Abstract
Intradermal infection of methicillin-resistant Staphylococcus aureus (MRSA) in burned mice was pathogenically analyzed. An abscess was formed in normal mice intradermally infected with 108 CFU/mouse of MRSA, and all of these mice survived after the infection; however, abscess formation was not demonstrated to occur in burned mice similarly exposed to the pathogen, and all of these mice died within 5 days of infection. In burned mice, MRSA infected at the burn site intradermal tissues spread quickly throughout the whole body, while in normal mice, the pathogen remained localized at the infection site. Macrophages (Mφ) isolated from the infection site tissues of normal mice produced interleukin-12 (IL-12) but not IL-10 and were characterized as M1Mφ. These M1Mφ were not isolated from the infection site tissues of burned mice. When normal-mouse infection site tissue Mφ were adoptively transferred to burned mice at the MRSA infection site, an abscess formed, and the infection did not develop into sepsis. In contrast, an abscess did not form and sepsis developed in normal mice that were inoculated with burned-mouse infection site tissue Mφ. These Mφ produced IL-10 but not IL-12 and were characterized as M2Mφ. These results indicate that abscess formation is a major mechanism of host resistance against intradermal MRSA infection. M1Mφ in the tissues surrounding the infection site play a pivotal role in abscess formation; however, the abscess is not formed in burned mice where M2Mφ predominate. M2Mφ have been described as inhibitor cells for Mφ conversion from resident Mφ to M1Mφ.
Infection is the major cause of morbidity and mortality in severely burned patients (27, 33). Methicillin-resistant Staphylococcus aureus (MRSA) is known as a typical pathogen in such infections. Generally, healthy individuals are resistant against MRSA infection; however, severely burned patients with greatly suppressed immune responses are particularly susceptible to MRSA infection. In severely burned patients, MRSA was represented in 40% of the wounds, and 14% to 17% of all wounds became infected once they were colonized with MRSA (11, 14, 25). Therefore, intervention targeting the host's antibacterial immune responses seems to be critical for successful regulation of MRSA infection.
Recently, defects in antibacterial innate immunities have been demonstrated to occur in patients and animals following severe burn injuries (5). Innate immunity is the major host defense against early burn wound infection with MRSA (10), and classically activated macrophages (Mφ) (M1Mφ [interleukin-12-positive {IL-12+} IL-10− Mφ]) have been identified as a major effector cell in host antimicrobial innate immunities (6, 19, 21). M1Mφ kill bacteria through the production of lysosomal enzymes, reactive oxygen intermediates, reactive nitrogen intermediates, and antimicrobial peptides (12, 21, 23). In general, resident Mφ (IL-12− IL-10− Mφ, isolatable from healthy donors) convert to M1Mφ following stimulation with invasive pathogens via pattern recognition receptors (19). Contrarily, a majority of thermally injured hosts are shown to be carriers of alternatively activated macrophages (M2Mφ [IL-12− IL-10+ Mφ]) (15, 16, 32). M2Mφ have reduced abilities to kill bacteria, and soluble factors released from M2Mφ inhibit pathogen-stimulated macrophage conversion from resident Mφ to M1Mφ (15).
Bacterial replication causes tissue destruction, and an abscess is commonly formed through this necrotizing process (18). An abscess, defined as a circumscribed collection of pus, is a very important host antibacterial defense against intradermal MRSA infection (8). The well-developed abscess has a wall or capsule of fibrous tissue separating it from the surrounding tissue. Histologically, the abscess lesions consist of accumulating leukocytes with live bacteria (26). In recent studies (7, 17, 26), abscess formation was caused by the accumulation of phagocytic Mφ to eliminate the pathogen. In this study, the influence of severe burn injury on abscess formation and bacterial growth at the infection site tissue was investigated for burned mice intradermally infected with MRSA. In the results, an abscess was not formed in burned mice, unlike in normal mice, after intradermal infection with MRSA. MRSA infected in burned mice at local intradermal tissues spread throughout the whole body quickly. In normal mice, IL-12-producing and IL-10-nonproducing Mφ (M1Mφ) were characterized as effector cells for abscess formation. After intradermal infection with MRSA, an abscess formed in burned mice that were inoculated with infection site tissue Mφ (M1Mφ). These results indicate that M1Mφ appearing in response to the MRSA infection are major effector cells in the host antibacterial defense. Through abscess formation, M1Mφ work to control the bacterial growth at the infection site and to inhibit the dissemination of the pathogen. M2Mφ inhibit Mφ conversion from resident Mφ to M1Mφ following bacterial stimulation. Therefore, an abscess is not formed in hosts where M2Mφ predominate, which results in the development of sepsis from local infections.
MATERIALS AND METHODS
Mice.
Eight- to 10-week-old male BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) were used in these experiments. Experimental protocols for animal studies were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston (IACUC approval number 01-04-010).
MRSA, reagents, antibodies, and media.
A vancomycin-sensitive strain of MRSA (biotype 21777), isolated from a clinical specimen of a burn patient in Shriners Hospital for Children, was utilized throughout the study. This strain was grown on mannitol salt agar supplemented with oxacillin for 18 h at 37°C under aerobic conditions. Two strains of MRSA (strain BAA-44 and strain 33591) and a strain of methicillin-sensitive Staphylococcus aureus (MSSA) (strain 29213) were purchased form the American Type Culture Collection (Manassas, VA). Fluorescein isothiocyanate (FITC)-conjugated anti-CD206 (mannose receptor) monoclonal antibody (MAb) was purchased from AbD Serotec (Raleigh, NC). Purified anti-IL-12 MAb, streptavidin particles plus DM, Cytofix/Cytoperm solution, and phycoerythrin (PE)-conjugated anti-IL-12 and anti-IL-10 MAbs were purchased from BD Biosciences (San Jose, CA). Biotin-conjugated anti-F4/80 MAb was purchased from eBioscience (San Diego, CA). Collagenase (type I) and protease inhibitor cocktail were purchased from Sigma-Aldrich. PE-conjugated anti-CXCL9 MAb and ELISA MAX standard kits for IL-10, IL-12, tumor necrosis factor alpha (TNF-α), and IL-1β were purchased from Biolegend (San Diego, CA). TRIzol reagent was purchased from Invitrogen (Carlsbad, VA). RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) (complete medium) was used as the medium for Mφ cultivation.
Burn injury.
Burned mice were created according to our previously reported protocol (32). Thus, mice were anesthetized with pentobarbital (40 mg/kg of body weight, intraperitoneally [i.p.]), and electric clippers were used to shave the hair on the back of each mouse from the groin to the axilla. The mice were then exposed to a gas flame for 9 s after the window of a custom-made insulated mold (with a 4- by 5-cm window) was pressed firmly against the shaved back. A Bunsen burner equipped with a flame-dispersing cap was used as the source of the gas flame. This procedure consistently produced a third-degree burn on approximately 25% of the total body surface area (TBSA) for a 26-g mouse (32). Immediately after thermal injury, physiologic saline (1 ml per mouse, i.p.) was administered for fluid resuscitation. Deaths within 5 days of 25% TBSA flame burn were not demonstrated after our burn procedure. As controls, mice were anesthetized and shaved but were not exposed to the gas flame. They also received physiologic saline (1 ml per mouse, i.p.).
Mouse model of MRSA abscess formation.
MRSA (108 CFU/mouse at 0.2 ml) was intradermally injected into the left groins of mice 30 min after burn injury (4). Abscess formation was determined by the presence of pustular exudates, fascial adhesions, skin thickening, and skin necrosis. Abscess volume was measured by a microcaliper, and the abscess volume was expressed in mm3 (length by width by height). In some experiments, tissues surrounding the infection site (15- by 15-mm window) were surgically removed and homogenized on ice. The number of viable bacteria per gram tissue was determined by a standard colony-counting method. Furthermore, blood specimens were obtained from normal and burned mice 1 to 3 days after MRSA infection. The number of bacteria per 1 ml blood was determined by a standard colony-forming method. Serum fractions of the blood were assayed for TNF-α and IL-1β by an enzyme-linked immunosorbent assay (ELISA) in accordance with the manufacturer's instructions.
Treatment of IL-10 antisense ODN or anti-IL-12 MAb.
Phosphothioate-modified antisense or scrambled oligodeoxynucleotides (ODN) were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in water. IL-10 antisense ODN had the following sequence and positions: 5′-CATTTCCGATAAGGCTTGG-3′. Twenty-four and 12 h before infection, mice were treated subcutaneously with 6 μg/mouse of IL-10 antisense ODN. They were again treated 0.1 and 12 h after infection with IL-10 antisense ODN. As control groups, normal and burned mice were treated subcutaneously with 6 μg/mouse of scrambled ODN. Also, normal mice were infected with MRSA intradermally, and these mice were then treated subcutaneously with anti-IL-12 MAb (10 μg/mouse) 0.1 h, 24 h, and 48 h after infection.
Infection site tissue Mφ. (i) Isolation.
Mφ were isolated from the tissue surrounding the infection site as previously described (34). Three days after intradermal infection with MRSA, the skin and fascia from around the injection site (15 by 15 mm) were aseptically removed and put in culture dishes containing complete medium and 25 mM HEPES. The tissues obtained were cut into small pieces with a scalpel and suspended in Hanks' balanced salt solution with 0.5% collagenase and incubated in a water bath at 37°C for 40 min with frequent agitation. The cell suspensions were then centrifuged (1,300 rpm for 5 min). Next, the remaining cells were adjusted to 1 × 107 cells/ml in IMag buffer and treated with 5 μg/ml of biotin-conjugated anti-mouse F4/80 MAb. After being washed with IMag buffer, the cell suspension was mixed with streptavidin particles plus DM at a ratio of one cell to five beads for 40 min at 4°C. F4/80+ cells were magnetically separated to the side of the tube, and the supernatant was eliminated (28). F4/80+ cells were shown to be 95% pure when they were analyzed by flow cytometry using FITC-conjugated anti-F4/80+ MAb purchased from AbD Serotec (Raleigh, NC). Utilizing this procedure, 5 × 105 Mφ were isolated from the infection site tissue of a burned mouse and 1 × 106 Mφ were isolated from the same tissue of a normal mouse.
(ii) Adoptive transfer.
Normal-mouse infection site tissue Mφ (NISMφ) (1 × 106 cells/mouse) were intradermally injected into normal mice at 3 different sites surrounding the infection site. Also, burned mice 30 min after MRSA infection were inoculated intradermally with 1 × 106 cells/mouse of NISMφ. Similarly, burned-mouse infection site tissue Mφ (BISMφ) (1 × 106 cells/mouse) were intradermally injected into normal mice at 3 different sites surrounding the infection site. Also, after MRSA infection, burned mice were inoculated intradermally with 1 × 106 cells/mouse of BISMφ. The severity of infection in these groups of mice was evaluated by the growth of MRSA in spleens and the abscess formation, as mentioned above.
Mφ from the heat-killed-MRSA stimulation site tissues. (i) Isolation.
Normal and burned mice were intradermally stimulated with heat-killed MRSA (corresponding to 3 × 108 CFU/ml of live bacteria). Three days after stimulation, Mφ were isolated from the stimulated site tissues, as mentioned above.
(ii) Adoptive transfer.
Mφ (1 × 106 cells/mouse) isolated from the stimulation site tissues of normal mice were adoptively transferred to the tissue surrounding the MRSA infection sites of burned mice. Also, Mφ (1 × 106 cells/mouse) isolated from the stimulation site tissues of the burned mice were adoptively transferred to the tissue surrounding the MRSA infection sites of the normal mice.
Definitions of M1Mφ and M2Mφ.
Mφ with abilities to produce IL-12 (but not IL-10) and to express inducible nitric oxide synthase (iNOS) mRNA and with a lack of mannose receptor and FIZZ1 mRNAs were considered to be M1Mφ. Also, Mφ with abilities to produce IL-10 (but not IL-12) and to express mannose receptor and FIZZ1 mRNAs and with a lack of iNOS mRNA were considered to be M2Mφ (6, 24). For reverse transcription-PCR (RT-PCR), RNA was extracted from Mφ by TRIzol in accordance with the manufacturer's recommendations. Within the experiment, each sample was normalized by the amount of isolated RNA. Then, this RNA was turned back into cDNA through the reverse transcription of mRNA. PCR was conducted using the following synthesized oligonucleotide primers: for iNOS, 5′-CCCTCCAGTGTCGGGAGCA-3′ (forward [F]) and 5′-TGCTTGTCACCACCAGCAGT-3′ (reverse [R]); for mannose receptor, 5′-CCATCGAGACTGCTGCTGAG-3′ (F) and 5′-AGCCCTTGGGTTGAGGATCC-3′ (R); and for FIZZ1, 5′-TCCCAGTGAATACTGATGAGA-3′ (F) and 5′-CCACTCTGGATCTCCCAAGA-3′ (R). With the use of a thermal cycler (GeneAmp PCR System 9600), 35 cycles of PCR were performed at 94°C for 15 s, at 60°C for 15 s, and at 72°C for 20 s. The predicted products were run on 2% agarose gels containing ethidium bromide.
Measurement of IL-12 and IL-10.
Without any stimulation, 5 × 105 cells/ml of Mφ were cultured individually. Culture fluids harvested 48 h after cultivation were assayed for IL-12 (a biomarker of M1Mφ) and IL-10 (a biomarker of M2Mφ) by ELISA in accordance with the manufacturer's instructions. In some experiments, tissues were chopped and homogenized in phosphate-buffered saline (PBS) supplemented with 1% of protease inhibitor cocktail. After centrifugation (6,500 rpm for 5 min), the supernatants harvested were assayed for IL-12 and IL-10 by use of ELISA. In our assay system, the detection limits were 2 pg/ml for IL-12 and 30 pg/ml for IL-10.
Flow cytometric analysis.
NISMφ and BISMφ were immediately stained with FITC-conjugated anti-mannose receptor MAb. For intracellular staining for IL-12, IL-10, and CXCL9, these Mφ were immediately incubated with Cytofix/Cytoperm solution at 4°C for 20 min. After being washed, the cells were incubated with PE-conjugated anti-IL-12, anti-IL-10, or anti-CXCL9 MAb or isotype control MAb at 4°C for 30 min. After being washed, the cells were analyzed with a FACSCanto flow cytometer.
Statistical analysis.
The results obtained were analyzed statistically using an analysis-of-variance (ANOVA) test. Kaplan-Mayer curves were constructed, and a log rank comparison test of the groups was used to calculate P values. All calculations were performed using the program Statview 4.5 from Brain Power (Calabasas, CA). The result was considered significant if the probability value was lower than 0.05.
RESULTS
Abscess formation and bacterial growth in mice intradermally infected with MRSA.
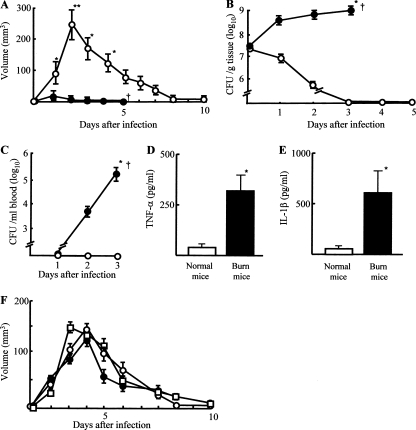
One to 10 days after intradermal infection with MRSA (108 CFU/mouse), abscess formation was observed in normal and burned mice. The volume of the abscess formed at the infection site was measured daily by a microcaliper. Also, the skin and fascia at the MRSA infection sites (15 by 15 mm) were surgically excised from both groups of mice 1 to 5 days after infection and homogenized. The number of bacteria in tissue homogenates was determined by the standard colony counting assay. The obtained results are shown in Fig. 1. For normal mice, abscesses were first demonstrated 1 day after MRSA infection, reached the maximum volume (234 mm3 average) 2 days after the infection, and then gradually diminished. In burned mice, however, only trace appearances of abscesses (5 mm3 average) were demonstrated at 1 day after MRSA infection, and then these abscesses disappeared within 3 days of the infection (Fig. 1A). MRSA grew logarithmically in the infection site tissues of burned mice, and all of these mice died within 5 days of the infection. In normal mice, with abscess formation, MRSA did not grow significantly, and the pathogen disappeared from the tissues surrounding the abscess sites within 3 days of the infection (Fig. 1B). A significant number of MRSA organisms were not detected in the blood samples of normal mice, while 7.0 × 103 to 1.3 × 105 CFU/ml of the pathogen were detected in the blood samples of burned mice 2 to 3 days after the infection (Fig. 1C). TNF-α and IL-1β were detected in the sera of burned mice 3 days after MRSA infection, but these cytokines were not detected in the sera of normal mice (Fig. 1D and E). These results indicate that, in contrast to what is observed in normal mice, significantly volumed abscesses are not formed in burned mice intradermally exposed to MRSA. The pathogen grew logarithmically at the infection site tissues of burned mice and spread throughout the whole body. In the results, similar abscess formations were demonstrated to occur in normal mice intradermally infected with 4 different strains of Staphylococcus aureus, including 2 strains of MRSA purchased from the ATCC, a strain of MSSA purchased from ATCC, and our hospital-isolated MRSA strain (Fig. 1F).
FIG. 1.
Abscess formation and growth of bacteria in mice intradermally infected with MRSA. (A) Abscess formation. One to 10 days after intradermal infection of MRSA at a dose of 108 CFU/mouse, abscess formation in normal mice (n = 3; open circles) and burned mice (n = 3; filled circles) was tested. The abscess volume was expressed in mm3 (length by width by height). Data are means ± standard errors of the means (SEM) of results from three different experiments. *, P < 0.01; **, P < 0.001; †, dead. (B) Numbers of MRSA in the infection site tissues. Following the same MRSA infection shown in panel A, the MRSA infection site tissues (15 by 15 mm) were excised from normal mice (n = 3; open circles) and burned mice (n = 3; filled circles) 1 to 5 days after infection. Tissues removed were homogenized, and the numbers of bacteria in these homogenates were determined using a standard colony-counting assay. Data are means ± SEM of results from three different experiments. *, P < 0.01; †,died. (C) Growth of MRSA in blood. Blood specimens were obtained from normal (n = 3; open circles) and burned (n = 3; filled circles) mice 1 to 3 days after MRSA infection. The numbers of bacteria in blood samples were determined using a standard colony-forming assay. Data are means ± SEM of results from three different experiments. *, P < 0.01; †, dead. (D, E) Amounts of TNF-α and IL-1β in the sera of normal and burned mice infected with MRSA. Serum specimens were obtained from normal (n = 3) and burned (n = 3) mice 3 days after intradermal infection with 108 CFU/mouse of MRSA. Amounts of TNF-α (D) and IL-1β (E) were measured using ELISA. Data are means ± SEM of results from three different experiments. *, P < 0.01. (F) Abscess formation in mice infected with 3 different strains of Staphylococcus aureus. Normal mice were intradermally infected with 108 CFU/mouse of the BAA-44 strain of MRSA (n = 3; open circles), the 33591 strain of MRSA (n = 3; filled circles), or the 29213 strain of MSSA (n = 3; open squares). The volumes of abscesses formed in these mice were measured. Data are means ± SEM of results from three different experiments.
Survival of normal and burned mice intradermally infected with MRSA.
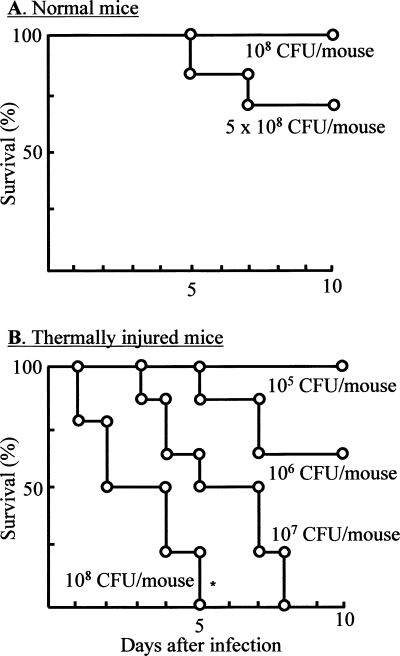
The resistance of normal and burned mice to intradermal infection with MRSA was examined. Four groups of burned mice (12 mice each) were intradermally infected with 105 to 108 CFU/mouse of MRSA, and their survival was observed daily for 10 days after the infection. Normal mice infected with the pathogen at doses of 1 × 108 to 5 × 108 CFU/mouse were utilized as controls. The results obtained are shown in Fig. 2A (for normal mice) and B (for burned mice). While all of the normal mice survived after intradermal infection with 108 CFU/mouse of MRSA, all burned mice exposed to the same number of MRSA died within 5 days of the infection. One 50% lethal dose (LD50) was shown to occur in burned mice infected with 106 CFU/mouse of MRSA. These results indicate that intradermally infected MRSA spread throughout the whole body and caused sepsis that resulted in the high mortality rates among burned mice, while anti-MRSA resistance is shown to occur in normal mice similarly exposed to the same pathogen.
FIG. 2.
Survival of mice intradermally infected with MRSA. Normal mice (A) (12 mice per group) and burned mice (B) (12 mice per group) were intradermally infected with MRSA at doses of 1 × 108 to 5 × 108 CFU/mouse (A) or 105 to 108 CFU/mouse (B). These mice were observed daily for 10 days to determine their survival rates. *, P < 0.05 for comparison with normal mice infected with the same number of MRSA. Data are means ± SEM of results from three different experiments.
Two different Mφ subsets isolated from MRSA infection site tissues.
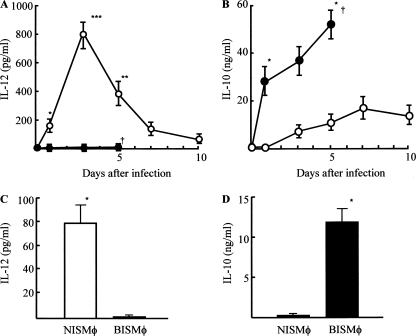
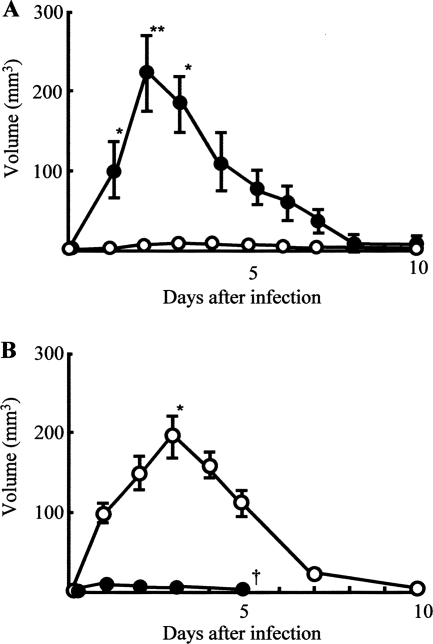
Next, we addressed the questions as to why the lack of abscess formation allows the pathogen to grow rapidly in burned mice intradermally infected with MRSA and, with abscess formation, why the same infection is completely controlled in normal mice. The presence of Mφ in an abscess has been histologically demonstrated (26). Also, antibacterial effector cells (M1Mφ) are known as IL-12+ IL-10− Mφ (2, 19, 21), and inhibitor cells on the Mφ conversion from resident Mφ to M1Mφ are characterized as IL-12− IL-10+ Mφ (M2Mφ) (16). Therefore, as a first-step experiment, MRSA infection site tissue homogenates were prepared from normal and burned mice, and IL-12 and IL-10 contents in the homogenates were measured using ELISA. As shown in Fig. 3A and B, IL-12 was detected in infection site tissue homogenates of normal mice, while this cytokine was not detected in the same homogenates prepared from burned mice. In contrast, IL-10 was detected in infection site tissue homogenates of burned mice, while this cytokine was not demonstrated to occur in the homogenates derived from normal mice. Therefore, the role of IL-12 and IL-10 in abscess formation in normal and burned mice was examined after intradermal infection of MRSA. When normal mice were treated with anti-IL-12 MAb before and after MRSA infection, an abscess was not demonstrated. Contrarily, abscesses formed in burned mice infected with MRSA when they were treated with IL-10 antisense ODN (Fig. 4A and B). These results suggest that Mφ with abilities to produce IL-10 (burned mice) or IL-12 (normal mice) are involved in the abscess formation resulting from an intradermal infection of MRSA.
FIG. 3.
Detection of IL-12 and IL-10 in homogenates of MRSA infection site tissues and production of IL-12 and IL-10 by Mφ isolated from MRSA infection site tissues. A square (15 by 15 mm) of infection site tissue was excised from normal mice (n = 6; open circles) or burned mice (n = 6; filled circles) 1, 3, 5, 7, and 10 days after the MRSA infection (108 CFU/mouse). Tissues were homogenized, and supernatants obtained were assayed for IL-12 (A) and IL-10 (B) by ELISA. Data are means ± SEM of results from three different experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; †, dead. Three days after intradermal infection of MRSA (108 CFU/mouse), Mφ were isolated from the infection site tissues of normal and burned mice (3 mice each). Then, cells obtained were adjusted to 5 × 105 cells/ml and cultured for 48 h. Culture fluids obtained were tested for IL-12 (C) and IL-10 (D) by use of ELISA. Data are means ± SEM of results from three different experiments. *, P < 0.001.
FIG. 4.
Effect of anti-IL-12 MAb or IL-10 antisense ODN on abscess formation in mice infected with MRSA. (A) Normal mice were infected with 108 CFU/mouse of MRSA intradermally. The mice were treated subcutaneously with anti-IL-12 MAb (10 μg/mouse) 0.1, 24, and 48 h after infection (open circles; n = 3). A control group of normal mice was injected subcutaneously with the same dose of saline (filled circles; n = 3). The sizes of the abscesses in the mice were calculated by a microcaliper 1 to 10 days after infection. The volume of the abscess was expressed in mm3 (length by width by height). Data are presented as the mean abscess volume ± SEM. *, P < 0.05; **, P < 0.01. (B) Burned mice were intradermally infected with MRSA (108 CFU/mouse). Twenty-four and 12 h before infection, the mice were treated subcutaneously with 6 μg/mouse of IL-10 antisense ODN. They were again treated 0.1 and 12 h after infection with 6 μg/mouse of IL-10 antisense ODN (open circles; n = 3). A control group of burned mice was treated subcutaneously with 6 μg/mouse of scrambled ODN (filled circles; n = 3). The abscess sizes were measured by a microcaliper 1 to 10 days after infection. The volume of the abscess was expressed in mm3 (length by width by height). Data are presented as the mean abscess volume ± SEM. *, P < 0.05.
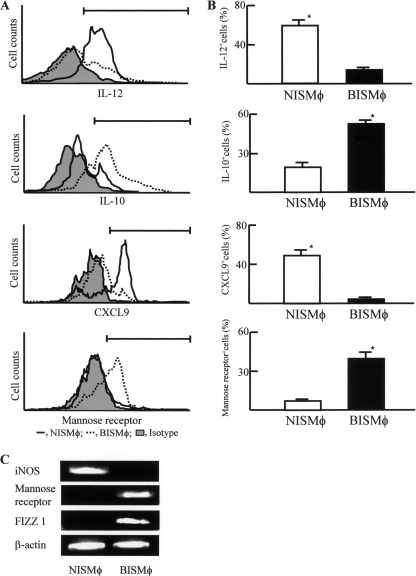
Therefore, in the next experiments we tried to isolate Mφ from redundant MRSA infection sites. Mφ were isolated from digested tissues of MRSA infection sites of normal and burned mice 3 days after the MRSA infection (108 CFU/mouse). Mφ preparations (5 × 105 cells/ml) were cultured for 48 h, and culture fluids harvested were assayed for IL-12 and IL-10. The results obtained are shown in Fig. 3C and D. NISMφ were shown to be IL-12 producer and IL-10 nonproducer cells, while BISMφ were shown to be IL-10 producer and IL-12 nonproducer cells. When these Mφ were analyzed for IL-12, IL-10, CXCL9, or mannose receptor by flow cytometry, NISMφ were shown to be IL-12+ IL-10− CXCL9+ mannose receptor-negative (mannose receptor−) Mφ and BISMφ were shown to be IL-12− IL-10+ CXCL9− mannose receptor+ Mφ (Fig. 5A and B). In addition, Mφ isolated from infection site tissues of normal mice expressed iNOS mRNA, and Mφ from infection site tissues of burned mice expressed mannose receptor and FIZZ1 mRNAs (Fig. 5C). These results indicate that IL-12 producer/IL-10 nonproducer Mφ expressing iNOS mRNA (M1Mφ) are present at the infection site tissues of normal mice intradermally exposed to MRSA. In contrast, IL-10 producer/IL-12 nonproducer Mφ expressing mannose receptor and FIZZ1 mRNAs (M2Mφ) are present at the infection site tissues of burned mice intradermally exposed to MRSA. These results strongly suggest that M1Mφ play a role on MRSA abscess formation.
FIG. 5.
Properties of Mφ accumulated in tissues surrounding the MRSA infection site tissues. (A, B) Expression of mannose receptor and intracellular expression of IL-12, IL-10, and CXCL9 in Mφ. NISMφ (black line) and BISMφ (dotted line) were analyzed for mannose receptor or intracellular IL-12, IL-10, and CXCL9 by flow cytometry. The shaded histogram represents the isotype control. Representative data from 3 independent experiments are shown in panel A. The percentages of positive cells in both groups are shown in panel B. Data are means ± SEM of results from three different experiments. (C) Expression of iNOS, mannose receptor, and FIZZ1 mRNAs by Mφ isolated from MRSA infection site tissue. Mφ, isolated from the tissue surrounding the infection site (108 CFU/mouse), were analyzed for iNOS, mannose receptor, and FIZZ1 mRNAs. Representative data from 3 independent experiments are shown in panel C.
Growth of MRSA in spleens of mice inoculated with two different Mφ preparations.
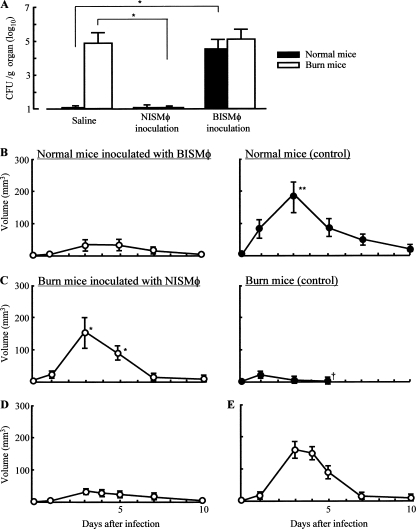
After intradermal infection with MRSA, bacterial growth in normal and burned mice inoculated with 2 different sources of tissue Mφ was examined. Three days after the infection, Mφ were isolated from infection site tissues of normal and burned mice. Then, these Mφ preparations (1 × 106 cells/mouse) were adoptively transferred to additional groups of normal and burned mice (6 mice each) previously exposed to 108 CFU/mouse of MRSA. Three days after infection, the spleens were removed, and the number of pathogens in these organs was determined by a standard colony-counting assay. In the results, bacterial growth was shown to occur in the spleens of normal mice inoculated with infection site tissue Mφ isolated from burned mice. In contrast, MRSA did not grow in the spleens of burned mice inoculated with normal mouse tissue Mφ. The growth of MRSA was not demonstrated to occur in the spleens of normal mice intradermally infected with 108 CFU/mouse of MRSA. Bacteria did grow in the spleens of burned mice similarly infected with MRSA (Fig. 6A).
FIG. 6.
Pathogenic role of BISMφ and NISMφ in growth of MRSA and abscess formation in mice infected with MRSA intradermally. (A) Growth of MRSA in spleens of normal and burned mice inoculated with Mφ. Three days after infection with 108 CFU/mouse of MRSA, Mφ were isolated from tissues surrounding the MRSA infection sites of normal and burned mice. Additional groups of normal and burned mice previously infected with MRSA were inoculated with these Mφ preparations (1 × 106 cells/mouse). Three days after the infection, spleens were removed from both groups of mice and homogenized. Numbers of bacteria in the homogenates were determined using a standard colony-counting assay. Data are means ± SEM of results from three different experiments. *, P < 0.01. (B, C) Abscess formation in normal or burned mice inoculated with NISMφ or BISMφ. After intradermal infection of MRSA (108 CFU/mouse), normal and burned mice were inoculated with 1 × 106 cells/mouse of BISMφ (Β) and NISMφ (C), respectively. As controls, both groups of mice were injected with 0.3 ml/mouse of saline. One to 10 days after the infection, abscess formation in all groups of mice was examined. Data are means ± SEM of results from three different experiments. *, P < 0.01; †, dead. (D, E) Normal and burned mice were intradermally stimulated with heat-killed MRSA (corresponding to 3 × 108 CFU/ml of live bacteria). Three days after stimulation, Mφ were isolated from stimulated site tissues. Mφ (1 × 106 cells/mouse) isolated from the stimulation site tissues of normal mice were adoptively transferred to tissues surrounding the MRSA infection sites of burned mice (D). Also, Mφ (1 × 106 cells/mouse) isolated from the stimulation site tissues of burned mice were adoptively transferred to tissues surrounding the MRSA infection sites of normal mice. One to 10 days after the infection, all recipient mice (n = 3) were tested for abscess formation (E). Data are presented as the mean abscess volume ± SEM.
Furthermore, the effect of tissue Mφ inoculation on abscess formation in normal and burned mice intradermally infected with MRSA was examined. Tissue Mφ (1 × 106 cells/mouse), isolated from the MRSA infection sites of normal and burned mice, were intradermally inoculated into additional groups of normal and burned mice (6 mice each), which were previously exposed to 108 CFU/mouse of MRSA intradermally. When normal mice inoculated with BISMφ were infected with MRSA, a significant abscess did not form (Fig. 6B). However, an abscess was formed in burned mice inoculated with NISMφ (Fig. 6C).
Mφ transferred from MRSA-infected donors may be carriers of the pathogen. To avoid any confusion, we performed the same Mφ transfer experiments utilizing heat-killed MRSA. Thus, normal and burned mice were intradermally injected with 3 × 108 CFU/mouse of heat-killed MRSA. Three days later, Mφ were isolated from the injection site tissues of these mice. Then, the Mφ preparation (1 × 106 cells/mouse) derived from the normal mice was transferred to the MRSA infection sites of the burned mice 5 min after the MRSA infection. Also, the Mφ preparation (1 × 106 cells/mouse) derived from burned mice was transferred to the MRSA infection sites of normal mice 5 min after the MRSA infection. One to 10 days after the infection, all recipients were examined for abscess formation. In the results, abscess formation was demonstrated to occur in the burned mice inoculated with the Mφ preparation derived from normal mice, while it was not demonstrated to occur in normal mice inoculated with the Mφ preparation derived from burned mice (Fig. 6D and E). These results suggest that the role of the pathogen being moved from donors to recipients by Mφ transfer on the abscess formation in the recipient mice may be minimal.
DISCUSSION
Staphylococcus aureus (or MRSA) is a pathogen frequently isolated from thermally injured patients (27). In general, the typical pathological manifestation of staphylococcal disease is abscess formation. However, in thermally injured patients, local MRSA infection frequently spreads throughout the whole body and causes sepsis (3). Whether an infection is contained or spreads depends on host antimicrobial resistances (18).
Leukocytes are the primary effector cells on host antimicrobial defense against MRSA infection. The migration of leukocytes to the site of infection results from the orchestrated expression of adhesion molecules on endothelial cells. This process is triggered by bacteria and tissue Mφ (35). Generally, Mφ appear in abscesses of individuals with infection (7, 17, 26). Therefore, in this paper, we investigated the pathogenetic role of these Mφ accumulated in the infection site tissue by use of a mouse model of burn injury with intradermal infection of MRSA.
In our studies, an abscess was formed at the infection sites of normal mice intradermally infected with 108 CFU/mouse of MRSA, and all of these mice survived after the infection. The pathogen did not grow significantly in the infection site tissues of these mice and therefore did not spread throughout the whole body. Sepsis evidenced by systemic inflammatory responses (increased levels of TNF-α, IL-1β, and nitric oxide in sera) and positive blood cultures was not demonstrated to occur in normal mice intradermally exposed to MRSA. However, abscess formation was not shown to occur in burned mice similarly exposed to the pathogen. Biomarkers of sepsis (systemic inflammatory responses and positive blood cultures) were detected in these burned mice intradermally infected with MRSA. Thus, significantly volumed abscesses were not formed in burned mice intradermally infected with MRSA, and in these mice, the pathogen grew logarithmically in the infection site tissues.
Following stimulation with microbes, microbial products, or cytokines, resident Mφ convert to M1Mφ, an effector cell in host antibacterial innate immunity (19). M1Mφ exhibit (i) high oxygen consumption, (ii) the ability to kill pathogens, (iii) the ability to express iNOS, and (iv) the ability to secrete Th1 response-associated cytokines/chemokines (IFN-γ, IL-12, IL-18, CCL3, CCL5, and CXCL9) (19). However, M1Mφ are not generated in burned mice even when these mice are stimulated with bacteria, bacterial products, or typical M1Mφ inducers (16). In this paper, M1Mφ (IL-12+ IL-10− Mφ) were detected in tissues surrounding the infection sites of normal mice intradermally infected with MRSA. However, these Mφ were not demonstrated to occur in MRSA infection site tissues of burned mice. M2Mφ (IL-12− IL-10+ Mφ) were detected in tissues surrounding the infection sites of burned mice. In our previous studies (15, 32), M2Mφ have been demonstrated to occur in peritoneal cavities and spleens of mice 2 to 28 days after burn injury. M2Mφ preferentially express mannose receptor and FIZZ1 mRNAs. They also produce arginase, IL-1 receptor antagonist, transforming growth factor β (TGF-β), IL-10, and CCL17 (6, 19). CCL17 and IL-10 released from M2Mφ are inhibitory on the Mφ conversion from resident Mφ to M1Mφ (15).
More recently, the presence of three different subtypes of M2Mφ (M2aMφ, M2bMφ, and M2cMφ) has been reported (2, 19, 21). These subsets are distinguished from each other by their expressed genes and chemokine-producing profiles. Thus, CCL17-producing Mφ with the FIZZ1 gene are identified as M2aMφ, CCL1-producing Mφ with the SPHK1 gene are classified as M2bMφ, and CXCL13-producing Mφ with the FIZZ1 gene are recognized as M2cMφ. All three M2Mφ subtypes inhibit the Mφ conversion from resident Mφ to M1Mφ. The subtypes of M2Mφ generated in MRSA infection site tissues will be characterized in future experiments.
In previous papers (9, 13), IL-12 and IL-10 were shown to play a role in the clearance of MRSA infected systemically. However, the role of these cytokines in the clearance of locally infected MRSA is still unclear. After intradermal infection of MRSA, abscesses formed in burned mice treated with IL-10 antisense ODN, while an abscess did not form in normal mice treated with anti-IL-12 MAb (Fig. 4A and B).
NISMφ were shown to be IL-12 producer cells (but not IL-10 producer cells), and BISMφ were identified as IL-10 producer cells (but not IL-12 producer cells) (Fig. 3C and D). Abscesses were formed in normal mice and burned mice inoculated with NISMφ, while an abscess was not formed in burned mice and normal mice inoculated with BISMφ (Fig. 6B and C). A variety of cells, including T cells, B cells, monocytes, Mφ, dendritic cells, granulocytes, keratinocytes, and epithelial cells, have been reported to be sources of IL-10 (22). On the other hand, the main producers of IL-12 are phagocytes (monocytes, Mφ, dendritic cells, and neutrophils) and B cells (31). In the present study, IL-10-producing Mφ were shown to be important in the infectious complications stemming from intradermal MRSA infection. Also, IL-12-producing Mφ were shown to be important effectors on the host's antibacterial defense against MRSA local infection. Furthermore, after treatment with IL-10 antisense ODN, abscesses were formed in normal mice inoculated with BISMφ, while an abscess was not formed in burned mice inoculated with NISMφ after treatment with anti-IL-12 MAb (unpublished data). With these results taken all together, we concluded that IL-10 produced by BISMφ and IL-12 produced by NISMφ had a role in abscess formation or lack thereof.
In this study, the bactericidal activity of Mφ was shown to be important for abscess formation and the prevention of MRSA dissemination. An abscess was not formed at MRSA infection site tissues of burned mice. Nevertheless, abscess formation was demonstrated to occur in burned mice inoculated with M1Mφ at the infection site. These M1Mφ were isolated from MRSA infection site tissues of normal mice. Also, MRSA intradermally infected did not grow in burned mice that were inoculated with M1Mφ. On the other hand, abscess formation was not demonstrated to occur in normal mice inoculated with M2Mφ at the infection site, and MRSA grew logarithmically in tissues surrounding the infection sites of normal mice that were inoculated with M2Mφ. These M2Mφ were isolated from the MRSA infection site tissues of burned mice.
In adaptive immunity, Th1 response is important in the host's antibacterial resistance against MRSA sepsis. In contrast, in antibacterial innate immunity, polymorphonuclear neutrophils (PMN) (1, 20) and macrophages (Mφ) (16, 29) have been described as important effector cells (30). In our mouse model of intradermal MRSA infection, Mφ were shown to play a role in the abscess formation. Abscess formation is a means of suppressing the spreading of MRSA present at the local infection site.
A pathogenic role for Mφ in abscess formation was studied. In a mouse model of thermal injury, an intradermal dose of MRSA did not spread to the whole body of mice when an abscess was formed at the infection sites of these mice. So far, we only have data obtained from the murine model. At this time, we have no data for severely burned patients. For clarification in this regard, future studies are needed.
Acknowledgments
This work was supported by grant 8840 from Shriners of North America. A.A. was supported by the James W. McLaughlin Fellowship Fund.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 2 August 2010.
REFERENCES
- 1.Bamberger, D., K. Bettin, and D. Gerding. 1987. Neutrophil localization in acute and chronic experimental abscesses. J. Lab. Clin. Med. 109:389-395. [PubMed] [Google Scholar]
- 2.Benoit, M., B. Desnues, and J. Mege. 2008. Macrophage polarization in bacterial infections. J. Immunol. 181:3733-3739. [DOI] [PubMed] [Google Scholar]
- 3.Branski, L., A. Al-Mousawi, H. Rivero, M. Jeschke, A. Sanford, and D. Herndon. 2009. Emerging infections in burns. Surg. Infect. (Larchmt.) 10:389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunce, C., L. Wheeler, G. Reed, J. Musser, and N. Barg. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church, D., S. Elsayed, O. Reid, B. Winston, and R. Lindsay. 2006. Burn wound infections. Clin. Microbiol. Rev. 19:403-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards, J., X. Zhang, K. Frauwirth, and D. Mosser. 2006. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 80:1298-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Firth, C., A. Laing, S. Baird, J. Pearson, and S. Gieseg. 2008. Inflammatory sites as a source of plasma neopterin: measurement of high levels of neopterin and markers of oxidative stress in pus drained from human abscesses. Clin. Biochem. 41:1078-1083. [DOI] [PubMed] [Google Scholar]
- 8.Gerber, J., S. Coffin, S. Smathers, and T. Zaoutis. 2009. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children's hospitals in the United States. Clin. Infect. Dis. 49:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gjertsson, I., O. Hultgren, and A. Tarkowski. 2002. Interleukin-10 ameliorates the outcome of Staphylococcus aureus arthritis by promoting bacterial clearance. Clin. Exp. Immunol. 130:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison, C. 2009. Innate immunity as a key element in host defense against methicillin resistant Staphylococcus aureus. Minerva Pediatr. 61:503-514. [PubMed] [Google Scholar]
- 11.Heggers, J., L. Phillips, J. Boertman, J. Carethers, M. Weingarten, C. Lentz, J. Hayden, and M. Robson. 1988. The epidemiology of methicillin-resistant Staphylococcus aureus in a burn center. J. Burn Care Rehabil. 9:610-612. [DOI] [PubMed] [Google Scholar]
- 12.Houghton, A., W. Hartzell, C. Robbins, F. Gomis-Rüth, and S. Shapiro. 2009. Macrophage elastase kills bacteria within murine macrophages. Nature 460:637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hultgren, O., M. Stenson, and A. Tarkowski. 2001. Role of IL-12 in Staphylococcus aureus-triggered arthritis and sepsis. Arthritis Res. 3:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunt, J., G. Purdue, and D. Tuggle. 1988. Morbidity and mortality of an endemic pathogen: methicillin-resistant Staphylococcus aureus. Am. J. Surg. 156:524-528. [DOI] [PubMed] [Google Scholar]
- 15.Katakura, T., M. Miyazaki, M. Kobayashi, D. N. Herndon, and F. Suzuki. 2004. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J. Immunol. 172:1407-1413. [DOI] [PubMed] [Google Scholar]
- 16.Katakura, T., T. Yoshida, M. Kobayashi, D. N. Herndon, and F. Suzuki. 2005. Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin. Exp. Immunol. 142:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, J., J. Sohn, H. Jung, S. Kim, K. Lee, and H. Kang. 2008. Pace of macrophage recruitment during different stages of soft tissue infection: semi-quantitative evaluation by in vivo magnetic resonance imaging. Eur. Radiol. 18:2033-2039. [DOI] [PubMed] [Google Scholar]
- 18.Lowy, F. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25:677-686. [DOI] [PubMed] [Google Scholar]
- 20.Mölne, L., M. Verdrengh, and A. Tarkowski. 2000. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect. Immun. 68:6162-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosser, D. 2003. The many faces of macrophage activation. J. Leukoc. Biol. 73:209-212. [DOI] [PubMed] [Google Scholar]
- 22.Mosser, D., and X. Zhang. 2008. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 226:205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naito, M. 2008. Macrophage differentiation and function in health and disease. Pathol. Int. 58:143-155. [DOI] [PubMed] [Google Scholar]
- 24.Porta, C., M. Rimoldi, G. Raes, L. Brys, P. Ghezzi, D. Di Liberto, F. Dieli, S. Ghisletti, G. Natoli, P. De Baetselier, A. Mantovani, and A. Sica. 2009. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc. Natl. Acad. Sci. U. S. A. 106:14978-14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ransjö, U., M. Malm, A. Hambraeus, G. Artursson, and A. Hedlund. 1989. Methicillin-resistant Staphylococcus aureus in two burn units: clinical significance and epidemiological control. J. Hosp. Infect. 13:355-365. [DOI] [PubMed] [Google Scholar]
- 26.Saenz, A., A. Koreishi, A. Rosenberg, and R. Kradin. 2009. Immune cell subsets in necrotizing fasciitis: an immunohistochemical analysis. Virchows Arch. 455:87-92. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan, R. 2005. Sepsis in pediatric burn patients. Pediatr. Crit. Care Med. 6:S112-S119. [DOI] [PubMed] [Google Scholar]
- 28.Shigematsu, K., A. Asai, M. Kobayashi, D. N. Herndon, and F. Suzuki. 2009. Enterococcus faecalis translocation in mice with severe burn injury: a pathogenic role of CCL2 and alternatively activated macrophages (M2aMφ and M2cMφ). J. Leukoc. Biol. 86:999-1005. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka, H., S. Miyazaki, Y. Sumiyama, and T. Kakiuchi. 2004. Role of macrophages in a mouse model of postoperative MRSA enteritis. J. Surg. Res. 118:114-121. [DOI] [PubMed] [Google Scholar]
- 30.Tosi, M. 2005. Innate immune responses to infection. J. Allergy Clin. Immunol. 116:241-249; quiz 250. [DOI] [PubMed] [Google Scholar]
- 31.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 32.Tsuda, Y., H. Takahashi, M. Kobayashi, T. Hanafusa, D. N. Herndon, and F. Suzuki. 2004. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 21:215-226. [DOI] [PubMed] [Google Scholar]
- 33.Vostrugina, K., D. Gudaviciene, and A. Vitkauskiene. 2006. Bacteremias in patients with severe burn trauma. Medicina (Kaunas) 42:576-579. [PubMed] [Google Scholar]
- 34.Wang, W., K. Keller, and K. Chadee. 1992. Modulation of tumor necrosis factor production by macrophages in Entamoeba histolytica infection. Infect. Immun. 60:3169-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao, L., J. Berman, S. Factor, and F. Lowy. 1997. Correlation of histopathologic and bacteriologic changes with cytokine expression in an experimental murine model of bacteremic Staphylococcus aureus infection. Infect. Immun. 65:3889-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]