Abstract
The eukaryotic translation initiation factor 4E (eIF4E) is frequently overexpressed in human cancers and is associated with cellular transformation, tumorigenesis, and metastatic progression. It is known that Mnks can phosphorylate eIF4E. Protein phosphatase 2A (PP2A) functions as a tumor suppressor, and it was previously suggested to regulate eIF4E phosphorylation. However, how PP2A regulates eIF4E phosphorylation has not been fully addressed. In this study, we have not only validated the role of PP2A in regulation of eIF4E phosphorylation but also demonstrated the mechanism underlying this process. Inhibition of PP2A using either okadaic acid or PP2A small interfering RNA (siRNA) increased eIF4E phosphorylation, which could be abolished by the presence of the Mnk inhibitor CGP57380 or deficiency of Mnk genes. Thus, Mnks are involved in PP2A-mediated regulation of eIF4E phosphorylation. Moreover, a dephosphorylation assay revealed that PP2A could directly dephosphorylate Mnk1 and eIF4E. m7GTP pull-down assay detected more eIF4G and phospho-eIF4E and less 4EBP-1 in PP2A siRNA-transfected cells than in control siRNA-transfected cells, indicating an increased cap binding of eIF4F complex. Accordingly, okadaic acid treatment or PP2A knockdown increased the levels of c-Myc and Mcl-1, which are proteins known to be regulated by a cap-dependent translation mechanism. Taken together, we conclude that PP2A negatively regulates eIF4E phosphorylation and eIF4F complex assembly through dephosphorylation of Mnk and eIF4E, thus suggesting a novel mechanism by which PP2A exerts its tumor-suppressive function.
Introduction
Protein translational control is an important strategy by which eukaryotic cells regulate gene expression. A prime target of translational control is eukaryotic translation initiation factor 4E (eIF4E), which recognizes and binds to the 7-methylguanosine cap structure present at the 5′ untranslated regions of cellular mRNA and delivers these mRNA to the eIF4F translation initiation complex. Assembly of the eIF4F complex is dependent on eIF4E availability. Given that eIF4E is the least abundant among the initiator factors involved in eIF4F complex, eIF4E is the rate limiting factor for cap-dependent translation initiation [1]. Consequently, changes in eIF4E levels profoundly affect translation rates of certain proteins, particularly those related to cell growth and survival involved in oncogenesis (e.g., c-Myc, cyclin D1, hypoxia-inducible factor 1, andMcl-1), which, under normal cellular conditions, are translationally repressed. eIF4E expression is frequently elevated in many types of cancers and is associated with malignant progression. Inhibition of eIF4E effectively suppresses cellular transformation and tumor growth, invasiveness, and metastasis [2–4].
eIF4E is regulated by phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin and mitogen-activated protein kinase (MAPK)/Mnk signaling and may act as a convergence point of these pathways. The former enhances eIF4E activity through release from the 4E-BPs [1,5,6], whereas the latter can increase eIF4E phosphorylation (usually at Ser209) through Mnk1/2 [7]. The biologic significance of eIF4E phosphorylation is not completely understood. However, it has been suggested that phosphorylation of eIF4E may increase its affinity for the cap of mRNA and may also favor its entry into initiation complexes [6–8]. A recent study using genetically engineered mouse models has clearly shown that Mnk-mediated eIF4E phosphorylation is absolutely required for eIF4E's oncogenic action [9].
Dynamic phosphorylation and dephosphorylation of proteins are fundamental mechanisms used by cells to transduce signals. Protein phosphatase 2A (PP2A) is the major protein serine/threonine phosphatase that modulates, particularly downregulates, activated protein kinases in eukaryotic cells. PP2A controls the activities of some major protein kinases involved in several important cell signaling pathways including PI3K/Akt, Raf/MAPK/ERK kinase (MEK)/extracellular signal-regulated kinase (ERK), and mammalian target of rapamycin/p70S6K [10]. The core enzyme of PP2A comprises a 36-kDa catalytic subunit (PP2Ac or C) that is always associated with a 65-kDa scaffolding subunit (PR65 or A), which modulates its enzymatic properties, substrate specificity, and subcellular localization [10].
PP2A is considered to be a tumor suppressor. Inhibition of PP2A activity cooperates with other oncogenic changes to cause transformation of human cells [10–12]. However, the molecular mechanisms by which PP2A exerts its tumor suppressive function have not been fully elucidated. Whereas multiple pathways have been shown to be regulated by PP2A in transformation [10,13], regulation of Mnk/eIF4E signaling by PP2A has not been fully demonstrated except for previous studies showing that the PP2A inhibitor okadaic acid (OA) increased eIF4E phosphorylation [14–16]. In the current study, we investigated the role of PP2A in regulation of eIF4E phosphorylation and eIF4F assembly or activity and mechanisms underlying this process. We have shown that PP2A negatively regulates eIF4E phosphorylation and eIF4F assembly through dephosphorylating Mnk and eIF4E proteins. Our findings thus suggest a novel mechanism by which PP2A suppresses transformation of human cells.
Materials and Methods
Reagents
OA, LY294002, and U0126 were purchased from LC laboratory (Woburn, MA). SB203580 was purchased from Biomol (Plymouth Meeting, PA). 4-Amino-5-(4-fluoroanilino)-pyrazolo[3,4-d]pyrimidine (CGP57380) was purchased from Calbiochem (San Diego, CA). These agents were dissolved in DMSO at a concentration of 20 mM, and aliquots were stored at -80°C. Antibodies against phospho-Mnk1 (p-Mnk1; Thr197/202), eIF4G, eIF4E, 4EBP-1, phospho-4EBP-1 (p-4EBP-1; T37/46), phospho-eIF4E (p-eIF4E; Ser209), and Mcl-1 were purchased from Cell Signaling Technology, Inc (Beverly, MA). Anti-PP2Ac antibody (clone 1D6) was purchased from Millipore/Upstate (Billerica, MA). Mouse monoclonal anti-c-Myc antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Mouse monoclonal antiactin antibody was purchased from Sigma Chemical Co. (St Louis, MO). Mouse and rabbit c-Myc tag antibodies were purchased from Genscript (Piscataway, NJ) and Cell Signaling Technology, Inc, respectively. 7-Methyl GTP (m7GTP)-sepharose 4B was purchased from GE Healthcare Biosciences (Pittsburgh, PA).
Plasmids
pcDNA3-myc-Mnk1 [17] was kindly provided by Dr E. L. Martin (Hospital Ramón y Cajal,Madrid, Spain). pcDNA-PP2Ac [18] was generously provided by Dr D. Pallas (Emory University, Atlanta, GA). To make the flag-eIF4E expression construct, RT-PCR was used to amplify the eIF4E coding region from total cellular RNA extracted from H157 cells using the following primers: forward 5′-GCAAGCTTATGGCGACTGTCGAACCGG-3′ and reverse 5′-GCGAATTCTTAAACAACAAACCTATTTTTAG-3′. The final PCR product was then cloned into p3xflag-Myc-CMV-26 expression vector from Sigma Chemical Co. through HindIII and EcoRI cloning sites. The insert was confirmed by DNA sequencing.
Cell Lines and Cell Culture
The human lung cancer cell lines used in this study were purchased from the American Type Culture Collection (Manassas, VA). HEK293 cells were obtained from Dr K. Ye (Emory University). Wild type, Mnk1 knockout (1KO), Mnk2 knockout (2KO), and Mnk1/Mnk2 double-knockout (DKO) murine embryonic fibroblasts were described previously [19,20]. These cell lines were grown in monolayer culture in RPMI 1640 medium or Dulbecco's modified Eagle medium supplemented with 5% fetal bovine serum at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air.
Western Blot Analysis
Whole-cell protein lysates were prepared and analyzed by Western blot analysis as described previously [21].
m7GTP Pull-down for Analysis of eIF4F Complex Formation
eIF4F complex in cell extracts was detected using affinity chromatography m7GTP-sepharose as described previously [22].
Small Interfering RNA and Transfection
Human PP2Ac small interfering RNA (siRNA; sc-43509), which consists of a pool of three target-specific 19 to 25 nt siRNA, was purchased from Santa Cruz Biotechnology, Inc. The nonsilencing control siRNA and transfection of the siRNA were described previously [21].
Immunoprecipitation and Dephosphorylation Assay
In 10-cm dishes, HEK293 cells were transiently transfected with pcDNA3 or pcDNA3-myc-Mnk1 (for Mnk1 dephosphorylation) or with pCMV-flag or pCMV-flag-eIF4E (for eIF4E dephosphorylation) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Twenty-four hours later, the cells were lysed inCHAPS lysis buffer containing 50 mM Tris-HCl (pH7.5), 150 mM NaCl, 1 mM EDTA, 0.3% CHAPS, and protease inhibitors. For in vitro Mnk dephosphorylation assay, the clear supernatant expressing myc-Mnk1 was split equally into two tubes: for a PP2A sample, 1 µg of anti-myc-tag and 1 µg of anti-PP2Ac antibodies were added; for a non-PP2A sample, 1 µg of anti-myc-tag and 1 µg of mouse IgG antibodies were added. For the vector control sample, equal lysate and 1 µg of anti-myc-tag and 1 µg of mouse IgG were used. All immunoprecipitation (IP) samples were mixed with 40 µl of protein G bead and then incubated with constant shaking overnight at 4°C. After IP, the beads were fully washed three times with the same lysis buffer and cold 1x PBS and then washed twice with dephosphorylation assay buffer containing 20 mM HEPES (pH 7.0), 0.1 mM DTT, 1 mM MnCl2, 0.1 mg/ml BSA, and protease inhibitors. The same procedure was used for in vitro eIF4E dephosphorylation assay except for using anti-flag-tag antibody to replace anti-myc-tag antibody in each aforementioned step. The phosphatase reactions were carried out in the same dephosphorylation buffer for 16 hours at 30°C. Reactions were terminated by adding SDS gel loading dye, and samples were run on 10% SDS-PAGE gels. p-Mnk1 (T197/202) and p-eIF4E (S209) antibodies were used to detect the phosphorylation levels of Mnk1 and eIF4E, respectively.
Results
PP2A Negatively Regulates eIF4E Phosphorylation
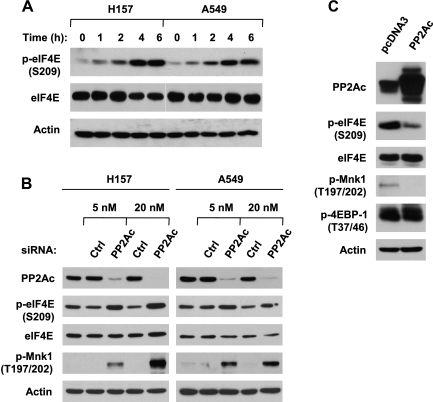
To determine whether PP2A regulates eIF4E phosphorylation, we treated two lung cancer cell lines with the PP2A inhibitor OA at 100 nM, a concentration used to selectively inhibit PP2A activity in the literature [23,24], for various times and then detected its effects on eIF4E phosphorylation at Ser209. In these tested cell lines, OA induced a time-dependent increase in p-eIF4E levels within the tested time ranges (0–6 hours; Figure 1A). These results thus validate previous observations that OA inhibits eIF4E phosphorylation [14–16]. Moreover, we further confirmed our finding on OA-induced eIF4E phosphorylation by inhibiting PP2A through specific knockdown of PP2Ac with PP2Ac siRNA. As presented in Figure 1B, knockdown of PP2Ac with either 5 or 20 nM PP2Ac siRNA substantially increased p-eIF4E levels in both H157 and A549 cell lines. Collectively, these data clearly indicate that inhibition of PP2A increases eIF4E phosphorylation. Furthermore, we expressed exogenous PP2Ac in HEK293 cells to determine whether enforced activation of PP2A inhibits eIF4E phosphorylation. Indeed, we detected much less p-eIF4E in cells transfected with PP2Ac than in cells transfected with empty vector (Figure 1C), demonstrating that overexpression of PP2Ac inhibits eIF4E phosphorylaiton. Taking all data together, we conclude that PP2A negatively regulates eIF4E phosphorylation.
Figure 1.
Inhibition of PP2A with either OA (A) or PP2A siRNA (B) increases eIF4E phosphorylation, whereas overexpression of ectopic PP2Ac (C) inhibits eIF4E phosphorylation. (A) The indicated cell lines were treated with 100 nM OA for the given times. (B) The indicated cell lines were transfected with the given concentrations of control or PP2Ac siRNA for 48 hours. (C) HEK293 cells were transfected with the given plasmids for 48 hours. After the aforementioned treatments, the cells were subjected to preparation of whole-cell protein lysates and subsequent detection of the indicated proteins by Western blot analysis.
PP2A Regulates eIF4E Phosphorylation through a Mnk-Dependent Mechanism
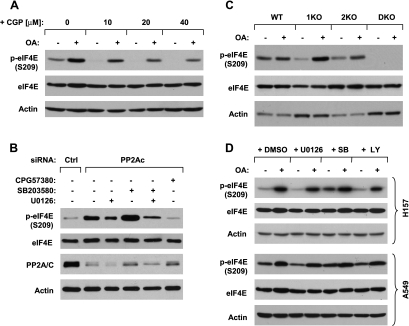
It is well known that the MAPK-activated protein kinases Mnks, particularly Mnk1, regulate eIF4E activation through its phosphorylation at serine 209 [8,19]. In our previously mentioned PP2A knockdown experiment, p-Mnk1 (Thr197/202) levels were also increased in cells in which PP2Ac was reduced (Figure 1B). In agreement, overexpression of PP2Ac also reduced the levels of p-Mnk1 (Figure 1C). These results suggest that Mnks might be involved in PP2A regulation of eIF4E phosphorylation. Thus, we next determined whether Mnks are involved in PP2A inhibition-induced eIF4E phosphorylation. To this end, we first examined the effect of the presence of the Mnk inhibitor CGP57380 on OA-induced eIF4E phosphorylation. The presence of CGP57380 at concentrations ranging from 10 to 40 µM reduced basal levels of p-eIF4E and blocked OA-induced increase in eIF4E phosphorylation in a concentration-dependent manner. CGP57380 at 20 or 40 µM almost abolished the ability of OA to increase p-eIF4E levels (Figure 2A). Consistently, knockdown of PP2A with PP2Ac siRNA increased p-eIF4E levels in the absence, but not in the presence, of CGP57380 (Figure 2B). These data together indicate that Mnks are involved in PP2A inhibition-induced increase in eIF4E phosphorylation.
Figure 2.
Inhibition of PP2A induces Mnk-dependent eIF4E phosphorylation (A-C) independent of ERK, p38, or PI3 kinase (B and D). (A) H157 cells were pretreated with the indicated concentrations of CGP57380 for 30 minutes and then cotreated with 100 nM OA for an additional 4 hours. (B) H157 cells were plated on six-well plates and, the next day, were transfected with 20 nM control (Ctrl) or PP2Ac siRNA. After 20 hours, the cells were exposed to 20 µM of the indicated inhibitors for an additional 4 hours. (C) The indicated cell lines were treated with 100 nM OA for 4 hours. (D) The indicated cell lines were pretreated with 20 µM U0126, SB203580, or LY294002 for 30 minutes and then cotreated with 100 nM OA for an additional 4 hours. After the aforementioned treatments, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis.
Moreover, we determined the effects of OA on eIF4E phosphorylation in murine embryonic fibroblasts deficient inMnk1 (1KO), Mnk2 (2KO), or both Mnk1 and Mnk2 (DKO). We noted that neither knockout of Mnk1 (1KO) nor knockout of Mnk2 (2KO) alone eliminated basal levels of p-eIF4E (although both did reduce basal levels of p-eIF4E) or blocked OA-induced increase in eIF4E phosphorylation; however, the double knockout of both Mnk1 and Mnk2 (DKO) abolished not only basal levels of p-eIF4E but also OA-induced eIF4E phosphorylation (Figure 2C). These data suggest that both Mnk1 and Mnk2 regulate eIF4E phosphorylation and are required for mediating PP2A inhibition-induced eIF4E phosphorylation.
PP2A Regulates eIF4E Phosphorylation Independently of the p38 MAPK Pathway
It has been shown that Mnks are regulated by ERK and p38 signaling pathways [6], whereas PP2A regulates ERK and p38 activity [10,25,26].We therefore further investigated whether ERK and p38 are involved in PP2A inhibition-induced eIF4E phosphorylation. The presence of the MEK inhibitor U0126 or the p38 inhibitor SB203580 alone failed to block OA-induced increase in eIF4E phosphorylation in both H157 and A549 cell lines (Figure 2D). In the experiment with PP2Ac siRNA, the presence of U0126, but not SB203580, partially inhibited knockdown of PP2A-induced increase in eIF4E phosphorylation. The combination of U0126 and SB203580 had similar results to U0126 alone in affecting knockdown of PP2A-induced p-eIF4E increase (Figure 2B). Taken together, PP2A inhibition-induced eIF4E phosphorylation does not require activation of p38 signaling pathway. Although we cannot rule out the possible role of the MEK/ERK signaling in this process, it is unlikely that this signaling pathway plays a major role in mediating PP2A inhibition-induced eIF4E phosphorylation. In addition, we looked at the effect of PI3K/Akt inhibition on OA-induced p-eIF4E increase and found that the PI3K inhibitor LY294002 failed to block OA-induced increase in eIF4E phosphorylation (Figure 2D), indicating a mechanism independent of PI3K/Akt.
PP2A Dephosphorylates Both Mnk and eIF4E
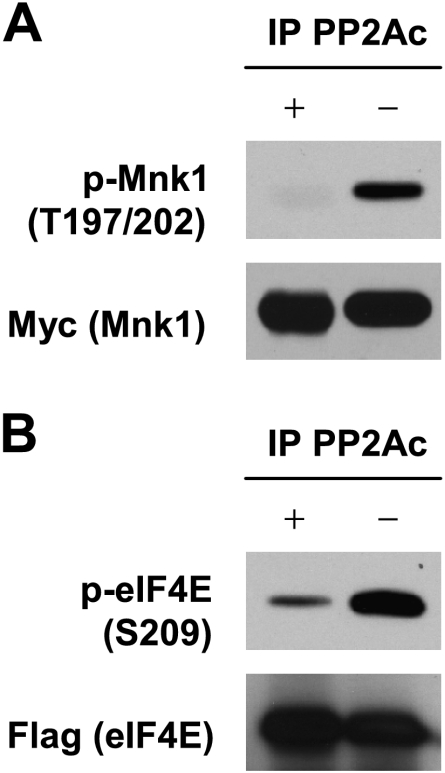
The aforementioned results led us to determine whether PP2A directly dephosphorylates Mnks and/or eIF4E. To this end, we conducted a biochemical dephosphorylation assay. Specifically, we transfected the target gene, Mnk1 or eIF4E, into HEK293 cells and then co-pulled down the target protein with a tag antibody and endogenous PP2A enzyme with PP2Ac-specific antibody for a subsequent dephosphorylation assay using the immunoprecipitated human PP2A as a source of PP2A enzyme. As presented in Figure 3A, p-Mnk1 levels were much lower in cellular materials coimmunoprecipitated with anti- Myc (i.e., for Myc-Mnk1) and anti-PP2Ac (for endogenous PP2A) antibodies than those without the inclusion of anti-PP2Ac antibody, indicating that PP2A functions as a Mnk phosphatase to directly dephosphorylate Mnk1. At the same time, we also determined whether PP2A directly dephosphorylates eIF4E through the same approach. As shown in Figure 3B, p-eIF4E levels were substantially reduced in cellular materials coimmunoprecipitated with anti-flag (i.e., for flag-eIF4E) and anti-PP2Ac antibodies compared with those without the inclusion of anti-PP2Ac antibody, indicating that PP2A also functions as an eIF4E phosphatase to directly dephosphorylate eIF4E. Thus, these data indicate that PP2A negatively regulates eIF4E phosphorylation through direct dephosphorylation of both Mnk and eIF4E.
Figure 3.
PP2A dephosphorylates Mnk1 (A) and eIF4E (B). (A) Cell lysates were prepared from HEK293 cells transfected with pcDNA3 or pcDNA3-myc-Mnk1 and followed by IP with anti-Myc tag plus anti-IgG antibodies (without PP2A) or anti-Myc tag plus anti-PP2Ac antibodies (with PP2A). (B) Cell lysates were prepared from HEK293 cells transfected with pCMV-flag or pCMV-flag-eIF4E and followed by IP with anti-flag-tag plus anti-IgG antibodies (without PP2A) or anti-flag-tag plus anti-PP2Ac antibodies (with PP2A). After the aforementioned procedures, the immunoprecipitates were then subjected to in vitro dephosphorylation assay, in which the endogenous PP2A was pulled down with anti-PP2Ac antibody as a source of PP2A, as described in detail in Materials and Methods, followed by Western blot analysis to detect p-Mnk1 (T197/202) (A) and p-eIF4E (S209) (B), respectively.
Inhibition of PP2A Enhances eIF4F Complex Assembly
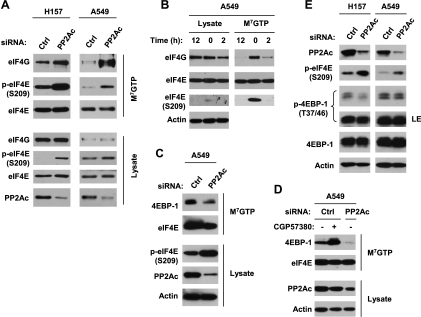
The role of eIF4E phosphorylation in regulation of initiation complex formation and cap-dependent translation is controversial [27], although some studies have shown that phosphorylation of eIF4E increases eIF4E's affinity for the cap of mRNA and may also favor its entry into initiation complexes [6–8]. Thus, we determined the effect of PP2A inhibition on the formation of the initiation complex eIF4F. To this end, we used m7GTP to pull down the eIF4F complex and then analyzed the eIF4F assembly. As shown in Figure 4A, the amount of eIF4G in the eIF4F complex was drastically increased in PP2Ac siRNA-transfected cells compared with control siRNA-transfected cells. Knockdown of PP2Ac increased p-eIF4E levels not only in whole cell lysates but also in m7GTP-pulled down samples. These results clearly indicate that inhibition of PP2A increases the association of eIF4E with eIF4G, that is, increases eIF4F activity. To further demonstrate the effect of eIF4E phosphorylation on eIF4F assembly, we also determined the effect of the Mnk inhibitor CGP57380, which inhibits eIF4E phosphorylation, on eIF4F assembly. As presented in Figure 4B, CGP57380 caused substantial reduction of eIF4G in the eIF4F complex while drastically inhibiting eIF4E phosphorylation. These data suggest that induction of eIF4E phosphorylation (e.g., by PP2A knockdown) indeed increases eIF4F assembly or activity.
Figure 4.
Effect of inhibition of PP2A (A and C-E) or Mnk (B and D) on the assembly of eIF4E with eIF4G (A and B), 4EBP-1 and eIF4E interaction (C and D), and 4EBP-1 phosphorylation (E). (A, C, and D) The indicated cell lines were transfected with 20 nM of control (Ctrl) or PP2Ac siRNA for 48 hours. (B) A549 cells were treated with 20 µM CGP57380 for the given times. After the aforementioned treatments, the whole-cell protein lysates prepared from these cells were subjected to m7GTP pull-down and subsequent detection of the given proteins by Western blot analysis. (E) The given cell lines were transfected with 5 nM of control or PP2Ac siRNA for 48 hours. The cells were then subjected to preparation of whole-cell protein lysates and subsequent detection of the indicated proteins by Western blot analysis. LE indicates long exposure.
Inhibition of PP2A Decreases 4EBP-1 Interaction with eIF4E
To further demonstrate the effect of eIF4E phosphorylation on eIF4F assembly, we determined whether inhibition of PP2A affects eIF4E and 4EBP-1 interaction given that 4EBP-1 is a key protein that binds to eIF4E and restricts its availability to form eIF4F [28]. In the m7GTP pull-down assay, we detected less 4EBP-1 in PP2Ac siRNA-transfected cells than in control siRNA-transfected cells (Figure 4, C and D), indicating that PP2A inhibition reduces the association of 4EBP-1 with eIF4E. In the cells treated with CGP57380, we detected much more 4EBP-1 than in cells exposed to DMSO control (Figure 4D), suggesting that inhibition of eIF4E phosphorylation may promote the association of 4EBP-1 with eIF4E. Collectively, we suggest that PP2A inhibition increases eIF4E phosphorylation, resulting in decreased association between 4EBP-1 and eIF4E.
Inhibition of PP2A Does Not Increase 4EBP-1 Phosphorylation
Moreover, we determined whether inhibition of PP2A increases 4EBP-1 phosphorylation, leading to increased eIF4F assembly. To this end, we analyzed the effect of PP2A inhibition on 4EBP-1 phosphorylation. As presented in Figure 4E, knockdown of PP2A did not increase p-4EBP-1 levels, whereas p-eIF4E levels were substantially elevated. Therefore, it is clear that PP2A inhibition increases eIF4E phosphorylation without enhancing 4EBP-1 phosphorylation. In agreement, we also looked at p-4EBP-1 levels in cells expressing ectopic PP2Ac and found that p-4EBP-1 levels were not decreased compared with the control cells (Figure 1C). These data again support the notion that PP2A negatively regulates phosphorylation of eIF4E but not 4EBP-1.
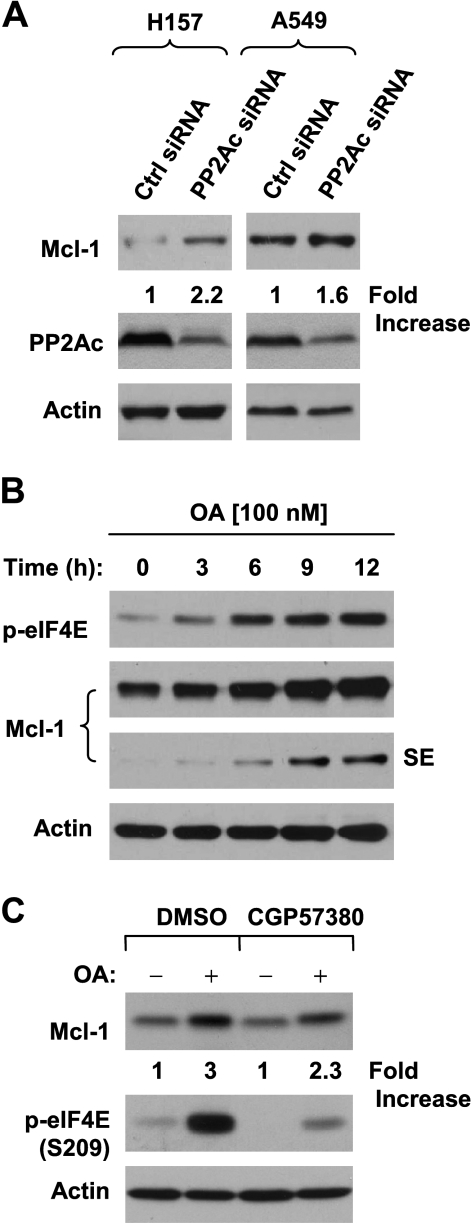
eIF4E Phosphorylation in Part Contributes to PP2A inhibition-Induced Mcl-1 and c-Myc Elevation
It has been recently shown that phosphorylated eIF4E promotes tumorigenesis primarily by suppressing apoptosis, and accordingly, the antiapoptotic protein Mcl-1 is one target of both p-eIF4E and Mnk1 [9]. Thus, we further analyzed Mcl-1 levels in cells treated with OA or transfected with PP2Ac siRNA. In both H157 and A549 cells, knockdown of PP2Ac increased the levels of Mcl-1 (Figure 5A). Moreover, OA treatment also increased Mcl-1 levels (Figure 5B). These results indicate that inhibition of PP2A elevates Mcl-1 levels. Furthermore, we determined whether eIF4E phosphorylation is involved in this process by examining the effect of CGP57380 onMcl-1 expression induced by PP2A inhibition. As presented in Figure 5C, the presence of CGP57380 blocked eIF4E phosphorylation, but only weakly or partially inhibited OA-induced Mcl-1 elevation (approximately by 23%).
Figure 5.
PP2A knockdown (A) and OA treatment (B) increases Mcl-1 levels, in part through increasing eIF4E phosphorylation (C). (A) The indicated cell lines were transfected with 20 nM control (Ctrl) or PP2Ac siRNA for 48 hours. (B) H157 cells were treated with 100 nM OA for the indicated times. (C) H157 cells were pretreated with 40 µM CGP57380 for 2 hours and then cotreated with 100 nM OA for additional 6 hours. After the aforementioned treatments, the cells were harvested for the preparation of whole-cell protein lysates and subsequent Western blot analysis. Protein levels were quantitated with ImageJ software (National Institutes of Health, Bethesda, MD). SE indicates short exposure.
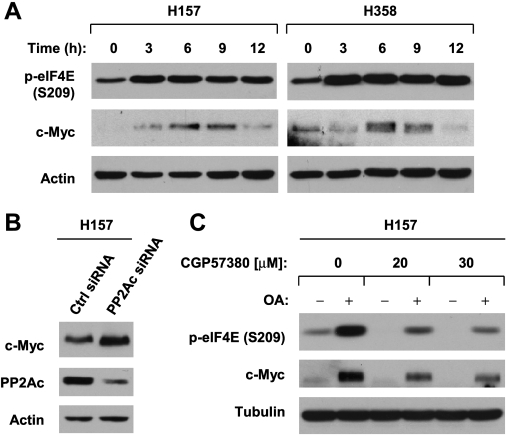
c-Myc is another protein known to be regulated by cap-dependent translation [29] and PP2A [30,31]. Similar toMcl-1, bothOA treatment and transfection of PP2Ac siRNA increased c-Myc protein levels (Figure 6, A and B). The presence of CGP57380 substantially abrogated OA-induced increase in c-Myc, albeit not completely, while abolishing OA-induced eIF4E phosphorylation (Figure 6C). Thus, the data suggest that eIF4E phosphorylation partly contributes to PP2A inhibition-induced c-Myc elevation.
Figure 6.
OA treatment (A) or PP2A knockdown (B) increases c-Myc expression, in part through increasing eIF4E phosphorylation (C). (A) The indicated cell lines were treated with 100 nM OA for the given times. (B) H157 cells were transfected with 20 nM control (Ctrl) or PP2Ac siRNA for 48 hours. (C) H157 cells were pretreated with 20 or 30 µM CGP57380 for 30 minutes and then cotreated with 100 nM OA for an additional 6 hours. After the aforementioned treatments, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis.
Discussion
In this study, we found that both treatment of cancer cells with the small molecule PP2A inhibitor OA and transfection of cancer cells with PP2Ac siRNA increased eIF4E phosphorylation at Ser209 (Figure 1, A and B). Complementarily, overexpression of PP2Ac substantially decreased p-eIF4E levels (Figure 1C). Collectively, our findings clearly demonstrate that PP2A inhibits eIF4E phosphorylation.
Mnks, particularly Mnk1, are kinases known to phosphorylate eIF4E at Ser209 [8,19]. In this study, we further showed that the presence of Mnk inhibitor CGP57380 and deficiency of both Mnk1 and Mnk2 genes abolished eIF4E phosphorylation induced by OA or PP2Ac siRNA (Figure 2). These results indicate that PP2A inhibition-induced eIF4E phosphorylation is an Mnk-dependent event.We then addressed the question of what the underlying mechanisms are. Through the dephosphorylation assay, we further showed that PP2A could directly dephosphorylate both Mnk1 and eIF4E (Figure 3), indicating that PP2A functions as the phosphatase of Mnk1 and eIF4E. Thus, we conclude that PP2A negatively regulates eIF4E phosphorylation through direct dephosphorylation of both Mnk1 and eIF4E. Our current findings not only confirm previous observations that PP2A directly dephosphorylates eIF4E in vitro [16] but also demonstrate the role of PP2A in direct dephosphorylation of Mnk1. To the best of our knowledge, PP2A dephosphorylation of Mnk1 has not been reported previously.
The effect of eIF4E phosphorylation on cap-dependent translation initiation remains controversial [27]. Increased eIF4E phosphorylation by OA-induced inhibition of PP2A was shown to have no effect on eIF4E binding to the mRNA cap [15,16], whereas eIF4E phosphorylation was shown to be associated with increased protein translation [14,32,33]. In our study, we found that siRNA-mediated knockdown of PP2A increased the amount of eIF4G in the eIF4F complex pulled down by the m7GTP (Figure 4A), indicating that PP2A inhibition increases eIF4F assembly. Complementarily, inhibition of eIF4E with CGP57380 decreased the amount of eIF4G in the eIF4F complex (Figure 4B), indicating that eIF4E phosphorylation does affect eIF4F assembly. Moreover,we found that inhibition of PP2Awith bothOA and PP2Ac siRNA increased the levels of Mcl-1 and c-Myc (Figures 5 and 6), two proteins known to be regulated by the cap-dependent mechanism [9,29]. Importantly, the elevation of both Mcl-1 and c-Myc is partially mediated by increased eIF4E phosphorylation because the presence of CGP57380 partially inhibited the elevation of both Mcl-1 and c-Myc induced by PP2A inhibition (Figures 5 and 6). Collectively, it is reasonable to conclude that PP2A negatively regulates eIF4F assembly and cap-dependent translation through inhibition of eIF4E phosphorylation.
4EBP-1 is a key protein that binds to eIF4E and restricts its availability to form eIF4F. On phosphorylation, 4EBP-1 dissociates from eIF4E, allowing eIF4E to bind to the mRNA cap structure of a given gene and to recruit eIF4G [28]. Thus, it is possible that inhibition of PP2A also increases 4EBP-1 phosphorylation, leading to increased eIF4F assembly. In our study, we found that inhibition of PP2A with PP2Ac siRNA did not increase 4EBP-1 phosphorylation (Figure 4E). Likewise, enforced activation of PP2A by overexpression of ectopic PP2Ac failed to decrease 4EBP-1 phosphorylation (Figure 1D). These data together suggest that PP2A does not regulate 4EBP-1 phosphorylation. Accordingly, we think that PP2A inhibition-mediated increase in eIF4F assembly is unlikely due to the alteration of 4EBP-1 phosphorylation.
With m7GTP pull-down assay, we observed a reduced level of 4EBP-1 bound to eIF4E in the cells transfected with PP2Ac siRNA and an increased amount of 4EBP1 associated with eIF4E in the cells treated with CGP57380 (Figure 4, C and D). These results indicate that eIF4E phosphorylation alters its binding to 4EBP-1. Therefore, it is likely that inhibition of PP2A causes increased eIF4E phosphorylation, resulting in the dissociation of eIF4E from 4EBP-1 and in the subsequent increase in eIF4F assembly.
Although the expressions of Mcl-1 and c-Myc are regulated by a cap-dependent translation mechanism, their protein levels are also regulated by protein stabilization through PP2A-mediated inhibition of protein phosphorylation [30,31,34]. Thus, it is generally thought that PP2A inhibition elevates the levels of Mcl-1 and c-Myc through increasing their stabilities. The current study in fact reveals an additional mechanism that contributes to PP2A inhibition-induced increase in Mcl-1, and particularly c-Myc; that is, PP2A inhibition increases eIF4E phosphorylation and eIF4F assembly, leading to increased protein translation of Mcl-1 and c-Myc in addition to increasing their protein stabilization. We need to point out that c-Myc seems more susceptible than Mcl-1 to this regulation because CGP57380 had a much more profound effect on blocking OA-induced c-Myc elevation than on blocking Mcl-1 increase by OA (Figures 5 and 6).
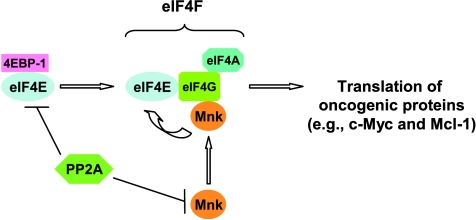
In summary, the current study demonstrates that PP2A negatively regulates eIF4E phosphorylation and eIF4F assembly through direct dephosphorylation of both Mnks and eIF4E, partially leading to reduction of certain oncogenic proteins (e.g., Mcl-1 and c-Myc; Figure 7). Our findings in this regard increase our understanding of eIF4E phosphorylation and its effect on the regulation of cap-dependent translation initiation. Given that inactivation of PP2A is an essential step for fully transforming a normal cell into a cancer cell [10], whereas eIF4E phosphorylation is an essential step for eIF4E's oncogenic function [9], our findings may imply that negative regulation of eIF4E phosphorylation and eIF4F activity by PP2A is an important mechanism by which PP2A exerts its tumor-suppressive function. Our findings thus warrant further investigation in this direction.
Figure 7.
Schematic model for PP2A regulation of Mnk/eIF4E signaling and eIF4F assembly. PP2A directly dephosphorylates Mnks and eIF4E, leading to a decrease in eIF4E phosphorylation, which enhances association of eIF4E with 4EBP-1 and subsequently inhibits eIF4F assembly. Accordingly, the expression of certain oncogenic proteins (e.g., Mcl-1 and c-Myc) is reduced in part through this mechanism.
Acknowledgments
The authors thank E. L. Martin and D. Pallas for providing plasmids and A. Hammond for editing the article.
Abbreviations
- eIF4E
eukaryotic translation initiation factor 4E
- m7GTP
7-methyl GTP
- PP2A
protein phosphatase 2A
- OA
okadaic acid
- siRNA
small interfering RNA
Footnotes
This study was supported by the Georgia Cancer Coalition Distinguished Cancer Scholar award (to S.-Y.S.) and National Institutes of Health/National Cancer Institute PO1 CA116676 (Project 1 to F.R.K. and S.-Y.S.) and R01 CA118450 (S.-Y.S.). F.R.K. and S.-Y.S. are Georgia Cancer Coalition Distinguished Cancer Scholars.
References
- 1.Goodfellow IG, Roberts LO. Eukaryotic initiation factor 4E. Int J Biochem Cell Biol. 2008;40:2675–2680. doi: 10.1016/j.biocel.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thumma SC, Kratzke RA. Translational control: a target for cancer therapy. Cancer Lett. 2007;258:1–8. doi: 10.1016/j.canlet.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–634. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- 4.Clemens MJ. Targets and mechanisms for the regulation of translation in malignant transformation. Oncogene. 2004;23:3180–3188. doi: 10.1038/sj.onc.1207544. [DOI] [PubMed] [Google Scholar]
- 5.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 6.Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 7.Pyronnet S. Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1. Biochem Pharmacol. 2000;60:1237–1243. doi: 10.1016/s0006-2952(00)00429-9. [DOI] [PubMed] [Google Scholar]
- 8.Mahalingam M, Cooper JA. Phosphorylation of mammalian eIF4E by Mnk1 and Mnk2: tantalizing prospects for a role in translation. Prog Mol Subcell Biol. 2001;27:132–142. [PubMed] [Google Scholar]
- 9.Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westermarck J, Hahn WC. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med. 2008;14:152–160. doi: 10.1016/j.molmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Van Hoof C, Goris J. PP2A fulfills its promises as tumor suppressor: which subunits are important? Cancer Cell. 2004;5:105–106. doi: 10.1016/s1535-6108(04)00027-3. [DOI] [PubMed] [Google Scholar]
- 12.Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell. 2007;130:21–24. doi: 10.1016/j.cell.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Rychlik W, Rush JS, Rhoads RE, Waechter CJ. Increased rate of phosphorylation-dephosphorylation of the translational initiation factor eIF-4E correlates with the induction of protein and glycoprotein biosynthesis in activated B lymphocytes. J Biol Chem. 1990;265:19467–19471. [PubMed] [Google Scholar]
- 15.Bu X, Haas DW, Hagedorn CH. Novel phosphorylation sites of eukaryotic initiation factor-4F and evidence that phosphorylation stabilizes interactions of the p25 and p220 subunits. J Biol Chem. 1993;268:4975–4978. [PubMed] [Google Scholar]
- 16.Bu X, Hagedorn CH. Phosphoprotein phosphatase 2A dephosphorylates eIF-4E and does not alter binding to the mRNA cap. FEBS Lett. 1992;301:15–18. doi: 10.1016/0014-5793(92)80200-z. [DOI] [PubMed] [Google Scholar]
- 17.O'Loghlen A, Gonzalez VM, Pineiro D, Perez-Morgado MI, Salinas M, Martin ME. Identification and molecular characterization of Mnk1b, a splice variant of human MAP kinase-interacting kinase Mnk1. Exp Cell Res. 2004;299:343–355. doi: 10.1016/j.yexcr.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Ogris E, Gibson DM, Pallas DC. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene. 1997;15:911–917. doi: 10.1038/sj.onc.1201259. [DOI] [PubMed] [Google Scholar]
- 19.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24:6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Yue P, Chan CB, Ye K, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Fu H, Khuri FR, Sun SY. Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3 kinase-dependent and Mnk-mediated eIF4E phosphorylation. Mol Cell Biol. 2007;27:7405–7413. doi: 10.1128/MCB.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–1780. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Proud CG. Methods for studying signal-dependent regulation of translation factor activity. Methods Enzymol. 2007;431:113–142. doi: 10.1016/S0076-6879(07)31007-0. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Chen L, Luo Y, Chen W, Zhou H, Xu B, Han X, Shen T, Huang S. Rapamycin inhibits IGF-1 stimulated cell motility through PP2A pathway. PLoS One. 2010;5:e10578. doi: 10.1371/journal.pone.0010578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing H, Vanderford NL, Sarge KD. The TBP-PP2A mitotic complex bookmarks genes by preventing condensin action. Nat Cell Biol. 2008;10:1318–1323. doi: 10.1038/ncb1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarado-Kristensson M, Andersson T. Protein phosphatase 2A regulates apoptosis in neutrophils by dephosphorylating both p38 MAPK and its substrate caspase 3. J Biol Chem. 2005;280:6238–6244. doi: 10.1074/jbc.M409718200. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Hofmann PA. Protein phosphatase 2A-mediated cross-talk between p38 MAPK and ERK in apoptosis of cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;286:H2204–H2212. doi: 10.1152/ajpheart.01050.2003. [DOI] [PubMed] [Google Scholar]
- 27.Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002;269:5350–5359. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 29.Bjornsti MA, Houghton PJ. Lost in translation: dysregulation of capdependent translation and cancer. Cancer Cell. 2004;5:519–523. doi: 10.1016/j.ccr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Arnold HK, Sears RC. Protein phosphatase 2A regulatory subunit B56α associates with c-myc and negatively regulates c-myc accumulation. Mol Cell Biol. 2006;26:2832–2844. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 32.Joshi-Barve S, Rychlik W, Rhoads RE. Alteration of the major phosphorylation site of eukaryotic protein synthesis initiation factor 4E prevents its association with the 48 S initiation complex. J Biol Chem. 1990;265:2979–2983. [PubMed] [Google Scholar]
- 33.Morley SJ, Dever TE, Etchison D, Traugh JA. Phosphorylation of eIF-4F by protein kinase C or multipotential S6 kinase stimulates protein synthesis at initiation. J Biol Chem. 1991;266:4669–4672. [PubMed] [Google Scholar]
- 34.Wang CY, Lin YS, Su WC, Chen CL, Lin CF. Glycogen synthase kinase-3 and Omi/HtrA2 induce annexin A2 cleavage followed by cell cycle inhibition and apoptosis. Mol Biol Cell. 2009;20:4153–4161. doi: 10.1091/mbc.E09-02-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]