Abstract
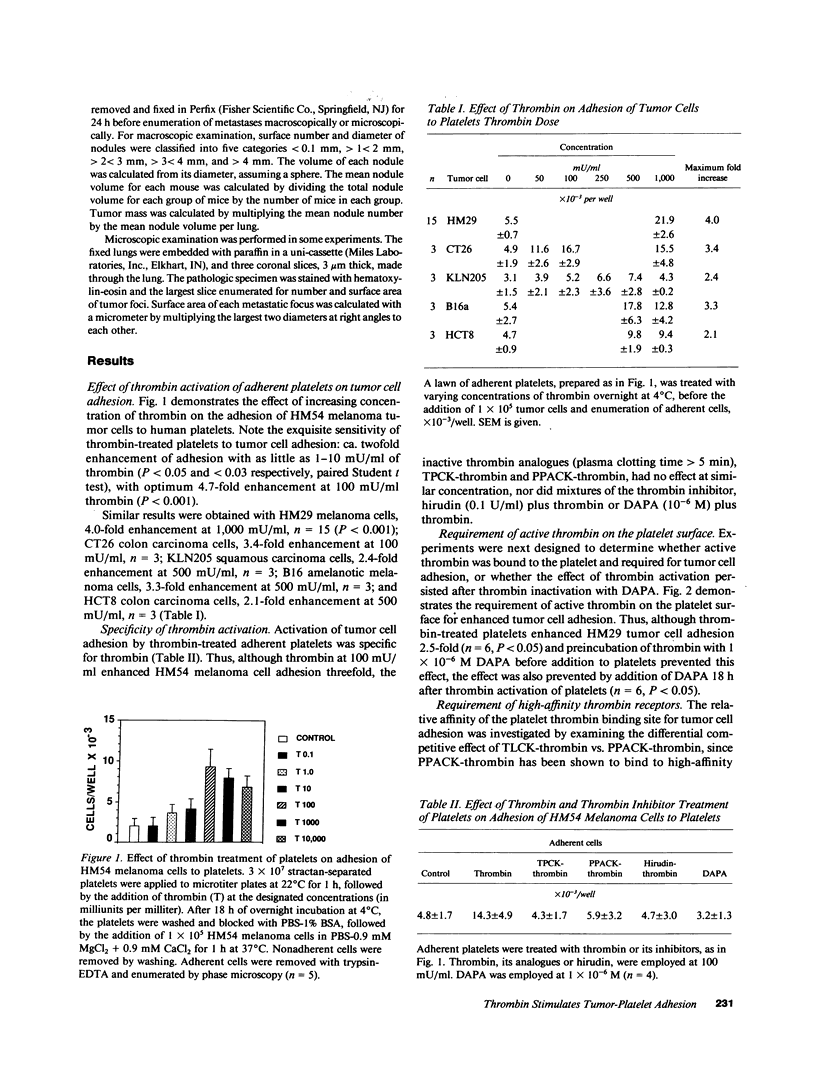
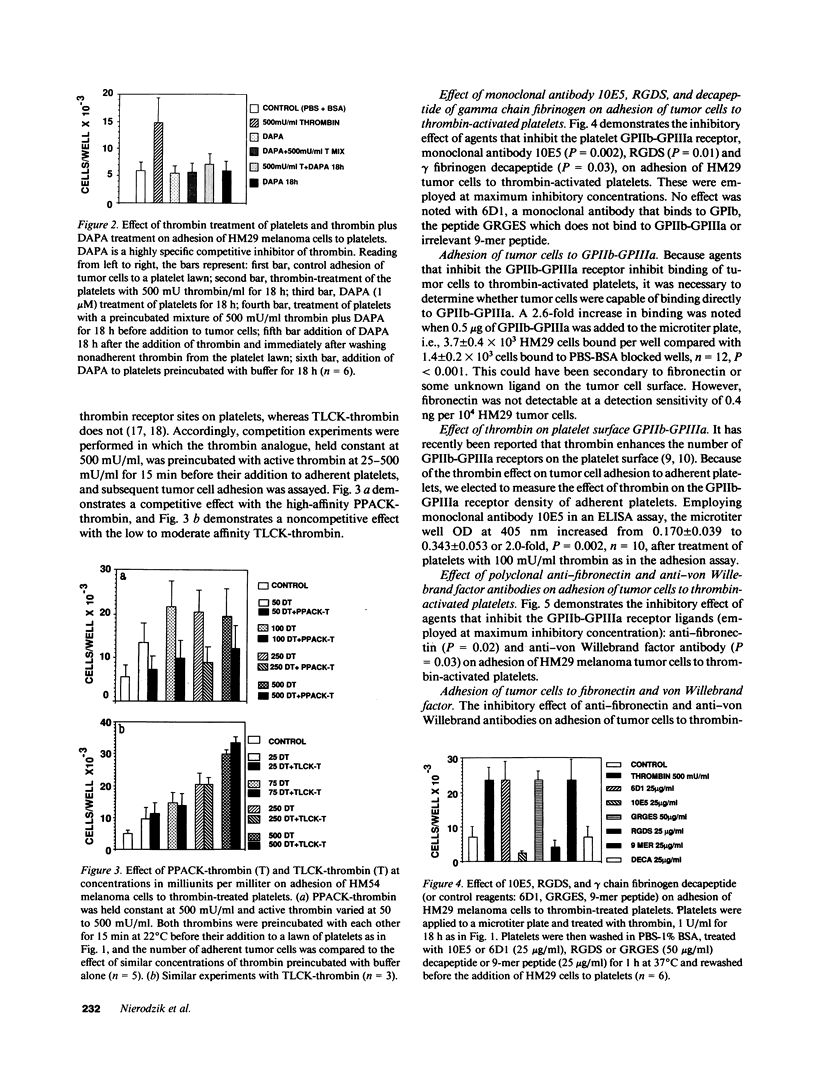
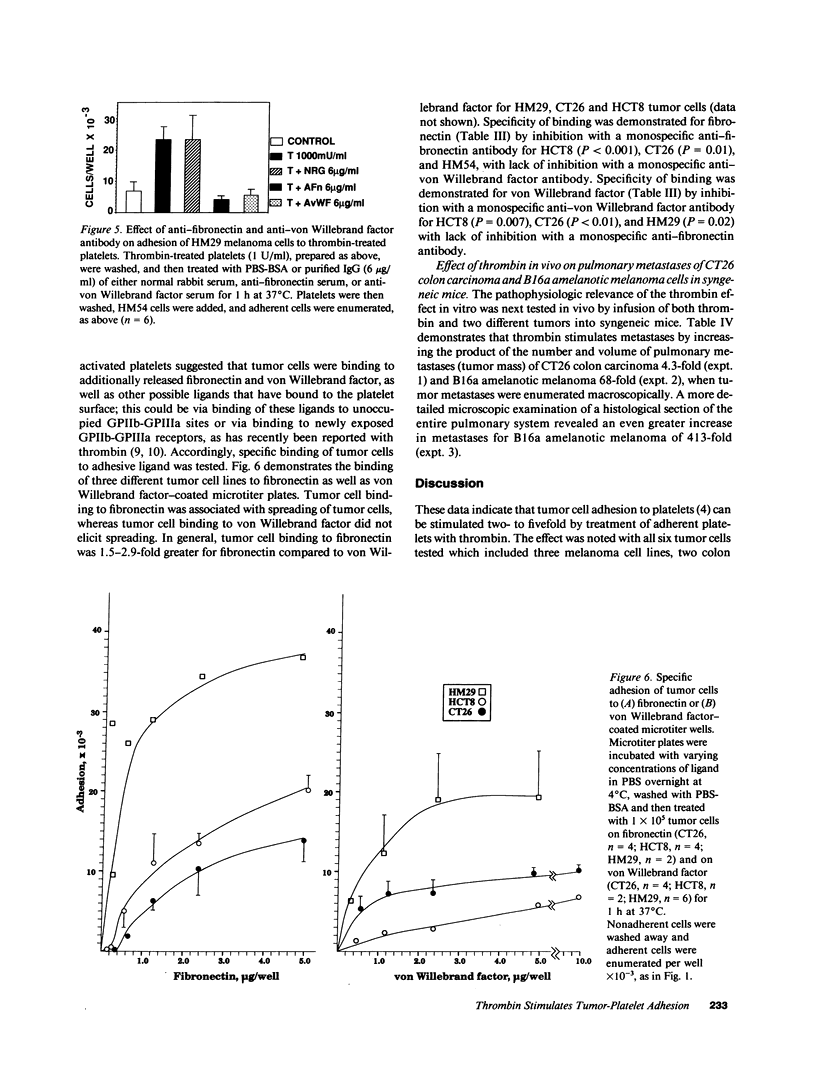
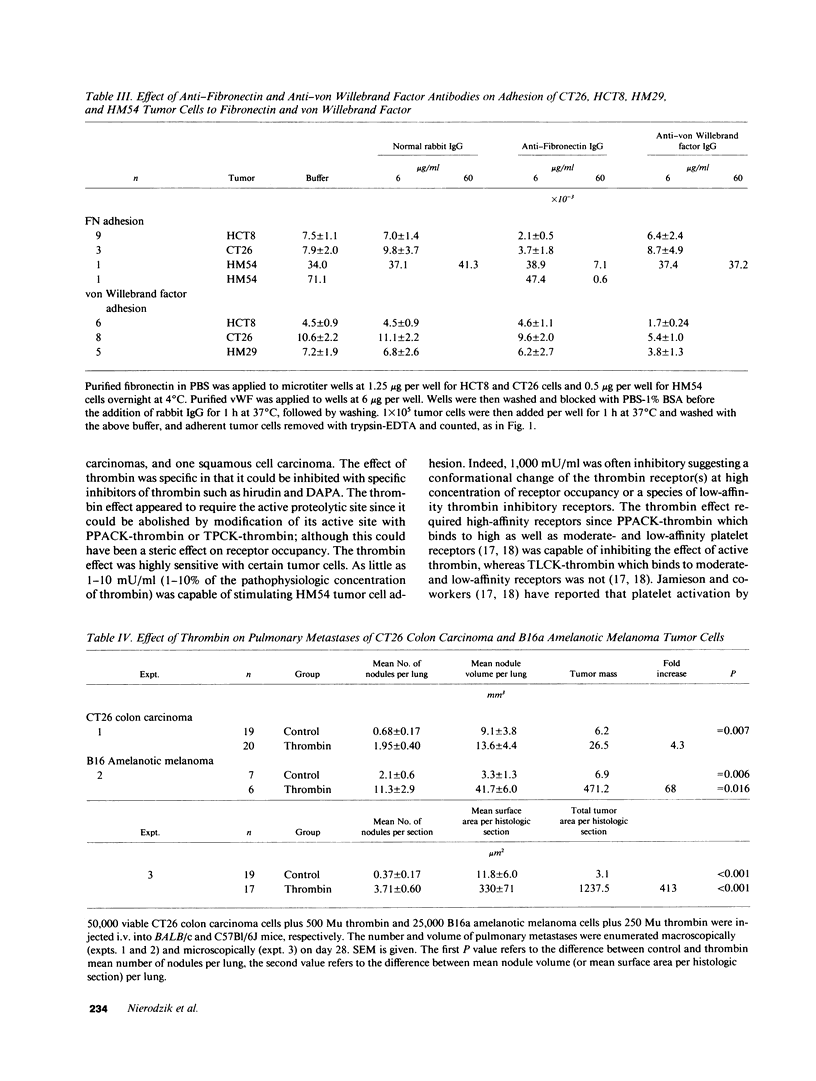
Recent studies have revealed a role for platelets and the platelet-adhesive proteins, fibronectin and von Willebrand factor (vWF) in platelet-tumor cell interaction in vitro and metastasis in vivo. The present report documents the effect of thrombin treatment of platelets on this interaction in vitro and in vivo. In vitro, thrombin at 100-1,000 mU/ml maximally stimulated the adhesion of six different tumor cell lines from three different species two- to fivefold. As little as 1-10 mU/ml was effective. The effect of thrombin was specific (inhibitable by hirudin, dansyl-arginine N-(3-ethyl-1,5 pentanediyl) amide and unreactive with the inactive thrombin analogue N-P-tosyl-L-phenylchloromethylketone-thrombin and D-phenylalanyl-L-propyl-L-arginine chloromethylketone-thrombin (PPACK-thrombin), and required high-affinity thrombin receptors (competition with PPACK-thrombin but not with N-P-tosyl-L-lysine-chloromethyl-ketone-thrombin). Functionally active thrombin was required on the platelet surface. Binding of tumor cells to thrombin-activated platelets was inhibitable by agents known to interfere with the platelet GPIIb-GPIIIa integrin: monoclonal antibody 10E5, tetrapeptide RGDS and gamma chain fibrinogen decapeptide LGGAKQAGDV, as well as polyclonal antibodies against the platelet adhesive ligands, fibronectin and vWF. In vivo, thrombin at 250-500 mU per animal increased murine pulmonary metastases fourfold with CT26 colon carcinoma cells and 68-413-fold with B16 amelanotic melanoma cells. Thus, thrombin amplifies tumor-platelet adhesion in vitro two- to fivefold via occupancy of high-affinity platelet thrombin receptors, and modulation of GPIIb-GPIIIa adhesion via an RGD-dependent mechanism. In vivo, thrombin enhances tumor metastases 4-413-fold with two different tumor cell lines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Larjava H., Yamada K. M. Differences in the biosynthesis and localization of the fibronectin receptor in normal and transformed cultured human cells. Cancer Res. 1990 Mar 1;50(5):1601–1607. [PubMed] [Google Scholar]
- Cavanaugh P. G., Sloane B. F., Honn K. V. Role of the coagulation system in tumor-cell-induced platelet aggregation and metastasis. Haemostasis. 1988;18(1):37–46. doi: 10.1159/000215781. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Smith J. W., Cooper H. M., Quaranta V. A novel vitronectin receptor integrin (alpha v beta x) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989 Apr 7;57(1):59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Leung B., Phillips D. R. A method for purifying the platelet membrane glycoprotein IIb-IIIa complex. Anal Biochem. 1985 Nov 15;151(1):169–177. doi: 10.1016/0003-2697(85)90067-3. [DOI] [PubMed] [Google Scholar]
- Gasic G. J., Gasic T. B., Galanti N., Johnson T., Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973 May;11(3):704–718. doi: 10.1002/ijc.2910110322. [DOI] [PubMed] [Google Scholar]
- Gasic G. J., Gasic T. B., Stewart C. C. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A. 1968 Sep;61(1):46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen K. R., Argraves W. S., Pierschbacher M. D., Ruoslahti E. Inhibition of in vitro tumor cell invasion by Arg-Gly-Asp-containing synthetic peptides. J Cell Biol. 1988 Mar;106(3):925–930. doi: 10.1083/jcb.106.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi I. M., Hatfield J. S., Fitzgerald L. A., Newcombe M., Taylor J. D., Honn K. V. Role of tumor cell glycoproteins immunologically related to glycoproteins Ib and IIb/IIIa in tumor cell-platelet and tumor cell-matrix interactions. FASEB J. 1988 May;2(8):2385–2395. doi: 10.1096/fasebj.2.8.2452113. [DOI] [PubMed] [Google Scholar]
- Harmon J. T., Jamieson G. A. Activation of platelets by alpha-thrombin is a receptor-mediated event. D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone-thrombin, but not N alpha-tosyl-L-lysine chloromethyl ketone-thrombin, binds to the high affinity thrombin receptor. J Biol Chem. 1986 Dec 5;261(34):15928–15933. [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A. The activation of platelets by thrombin: a model for activation by high and moderate affinity receptor pathways. Prog Clin Biol Res. 1988;283:137–158. [PubMed] [Google Scholar]
- Kaneko T., LePage G. A. Growth characteristics and drug responses of a murine lung carcinoma in vitro and in vivo. Cancer Res. 1978 Jul;38(7):2084–2090. [PubMed] [Google Scholar]
- Karpatkin S., Ambrogio C., Pearlstein E. Lack of effect of in vivo prostacyclin on the development of pulmonary metastases in mice following intravenous injection of CT26 colon carcinoma, Lewis lung carcinoma, or B16 amelanotic melanoma cells. Cancer Res. 1984 Sep;44(9):3880–3883. [PubMed] [Google Scholar]
- Karpatkin S., Pearlstein E., Ambrogio C., Coller B. S. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988 Apr;81(4):1012–1019. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., McDonald K. A., Crowley E., Ramos D. M., Damsky C. H. Melanoma cell adhesion to basement membrane mediated by integrin-related complexes. Cancer Res. 1989 Jan 15;49(2):393–402. [PubMed] [Google Scholar]
- Lahav J., Hynes R. O. Involvement of fibronectin, Von Willebrand factor, and fibrinogen in platelet interaction with solid substrata. J Supramol Struct Cell Biochem. 1981;17(4):299–311. doi: 10.1002/jsscb.380170402. [DOI] [PubMed] [Google Scholar]
- Lerner W. A., Pearlstein E., Ambrogio C., Karpatkin S. A new mechanism for tumor induced platelet aggregation. Comparison with mechanisms shared by other tumor with possible pharmacologic strategy toward prevention of metastases. Int J Cancer. 1983 Apr 15;31(4):463–469. doi: 10.1002/ijc.2910310411. [DOI] [PubMed] [Google Scholar]
- Lyman B., Rosenberg L., Karpatkin S. Biochemical and biophysical aspects of human platelet adhesion to collagen fibers. J Clin Invest. 1971 Sep;50(9):1854–1863. doi: 10.1172/JCI106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey C., Johnson A. J., Harris J. U., Wang M. T., Swain S. Isolation of fibrinogen-fibrin related antigen from human plasma by immuno-affinity chromatography: its characterization in normal subjects and in defibrinating patients with abruptio placentae and disseminated cancer. Br J Haematol. 1980 Apr;44(4):655–670. doi: 10.1111/j.1365-2141.1980.tb08720.x. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Prendergast F. G., Mann K. G. Interactions of a fluorescent active-site-directed inhibitor of thrombin: dansylarginine N-(3-ethyl-1,5-pentanediyl)amide. Biochemistry. 1979 Mar 20;18(6):996–1003. doi: 10.1021/bi00573a010. [DOI] [PubMed] [Google Scholar]
- Niiya K., Hodson E., Bader R., Byers-Ward V., Koziol J. A., Plow E. F., Ruggeri Z. M. Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation. Relationship to the binding of fibrinogen and platelet aggregation. Blood. 1987 Aug;70(2):475–483. [PubMed] [Google Scholar]
- Pearlstein E., Ambrogio C., Karpatkin S. Effect of antiplatelet antibody on the development of pulmonary metastases following injection of CT26 colon adenocarcinoma, Lewis lung carcinoma, and B16 amelanotic melanoma tumor cells into mice. Cancer Res. 1984 Sep;44(9):3884–3887. [PubMed] [Google Scholar]
- Peuscher F. W., Cleton F. J., Armstrong L., Stoepman-van Dalen E. A., van Mourik J. A., van Aken W. G. Significance of plasma fibrinopeptide A (fpA) in patients with malignancy. J Lab Clin Med. 1980 Jul;96(1):5–14. [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Plow E. F., Srouji A. H., Meyer D., Marguerie G., Ginsberg M. H. Evidence that three adhesive proteins interact with a common recognition site on activated platelets. J Biol Chem. 1984 May 10;259(9):5388–5391. [PubMed] [Google Scholar]
- Rickles F. R., Edwards R. L., Barb C., Cronlund M. Abnormalities of blood coagulation in patients with cancer. Fibrinopeptide A generation and tumor growth. Cancer. 1983 Jan 15;51(2):301–307. doi: 10.1002/1097-0142(19830115)51:2<301::aid-cncr2820510223>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Rieber M., Gross A., Rieber M. S. Relationship of a Mr 140 fibronectin receptor and other adhesion-related glycoproteins to tumor cell-cell interaction. Cancer Res. 1987 Oct 1;47(19):5127–5131. [PubMed] [Google Scholar]
- Shulman S., Karpatkin S. Crossed immunoelectrophoresis of human platelet membranes. Diminished major antigen in Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Biol Chem. 1980 May 10;255(9):4320–4327. [PubMed] [Google Scholar]
- Thorell L., Blombäck B. Purification of the factor VIII complex. Thromb Res. 1984 Aug 15;35(4):431–450. doi: 10.1016/0049-3848(84)90235-4. [DOI] [PubMed] [Google Scholar]
- Woods V. L., Jr, Wolff L. E., Keller D. M. Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complex which may be accessible to some but not other extracellular proteins. J Biol Chem. 1986 Nov 15;261(32):15242–15251. [PubMed] [Google Scholar]
- Workman E. F., Jr, White G. C., 2nd, Lundblad R. L. Structure-function relationships in the interaction of alpha-thrombin with blood platelets. J Biol Chem. 1977 Oct 25;252(20):7118–7123. [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylänne J., Virtanen I. The Mr 140,000 fibronectin receptor complex in normal and virus-transformed human fibroblasts and in fibrosarcoma cells: identical localization and function. Int J Cancer. 1989 Jun 15;43(6):1126–1136. doi: 10.1002/ijc.2910430628. [DOI] [PubMed] [Google Scholar]
- Yoda Y., Abe T. Fibrinopeptide A (FPA) level and fibrinogen kinetics in patients with malignant disease. Thromb Haemost. 1981 Dec 23;46(4):706–709. [PubMed] [Google Scholar]



