Abstract
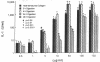
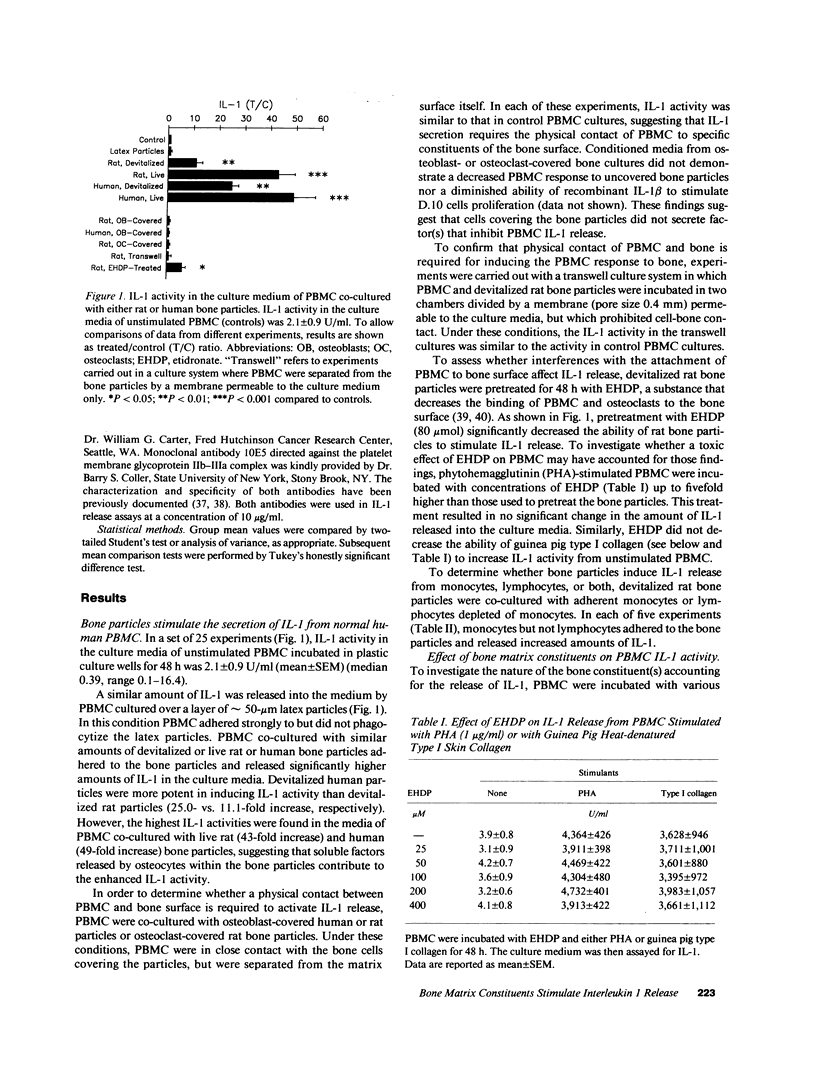
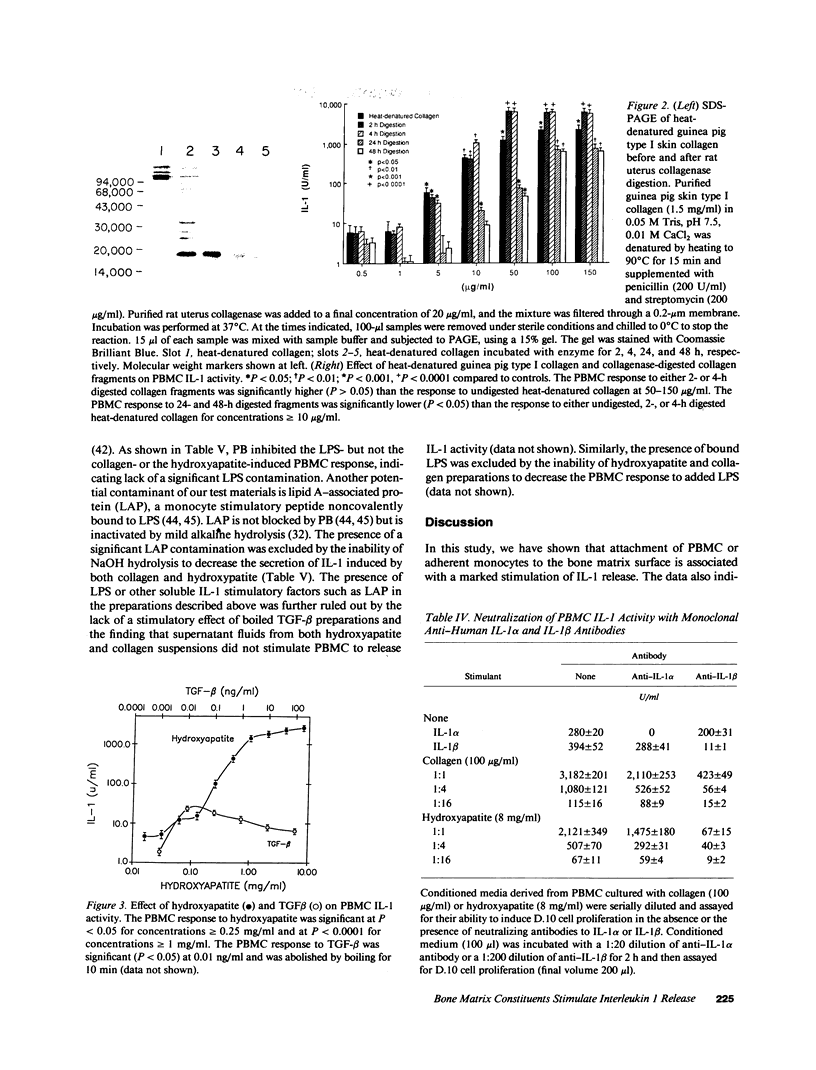
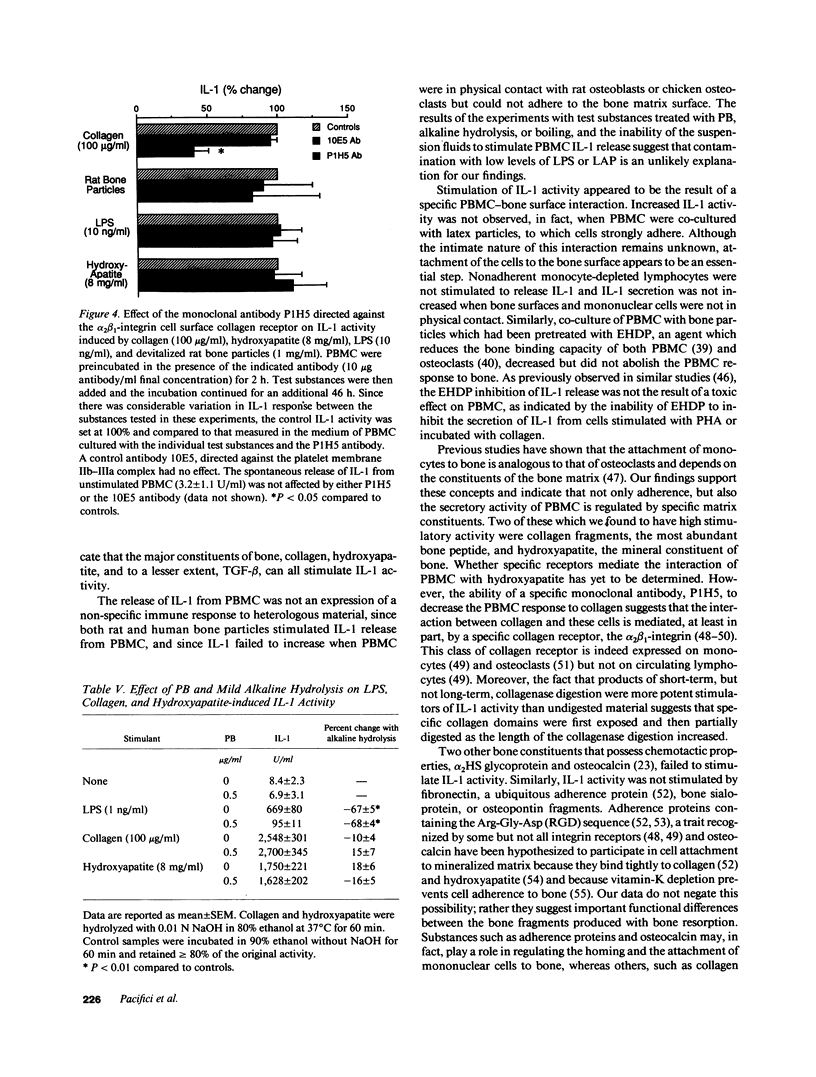
To test the hypothesis that mononuclear cells are stimulated to release interleukin 1 (IL-1) by bone fragments released in the bone microenvironment during the remodeling cycle, we have investigated the effects of bone matrix and some of its constituents on IL-1 secretin from peripheral blood mononuclear cells (PBMC). Increases in IL-1 activity were observed when either PBMC or adherent monocytes, but not lymphocytes depleted of monocytes, were co-cultured with either human or rat bone particles but not with latex particles of similar size. Co-culture of PBMC with bone particles in a transwell system where the cells were physically separated from the bone particles, or with osteoblast- or osteoclast-covered bone particles, did not stimulate IL-1 release, indicating that a physical contact between PBMC and the bone surface is required for eliciting IL-1 release. This was confirmed by the finding of a lower stimulatory effect of bone particles pretreated with etidronate, a bisphosphonate which decreases the bone binding capacity of PBMC. Constituents of bone matrix, such as collagen fragments, hydroxyproline, and, to a lesser extent, transforming growth factor-beta, but not osteocalcin, alpha 2HS glycoprotein, fragments of either bone sialoprotein or osteopontin, and fibronectin, stimulated PBMC IL-1 release in a dose-dependent fashion. Collagen-stimulated IL-1 release was partially and specifically inhibited by a monoclonal antibody directed against the alpha 2 beta 1-integrin cell surface collagen receptor. These data demonstrate that products of bone resorption, known to be chemotactic for mononuclear cells, stimulate PBMC IL-1 activity. These findings may help explain previous documentation of increased IL-1 secretion by circulating monocytes obtained from patients with high turnover osteoporosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair H. C., Kahn A. J., Crouch E. C., Jeffrey J. J., Teitelbaum S. L. Isolated osteoclasts resorb the organic and inorganic components of bone. J Cell Biol. 1986 Apr;102(4):1164–1172. doi: 10.1083/jcb.102.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonucci E. The organic-inorganic relationships in bone matrix undergoing osteoclastic resorption. Calcif Tissue Res. 1974;16(1):13–36. doi: 10.1007/BF02008210. [DOI] [PubMed] [Google Scholar]
- Boyce B. F., Aufdemorte T. B., Garrett I. R., Yates A. J., Mundy G. R. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989 Sep;125(3):1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Canalis E. Effect of platelet-derived growth factor on DNA and protein synthesis in cultured rat calvaria. Metabolism. 1981 Oct;30(10):970–975. doi: 10.1016/0026-0495(81)90094-9. [DOI] [PubMed] [Google Scholar]
- Canalis E. Interleukin-1 has independent effects on deoxyribonucleic acid and collagen synthesis in cultures of rat calvariae. Endocrinology. 1986 Jan;118(1):74–81. doi: 10.1210/endo-118-1-74. [DOI] [PubMed] [Google Scholar]
- Carano A., Teitelbaum S. L., Konsek J. D., Schlesinger P. H., Blair H. C. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J Clin Invest. 1990 Feb;85(2):456–461. doi: 10.1172/JCI114459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985 Jul;76(1):101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comite F., Delman M., Hutchinson-Williams K., DeCherney A. H., Jensen P. Reduced bone mass in reproductive-aged women with endometriosis. J Clin Endocrinol Metab. 1989 Oct;69(4):837–842. doi: 10.1210/jcem-69-4-837. [DOI] [PubMed] [Google Scholar]
- Damais C., Jupin C., Parant M., Chedid L. Induction of human interleukin-1 production by polymyxin B. J Immunol Methods. 1987 Jul 16;101(1):51–56. doi: 10.1016/0022-1759(87)90215-8. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Doe W. F., Yang S. T., Morrison D. C., Betz S. J., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. II. Evidence for differentiation signals delivered by lipid A and by a protein rich fraction of lipopolysaccharides. J Exp Med. 1978 Aug 1;148(2):557–568. doi: 10.1084/jem.148.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evêquoz V., Trechsel U., Fleisch H. Effect of bisphosphonates on production of interleukin 1-like activity by macrophages and its effect on rabbit chondrocytes. Bone. 1985;6(6):439–444. doi: 10.1016/8756-3282(85)90221-2. [DOI] [PubMed] [Google Scholar]
- Fakih H., Baggett B., Holtz G., Tsang K. Y., Lee J. C., Williamson H. O. Interleukin-1: a possible role in the infertility associated with endometriosis. Fertil Steril. 1987 Feb;47(2):213–217. [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Mizel S. B. T-Cell lymphoma model for the analysis of interleukin 1-mediated T-cell activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1133–1137. doi: 10.1073/pnas.78.2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen M., Mundy G. R. Actions of recombinant interleukin 1, interleukin 2, and interferon-gamma on bone resorption in vitro. J Immunol. 1986 Apr 1;136(7):2478–2482. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Russell R. G. Stimulation of the proliferation of human bone cells in vitro by human monocyte products with interleukin-1 activity. J Clin Invest. 1985 Apr;75(4):1223–1229. doi: 10.1172/JCI111819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Amano S., Nakada K., Ohmori Y., Miyoshi T., Hirose K., Kitano S. Biological characterization of interleukin-1-like cytokine produced by cultured bone cells from newborn mouse calvaria. Calcif Tissue Int. 1987 Jul;41(1):31–37. doi: 10.1007/BF02555128. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. Adhesive protein receptors on hematopoietic cells. Immunol Today. 1988 Apr;9(4):109–113. doi: 10.1016/0167-5699(88)91280-7. [DOI] [PubMed] [Google Scholar]
- Hurley M. M., Fall P., Harrison J. R., Petersen D. N., Kream B. E., Raisz L. G. Effects of transforming growth factor alpha and interleukin-1 on DNA synthesis, collagen synthesis, procollagen mRNA levels, and prostaglandin E2 production in cultured fetal rat calvaria. J Bone Miner Res. 1989 Oct;4(5):731–736. doi: 10.1002/jbmr.5650040512. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Fibronectins. Sci Am. 1986 Jun;254(6):42–51. doi: 10.1038/scientificamerican0686-42. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ikeda E., Kusaka M., Hakeda Y., Yokota K., Kumegawa M., Yamamoto S. Effect of interleukin 1 beta on osteoblastic clone MC3T3-E1 cells. Calcif Tissue Int. 1988 Sep;43(3):162–166. doi: 10.1007/BF02571314. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T., Horowitz M., Lee F., Robb R., Flood P. M. Autocrine growth of T cells independent of interleukin 2: identification of interleukin 4 (IL 4, BSF-1) as an autocrine growth factor for a cloned antigen-specific helper T cell. J Immunol. 1987 Jun 15;138(12):4280–4287. [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J. B., Dunn K., Key L. L., Jr In vitro degradation of bone particles by human monocytes is decreased with the depletion of the vitamin K-dependent bone protein from the matrix. Endocrinology. 1986 Apr;118(4):1636–1642. doi: 10.1210/endo-118-4-1636. [DOI] [PubMed] [Google Scholar]
- Lorenzo J. A., Sousa S. L., Alander C., Raisz L. G., Dinarello C. A. Comparison of the bone-resorbing activity in the supernatants from phytohemagglutinin-stimulated human peripheral blood mononuclear cells with that of cytokines through the use of an antiserum to interleukin 1. Endocrinology. 1987 Sep;121(3):1164–1170. doi: 10.1210/endo-121-3-1164. [DOI] [PubMed] [Google Scholar]
- Malone J. D., Richards M. alpha 2HS glycoprotein is chemotactic for mononuclear phagocytes. J Cell Physiol. 1987 Jul;132(1):118–124. doi: 10.1002/jcp.1041320116. [DOI] [PubMed] [Google Scholar]
- Malone J. D., Teitelbaum S. L., Griffin G. L., Senior R. M., Kahn A. J. Recruitment of osteoclast precursors by purified bone matrix constituents. J Cell Biol. 1982 Jan;92(1):227–230. doi: 10.1083/jcb.92.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Curry B. J. The use of polymyxin B and C3H/HeJ mouse spleen cells as criteria for endotoxin contamination. J Immunol Methods. 1979 May 10;27(1):83–92. doi: 10.1016/0022-1759(79)90241-2. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Varani J., Orr W., Gondek M. D., Ward P. A. Resorbing bone is chemotactic for monocytes. Nature. 1978 Sep 14;275(5676):132–135. doi: 10.1038/275132a0. [DOI] [PubMed] [Google Scholar]
- Niwa M., Milner K. C., Ribi E., Rudbach J. A. Alteration of physical, chemical, and biological properties of endotoxin by treatment with mild alkali. J Bacteriol. 1969 Mar;97(3):1069–1077. doi: 10.1128/jb.97.3.1069-1077.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osdoby P., Martini M., Caplan A. I. The development of long bones of the limb: cell and matrix interactions of osteoclasts and monocytes. Prog Clin Biol Res. 1982;110(Pt B):229–238. [PubMed] [Google Scholar]
- Pacifici R., Rifas L., McCracken R., Vered I., McMurtry C., Avioli L. V., Peck W. A. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2398–2402. doi: 10.1073/pnas.86.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R., Rifas L., Teitelbaum S., Slatopolsky E., McCracken R., Bergfeld M., Lee W., Avioli L. V., Peck W. A. Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4616–4620. doi: 10.1073/pnas.84.13.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J., Mundy G. R. Modulation of type beta transforming growth factor activity in bone cultures by osteotropic hormones. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2024–2028. doi: 10.1073/pnas.84.7.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polan M. L., Daniele A., Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988 Jun;49(6):964–968. [PubMed] [Google Scholar]
- Polan M. L., Loukides J., Nelson P., Carding S., Diamond M., Walsh A., Bottomly K. Progesterone and estradiol modulate interleukin-1 beta messenger ribonucleic acid levels in cultured human peripheral monocytes. J Clin Endocrinol Metab. 1989 Dec;69(6):1200–1206. doi: 10.1210/jcem-69-6-1200. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Healy A. M., Byrne M., Krane S. M. 1,25-Dihydroxyvitamin D3 induces collagen binding to the human monocyte line U937. J Clin Invest. 1987 Oct;80(4):962–969. doi: 10.1172/JCI113189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Otsuka A. A., Poser J. W., Kristaponis J., Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976 May;73(5):1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini M., Boyce B., Aufdemorte T., Bonewald L., Mundy G. R. Infusions of recombinant human interleukins 1 alpha and 1 beta cause hypercalcemia in normal mice. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5235–5239. doi: 10.1073/pnas.85.14.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook P. N., Reeve J. Bone disease in rheumatoid arthritis. Clin Sci (Lond) 1988 Mar;74(3):225–230. doi: 10.1042/cs0740225. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Rooney M. L., Desjardins L. A., Heeley J. D. Interleukin-1 beta is a potent inhibitor of bone formation in vitro. J Bone Miner Res. 1987 Dec;2(6):559–565. doi: 10.1002/jbmr.5650020612. [DOI] [PubMed] [Google Scholar]
- Tan P., Shore A., Leary P., Keystone E. C. Interleukin abnormalities in recently active rheumatoid arthritis. J Rheumatol. 1984 Oct;11(5):593–596. [PubMed] [Google Scholar]
- Teitelbaum S. L., Stewart C. C., Kahn A. J. Rodent peritoneal macrophages as bone resorbing cells. Calcif Tissue Int. 1979 Jul 3;27(3):255–261. doi: 10.1007/BF02441194. [DOI] [PubMed] [Google Scholar]
- Thomson B. M., Saklatvala J., Chambers T. J. Osteoblasts mediate interleukin 1 stimulation of bone resorption by rat osteoclasts. J Exp Med. 1986 Jul 1;164(1):104–112. doi: 10.1084/jem.164.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Grant G. A., Sacchettini J. C., Roswit W. T., Jeffrey J. J. The gelatinolytic activity of rat uterus collagenase. J Biol Chem. 1985 Nov 5;260(25):13601–13606. [PubMed] [Google Scholar]
- Wood D. D., Cameron P. M. The relationship between bacterial endotoxin and human B cell-activating factor. J Immunol. 1978 Jul;121(1):53–60. [PubMed] [Google Scholar]
- Zambonin Zallone A., Teti A., Primavera M. V. Isolated osteoclasts in primary culture: first observations on structure and survival in culture media. Anat Embryol (Berl) 1982 Dec;165(3):405–413. doi: 10.1007/BF00305576. [DOI] [PubMed] [Google Scholar]