Assessment of disease severity as well as monitoring patients over time remains a major challenge for pediatricians taking care of the growing number of children with nonalcoholic fatty liver disease (NAFLD)(1). Although, liver biopsy is still considered the goal standard, there is an urgent need to develop non-invasive reliable tests(2). A liver biopsy provides important information regarding the degree of liver damage, changes in the overall liver architecture, as well as severity of inflammatory activity and fibrosis. However, a liver biopsy is an invasive test that is poorly suited as a diagnostic modality in such a prevalent condition because of its expense and risks of complications(3). The currently available noninvasive tests mainly serum aminotransferases (ALT and AST) and liver ultrasound have two central limitations: the lack of sensitivity and specificity to distinguish nonalcoholic steatohepatitis (NASH), the more serious form of NAFLD from hepatic steatosis, and to stage the presence and extent of liver fibrosis. Thus, identify and validate potential novel noninvasive biomarkers has been recently the focus of intense research. In general terms diagnostics development in NAFLD have been divided in two major groups: those directed to establish the diagnosis of NASH and those directed to detect and quantify the presence of fibrosis. While different approaches have been taken for developing these tests, the growing understanding of the pathophysiologic mechanisms involved in disease progression in NAFLD have allowed to test several mechanism-based biomarkers target at specific pathways involved in liver damage and disease progression to NASH (Fig. 1).
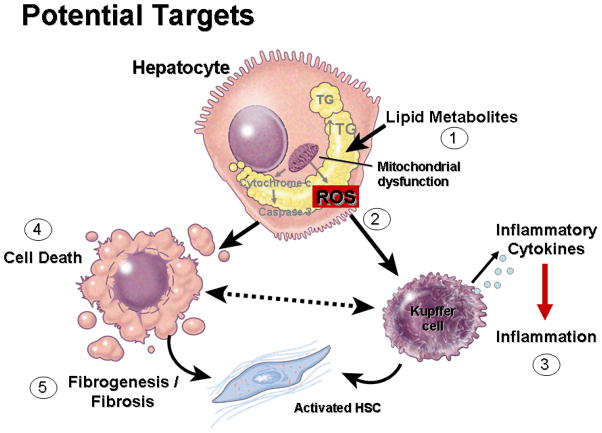
Figure 1. Diagnostic Target Discovery in NAFLD.
The understanding of pathophysiologic pathways involved in liver damage and fibrosis in NAFLD can be used to develop mechanism-base biomarkers. In the context of lipid overloading of hepatocyte the different lipid types and metabolites accumulating may play an important role in triggering lipotoxicity (1) and NASH development. Mitochondrial dysfunction is a key feature of NASH and different mechanisms have been proposed including an increased production of ROS (2), and mitochondrial outer membrane permeabilization, resulting in a cascade of events leading to inflammation (3), hepatocellular apoptosis (4), fibrogenesis and fibrosis (5). Abbreviations: ROS, reactive oxygene species; HSC, activated stellate cells.
In this issue of JPGN, Fitzpatrick et al (4) present the results from a study examining a group of previously proposed mechanism-based biomarkers for NASH diagnosis including the cytokeratin 18 (CK-18) fragments, adiponectin, and high-sensitivity C-reactive protein (hs-CRP), or fibrosis staging including hyaluronic acid and leptin. The study population consisted of 45 children with biopsy-proven NAFLD and 13 age-matched controls. Measurements were done on a blood sample taken on the day of liver biopsy or within 3 months of the liver biopsy. Children with NAFLD showed considerably elevated levels of the CK-18 fragments as compared to healthy controls. In addition those with established NASH showed significantly higher numbers versus those with hepatic steatosis or borderline disease. As a diagnostic test, the CK-18 fragments showed an area under the receiver operating characteristic (ROC) curve of 0.85 with a sensitivity and a specificity for NASH diagnosis of 84% and 88%, respectively. These results are consistent to those previously reported in adult NAFLD patients(5–8) and suggest measuring markers of cell death and in particular circulating CK-18 fragments may become a clinically valuable tool in the diagnosis and monitoring of children with NAFLD. Neither serum adiponectin levels nor hsCRP could distinguish between subjects with NASH and those with hepatic steatosis or borderline disease. Although a state of chronic inflammation and an imbalance in adipocytokines such as adiponectin, TNF-α, and interleukin-6 (IL-6) have been linked to NASH development, measuring the circulating levels of these markers in isolation do not appear to have the sensitivity or specificity to distinguish patients with hepatic steatosis from those with NASH(9–12).
From the markers of fibrosis tested, leptin but not hyaluronic acid levels were significantly higher in those patients with clinically relevant fibrosis (stage ≥ 2) compared to those with no or minimal fibrosis. Quantification of leptin levels performed better that a simple panel marker of fibrosis the APRI test that is calculated by determining the serum AST to platelet ratio and has been shown to be a good predictor of severe stage 3 fibrosis or cirrhosis in adult patients with a variety of chronic liver diseases including NAFLD(13, 14). However, no data was reported regarding the diagnostic utility of leptin for fibrosis staging such as ROC curves, specificities or sensitivities and no comparisons were made with more promising techniques for staging disease in pediatric NAFLD such as tissue elastography or the ELF test (15, 16).
The advantages of this study include (1) the availability of well-characterized biopsy-proven children with the full spectrum of NAFLD, and (2) the study of plasma-based biomarkers which appear relevant in the pathogenesis of NAFLD. Potential limitations include (1) the inclusion of a highly selected group of children evaluated at a tertiary level pediatric Hepatology unit, (2) the absence of reported imaging studies or liver biopsies in control subjects to exclude NAFLD, (3) the possibility that the values of the different markers could be influenced by obesity/insulin resistance/metabolic abnormalities acting as confounders of the association described with NASH and/or fibrosis, and (4) lack of data on liver histology specimen size and portal tract number to determine the potential effect of sampling variability in diagnosing NASH and staging fibrosis.
A key issue that is specific for biomarkers development in pediatric NAFLD and relates to the distinct histological features of this condition in children is worth of discussion. The authors used the NAFLD activity score (NAS) to classified children into either NASH or hepatic steatosis groups. This resulted in a large number of children, close to 40% that could not be classified into any of these two categories and end up in a gray area of so call “borderline disease”. As stated by the authors, the majority of children showed the presence of portal-based disease which does not form part of the NAS. These results confirm again the observations from previous studies that the NAS does not correlate as well with pediatric NAFLD as with adults, and thus, by itself, is not a means of obtaining a “diagnosis”(17, 18). These results also suggest the need for a more reproducible scoring system, perhaps a modified pediatric NAS, to interpret liver histology in pediatric cases of NAFLD that would allow for a better separation of cases. Development of such a score may facilitate both identification of specific biomarkers as well as novel therapeutic strategies for pediatric NAFLD.
With the growing epidemic of NAFLD in children there is an urgent need to identify, develop and validate noninvasive simple and reproducible biomarkers for assessing disease severity and monitoring progression over time(19). The work of Fitzpatrick and colleagues has helped to set the stage for this new era of mechanism-based biomarkers in pediatric NAFLD. Additional larger studies are required before we can know how these markers will perform in pediatric clinics.
Acknowledgments
This work was supported by NIH grants (DK076852) and (DK082451) to AEF and grants from “Bambino Gesù” Children’s Hospital and Research Institute, Rome, Italy to VN.
References
- 1.Nobili V, Day C. Childhood NAFLD: a ticking time-bomb? Gut. 2009;58(11):1442. doi: 10.1136/gut.2009.184465. [DOI] [PubMed] [Google Scholar]
- 2.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46(2):582–9. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 3.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28(4):386–95. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick E, Mitry R, Quaglia A, Hussain M, deBruyne R, Dhawan A. Serum level of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. JPGN-Europe-D-09-00386. doi: 10.1097/MPG.0b013e3181e376be. (In press) [DOI] [PubMed] [Google Scholar]
- 5.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–8. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M, et al. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH) Obes Surg. 2008;18(11):1430–7. doi: 10.1007/s11695-008-9506-y. [DOI] [PubMed] [Google Scholar]
- 7.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44(1):27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 8.Malik R, Chang M, Bhaskar K, Nasser I, Curry M, Schuppan D, et al. The clinical utility of biomarkers and the nonalcoholic steatohepatitis CRN liver biopsy scoring system in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24(4):564–8. doi: 10.1111/j.1440-1746.2008.05731.x. [DOI] [PubMed] [Google Scholar]
- 9.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90(6):3498–504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 10.Oruc N, Ozutemiz O, Yuce G, Akarca US, Ersoz G, Gunsar F, et al. Serum procalcitonin and CRP levels in non-alcoholic fatty liver disease: a case control study. BMC Gastroenterol. 2009;9:16. doi: 10.1186/1471-230X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui JM, Farrell GC, Kench JG, George J. High sensitivity C-reactive protein values do not reliably predict the severity of histological changes in NAFLD. Hepatology. 2004;39(5):1458–9. doi: 10.1002/hep.20223. [DOI] [PubMed] [Google Scholar]
- 12.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40(1):46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 13.Adler M, Gulbis B, Moreno C, Evrard S, Verset G, Golstein P, et al. The predictive value of FIB-4 versus FibroTest, APRI, FibroIndex and Forns index to noninvasively estimate fibrosis in hepatitis C and nonhepatitis C liver diseases. Hepatology. 2008;47(2):762–3. doi: 10.1002/hep.22085. [DOI] [PubMed] [Google Scholar]
- 14.Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–50. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48(2):442–8. doi: 10.1002/hep.22376. [DOI] [PubMed] [Google Scholar]
- 16.Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136(1):160–7. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Carter-Kent C, Yerian LM, Brunt EM, Angulo P, Kohli R, Ling SC, et al. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology. 2009;50(4):1113–20. doi: 10.1002/hep.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58(11):1538–44. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]