/ml) and 2 mm l‐glutamine (herein referred to as full growth medium, FGM). During transient transfection of cells, Opti‐MEM supplemented with 6 μg/ml of Polybrene® was used (Invitrogen, herein referred to as transfection medium). Rhek‐1A was chosen because it is derived from human skin keratinocytes as is cholesteatoma.
Full‐length Id1 cDNA was cloned into a protein‐expressing vector (pEGFP; Clontech, Mountain View, CA) using standard protocols as described previously (11); functionality of this Id1 construct has recently been verified (12). I kappa B alpha mutant (IκBαM, a kind gift from Dr Inder Verma of the Salk Institute, La Jolla, CA), in which serine at position 36 had been changed to alanine, thus preventing phosphorylation and subsequent proteasomal degradation in response to stimuli (13, 14), is a dominant‐negative inhibitor of NF‐κB activity. Pyrrolidine dithiocarbamate (PDTC), a proteasome inhibitor purchased from Calbiochem, was used as a potent inhibitor of NF‐κB (15) and proven as an effective inhibitor of the NF‐κB activity in keratinocytes (16). Cyclin D1 reporters were generous gifts of Dr Richard Pestell (Department of Developmental and Molecular Biology, Albert Einstein College of Medicine, Bronx, NY). They contain wild‐type and mutant cyclin D1 reporter constructs, as described previously (17). Briefly, wild‐type cyclin D1 reporter construct consisted of a fragment of cyclin D1 promoter sequence (from −1745 to 0) flanking the cyclin D1 gene. Mutant cyclin D1 (CDmt) reporter construct has a fragment of cyclin D1 promoter sequence (from −66 to 0) with NF‐κB binding site truncated. NF‐κB reporter, provided by Dr Keith Brown at the National Institute of Allergy and Infectious Diseases, contains three repeats of κB sites for immunoglobulin κ‐light chain (18). β‐galactosidase (β‐gal) reporter was purchased from Stratagene and used as internal control for NF‐κB and cyclin D1 reporters. Id1 signalling pathway and relationship among IκBαM, NF‐κB (p65 and p50 heterodimer), PDTC, cyclin D1 and their effectors are schematically presented in Fig. 1.
Figure 1.
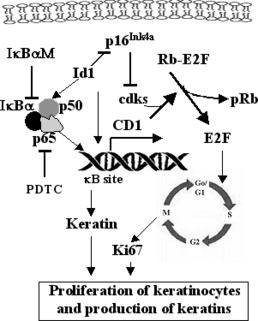
Schematic representation of Id1‐induced cell proliferation and keratin 10 production pathways in keratinocytes. Id1 activates translocation of NF‐κB subunit p65 into the nucleus and increases transcription of cyclin D1 (CD1). CD1, together with cyclin‐dependent kinases (cdk 4/6), phosphorylates retinoblastoma (Rb) protein that releases E2F from the Rb‐E2F complex. E2F, a transcription factor that drives the G0 to S phase transition of cells, promotes cell cycle progression. Ki67 is a proliferating cell antigen active from G1 to M phase; with expression of Ki67, a cell proliferates. In addition, phosphorylation of NF‐κB subunit p65 and transcription of p65 by Id1 increases production of keratins. Id1‐induced down‐regulation of p16Ink4a, an inhibitor of cdks, also promotes cell cycle progression. IκBαM inhibits NF‐κB activity by forming a firm complex with p65 and p50, whereas PDTC inhibits NF‐κB activity by suppressing formation and degradation of IκB (an inhibitor of NF‐κB). NF‐κB, consists of a heterodimer: p65 and p50; T‐bar, indicating inhibition; arrow, symbolizing up‐regulation.
Immunohistochemistry
Cholesteatoma and control specimens were fixed in 10% formalin, embedded in paraffin wax and cut to a thickness of 5 μm. Sections were deparaffinized, and incubated with primary antibodies to Id1 (rabbit anti‐human Id1; Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), keratin 10 (rabbit anti‐human K10; NeoMarker, Fremont, CA, USA; mouse anti‐human pan‐cytokeratin, Abcam, Cambridge, MA, USA), ‘activated’ NF‐κB (anti‐NF‐κB subunit p65; Chemicon, Temecula, CA, USA), cyclin D1 (BD Sciences, Franklin lakes, NJ, USA) and Ki67 (Abcam), for 90 min followed by secondary antibodies conjugated to fluorescein isothiocyanate (FITC) or tetramethylrhodamine isothiocyanate (TRITC), using protocols described previously (19). Tissue sections incubated with non‐specific antibodies (mouse or rabbit IgG isotope controls from Zymed) or antibody absorbed with specific antigen (Id1 blocking peptide, Santa Cruz Biotechnology Inc, 5‐fold excess) at 4 °C overnight, or at room temperature for 2 h, served as controls.
Incorporation of [3H]dT and trypan blue exclusion
Cells were cultured in 24‐well plates until 40% confluent, transfected with Id1 and empty vector, respectively, at concentration of 1.4 μg/ml for 16 h in transfection medium, recovered in FGM for 24 h after transfection, incubated with [3H]dT (1 μCi/well) for 5, 10 and 24 h respectively, to measure radioactivity in a scintillation counter, as previously described (20). Simultaneously, cells in duplicate 24‐well plates were harvested for cell counts using the trypan blue exclusion technique. Radioactivity per well was divided by cell number in a duplicated well yielding radioactivity on average per cell. Results are presented as CPM/104 cells for [3H]dT incorporation. Cell population growth rate (0 versus 5, 10 and 24 h respectively) was calculated using total cell numbers at 0 h against 5, 10 and 24 h respectively.
Luciferase assays
Cells were cultured in six‐well plates until 60% confluent, transfected with empty vector, Id1, p65 and Id1+p65, co‐transfected with NF‐κB/β‐galactosidase reporters and cyclin D1/β‐galactosidase reporters, respectively, at 1.4 μg/ml for 7–16 h, recovered in FGM for 24 h, and harvested for luciferase assay, as previously described (21). Activities of target luciferase reporters versus β‐galactosidase are presented as relative luciferase activity (RLA).
Western blot analysis
Cells were cultured in T‐75 flasks up to 60% confluence, transfected with empty vector, Id1, p65, IκBαM, Id1+p65, Id1+ IκBαM and Id1+PDTC, respectively, in transfection medium, for 16 h, recovered in FGM for 24 h, and harvested for cytosol and nuclear protein isolation. Forty micrograms of cytosol or nuclear protein was electrophoresed and blotted on a nitrocellulose membrane. Specific antigens (cyclin D1, keratin 10, p16Ink4a and GAPDH) on the membrane were detected by respective antibodies (anti‐cyclin D1, BD Biosciences product; anti‐K10 and anti‐p16Ink4a, BioMarkers product; anti‐GAPDH, Novus Biologicals, Littleton, CO) using the ECL kit (Amersham Biosciences) according to the manufacturers’ instructions.
Statistical analysis
Student’s t‐test for unequal variances was used for evaluation of two‐group studies, whereas analysis of variance (ANOVA) was used for evaluation of multiple‐group studies. P values <0.05 were considered significant.
Results
Id1, NF‐κB, cyclin D1, Ki67 and keratin 10 were highly expressed in human middle ear cholesteatoma specimens
By immunohistochemistry, Id1, NF‐κB, cyclin D1, Ki67 and keratin 10 were detected in the epithelial layer of cholesteatoma specimens (11 of 14 specimens). Three of 14 middle ear specimens contained onion peel‐like substances but no epithelial layer. Normal middle ear mucosa showed no staining for Id1 and activated NF‐κB in the epithelial layer (Fig. 2a,b), whereas normal head and neck skin showed baseline expression of Id1 and NF‐κB in the basal cell layer (limited to few basal cells, data not shown). Unlike normal head and neck skin specimens, Id1 and NF‐κB were extensively expressed in the cholesteatoma specimens. Id1 and NF‐κB, NF‐κB and cyclin D1, and cyclin D1 and Ki67 antigens were co‐expressed in the basal cells (Fig. 2d–h, nuclei) of cholesteatoma, whereas NF‐κB and keratin 10 were co‐expressed in the suprabasal cell layer (Fig. 2i, nuclei and cytosol) of the cholesteatomatous epithelium.
Figure 2.
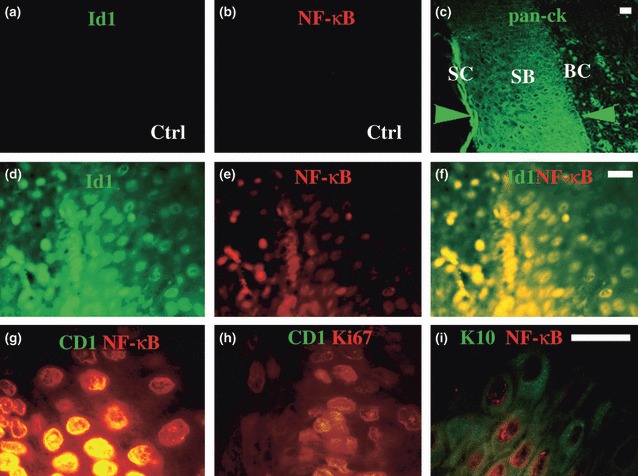
Id1, NF‐κB, cyclin D1 and Ki67 are highly expressed in cholesteatoma tissues. Normal middle ear epithelium was negative for Id1 (a) and activated NF‐κB (b), whereas cholesteatoma produced abundant pan‐cytokeratins (pan‐ck) in the basal cell (BC), and suprabasal (SB) and stratum corneum (SC) layers (c, between green arrowheads). Id1 (d) and activated NF‐κB (e) were co‐expressed in the basal cell (BC) layer (f) of cholesteatoma tissues. Similarly, activated NF‐κB and cyclin D1 (g) as well as cyclin D1 and Ki67 (h) were co‐expressed in the basal layer; while keratin 10 and NF‐κB (i) were co‐expressed in the suprabasal layer of cholesteatoma epithelium. Bar = 10 μm applying to the same row; Ctrl, control middle ear tissue (a and b); and cholesteatoma tissue (c–i).
Id1 induces proliferation of keratinocytes via NF‐κB in vitro
To study the role of Id1 in cholesteatomatous epithelium, Id1 was transfected into Rhek‐1A cells and cell proliferation was studied by [3H]dT incorporation and cell population growth rate methods. Id1 significantly increased DNA synthesis (Fig. 3A) and cell population growth rate (Fig. 3B), associated with increase of NF‐κB luciferase activity (Fig. 3C) and translocation of NF‐κB from the cytosol to nuclei (Fig. 3D) compared to controls.
Figure 3.
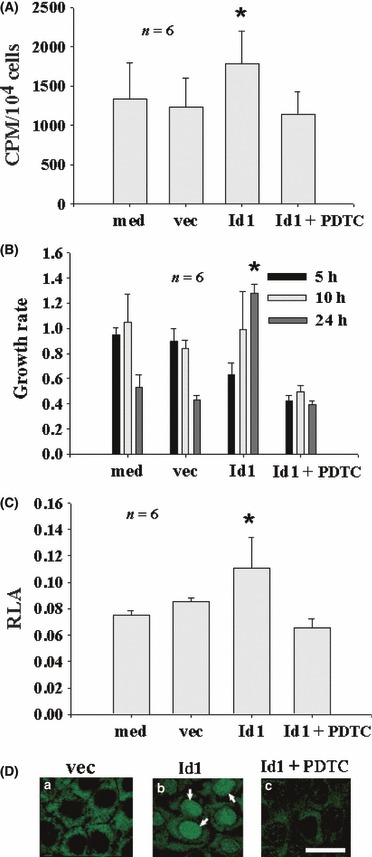
Id1 induces proliferation of keratinocytes via NF‐κB in vitro. (A) Id1 significantly increased DNA synthesis (CPM, counts per minute) compared with vec, whereas PDTC eliminated the effect of Id on DNA synthesis of keratinocytes. (B) Id1 increased cell population growth rate in a time‐dependent manner. At 24 h, Id1 significantly increased growth rate compared to vec. PDTC significantly inhibited the effect of Id1 on growth rate. Note that empty vector‐transfected cells had high growth rate at 5 h but declined thereafter, whereas Id1‐transfected cells had low growth rate at 5 h but increased thereafter. (C) Id1 significantly increased promoter activity of NF‐κB (RLA, relative luciferase activity) compared to vec and PDTC eliminated the effect of Id1 on promoter activity of NF‐κB. (D) Id1 increased NF‐κB translocation into nuclei of Rhek‐1A keratinocytes after 1 h transfection of Id1 compared to vec (arrows in panel b pointing to the nuclei of Rhek‐1A keratinocytes, positive for activated NF‐κB after staining with anti‐activated NF‐κB). Bar = 10 μm, applying to the same row in (D). med, medium alone as blank control; vec, empty vector as transfection control; *P < 0.05.
Id1 up‐regulates cyclin D1 via NF‐κB in keratinocytes
Immunohistochemical data demonstrated co‐expression of NF‐κB and cyclin D1 in basal cells of cholesteatoma tissues. To study whether Id1 promoted proliferation of keratinocytes via the NF‐κB/cyclin D1 pathway and provided a link to aggressive growth of keratinocytes in cholesteatoma, we focused on this pathway using luciferase assays. Specifically, Rhek‐1A cells were transfected with Id1, p65 and Id1+p65, respectively, co‐transfected with wild‐type or mutant cyclin D1/β‐galactosidase reporters, and harvested for luciferase assays; Western blot analysis was carried out for evaluation of any potential link between NF‐κB and cyclin D1 in the presence or absence of Id1. It was demonstrated that Id1 significantly increased luciferase activity of wild‐type cyclin D1 reporters compared to empty vector (Fig. 4A, Id1 versus vec). Mutation of the NF‐κB binding site at the cyclin D1 promoter fully abrogated Id1‐induced cyclin D1 promoter activity (Fig. 4B, Id1 versus Id1+CDmt). This suggests that Id1 drives transcription of cyclin D1 via NF‐κB. In addition, p65 significantly increased Id1‐induced promoter activity of cyclin D1 (Id+p65 versus Id1), adding support to the notion that Id1 regulates cyclin D1 via NF‐κB.
Figure 4.
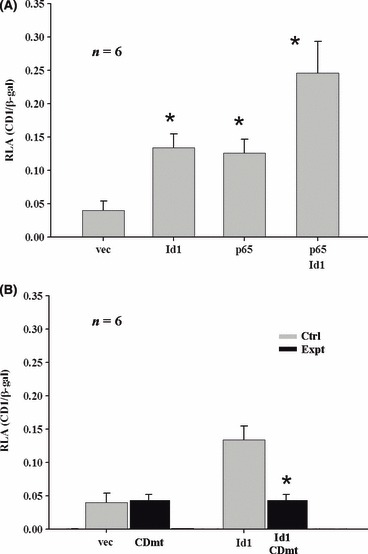
Id1 up‐regulates cyclin D1 via NF‐κB in keratinocytes in vitro. (A) Id1 significantly increased luciferase activity of cyclin D1 in Rhek‐1A cells (Id1 versus vec); p65 further increased Id1‐induced action (Id1+p65 versus Id1); p65 itself significantly increased luciferase activity of cyclin D1 (p65 versus vec); and Id1+p65 significantly increased luciferase activity of cyclin D1 compared with Id1 or p65 alone (Id1+p65 versus Id1 or p65). (B) Mutation of the NF‐κB‐binding site at the promoter of cyclin D1 (CDmt) fully abrogated the Id1‐indued cyclin D1 promoter activity (Id1 versus Id1+CDmt); and CDmt itself did not increase or decrease luciferase activity (CDmt versus vec). *P < 0.05 when compared with vec in (A) or Id1 in (B).
Id1 inhibited expression of p16Ink4a but induced expression of keratin 10 via an NF‐κB‐dependent mechanism in keratinocytes
p16Ink4a is an inhibitor of cyclin‐dependent kinases, preventing cells from entering into the cell cycle (22). To study whether Id1 inhibit would expression of p16Ink4a in keratinocytes, Id1 and empty vector were transfected into Rhek‐1A cells and expression of p16Ink4a and keratin 10 were evaluated by Western blot analysis. The data demonstrated that Id1 inhibited expression of p16Ink4a (Fig. 5, middle row, Id1 versus vec) but increased expression of keratin 10 (Fig. 5, top row, Id1 versus vec). The same applied to p65 and Id1+p65. IκBαM, a dominant‐negative inhibitor of NF‐κB, inhibited expression of keratin 10 but rescued expression of p16Ink4a. However, Id1 antagonized action of IκBαM on expression of keratin 10 and action of IκBαM on expression of p16Ink4a. To study whether Id1‐induced keratin 10 expression was also dependent upon NF‐κB, Id1+p65 as well as Id1+ IκBαM were co‐transfected into the cells. Results demonstrated that Id1+p65 strengthened expression of keratin 10 compared to p65 and Id1 alone, whereas IκBαM weakened expression of keratin 10 induced by Id1 compared to Id1.
Figure 5.
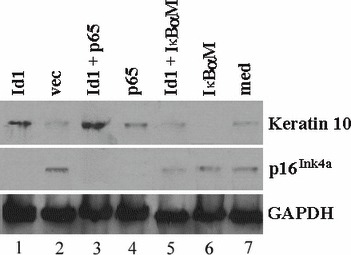
Id1 positively regulates expression of keratin 10 via NF‐κB but negatively regulates expression of p16 Ink4a in keratinocytes in vitro. Western blot analysis demonstrated that Id1 increased expression of keratin 10 (lane 1, top) compared with its control (lane 2, top). p65 further increased Id1‐induced expression of keratin 10 (lane 3 versus lane 1, top) but IκBαM abrogated Id1‐induced expression of keratin 10 (lane 5 versus lane 1, top). p65 itself also increased expression of keratin 10 (lane 4) compared with empty vector (lane 2, top). Id1, p65 and Id1+p65 inhibited expression of p16Ink4a (lanes 1, 3 and 4, middle) compared with empty vector (lane 2, middle) in Rhek‐1A cells. On the contrary, IκBαM increased expression of p16Ink4a (lane 6, middle) but Id1 overcame the action of IκBαM on expression of p16Ink4a (lane 5, middle). med, blank control (without any transfection). GAPDH, glyceraldehyde 3‐phosphate dehydrogenase (loading control).
Discussion
Id1 was originally recognized as a negative regulator of HLH DNA‐binding proteins (5). Later, it was shown to be involved in neurogenesis and angiogenesis of tumour xenografts (23). In this study, we demonstrate for the first time that Id1 was involved in mucosal infectious diseases (1) and related to proliferative middle ear diseases.
As expected, Id1 was linked to the aggressive growth of acquired cholesteatoma through the Id1→NF‐κB→cyclin D1→Ki67 signalling pathway (Fig. 1). First, Id1 activated NF‐κB. NF‐κB, in turn, activated transcription of cyclin D1. Cyclin D1 then increased progression of the cell cycles from G0 to S phase. In addition, Id1 inhibited expression of p16Ink4a, liberating cells from cell cycle inhibition. Id1 acting through NF‐κB/cyclin D1/Ki67 signalling pathway provided an explanation of why middle ear infection is linked to cholesteatoma. However, this experiment was performed in vitro using immortalized keratinocytes and whether the above signalling pathway occurs in cholesteatoma matrix remains to be further studied. At present, it is not clear whether physical damage and trauma to the tympanic membrane trigger expression of the Id family.
Numerous mechanisms are proposed to underlie acquired cholesteatoma, all of them invoke inflammation of keratinocytes in some way. We have demonstrated in this study that activation of NF‐κB is actively linked to cell proliferation in keratinocytes, which frequently originate from the external auditory canal skin. The molecular mechanism involves up‐regulation of cyclin D1 and down‐regulation of p16Ink4a in keratinocytes. The former is a cell cycle progression protein and the latter a cell cycle progression inhibitor. Through this mechanism, Id1 opens a window for keratinocytes to actively grow and proliferate. Our in vitro studies demonstrate that PDTC, an inhibitor of NF‐κB, blocks Id1‐induced proliferation of keratinocytes, indicating that Id1‐induced cell proliferation is dependent on NF‐κB.
Activity of the cyclin D/retinoblastoma (Rb) pathway leads to proliferation of cells (24). Cyclin D1, together with cyclin‐dependent kinases 4/6 (cdk 4/6), overcomes the function of Rb protein that promotes cell cycle progression. It is generally accepted that normal Rb function must be removed, one way or another, for a cell to divide and down‐regulation of p16Ink4a is one of the mechanisms for removing Rb function. Cyclin D1 is a well‐established positive regulator of early cell cycle progression, that is, transition from G0/G1 to S phase (25) through phosphorylation of Rb and dissociation of E2F (a checkpoint protein for S phase entry). Up‐regulation of cyclin D1 and removal of p16Ink4a inhibition in Rhek‐1A cells by Id1, potentiates population growth and proliferation of keratinocytes and in part explains the behaviour of keratinocytes in cholesteatoma – aggressive growth (26, 27, 28) that destroys the ossicular chain and temporal bone and causes serious complications such as deafness and intracranial lesions. Id1‐induced cyclin D1 up‐regulation and p16Ink4a down‐regulation may represent a disease mechanism for cholesteatoma epithelial growth under chronic inflammatory conditions.
Activation of NF‐κB not only increases proliferation of keratinocytes but also up‐regulates production of keratins. Keratin 10 is a product of mature keratinocytes (29, 30, 31) and is highly expressed in the external auditory canal skin. Abundant expression of keratin 10 suggests that keratinocytes in middle ear cholesteatoma originated from the external auditory canal epidermis possibly via a migration process under chronic inflammatory conditions (32). Biologically, cell migration is coupled with cell proliferation. Our data indicate that Id1 regulates NF‐κB, and NF‐κB, in turn, up‐regulates expression of keratin 10, which represents a pathological mechanism for accumulation of the onion peel‐like substances in cholesteatoma. p65 strengthens this process, whereas IκBαM attenuates it.
It is noted that NF‐κB at the suprabasal layer and above may not be related to cell population growth and proliferation because cells at the suprabasal layer and above do not grow and proliferate but commit to keratin production. Earlier studies point out that NF‐κB at the suprabasal layer and above may be related to survival and protection of cells from apoptosis (33, 34, 35). Why Id1‐induced NF‐κB in the basal layer leads to cell proliferation, while NF‐κB alone beyond the basal layer results in cell survival and resistance to apoptosis remains an interesting question. It is also not clear from this study how Id1 inhibits expression of p16Ink4a. It is warranted to address these questions in the future because of their importance in pathogenesis of cholesteatoma and physiology of the related epidermis.
Acknowledgements
This study is in part supported by the NIH grants (R01 DC008165, R01 Supplement # 00010055, and R03 CA107989) and the Minnesota Medical Foundation. We would like to express our thanks to Eileen P. Schlentz and Beverly Wuertz for their editorial assistance in the preparation of this manuscript.
References
- 1. Lin J, Tsuboi Y, Pan W, Giebink SG, Adams GL, Kim Y (2002) Analysis by cDNA microarrays of altered gene expression in middle ears of rats following penumococcal infection. Int. J. Pediatr. Otorhinolaryngol. 65, 203–211. [DOI] [PubMed] [Google Scholar]
- 2. Zhang QA, Hamajima Y, Zhang Q, Lin J (2008) Identification of Id1 in acquired middle ear cholesteatoma. Arch. Otolaryngol. 134, 306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Norton JD, Atherton GT (1998) Coupling of cell growth control and apoptosis functions of Id proteins. Mol. Cell. Biol. 18, 2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Norton JD, Deed RW, Craggs G, Sablitzky F (1998) Id helix‐loop‐helix proteins in cell growth and differentiation. Trends Cell Biol. 8, 58–65. [PubMed] [Google Scholar]
- 5. Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H (1990) The protein Id: a negative regulator of helix‐loop‐helix DNA binding proteins. Cell 61, 49–59. [DOI] [PubMed] [Google Scholar]
- 6. Norton JD (2000) ID helix‐loop‐helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci. 113, 3897–3905. [DOI] [PubMed] [Google Scholar]
- 7. Alani RM, Hasskarl J, Grace M, Hernandez MC, Israel MA, Munger K (1999) Immortalization of primary human keratinocytes by the helix‐loop‐helix protein, Id‐1. Proc. Natl. Acad. Sci. USA 96, 9637–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nickoloff BJ, Chaturvedi V, Bacon P, Qin JZ, Denning MF, Diaz MO (2000) Id‐1 delays senescence but does not immortalize keratinocytes. J. Biol. Chem. 275, 27501–27504. [DOI] [PubMed] [Google Scholar]
- 9. Hamajima Y, Toyama K, Zhao Z, Kim Y, Ondrey FG, Lin J (2003) Id1 induces the proliferation of middle ear epithelial cells in rats In: The Eighth International Symposium on Recent Advances in Otitis Media. Lim D.J., Bluestone C.D., Casselbrant M. (eds). Fort Lauderdale, FL, USA: BC Decker, 274–276. [Google Scholar]
- 10. Gamou S, Shimizu N (1987) Change in metabolic turnover is an alternate mechanism increasing cell surface epidermal growth factor receptor levels in tumor cells. J. Biol. Chem. 262, 6708–6713. [PubMed] [Google Scholar]
- 11. Ozeki M, Hamajima Y, Feng L, Ondrey FG, Zheng M, Schlentz EP et al. (2007) Id1 induces the proliferation of cochlear sensorineural epithelial cells via the NF‐kB/cyclin D1 pathway in vitro. J. Neurosci. Res. 85, 515–524. [DOI] [PubMed] [Google Scholar]
- 12. Lin J, Guan Z, Wang C, Feng L, Zheng Y, Granados E et al. (2010) Id1 regulates the survival of HNSCC via the NF‐kB/survivin and PI3K/Akt signaling pathways. Clin Cancer Res. 16, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM (1996) Suppression of TNF‐alpha‐induced apoptosis by NF‐kappaB. Science 274, 787–789. [DOI] [PubMed] [Google Scholar]
- 14. Gilmore TD, Koedood M, Piffat KA, White DW (1996) Rel/NF‐kappaB/IkappaB proteins and cancer. Oncogene 13, 1367–1378. [PubMed] [Google Scholar]
- 15. Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA (1992) Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 175, 1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chun KS, Cha HH, Shin JW, Na HK, Park KK, Chung WY et al. (2004) Nitric oxide induces expression of cyclooxygenase‐2 in mouse skin through activation of NF‐kappaB. Carcinogenesis 25, 445–454. [DOI] [PubMed] [Google Scholar]
- 17. Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A et al. (1995) Transforming p21ras mutants and c‐Ets‐2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270, 23589–23597. [DOI] [PubMed] [Google Scholar]
- 18. Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D (1993) The candidate proto‐oncogene bcl‐3 encodes a transcriptional coactivator that activates NF‐kappa B p50 homodimers. Genes Dev. 7, 1354–1363. [DOI] [PubMed] [Google Scholar]
- 19. Lin J, Tsprun V, Kawano H, Paparella MM, Zhang Z, Andway R et al. (2001) Characterization of mucins in human middle ear and eustachian tube. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L1157–L1167. [DOI] [PubMed] [Google Scholar]
- 20. Toyama K, Kim Y, Paparella MM, Lin J (2004) Temperature‐sensitive SV40 immortalized rat middle ear epithelial cells. Ann. Otol. Rhinol. Laryngol. 113, 967–974. [DOI] [PubMed] [Google Scholar]
- 21. Tsuchiya K, Kim Y, Ondrey FG, Lin J (2005) Characterization of a temperature‐sensitive mouse middle ear epithelial cell line. Acta Otolaryngol. 125, 823–829. [DOI] [PubMed] [Google Scholar]
- 22. Shapiro GI, Rollins BJ (1996) p16INK4A as a human tumor suppressor. Biochim. Biophys. Acta 1242, 165–169. [DOI] [PubMed] [Google Scholar]
- 23. Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R et al. (1999) Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401, 670–677. [DOI] [PubMed] [Google Scholar]
- 24. Weinberg RA (1995) The retinoblastoma protein and cell cycle control. Cell 81, 323–330. [DOI] [PubMed] [Google Scholar]
- 25. Sherr CJ (1993) Mammalian G1 cyclins. Cell 73, 1059–1065. [DOI] [PubMed] [Google Scholar]
- 26. Michaels L (1989) Biology of cholesteatoma. Otolaryngol. Clin. North Am. 22, 869–881. [PubMed] [Google Scholar]
- 27. Broekaert D, Coucke P, Leperque S, Ramaekers F, Van Muijen G, Boedts D et al. (1992) Immunohistochemical analysis of the cytokeratin expression in middle ear cholesteatoma and related epithelial tissues. Ann. Otol. Rhinol. Laryngol. 101, 931–938. [DOI] [PubMed] [Google Scholar]
- 28. Ergun S, Zheng X, Carlsoo B (1994) Antigen expression of epithelial markers, collagen IV and Ki67 in middle ear cholesteatoma. An immunohistochemical Study. Acta Otolaryngol. 114, 295–302. [DOI] [PubMed] [Google Scholar]
- 29. Roop DR, Krieg TM, Mehrel T, Cheng CK, Yuspa SH (1988) Transcriptional control of high molecular weight keratin gene expression in multistage mouse skin carcinogenesis. Cancer Res. 48, 3245–3252. [PubMed] [Google Scholar]
- 30. Stoler A, Kopan R, Duvic M, Fuchs E (1988) Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J. Cell Biol. 107, 427–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fuchs E (1990) Epidermal differentiation: the bare essentials. J. Cell Biol. 111, 2807–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sade J (1972) Epithelial invasion of intraossicular spaces. J. Laryngol. Otol. 86, 15–21. [DOI] [PubMed] [Google Scholar]
- 33. Seitz CS, Freiberg RA, Hinata K, Khavari PA (2000) NF‐kappaB determines localization and features of cell death in epidermis. J. Clin. Invest. 105, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaufman CK, Fuchs E (2000) It’s got you covered. NF‐kappaB in the epidermis. J. Cell Biol. 149, 999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qin JZ, Chaturvedi V, Denning MF, Choubey D, Diaz MO, Nickoloff BJ (1999) Role of NF‐kappaB in the apoptotic‐resistant phenotype of keratinocytes. J. Biol. Chem. 274, 37957–37964. [DOI] [PubMed] [Google Scholar]


