Abstract
Helicobacter pylori is a Gram-negative, spiral-shaped bacterium that infects more than 50% of the human population and can cause gastritis, peptic ulcer, or gastric malignancies. It is generally viewed as an extracellular microorganism. In a gentamicin protection assay on AGS or MKN45 cells, H. pylori could invade the epithelial cells and multiply within double-layer vesicles either on the plasma membrane or in the cytoplasm. A 5-fold increase in the number of bacteria was recultured from the infected cells at 12 h, compared with the number of invading cells at 2.5 h postinfection. The autophagic vesicles induced by H. pylori are the sites of replication and also of the degradation of the replicating bacteria after fusion with lysosomes. Many H. pylori bacteria in coccoid form associated with the plasma membrane can be released into culture. Only cell-penetrating antibiotics can enhance the intracellular killing of the replicating bacteria. The multiplication of H. pylori within cells provides a niche for its resistance to antibacterial therapy and has a significant impact on its biological life cycle.
Helicobacter pylori is a Gram-negative, flagellated, microaerophilic bacterium that selectively colonizes the gastric mucosa. It infects people worldwide and is correlated with socioeconomic conditions (24). The prevalence among middle-aged adults is over 80% in many developing countries. Overt disease, however, occurs in only 10 to 20% of infected individuals. The most common pathology associated with H. pylori infection is chronic active gastritis and peptic ulceration. A long-term chronic infection will increase the risk of gastric adenocarcinoma and mucosa-associated lymphoid-tissue lymphoma (19). Gastric mucosa is well protected against bacterial infections. However, H. pylori adapts and resides in the mucus and achieves attachment to epithelial cells, evasion of the immune responses, and persistent colonization in the stomach. It is not well understood why the immune system fails to clear H. pylori infection. Furthermore, the mechanisms controlling the induction and maintenance of the H. pylori-induced chronic inflammation are only partly understood.
Although H. pylori is generally viewed as a noninvasive pathogen, a number of in vivo and in vitro studies have shown that H. pylori is invasive, and it can reside in the vacuole in the cytoplasm or even replicate on the cell membrane to form a microcolony (2, 11, 25). This suggests that H. pylori can be considered a facultative intracellular organism (6, 20). We have reported that H. pylori can multiply in macrophages and bone marrow-derived dendritic cells with autophagy induction (27, 28). In this study, we further extended this line of research to epithelial cells and found that H. pylori could invade and replicate in epithelial cells. Thus, H. pylori can be considered an intracellular microorganism, and this has an impact on its own biological life cycle and its resistance to antibiotics.
MATERIALS AND METHODS
Bacterial strains and culture.
The H. pylori clinical isolates (HP238, HP917, HP1076, and HP1024) were obtained from the Department of Pathology, National Cheng Kung University Hospital. The babA, cagA, and vacA mutant strains derived from HP238 were used as previously described (26). ATCC 43504 and J99 were obtained from the American Type Culture Collection (ATCC). H. pylori bacteria were grown on CDC anaerobe 5% sheep blood agar plates (BBL, Becton-Dickinson) under microaerophilic conditions (5% O2, 10% CO2, 85% N2) in 85% humidity in a NuAire incubator (Plymouth, MN) at 37°C. Fresh plates were started from glycerol stocks and subcultured every 48 h.
Gentamicin protection assay.
AGS cells (human gastric adenocarcinoma epithelial cell line) were seeded to give 7 × 104 cells in F12 medium supplemented with 10% fetal bovine serum (FBS) per well in 12-well tissue culture plates. MKN45 cells were cultured in RPMI 1640 medium with 10% FBS. The plates were incubated at 37°C in 5% CO2 for 15 h. AGS cells were pulsed with the bacterial strains at a multiplicity of infection (MOI) of 50 at 37°C for 1 h and then washed two times and treated with 150 μg/ml gentamicin for 1.5 h to kill extracellular bacteria. The infected cells were washed two times and then incubated with gentamicin-containing (25 μg/ml) medium before the samples were harvested. After AGS cells were coincubated with H. pylori for 2.5, 6, 12, and 24 h postinfection (p.i.), the cells were lysed with 1 ml of 0.01% saponin in Dulbecco's phosphate-buffered saline (DPBS) and then plated on CDC plates with serial dilutions to determine the number of viable bacteria. Colonies were grown and counted after 6 days of culture.
Intracellular susceptibility to antibiotics.
Confluent AGS cell monolayers in 12-well tissue culture plates (7 × 104 cells/well) were pulsed with the bacterial strains at an MOI of 50 at 37°C for 1 h. After extracellular bacteria were killed by gentamicin, each antibiotic (clarithromycin, metronidazole, and amoxicillin) was added at a concentration corresponding to different multiples of the MIC. AGS cells were lysed with 1 ml of 0.01% saponin (in DPBS) at different times (2.5, 6, 12, or 24 h) p.i. and then plated on CDC plates with serial dilutions to determine the number of viable bacteria. Colonies were grown and counted after 6 days of culture. A clarithromycin MIC of >1 μg/ml and a metronidazole MIC of >8 μg/ml are considered to indicate antibiotic resistance.
Flow assay.
AGS cells were infected with the H. pylori at an MOI of 25 at 37°C for 1 h. After extracellular bacteria were killed by gentamicin, the infected cells were cultured in gentamicin-containing (25 μg/ml) or gentamicin-free medium beginning at 5.5 h. The infected cells were harvested at different times postinfection, and plasma membrane-associated H. pylori bacteria were stained with rabbit anti-H. pylori antibody (Dako) on ice for 30 min and then washed with washing buffer. The secondary antibodies used were fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody on ice for 30 min. The percentage of H. pylori bacteria on the plasma membrane was analyzed by a FACSCalibur flow cytometry system.
Immunofluorescence confocal microscopy.
AGS cells (3 × 105 cells) were seeded on coverslips in 24-well plates and incubated for 20 h. AGS cells were infected with H. pylori at an MOI of 50 at 37°C for 1 h. The infected cells were harvested at different times postinfection and fixed with 3% paraformaldehyde, pH 7.4, at room temperature for 10 min and then washed with phosphate-buffered saline three times. The primary antibodies used were rabbit anti-H. pylori antibody (ABR), mouse anti-H. pylori lipopolysaccharide ([LPS] Santa Cruz), rabbit anti-light chain 3 (LC3)-II antibody (Abgent), mouse anti-lysosome-associated membrane glycoprotein 1 ([LAMP1] (BD Pharmingen), and goat anti-human early endosome antigen 1 (EEA1) antibody (Santa Cruz). The secondary antibodies used were FITC-conjugated goat anti-rabbit, tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit, Alexa Fluor 488-conjugated goat anti-mouse, and Alexa Fluor 488-conjugated donkey anti-goat antibody. Samples were observed on an Olympus FV1000 confocal microscope for image acquisition.
Western blot analysis.
The cells were harvested at different times postinfection and lysed in lysis buffer (25 mM Tris, 137 mM NaCl, 10% [vol/vol] glycerol, 0.5% [wt/vol] sodium deoxycholate, 2 mM EDTA) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 30 μg/ml aprotinin, 1 mM sodium orthovanadate). Lysates were clarified by pipetting and vortexing at 4°C for 30 min. Insoluble material was removed by centrifugation at 12,000 rpm. Protein concentrations were determined by a Bio-Rad protein detection kit. Equal amounts of protein were loaded on a 12% SDS-PAGE gel and transferred to nitrocellulose membrane. Anti-Beclin 1 (Santa Cruz, CA), BNIP3 (BD Pharmingen), p62 (Abgent), and LC3-II (Abgent) antibodies were used to detect protein expression patterns. After incubation with peroxidase-conjugated secondary antibodies, the blots were visualized by enhancing chemiluminescence reagents (Perkin Elmer Life Sciences, Boston, MA).
Transmission electron microscopic examination.
For ultrastructural analysis, H. pylori-infected cells at different time points were fixed with 4% glutaraldehyde and postfixed in 1% OsO4. The cells were observed with transmission electron microscopy (Hitachi 7000; Japan).
RESULTS
The replication of H. pylori in epithelial cells.
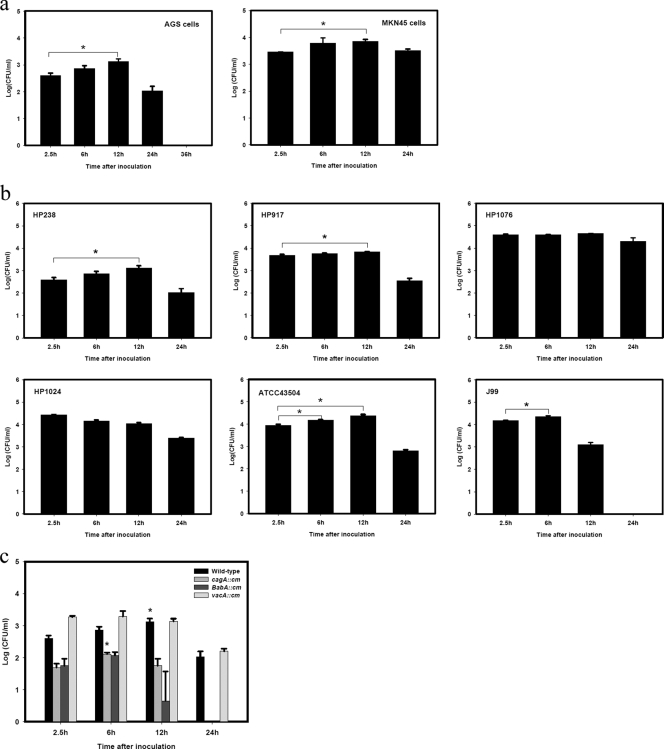
The epithelial cell line AGS was infected with H. pylori HP238 in a gentamicin protection assay. At various times postinfection (p.i.), AGS cells were collected and lysed, and viable H. pylori bacteria were recultured and plate counted on a CDC plate. The number of CFU of H. pylori represents the viable number of bacteria in the cells. As shown in Fig. 1a, the number of CFU at 2.5 h p.i. represents the amount of H. pylori that invades the AGS cells. This number increased gradually from 6 h to 12 h p.i., with a 5-fold increase in the number of CFU recultured from the infected cells at 12 h p.i. This indicates that the invading H. pylori can replicate in cells. The amount of viable H. pylori bacteria then decreased at 24 h p.i., and no viable H. pylori bacteria could be recovered at 36 h p.i., suggesting that they were gradually cleared in the infected AGS cells. Gentamicin is present throughout the culture period, so the viable H. pylori we detected can come only from the pelleted AGS cells, not from the culture supernatant. The increase in viable bacterial numbers between 2.5 h and 12 h p.i. therefore indicates that H. pylori can replicate in cells.
FIG. 1.
Multiplication of H. pylori in epithelial cells. (a) H. pylori infection of AGS or MKN45 cells. (b) Infection of AGS cells by various strains of H. pylori. (c) Infection of AGS cells by cagA, babA, and vacA mutants of H. pylori. Epithelial cell lines AGS or MKN45 were infected with HP238 or various strains or mutants at an MOI of 50 for 1 h. Gentamicin was present throughout the culture period. Recovery of viable cell-associated H. pylori (CFU) was determined by a gentamicin protection assay at various times postinfection. *, P < 0.05. Each experiment was repeated at least three times with the same results. Values are the means ± standard deviations from one representative experiment.
We used another epithelial cell line, MKN45, to verify that the intracellular replication of H. pylori is a common phenomenon. HP238 can also replicate in MKN cells (Fig. 1a). The capability of H. pylori to multiply in epithelial cells is not restricted to the HP238 Taiwanese isolate because other clinical isolates such as HP917 or even standard strains of ATCC 43504 or J99 can also replicate in AGS cells. On the other hand, HP1076 or HP1024 was not found to replicate (Fig. 1b). Furthermore, the ability to replicate within epithelial cells is not affected by the mutation of cagA because the cagA mutant is still found to multiply in epithelial cells (Fig. 1c). However, in comparison with the wild type, the cagA and babA mutants are cleared more quickly at 12 or 24 h p.i., suggesting that cagA and babA might participate in the survival within the cells. The vacA mutant invades cells more efficiently than the wild type.
Localization of H. pylori in infected AGS cells.
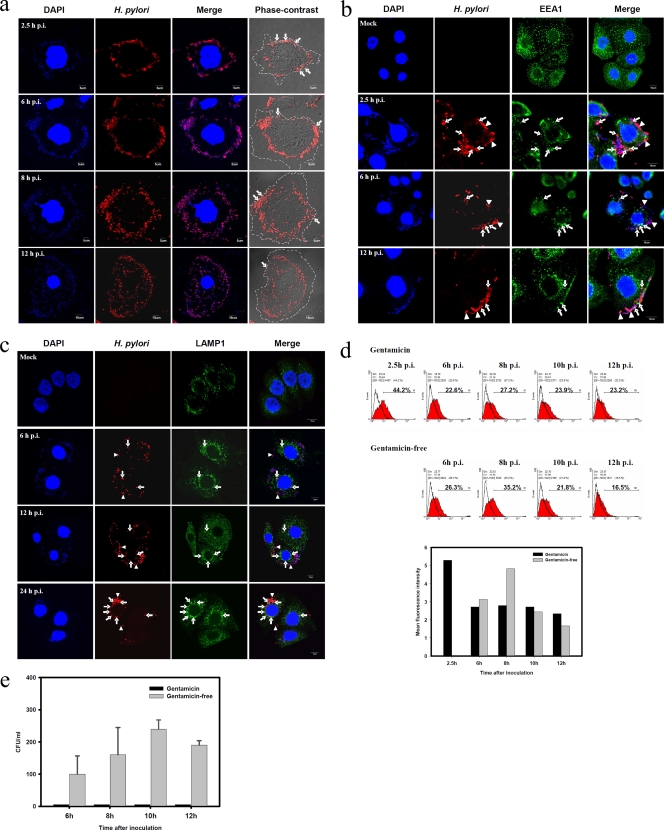
The replicating site of H. pylori was further determined. As shown in Fig. 2a, the invading H. pylori bacteria not killed by gentamicin were first localized on the periplasmic membrane at 2.5 h p.i. and then gradually moved into the cytoplasm as the infection proceeded. The numbers of bacteria increased, as revealed by anti-H. pylori antibody staining or tiny DAPI (4′,6′-diamidino-2-phenylindole)-staining bacterial dots, indicating that the bacteria are multiplying as well as moving from the membrane into the cytoplasm between 6 and 12 h p.i. Phase-contrast microscopy was used to define the boundary of the cell membrane. At 2.5 h to 12 h p.i. many H. pylori bacteria were plasma membrane-associated. But at 12 h p.i., more H. pylori bacteria were found to locate in the cytoplasm. The internalization of H. pylori in AGS cells was further characterized by the early endosomal marker EEA1 and lysosome marker LAMP1 colocalization under confocal microscopy. Some H. pylori bacteria resided first in the EEA1-positive (EEA1+) early endosome and then late in the LAMP1-positive (LAMP1+) lysosome. However, not all the H. pylori bacteria colocalized with the early endosome or lysosome (Fig. 2b and c). Flow cytometry was used to detect H. pylori on the cell surface without fixation and permeabilization of the infected AGS cells. At 2.5 h p.i., many H. pylori bacteria had adhered to the plasma membrane, as detected by the anti-H. pylori antibody on the infected AGS cells (Fig. 2d). There was a slight increase in H. pylori-positive AGS cells, from 22.6% at 6 h to 27.2%, at 8 h and a stable level of around 23% was maintained thereafter. However, if the infection was cultured in the absence of gentamicin, the H. pylori-positive AGS cells increased from 26.3% at 6 h to 35.2% at 8 h p.i., and the mean fluorescence intensity of the positive cells subtracted from that of mock-infected cells was also elevated from 3 at 6 h p.i. to 4.8 at 8 h p.i., suggesting that the number of H. pylori bacteria on the plasma membrane as recognized by anti-H. pylori antibody staining increased. Without gentamicin, the H. pylori-positive AGS cells gradually decreased to 16.5% at 12 h p.i. We suspected that some of the plasma membrane-associated H. pylori bacteria were released into the culture supernatant from the infected AGS cells. Indeed, we can recover the H. pylori from the supernatant of the AGS cell-free culture in the absence of gentamicin. In the gentamicin protection assay, a low dose of gentamicin (25 μg/ml) was included to kill the extracellular bacteria during the period of infection so that no H. pylori bacteria could be recovered from this culture supernatant (Fig. 2e).
FIG. 2.
Localization of replicating H. pylori in infected AGS cells. (a) Immunofluorescent staining of H. pylori in AGS cells. AGS cells were infected with HP238 at an MOI of 50 for 1 h. The infected cells were collected, fixed, and stained with anti-H. pylori antibody (red) and DAPI (blue) at various time points postinfection. The samples were observed with confocal or phase-contrast microscopy. The cell boundary is shown by dashed line. The plasma membrane-associated H. pylori bacteria are indicated by arrows. (b) Internalization of H. pylori on EEA1+ endosomes. AGS cells were infected with HP238 at an MOI of 20 for 1 h. The infected cells were collected, fixed, and stained with anti-H. pylori antibody (red) and anti-EEA1 antibody (green). The samples were observed with confocal microscopy. Arrows indicate the colocalization of H. pylori with the EEA1 early endosome marker. Arrowheads indicate no colocalization. (c) H. pylori on LAMP1+ lysosomes. As stated for panel b, the infected cells were stained with anti-H. pylori antibody (red) and anti-LAMP1 antibody (green). Arrows indicate the colocalization of H. pylori with the LAMP1 lysosome marker. Arrowheads indicate no colocalization. (d) Flow cytometric staining of H. pylori in AGS cells. AGS cells were infected with HP238 at an MOI of 25 for 1 h. Gentamicin (Gm; 150 μg/ml) was added to kill the extracellular bacteria. The infected cells were then cultured in gentamicin-containing medium (25 μg/ml) or gentamicin-free medium beginning at 5.5 h. At various time points, the infected cells were collected and stained with anti-H. pylori antibody without fixation. The mean fluorescence intensity of plasma membrane-associated-H. pylori in an arbitrary unit is shown to indicate the increased amount of anti-H. pylori staining. (e) Release of H. pylori into the culture supernatant. As stated for panel d, the supernatant from the infected AGS cells was collected for the recovery of the viable H. pylori (CFU). *, P < 0.05. Each experiment was repeated at least three times with the same results. Values are the means ± standard deviations from one representative experiment.
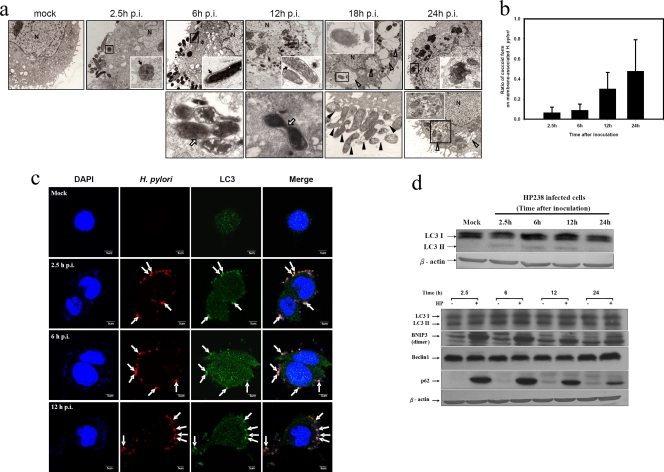
When transmission electron microscopy was used to examine the location of the invading H. pylori, many bacteria were found to be attached to the plasma membrane at an early time (Fig. 3a), a finding which is consistent with the anti-H. pylori antibody surface staining in flow cytometry. After invasion of the plasma membrane, all the H. pylori bacteria were enclosed by double-layer membrane vesicles. The dividing H. pylori bacteria were found in some double-layer vesicles on the plasma membrane (6 h p.i.). Increasing numbers of H. pylori bacteria were found in the cytoplasm as the time postinfection increased. The dividing H. pylori bacteria were also found in the autophagosome in the cytoplasm (12 h p.i.). Occasionally, onion-like vesicles were formed around the H. pylori. At a later time, multiple vacuoles were abundant, and the H. pylori-containing vesicles fused with lysosomes to digest the bacteria (18 h and 24 h p.i.). Moreover, some coccoid forms of H. pylori were present on the plasma membrane and were abundant at 18 h p.i. The coccoid form of H. pylori represents a transformation from the spiral one under the stress of the host cells. The ratio of the coccoid form of membrane-associated H. pylori increased as infection proceeded. H. pylori bacteria were attached to the plasma membrane, suggesting that they can freely exit the cell membrane or detach from the membrane and release into the culture supernatant, as shown in Fig. 2e.
FIG. 3.
Autophagy induction by H. pylori in AGS cells. (a) H. pylori resides and replicates in double-layer vesicles. AGS cells were infected with HP238 at an MOI of 50 for 1 h. The infected cells were collected, fixed, and observed with electron microscopy at various time points postinfection. The fate of H. pylori postinfection is shown in a time sequence, as indicated on the figure. N, nucleus; MV, multiple-layered vesicle. Black arrows indicate double-layer membranes, whereas white arrows indicate the replicating H. pylori bacteria. White arrowheads indicate the multiple vacuoles, whereas black arrowheads indicate the coccoid forms of H. pylori. (b) The ratio of spiral to coccoid forms on the infected cells at various time points postinfection is shown. (c) LC3 punctate formation colocalized with H. pylori. The arrows indicate the colocalization of LC3 and H. pylori-containing vesicles. (d) Autophagic flux was induced post-H. pylori infection. LC3-II conversion, BNIP3, and p62 were induced postinfection. Each experiment was repeated at least two times with the same result.
The induction of H. pylori-associated autophagosomes in infected epithelial cells.
The intracellular H. pylori bacteria were surrounded by a double-layer membrane which is the characteristic of autophagosome formation. H. pylori infection can induce autophagy. The autophagic marker LC3 punctate formations were colocalized with the H. pylori in the infected AGS cells. At an early time period (2.5 h or 6 h p.i.), most of the bacteria are plasma membrane associated, but their numbers increase in the cytoplasm at 12 h p.i. (Fig. 3b). Western blot analysis demonstrated the LC3-II conversion in H. pylori-infected AGS cells and BNIP3 and p62 were also induced (Fig. 3c). As shown above, that multiple vacuoles contain some degraded H. pylori bacteria within a single membrane, as shown by transmission electron microscopic observation at 18 h or 24 h p.i., suggests that autophagy is used by AGS cells to clear the invaded bacteria. We conclude that H. pylori infection induces the autophagic flux and that the double-layer autophagosome vesicles are the replicating sites; when they fuse with lysosomes, they become the digestion sites for the replicating H. pylori.
Antibiotic resistance of H. pylori-infected epithelial cells.
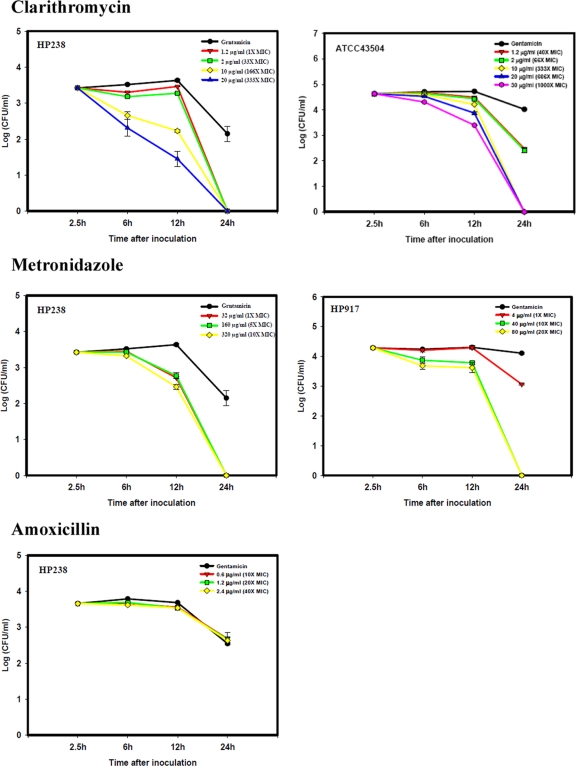
The effect of antibiotics on the intracellular multiplication of H. pylori was evaluated. For the gentamicin protection assay, gentamicin at 25 μg/ml was included in the culture. Various concentrations of clarithromycin, metronidazole, or amoxicillin were then used to replace the gentamicin at this stage. For HP238, the MIC of clarithromycin is 0.06 μg/ml. Higher doses (10 to 20 μg/ml, or 166 to 333 times the MIC) of clarithromycin are needed to inhibit the multiplication of H. pylori in AGS cells. Lower doses (1.2 to 2 μg/ml, or 20 to 33 times the MIC) are not effective (Fig. 4). This also applied to another clarithromycin-sensitive strain, ATCC 43504 (MIC of 0.03 μg/ml), where a higher dose of clarithromycin (at least 333 times the MIC) enhanced the intracellular killing of the bacteria. For metronidazole-resistant HP238 (MIC of 32 μg/ml) or metronidazole-sensitive HP917 (MIC of 4 μg/ml), a higher dose of metronidazole (10 to 20 times the MIC) enhanced the clearance of the invading H. pylori. On the other hand, amoxicillin, which like gentamicin is unable to penetrate into cells, even at 20 to 40 times the MIC (HP238; MIC of 0.06 μg/ml) did not have any effect. This suggests that the intracellular growth of H. pylori provides a niche to escape being killed by extracellular antibiotics. However, if the antibiotic can diffuse into the cells, such as with clarithromycin or metronidazole, there is still an inhibitory effect on the intracellular growth of H. pylori, but a higher dose of antibiotic is required.
FIG. 4.
The effect of antibiotic on the multiplication of H. pylori in AGS cells. AGS cells were infected with HP238 at an MOI of 50 for 1 h. Gentamicin (150 μg/ml) was added to kill the extracellular bacteria. The infected cells were then cultured in gentamicin-containing medium (25 μg/ml) or another antibiotic-containing medium beginning at 5.5 h. At various time points, the infected cells were collected and lysed for recovery of the viable cell-associated H. pylori (CFU). For HP238, the MICs of clarithromycin, metronidazole, and amoxicillin are 0.06 μg/ml, 32 μg/ml, and 0.06 μg/ml, respectively. For ATCC 43504, the MIC of clarithromycin is 0.03 μg/ml. For HP917, the MIC of metronidazole is 4 μg/ml. A clarithromycin MIC of >1 μg/ml and a metronidazole MIC of >8 μg/ml indicate resistance. Each experiment was repeated at least three times with the same results. Values are the means ± standard deviations from one representative experiment.
DISCUSSION
Using the gentamicin protection assay on AGS cells, we demonstrated that H. pylori can invade the epithelial cells and multiply either on the plasma membrane or in double-layer vesicles. The invasion rate is around 0.01 to 0.1% of the bacteria inoculated. The 5-fold CFU increment between 2.5 h and 12 h p.i. indicates that the bacteria can replicate in the cells. The doubling time of the cell-associated H. pylori is calculated to be 5 to 6 h. The autophagic vesicles induced by H. pylori provide the site for its replication. The multiple vacuoles that fused from the H. pylori-containing autophagic vesicles with lysosomes will degrade the bacteria. Many H. pylori bacteria in coccoid form associated with the plasma membrane. This study is consistent with the report of Tan et al. that H. pylori can form microcolonies on the plasma membrane and use it as the replicative niche, with a doubling time of 4 to 6 h (25). However, we further showed that these autophagic vesicles are the site not only of replication but also of degradation when they are fused into autolysosomes.
Although H. pylori is generally viewed as a noninvasive pathogen present only in the lumen of the stomach and attached to gastric epithelial cells, many studies have shown that H. pylori is invasive and can be considered a facultative intracellular organism (6, 20). However, no direct demonstration of its replication in cells has been provided by the conventional gentamicin protection assay. We have found that H. pylori can multiply not only in macrophages and bone marrow-derived dendritic cells (27, 28) but also in epithelial cells as shown in this study. The key to this finding is that the increase in the CFU count is transient, observed only from 6 to 12 h p.i., while most of the other studies have determined the bacterial number at 1 to 3 days p.i. Another difference is the strain of H. pylori used. This new finding has several implications for the biological life cycle of H. pylori in the host. H. pylori can be considered a kind of intracellular microorganism because it can invade cells, undergo replication within cells, and leave the infected cells for further spread. Kwok et al. reported the entry of H. pylori into the AGS cells via a zipper-like mechanism, involving intimate contact with AGS cell microvilli and surface membrane pseudopod structures (11). The internalized H. pylori bacteria are found in LAMP1-containing vacuoles. The CagA protein can be translocated to the plasma membrane and form rafts with the invading H. pylori (12).
Autophagy is a component of the innate cellular immune responses against not only intracellular but also extracellular microorganisms (4, 5, 8, 14, 18). After internalization of the microorganism, the autophagosomes are formed to degrade the ingested bacterium by the lysosomal killing mechanism. Several intracellular bacteria have developed different mechanisms to evade the autophagic surveillance in macrophages. The autophagic vesicles are induced, but their maturation into autophagolysosomes is arrested or delayed (1, 22). The autophagy is induced by H. pylori in either phagocytic THP-1 cells or dendritic cells (27, 28) or nonphagocytic AGS epithelial cells. All H. pylori bacteria reside within the autophagosome, with no escape from the autophagic vesicle. On invasion of epithelial cells, some of the H. pylori bacteria were internalized by EEA1+ early endosome followed by the LAMP1+ late endosome/lysosomes. Multiple-vesicle vacuoles formed at 18 or 24 h p.i. that resulted in the destruction of the bacteria by fusion with lysosomes. Amieva et al. have reported viable H. pylori bacteria in large cytoplasmic vacuoles (2). They reasoned that bacteria can repopulate the extracellular environment in parallel with the disappearance of intravacuolar bacteria in the absence of gentamicin, and the intravacuolar niche is a place for the release of the bacteria and the place for evasion of the hostile microenvironment of the host. Recently, they have also reported that the plasma membranes are the site for H. pylori to replicate (25). We found many degrading bacteria in the multiple vacuoles, especially at 24 h p.i. The autophagy inducer rapamycin enhanced the clearance of the H. pylori (data not shown). We reasoned that the autophagic flux will clear the H. pylori in the autophagosome after fusion with the lysosomes. The multiple vacuoles are the place for the destruction of the invading and replicating bacteria. With regard to the release of H. pylori into the culture supernatant from the infected AGS cells, as shown in Fig. 2e, it is the plasma membrane-associated H. pylori bacteria that are released and which can be recovered as viable bacteria in the absence of gentamicin. H. pylori is spiral in nature, but it transforms into a coccoid form under stress conditions (3). It is generally accepted that coccoid forms of H. pylori are viable but are not culturable. Many coccoid forms of H. pylori were found on the membrane of the infected AGS cells, and the ratio of coccoid to bacillary forms increased as infection proceeded (Fig. 3a).
Intracellular H. pylori collections have been demonstrated in a small subset of gastric epithelial progenitors after infection of acid-producing parietal cell-deficient mice (16, 17) or in preneoplastic or cancer tissue (15, 23). H. pylori bacteria were detectable in endosome, cytoplasm vacuole, or plasma membrane or in membrane-associated coccoid form, which is consistent with our observation that H. pylori is replicating in the autophagosome or plasma membrane.
A triple-therapy regimen for 7 to 14 days is used as the first-line treatment for H. pylori-infected patients, but the failure rate is around 20 to 30% (7, 9). The failure of antibiotic treatment has been assumed to be due to the selection of an antibiotic-resistant mutant. The second-line treatment with quadruple therapy or even a third-line levofloxacin-based empirical regimen can be used to retreat the failed patients, and high cumulative eradication rates can be achieved (9, 10, 13, 21, 26). Our finding that H. pylori is capable of multiplication within infected epithelial cells provides an explanation of why the treatment failure may not be exclusively caused by the recurrence of antibiotic-resistant mutants. More likely, it may be due to the existence of an ecological niche for the organism to survive both the hostile gastric environment and the antibacterial therapy. The dormant coccoid form on the plasma membrane is resistant to antibiotic and can spread to infect other cells in the absence of an effective concentration of antibiotic. Thus, the antibiotic concentration needs to be high and sustained longer as it not only is needed to kill the extracellular H. pylori but also must be capable of penetrating the epithelial cells to kill the intracellular H. pylori. Cell membrane-penetrating antibiotics such as clarithromycin or metronidazole are effective, whereas non-cell membrane-penetrating amoxicillin or gentamicin is not effective. Interestingly, when the H. pylori bacteria derived from the clarithromycin-treated AGS cells were recultured on the plate, the colony size was much smaller than colonies from metronidazole- or gentamicin-treated cells (data now shown). In summary, H. pylori usurps the autophagic vesicles as the site for replication, and the autolysosomes after fusion will also degrade the replicating bacteria. This raises a distinct potential for new anti-H. pylori drugs that target the autophagy to enhance the clearance of intracellular bacteria in addition to the conventional use of antibiotics.
Acknowledgments
This work was supported by grant NSC98-2320-B006-031-MY2 from the National Science Council, Taiwan.
Y.-T.C. and Y.-H.W. performed the experiments. J.-J.W. preformed construction of mutants. H.-Y.L. developed and designed the experiments.
We declare that we have no competing financial interests.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Allen, L. A. 2007. Phagocytosis and persistence of Helicobacter pylori. Cell Microbiol. 9:817-828. [DOI] [PubMed] [Google Scholar]
- 2.Amieva, M. R., N. R. Salama, L. S. Tompkins, and S. Falkow. 2002. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell Microbiol. 4:677-690. [DOI] [PubMed] [Google Scholar]
- 3.Benaissa, M., P. Babin, N. Quellard, L. Pezennec, Y. Cenatiempo, and J. L. Fauchere. 1996. Changes in Helicobacter pylori ultrastructure and antigens during conversion from the bacillary to the coccoid form. Infect. Immun. 64:2331-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deretic, V. 2006. Autophagy as an immune defense mechanism. Curr. Opin. Immunol. 18:1-8. [DOI] [PubMed] [Google Scholar]
- 5.Deretic, V., and B. Levine. 2009. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5:527-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois, A., and T. Boren. 2007. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 9:1108-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischbach, L. A., K. J. Goodman, M. Feldman, and C. Aragaki. 2002. Sources of variation of Helicobacter pylori treatment success in adults worldwide: a meta-analysis. Int. J. Epidemiol. 31:128-139. [DOI] [PubMed] [Google Scholar]
- 8.Flannagan, R. S., G. Cosio, and S. Grinstein. 2009. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 7:355-366. [DOI] [PubMed] [Google Scholar]
- 9.Gerrits, M. M., A. H. M. van Vliet, E. J. Kuipers, and J. G. Kusters. 2006. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect. Dis. 6:699-709. [DOI] [PubMed] [Google Scholar]
- 10.Houben, M. H. M. G., D. Van De Beek, E. F. Hensen, A. J. M. De Craen, E. A. J. Rauwas, and G. N. J. Tytgat. 1999. A systematic review of Helicobacter pylori eradication therapy—the impact of antimicrobial resistance on eradication rates. Aliment. Pharmacol. Ther. 13:1047-1055. [DOI] [PubMed] [Google Scholar]
- 11.Kwok, T., S. Backert, H. Schwarz, J. Berger, and T. F. Meyer. 2002. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a Zipper-like mechanism. Infect. Immun. 70:2108-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai, C. H., Y. C. Chang, S. Y. Du, H. J. Wang, C. H. Kuo, S. H. Fang, H. W. Fu, H. H. Lin, A. S. Chiang, and W. C. Wang. 2008. Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA-induced responses in AGS cells. Infect. Immun. 76:3293-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Megraud, F., and H. Lamouliatte. 2003. Review article: the treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 17:1333-1343. [DOI] [PubMed] [Google Scholar]
- 14.Miller, S., and J. Krijnse-Locker. 2008. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 6:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Necchi, V., M. E. Candusso, F. Tava, O. Luinetti, U. Ventura, R. Fiocca, V. Ricci, and E. Solcia. 2007. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology 132:1009-1023. [DOI] [PubMed] [Google Scholar]
- 16.Oh, J. D., H. Kling-Bäckhed, M. Giannakis, L. G. Engstrand, and J. I. Gordon. 2006. Interactions between gastric epithelial stem cells and Helicobacter pylori in the setting of chronic atrophic gastritis. Curr. Opin. Microbiol. 9:21-27. [DOI] [PubMed] [Google Scholar]
- 17.Oh, J. D., S. M. Karam, and J. I. Gordon. 2005. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc. Natl. Acad. Sci. U. S. A. 102:5186-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orvedahl, A., and B. Levine. 2009. Eating the enemy within: autophagy in infectious diseases. Cell Death Differ. 16:57-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peek, R. M. Jr., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28-37. [DOI] [PubMed] [Google Scholar]
- 20.Petersen, M. A., and K. A. Krogfelt. 2003. Helicobacter pylori: an invading microorganism? A review. FEMS Immunol. Med. Microbiol. 36:117-126. [DOI] [PubMed] [Google Scholar]
- 21.Rokkas, T., P. Sechopoulos, I. Robotis, G. Margantinis, and D. Pistiolas. 2009. Cumulative H. pylori eradication rates in clinical practice by adopting first- and second-line regimens proposed by the Maastricht III consensus and a third-line empirical regimen. Am. J. Gastroenterol. 104:21-25. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberger, C. M., and B. B. Finlay. 2003. Phagocytes sabotage: disruption of macrophage signaling by bacterial pathogens. Nat. Rev. Mol. Cell Biol. 4:385-396. [DOI] [PubMed] [Google Scholar]
- 23.Semino-Mora, C., S. Q. Doi, A. Marty, V. Simko, I. Carlstedt, and A. Dubois. 2003. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J. Infect. Dis. 187:1165-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 25.Tan, S., L. S. Tompkins, and M. R. Amieva. 2009. Helicobacter pylori usurps cell polarity to turn the cell surface into a replicative niche. PLoS Pathog. 5:e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Wouden, E. J., J. C. Thijs, A. A. van Zwet, W. J. Sluiter, and J. H. Kleibeuker. 1999. The influence of in vivo nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta-analysis. Am. J. Gastroenterol. 94:1751-1759. [DOI] [PubMed] [Google Scholar]
- 27.Wang, Y. H., J. J. Wu, and H. Y. Lei. 2009. The Autophagic induction in Helicobacter pylori-infected macrophage. Exp. Biol. Med. 34:171-180. [DOI] [PubMed] [Google Scholar]
- 28.Wang, Y. H. Wang, J. P. Gorvel, Y. T. Chu, J. J. Wu, and H. Y. Lei. 2010. Helicobacter pylori impairs murine dendritic cell responses to infection. PLoS One. doi: 10.1371/journal.pone.0010844. [DOI] [PMC free article] [PubMed]