Abstract
Vibrio cholerae is the causative agent of cholera, a severe diarrheal disease that remains endemic in many parts of the world and can cause outbreaks wherever sanitation and clean water systems break down. Prevention of disease could be achieved through improved sanitation and clean water provision supported by vaccination. V. cholerae serogroup O1 is the major cause of cholera; O1 serotypes Inaba and Ogawa have similar disease burdens, while O139 is the only non-O1 serogroup to cause epidemics. We showed previously that immunization of adult female mice with purified V. cholerae outer membrane vesicles (OMVs) elicits an antibody response that protect neonates from oral V. cholerae challenge and that suckling from an immunized dam accounts for the majority of protection from V. cholerae colonization. Here we report that lipopolysaccharide (LPS) is the major OMV protective antigen. Mucosal immunization with OMVs from Inaba or Ogawa provides significant cross-serotype protection from V. cholerae colonization, although serotype-specific antigens are dominant. OMVs from O1 or O139 do not provide cross-serogroup protection, but by immunization with a mixture of O1 and O139 OMVs, cross-serogroup protection was achieved. Neonatal protection is not associated with significant bacterial death but may involve inhibition of motility, as antibodies from OMV-immunized mice inhibit V. cholerae motility in vitro, with trends that parallel in vivo protection. Motility assays also reveal that a higher antibody titer is required to immobilize O139 compared to O1, a phenotype that is O139 capsule dependent.
Vibrio cholerae is the causative agent of the fecally-orally transmitted, severe secretory diarrheal disease cholera, which remains endemic in many parts of the world. The WHO reported 236,896 cholera cases worldwide in 2006, but the true disease burden is estimated to be in the millions (67). Oral or intravenous rehydration therapies are effective treatments to prevent cholera deaths, but in some regions these treatments are unavailable or poorly administered. Prevention of disease through improved sanitation complemented by vaccination could reduce the disease burden in regions where cholera is endemic and use of vaccination could help to contain or prevent isolated outbreaks.
Despite there being over 200 V. cholerae serogroups detectable in the aquatic environment, where V. cholerae is a natural resident, the O1 serogroup alone is the major cause of cholera. Currently, cholera outbreaks are caused by the El Tor biotype of O1, which in Bangladesh by 1989 had replaced the previously circulating classical biotype (49). The O1 serogroup includes two subtypes, serotypes Ogawa and Inaba, which differ only by the presence of a 2-O-methyl group in the Ogawa O antigen (37). O139 appeared in 1993 and to date is the only non-O1 serogroup to have caused a major cholera epidemic (23, 64). O139 appears to have arisen from O1 El Tor by acquisition of a new lipopolysaccharide (LPS) locus that contains genes that allow synthesis of a capsule that is not present in O1 (26).
The only cholera vaccine currently widely available and licensed for human use is composed of whole-cell heat- and formalin-killed V. cholerae O1 plus cholera toxin (CTX) B subunit (WCK-CTB) under the trade name Dukoral. Taken orally in two doses, or three doses for children aged 2 to 6 years, Dukoral provides moderate protection, about 50% protective efficacy over 3 years (24), and herd immunity can provide additional protection to the unvaccinated (6). The WCK-CTB vaccine is considered unsatisfactory due to “its two-dose regimen, short shelf-life, high cost and need for cold chain distribution” (27), with the inclusion of recombinant CTB being the costly component, leaving room for an improved cholera vaccine for use in developing countries (50). In Vietnam, a locally produced WCK vaccine that lacks CTB, making it more affordable, has had around 66% efficacy (78). A new version of the Vietnam vaccine, reformulated to meet WHO standards, has achieved 67% protective efficacy, even in children as young as 1 year old, in an area where cholera is endemic (74); it contains a mixture of O1 and O139 WCK V. cholerae and has proven to be immunogenic toward both serogroups, but with a stronger response to O1 than O139 (8). Live attenuated V. cholerae vaccines are also orally delivered and provide an interesting alternative approach, as reviewed in reference 66.
All Gram-negative bacteria observed to date, including V. cholerae, release outer membrane vesicles (OMVs) (14, 21). OMVs from Salmonella enterica subsp. I serovar Typhimurium have been shown to stimulate both adaptive and innate immune responses, and OMVs from several species are immunogenic and protective in mouse models of infection (2, 14, 45, 65). OMV-based intramuscularly delivered vaccines designed to protect against Neisseria meningitidis serogroup B infection have proved to be safe, immunogenic, and protective in human trials, as reviewed in reference 76.
In 1977, it was found that subcutaneous immunization of mice with V. cholerae-derived vesicles protected their neonates from a lethal V. cholerae challenge (30). This neonatal protection model is used because adult mice are resistant to acute small-intestinal colonization by V. cholerae. We have recently revisited this approach and reported that mucosal immunization of adult female mice with V. cholerae O1 Ogawa OMVs via the oral or intranasal (i.n.) route elicits an antibody response that significantly reduces small-intestinal colonization of suckling neonates challenged orally with V. cholerae (71). In addition, by using OMVs as a delivery vehicle, responses to heterologous antigens have been observed with mice without the need for additional adjuvants (22, 70). Therefore, OMVs may represent a versatile vaccine delivery system with natural mucosal adjuvant properties.
Many studies have shown that antibodies mediate protection from V. cholerae infection. In a household study, circulating levels of vibriocidal anti-V. cholerae IgG were found to inversely correlate with symptomatic or asymptomatic V. cholerae infection (55), although this association is not perfect (68). While another study of patients and their contacts found that high serum IgA (but not serum IgG) directed at V. cholerae antigens CtxB, TcpA, and LPS is associated with protection from infection (36). After oral or i.n. immunization of mice with V. cholerae OMVs, we detected anti-OMV IgG1, IgG2a, IgM, and IgA in adult mouse serum samples, as well as IgA (and to a lesser extent IgG1) in stool samples, IgG1 (and to a lesser extent IgA) in milk extracted from the stomachs of neonates suckling from immunized dams, and IgG1 and IgG2a in neonatal blood samples (70). Fostering of pups from sham-immunized to OMV-immunized dams indicated that suckling from an OMV-immunized dam accounted for the majority of protection from colonization upon V. cholerae challenge (70).
Here we present further characterization of the V. cholerae OMV vaccine. First, we have attempted to simplify the OMV immunization schedule from three doses to two. Second, V. cholerae OMV immunization to date had utilized OMVs from only an O1 Ogawa serotype strain. Here we investigate whether LPS and/or proteins are major V. cholerae OMV antigens and whether O1 Inaba, O1 Ogawa, or O139 OMVs alone or in combination are immunogenic and protective against homologous or heterologous V. cholerae challenge. Third, we investigate the mechanisms of neonatal protection. We find no evidence that protection is dependent on bacterial killing, whereas the ability of anti-OMV antibodies to inhibit motility of different V. cholerae strains in vitro reflects trends of in vivo protection, suggesting that inhibition of motility contributes to protection.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
V. cholerae was cultured in Luria-Bertani (LB) broth with shaking (250 rpm) or on LB broth agar plates, supplemented with 100 μg ml−1 streptomycin (Sm), 50 μg ml−1 ampicillin (Ap), or 50 μg ml−1 rifampin (Rif) as appropriate, at 37°C.
Wild-type V. cholerae strains used for OMV preparation, immunization, and/or neonatal challenge and strains deleted for, or with plasmid disruptions in, otnA, flaA, flrA, CTXφ, ompU, ompA, or hlyA that were used for OMV preparation (and for immunization in the case of otnA and CTXφ) are listed in Table 1. For deletion of hlyA (VCA0219), pCVD442-ΔhlyA was constructed, which is missing the entire hlyA open reading frame except for the start and stop codons, using splicing by overlap extension (SOE) PCR (41) and the pCVD442 XbaI restriction site. DNA manipulations were carried out as described previously (54). pCVD442-ΔhlyA was transformed into SM10λpir by electroporation, and exconjugates from the mating of SM10λpir pCVD442-ΔhlyA with AC53 were isolated. Plasmid excision was induced by counterselection of Sm-resistant and Ap-resistant (Smr Apr) isolates on 10% sucrose Sm plates to produce ΔhlyA Smr Aps mutant isolates as described previously (28). A plasmid disruption in flaA was made by using pGPflaA (54). Exconjugates between SM10λpir pGPflaA and AC53 were isolated on Sm Ap plates as described previously (53).
TABLE 1.
Plasmids and strains used in this study
| Plasmid or strain | V. cholerae biotype, serogroup, and/or serotype | Relevant genotype/resistance/description | Reference or source |
|---|---|---|---|
| Plasmids | |||
| pCVD442 | oriR6K mobRP4sacB, Apr | 28 | |
| pCVD442-ΔhlyA | ΔhlyA/VCA0219 of AC53 in pCVD442, Apr | This study | |
| pGP704 | oriR6K mobRP4, Apr | 53 | |
| pGPflaA | pGP704::flaA | 54 | |
| E. coli strain | |||
| SM10λpir | thi recA thr leu tonA lacY supE RP4-2-Tc::Mu λ::pir | 53 | |
| Wild-type V. cholerae strains | |||
| AC53 | El Tor O1 Ogawa | Spontaneous Smr mutant of E7946, a 1978 clinical isolate from Bahrain; hapR+, Smr-; used for previous OMV immunization studies (70, 71) | 52 |
| AC1006 | El Tor O1 Ogawa | 2001 clinical isolate from Bangladesh; hapR status unknown, Smr | Gift from F. Qadri (ICDDR,B) |
| AC51 | El Tor O1 Inaba | Spontaneous Smr mutant of C6709-1, a 1991 clinical isolate from Peru; hapR+, Smr | 82 |
| A1552 | El Tor O1 Inaba | Spontaneous Rifr mutant of 92A1552 clinical isolate from Latin America; hapR+, Rifr | 85 |
| MO10 | O139 | 1992 clinical isolate from India; hapR+, Smr | 83 |
| Other V. cholerae strains | |||
| otnA mutant | O139 | MO10 otnA::pGP704 (otnA also known as wbfF) acapsular mutant; hapR+, Apr Smr | 58 |
| Bah-2 | El Tor O1 Ogawa | AC53 ΔCTXφ, mutant with a deletion of the entire cholera toxin bacteriophage and its attachment sites; hapR+, Smr | 77 |
| AC3035 | EL Tor O1 Ogawa | AC53 pGPflaA; hapR+, Apr Smr | This study |
| ΔflrA mutant | El Tor O1 Inaba | AC51 ΔflrAhapR+, Smr | 54 |
| ΔompA mutant | El Tor O1 Inaba | A1552 ΔompA hapR+, Rifr | 72 |
| ΔompU mutant | El Tor O1 Ogawa | AC53 ΔompUhapR+, Smr | 71 |
| AC2915 | El Tor O1 Ogawa | AC53 ΔhlyAhapR+, Smr | This study |
Mice.
BALB/c mice (Charles River or Taconic Laboratories) were used in all experiments. Mice were housed in specific pathogen-free conditions with food and water ad libitum and monitored under the care of full-time staff and in accordance with the rules of the Department of Laboratory Animal Medicine at Tufts Medical Center. All adult animals were acclimated for at least 1 week before any procedures were carried out and were 7 to 8 weeks old at the time of immunization.
Preparation of OMVs and LPS from V. cholerae.
OMVs were prepared from late-exponential LB broth cultures of V. cholerae as described previously (71). Ultracentrifugation of a liter of culture supernatant resuspended in 500 μl of phosphate-buffered saline (PBS) gave a yield of ca. 4 mg ml−1 OMVs by protein content, estimated with the modified Lowry protein assay (Thermo Scientific Pierce). Amounts of OMVs given below are by protein amount.
LPS was purified from proteinase-K-treated overnight V. cholerae O1 Ogawa cultures by a method adapted from Hitchcock and Brown (39), including an additional phenol extraction using phase-lock gel light tubes (Eppendorf) and two washes with TM buffer (50 mM Tris [pH 7.5], 10 mM MgCl2) with centrifugation at 75,000 × g for 60 min. The concentration of the V. cholerae LPS was determined by comparison to a standard curve of commercially available Escherichia coli O26:B6 LPS (Sigma). LPS was separated on a 4 to 12% NuPage Bis-Tris gel (Invitrogen) and silver-stained with a SilverQuest staining kit according to the manufacturer's instructions (Invitrogen). A Fujifilm FLA-9000 scanner was used to quantify the amount of LPS silver staining in each lane.
ID50 determination with neonatal mice.
The 50% infective dose (ID50) for V. cholerae colonization used a limit of detection of greater than 100 CFU per small intestine. The ID50 for oral infection of naïve 5- to 6-day-old BALB/c neonates returned to their dams postinfection and euthanized after 24 h was previously shown to be ca. 200 CFU for V. cholerae strain AC53 (71). Similarly, with groups of 5 to 7 neonates per input dose, the ID50s for V. cholerae strains O1 Inaba A1552 and O139 MO10 were determined. The percentage of mice infected versus input dose was plotted, and the ID50 was estimated from this curve by nonlinear regression using the Hill equation, as described previously (57) (data not shown).
OMV immunization and neonatal challenge with V. cholerae.
The majority of immunizations were i.n. or by oral gavage (orally) at days 0, 14, and 28, each with 25 μg of OMVs in PBS as described previously (71). Where mixtures of OMVs were tested, the total amount of OMVs was kept at 25 μg per immunization, split equally between each type of OMV. Control mice were cohoused with immunized mice and were sham immunized with PBS, except for those represented by data shown in Fig. 1 for which control mice were left unimmunized. Mice were mated at day 41, and 5- to 6-day-old neonates were challenged with V. cholerae. In one case a shorter immunization protocol was followed to see whether two doses (at days 0 and 14) are sufficient for protection with mating at day 22. A summary of the experimental timelines for the three- and two-dose protocols are shown in Fig. 1A and B. To follow the immune response, tail bleeds were carried out preimmunization (day −1) and prior to mating (days 39 to 40), and animals were terminally bled by cardiac puncture at the end of the experiment (days 80 to 99). Pups, 5 or 6 days old, from OMV- or sham-immunized mice, were orally infected with ca. 500× the ID50 for each strain (105 CFU for AC53, 3.5 × 104 CFU for A1552, and 7.4 × 105 CFU for MO10). Bacteria for infection were taken from an LB plate (with appropriate antibiotics) into LB broth, diluted to the appropriate density, and plated for verification of the number of input CFU. A total of 50 μl was inoculated into the stomach of neonatal mice using 1-mm-diameter tubing attached to a 1-ml syringe via a 30-gauge needle. Adult and neonatal mice were anesthetized by inhalation of isoflurane (2.5%) prior to all i.n., oral-gavage, or intragastric inoculations. Neonates were sacrificed by cervical dislocation 24 h postinfection in all cases except for that represented by data shown in Fig. 5, for which harvests were carried out just 2, 4, or 1 h postinfection. Small intestines were harvested (for data shown in Fig. 5, stomachs and large intestines were also harvested for viable counts), homogenized in 1 ml LB broth containing 16% glycerol, diluted in LB broth, and plated on LB agar with appropriate antibiotics to determine viable counts. In the majority of cases, duplicates, each of 10 μl, were plated for each dilution (limit of detection, 50 CFU), but in some cases (Fig. 1 and 5), 100 μl was plated (limit of detection, 10 CFU). After challenge, suckling mouse stomachs were collected and pooled for each litter. Stomach contents were used for analysis of milk antibody titers and motility assays.
FIG. 1.
V. cholerae OMV two-dose immunization and challenge. Immunization and challenge timeline using three doses (A) or two doses (B). (C) Anti-O1 Ogawa OMV ELISA probed with preimmune (day −1) and terminal serum (day 57) from mice immunized with O1 Ogawa OMVs using the two-dose protocol and anti-mouse IgG1 secondary antibody. Immunization route was either i.n. or oral as indicated. Each symbol represents serum from one mouse. *, P < 0.05 significantly above preimmune serum (Mann-Whitney U tests). (D) Small intestinal viable counts 24 h after challenge with ca. 105 O1 Ogawa V. cholerae CFU (exact input does were 0.7 to 0.9 ×105) for neonates born to unimmunized (control) mice or mice immunized i.n. (i.n. OMVs) or orally (oral OMVs) with O1 Ogawa OMVs. Each symbol represents one neonate. *, P < 0.05, significant protection compared to controls is provided by i.n., but not oral, OMV immunization using a two-dose immunization protocol (Kruskal-Wallis and post hoc Dunn's multiple comparison tests). Bars, medians; dotted line, limit of detection.
Preparation of blood, milk, and stool samples.
Neonatal mouse stomach contents and adult stool and blood samples from immune or control mice were used for enzyme-linked immunosorbent assays (ELISAs), Western blot analyses, and/or motility assays. These samples were collected and processed as described previously (70), with minor modifications. In summary, adult mouse blood samples were collected in K3-EDTA anticoagulant-coated tubes (Greiner Bio-One) and centrifuged at 3,000 × g for 15 min, and the serum supernatant was diluted 4-fold with PBS and stored at −80°C. Stomachs removed from pups after infection were snap-frozen and stored at −80°C. Upon thawing, stomachs were cut open, stomach tissue was removed, and the contents (curdled milk) were weighed. A total of 300 μl of extraction buffer (PBS plus 1× protease inhibitor cocktail, EDTA-free; Roche) was added per 100 mg of stomach contents, and samples were disrupted by homogenization. Milk homogenate was centrifuged at 13,000 × g for 5 min, and the cleared supernatant (hereinafter termed milk) was extracted from between the upper lipid and lower insoluble fractions and was stored at −80°C until use. Sodium azide was not added to milk and serum samples so that they could be used for live bacterial motility assays. Stool samples were dried, weighed, and suspended in 1 ml of extraction buffer per 100 mg of stool. Stool samples were vortexed for 15 min at room temperature, insoluble material was removed by centrifugation at 13,000 × g for 5 min, and the cleared supernatant was stored at −80°C until use. Bovine calf serum (10%) was added to stool and milk samples during their preparation for ELISA analysis, rather than during initial extraction (70).
ELISAs.
Anti-OMV ELISAs were carried out as described previously (71). The limits of detection were 0.5 μg ml−1 for serum, 0.1 μg ml−1 for milk, and 0.02 μg ml−1 for stool. ELISAs with whole-cell killed bacteria used V. cholerae that was taken from LB plates (with appropriate antibiotics), suspended in PBS, and centrifuged at 5,000 × g for 10 min. Bacterial pellets were resuspended in PBS and diluted to an optical density at 600 nm (OD600) of 5 in PBS, and a sample was further diluted and plated on LB agar plates (with appropriate antibiotics) for the enumeration of input CFU. Aliquots of V. cholerae in PBS at an OD600 of 5 were then either killed by heating to 56°C for 30 min or centrifuged again, as described above, and resuspended in 1% formalin in PBS and fixed for 2 h at 37°C. Fixed bacteria were washed twice in PBS before mixing 1:1 with heated bacteria to create the “whole-cell killed” (WCK) preparation used subsequently. WCK samples were plated neat on LB agar to check for sterility and stored at −20°C until use.
Saturating amounts of OMVs (5 μg ml−1), WCK V. cholerae (108 prekilling CFU ml−1), or LPS (7 μg ml−1) were used to coat ELISA plates. The level at which saturation occurs was determined using serial dilutions coated on to ELISA plates and probed with anti-O1 Ogawa rabbit serum (Dako) diluted 1 in 500 in PBS plus 10% fetal bovine serum (PBSF) and an anti-rabbit-alkaline phosphatase secondary antibody (Dako), diluted 1 in 4,000 in PBSF.
Adsorption of anti-OMV serum with LPS used a 1:50 dilution of each serum in PBSF mixed overnight at 4°C with LPS at a final concentration of 25 μg ml−1. Control nonadsorbed serum samples were treated identically, but without addition of LPS. Samples were ultracentrifuged at 100,000 × g for 5 h. Supernatants were tested for levels of IgG1 binding to OMVs or LPS by ELISA as described.
Vibriocidal antibody titer.
Vibriocidal antibody titers were carried out essentially as described previously (63), but with the addition of 2 mM MgCl2 to the saline used to make serum and complement/bacterium mixture dilutions (10). Terminal and preimmune serum samples were heat inactivated at 56°C for 30 min prior to dilution (from 1 in 20 to 1 in 2,560). Mid-log cultures (1-in-1,000 subculture from overnight cultures and 3 h growth at 37°C with shaking at 250 rpm in LB broth with appropriate antibiotics) of V. cholerae were washed in saline plus 2 mM MgCl2 and mixed with 10% guinea pig complement (EMD Chemicals) with bacteria at the following final concentrations: O1 Ogawa AC53, 5 × 107 CFU ml−1; O1 Inaba A1552, 2.5 × 107 CFU ml−1; and O139 MO10, 4 × 106 CFU ml−1. A total of 25 μl of bacteria/complement mix was added to 25 μl of each serum dilution or saline plus 2 mM MgCl2 alone for 1 h at 37°C with gentle (40-rpm) shaking. A total of 150 μl of brain heart infusion (BHI) (with appropriate antibiotics) was added, plates were incubated for 3 h at 37°C with 40-rpm shaking, and the OD600 was read. The reciprocal vibriocidal titer is the highest dilution factor achieving at least 50% killing compared to controls with saline plus 2 mM MgCl2.
Silver-stained polyacrylamide gels and Western blotting.
A total of 5 μg of OMVs were separated by SDS-PAGE using 4 to 12% polyacrylamide NuPAGE Bis-Tris gels and MES (morpholineethanesulfonic acid) SDS running buffer (Invitrogen). Silver staining was carried out with the SilverQuest staining kit according to the manufacturer's instructions (Invitrogen), or gels were transferred to nitrocellulose membranes, blocked, and probed with anti-OMV serum as described previously (71), except that 5% milk rather than 10% was used for blocking and sera were diluted to 0.5 μg ml−1 anti-OMV IgG1 (determined by ELISA against the same type of OMVs used for immunization).
Proteinase K treatment of OMVs.
Proteinase K treatment of OMVs prior to the ELISA was carried out as follows. O1 Ogawa OMVs, or bovine serum albumin (BSA) as a control, in PBS at 2.5 mg ml−1 were stored at −20°C (no heat control) or overnight at 55°C with or without 200 μg ml−1 proteinase K (Sigma), with or without 0.2% SDS, in a total of 30 μl. SDS was used to disrupt OMV membranes. Following digestion, protease inhibitor α-toluenesulfonyl fluoride, benzylsulfonyl fluoride, phenylmethylsulfonyl fluoride (PMSF; Sigma) from a 200 mM stock in ethanol was added to all samples at a final concentration of 5 mM. A total of 10 μl (25 μg of OMVs) of each sample was separated by SDS-PAGE and silver stained to visualize the degree of protein degradation. An aliquot of each sample was diluted 1 in 500, to give 5 μg ml−1 original OMV content, in PBS and coated onto ELISA plates for probing with anti-O1 Ogawa OMV serum samples as described above. No residual proteinase K activity was detected (data not shown).
Motility assay.
For motility assays, milk or serum was mixed with V. cholerae. Immune milk or serum was diluted to give equal concentrations of anti-OMV IgG1 (0.25, 1, or 4 μg ml−1 as stated) in each sample, which was determined by ELISA. Control milk or serum samples from sham-immunized mice was diluted by the lowest dilution factor needed for the immune serum or milk in the same experiment; to obtain 4 μg ml−1 IgG1, one anti-OMV immune serum sample was diluted 1.1-fold, which was the lowest dilution factor applied to the control sera in any experiment. V. cholerae was taken from an LB plate (with appropriate antibiotics) and resuspended in LB broth at an OD600 of 0.05. A total of 30 μl of bacterial suspension was mixed with 15 μl of milk, serum, or PBS alone in a 96-well plate. After a 10-min incubation at room temperature, 5 μl was placed on a slide under a coverslip and viewed with a 20× objective by dark-field microscopy. Images of bacteria swimming under dark-field microscopy were taken with a 400-ms exposure using a Nikon 80i microscope and NIS elements software. Motile bacteria appear as swirls and lines in these long-exposure images, whereas stationary bacteria appear as bright spots. The number of motile bacteria per field in each image was counted by eye.
Statistical analysis.
In the majority of cases, the data presented are not normally distributed, due to biological variation or samples below the limit of detection for the assay. Therefore, nonparametric tests were used for all data analysis. Comparisons between two categorical variables were made using the Mann-Whitney U test. Comparisons between multiple categorical variables were made using the Kruskal-Wallis test and post hoc Dunn's multiple comparisons. Finally, correlations between two continuous variables were tested using the Spearman rank correlation test. Prism 5 for Mac OS was used for all statistical analyses. A P value of <0.05 was considered significant.
RESULTS
Two doses of OMVs intranasally, but not orally, provide protection.
Oral or i.n. immunization of adult female mice with V. cholerae O1 Ogawa OMVs using three doses (days 0, 14, and 28) (Fig. 1A), each of 25 μg (by protein content), had proved immunogenic and provided protection from neonatal challenge with V. cholerae O1 Ogawa (71). The oral immunization route is suggested to mimic natural infection, which is protective for at least 3 years in volunteers (47). The ideal cholera vaccine would require as few doses as possible, in order to maximize compliance and minimize cost, and would be mucosally delivered. To this end, we tested whether O1 Ogawa OMVs were immunogenic and efficacious using just two doses administered either orally or i.n. (Fig. 1B).
IgG1 is the major isotype induced upon OMV immunization of BALB/c mice (71). ELISAs showed significant anti-Ogawa OMV IgG1 responses for mice i.n. immunized with two doses (day 57 bleeds), but no detectable anti-OMV response for orally immunized mice (Fig. 1C). After a two-dose i.n. immunization, terminal IgG1 titers were well within the range of those obtained with a three-dose immunization regimen. For example, compare the i.n. two-dose (median, 102 μg ml−1; interquartile range, 66 to 113 μg ml−1) (Fig. 1C) and the three-dose (median, 75 μg ml−1; interquartile range, 60 to 95 μg ml−1) (see Fig. 4A) anti-OMV IgG1 titers for anti-O1 Ogawa immune sera determined by O1 Ogawa OMV ELISA. Anti-OMV IgG1 responses were below the limit of detection in preimmune sera or unimmunized control mouse sera at any time point and at day 21 for OMV-immunized mice, regardless of the route of immunization (Fig. 1C and data not shown).
Significant protection was provided to neonates challenged with O1 Ogawa (at ca. 500× the ID50) by the two-dose immunization scheme administered i.n., but not when delivered orally (Fig. 1D), consistent with the IgG1 titers (Fig. 1C). In the i.n. immunized group, small intestine viable counts were near or below the limit of detection for 13 of 20 mice, while 7 had a burden of more than 1,000 CFU. These different outcomes did not cluster with one particular dam or one challenge infection. A spread in the numbers of CFU is often seen with the three-dose immunization protocol and the relatively high challenge dose used in this study; see, for instance, the O1 Ogawa challenge of neonates born to O1 Ogawa OMV i.n. immunized mice represented in Fig. 4A, for which 3 of 14 mice had more than 1,000 CFU in the small intestine. As milk is the mediator of protection (70), the least protected individuals may have been less well fed than their littermates.
There have been safety issues raised regarding i.n. immunization, in particular risks of Bell's palsy with an enterotoxin adjuvant (56), whereas oral immunization has an excellent safety record. For this reason we wished to include the oral immunization route for continued characterization of the V. cholerae OMV vaccine. Therefore, despite achieving significant protection with a simplified two-dose i.n. immunization, for subsequent immunizations we continued to use the three-dose protocol (Fig. 1A), which is known to be protective via both oral and i.n. routes (71).
OMV immunization induces responses to conserved protein antigens.
Proteins in O1 Ogawa OMVs are recognized with anti-O1 Ogawa OMV-immunized mouse sera using Western blotting (71), but are these protein antigens conserved and detected in non-O1 Ogawa OMVs? OMVs were prepared from O1 Ogawa, O1 Inaba, O139, and an acapsular O139 mutant with a plasmid insertion in the otnA (also known as wbfF) gene, which codes for a transporter of capsule precursors (59). To our knowledge, this is the first time that OMVs have been isolated from an encapsulated V. cholerae serogroup. OMV yields were similar, judged by protein content using a Lowry assay, for each strain. OMVs were separated by SDS-PAGE, and gels were either silver-stained or transferred to nitrocellulose for Western blotting with serum from mice i.n. immunized with O1 Ogawa OMVs. O1 LPS is composed of 15 to 18 repeating units of the disaccharide perosamine (20, 73). As mentioned above, O1 Ogawa O antigen differs from O1 Inaba only by the presence of a methyl group (37). In contrast, O139 terminal O antigen is composed of a single hexasaccharide unit resulting in a short LPS (38). Silver staining of the different OMVs showed a range of proteins in each preparation and LPS profiles characteristic of each serogroup (Fig. 2A). The presence of a capsule in O139-derived OMVs, but not OMVs from the acapsular otnA mutant, was confirmed by Western blotting with anti-O139 OMV mouse serum (data not shown).
FIG. 2.
OMV immunization elicits responses to conserved proteins, but proteinase K-resistant antigens are dominant. A total 5 μg of OMVs derived from V. cholerae O1 Ogawa strain AC53 (Og), O1 Inaba strain A1552 (In), O139 strain MO10 (O139) or otnA acapsular O139 mutant (otnA) were separated by SDS-PAGE (NuPAGE Bis-Tris 4 to 12% gels) and either silver stained (A) or transferred to nitrocellulose and probed with immune serum (day 99 bleeds) from mice i.n. immunized with O1 Ogawa OMVs (B). (C and D) Twenty-five-milligram samples of O1 Ogawa OMVs were kept frozen (lane 1) or incubated overnight (o/n) at 55°C alone (lane 2) or in the presence of 0.2% SDS (lane 3), 200 μg ml−1 proteinase K (lane 4), or 0.2% SDS and 200 μg ml−1 proteinase K (lane 5). (C) The OMVs were separated by SDS-PAGE and silver stained. (D) After each treatment, OMVs were diluted to 5 μg ml−1 and used to coat ELISA plates that were probed for binding of anti-O1 Ogawa OMV (day 38) serum samples and anti-IgG1 secondary antibody. Each symbol represents serum from one O1 Ogawa OMV-immunized mouse. Bars = medians. In panels A to C, arrows show the positions of the lipid A-plus-core polysaccharide LPS precursor (A-core) and full-length O1 or O139 LPS molecules after the addition of their terminal O antigens (A and C) and two conserved OMV protein antigens (Conserved) and anti-O1 LPS signal detected by Western blotting (B).
Anti-O1 Ogawa OMV mouse serum recognized two conserved antigens at around 90 kDa and 52 kDa (Fig. 2B). The inclusion of 0.2% Triton in the Western blotting wash buffer inhibited the majority of anti-LPS signal, allowing selective viewing of protein responses. Preimmune sera and sera from sham-immunized control mice had no visible anti-OMV protein responses, consistent with a lack of anti-OMV Ig detectable by ELISA (data not shown). Sera from different individual immunized mice from one OMV immunization and mice immunized with different batches of O1 Ogawa OMVs or bleeds from the same mice at different time points after immunization were all tested against OMVs from different O-antigen types and in some cases from different OMV batches (data not shown). Although there was variation between the exact antigens visible in each blot, this variation could not be clearly attributed to one of the above variables more than another. In every case, the very strong (ca. 52-kDa) band was visible, along with one or more other antigenic proteins, whereas the signal at ca. 90 kDa was not always detectable. No reproducible serotype-specific differences in protein antigens were seen, consistent with the very similar genomes between O1 El Tor and O139 outside the LPS locus (43).
Candidate protein antigens were specifically ruled out as major OMV antigens by Western blotting of OMVs prepared from the Bah-2 (ΔCTXφ), AC3035 (pGPflaA), ΔflrA, ΔompU, ΔompA, and AC2915 (ΔhlyA) strains, described in Table 1, with anti-O1 Ogawa serum. These strains do not express cholera toxin A and B subunits (deleted for the entire CTXφ carrying the toxin genes), major flagellin FlaA, all flagellins (FlaA to -E, whose expression was disrupted by deletion of the master regulator flrA), outer membrane protein OmpU or OmpA, or hemolysin HlyA (data not shown). The identity of the major conserved 52-kDa protein antigen and whether it can provide any degree of OMV-mediated protection are currently under investigation.
LPS is the major OMV antigen.
Although we observed an antibody response to proteins in different OMVs by Western blotting (Fig. 2B), a number of lines of evidence suggest that LPS, rather than conserved proteins, is the dominant OMV antigen. First, a comparison of responses to purified O1 Ogawa LPS and O1 Ogawa OMVs by ELISA revealed that the vast majority of anti-O1 Ogawa OMV mouse antibodies recognize pure LPS and OMVs to similar extents (Table 2). This was true of IgG1 in serum and milk samples and IgA in serum and stool samples; in all cases there were highly significant strong positive correlations between O1 Ogawa OMV and LPS responses and almost a 1:1 relationship by linear regression (Table 2).
TABLE 2.
OMV and LPS ELISAs for antibodies from mice i.n. immunized with O1 Ogawa OMVs and results of LPS adsorption
| Sample, Igb | No. of samples | ELISA with OMV or LPS substrates (Ig mg ml−1 [serum] or mg g−1 [milk or stool]), median (interquartile range) |
rd,i | Linear regression (R2); slope (95% CI)f | |
|---|---|---|---|---|---|
| O1 Ogawa OMV | O1 Ogawa LPS | ||||
| Serum, IgG1a | 8 | 85.8 (45.0-250.6) | 137.5 (70.1-299.5)c | 1.000* | 0.986; 1.21 (1.07-1.36) |
| Milk, IgG1 | 8 | 8.2 (1.5-15.0) | 11.14 (3.0-20.1)c | 1.000* | 0.986; 1.36 (1.20-1.52) |
| Serum, IgAa | 6 | 3.3 (2.461-4.4) | 2.6 (2.3-4.2)c | 0.829e | 0.968; 1.24 (0.92-1.56) |
| Stool, IgA | 6 | 1.2 (0.2-2.0) | 1.4 (0.3-2.3)c | 0.886* | 0.998; 1.24 (0.93-1.56) |
| Serum, IgG1 unadsorbed | 3 | 107.6 (119.4-96.5) | 107.2 (125.7-107.1)c | ||
| Serum, IgG1 LPS adsorbedg | 3 | 0.8 (1.4-0.7) | Below detectionh | ||
Terminal bleed serum samples were tested.
Immunoglobulin tested using isotype-specific secondary antibodies.
No significant difference compared to OMV ELISA(Mann-Whitney U test).
r, Spearman correlation coefficient.
P = 0.058, not quite significant with Spearman rank correlation test.
95% CI, 95% confidence interval.
Overnight adsorption of 1-in-50 diluted serum with 25 μg ml−1 LPS.
Limit of detection, 0.5 μg ml−1 IgG1.
*, P < 0.05, Spearman rank correlation test.
Storage of OMVs at 37°C for up to 60 days (see Fig. S1 in the supplemental material) or treatment of OMVs with proteinase K (Fig. 2C and D) were used to further assess the stability of OMV antigens and the relative contributions of proteins and LPS as OMV antigens. Prolonged heating at 37°C resulted in some protein degradation as assessed by silver staining (see Fig. S1A in the supplemental material). A previous heat treatment of OMVs at 37°C for 1 month was not seen to reduce the protein content on Imperial blue (Pierce)-stained gels, (18, 71), whereas here we did observe a reduction in protein content after 30 days at 37°C but little further reduction at 60 days (see Fig. S1A). After prolonged heating of OMVs at 37°C, despite protein loss (see Fig. S1A), the heated OMVs bound similarly to anti-OMV antibodies by ELISA (see Fig. S1B). This suggests that prolonged heating of OMVs did not significantly reduce their antigenicity. Incubation at 55°C overnight had very little effect on OMV protein composition (Fig. 2C, compare lanes 1 and 2).
Proteinase K treatment of OMVs greatly reduced their protein content (Fig. 2C, compare lanes 2 and 4). However, unlike proteinase K treatment of a soluble protein substrate (bovine serum albumin [data not shown]), OMV proteins were not fully degraded by proteinase K (Fig. 2C, compare lanes 2 and 4). Upon addition of 0.2% SDS to disrupt OMV membranes, proteinase K was able to destroy all detectable proteins, leaving only the LPS signal by silver staining (Fig. 2C, lane 5). Alone, 0.2% SDS did not cause significant protein degradation (Fig. 2C, compare lanes 2 and 3). By coating treated OMVs on ELISA plates and probing with anti-O1 Ogawa serum, we found no effect on antigenicity of OMVs from either the addition of SDS alone (Fig. 2Dii, compare conditions 2 and 3) or proteinase K treatment without SDS (Fig. 2Di, compare conditions 2 and 4), which destroyed a subset of proteins (Fig. 2C, compare lanes 2 and 4). Even complete destruction of proteins in OMVs by proteinase K plus SDS caused only a small, statistically insignificant (analysis of variance [ANOVA] and Tukey's correction for multiple comparisons) reduction in binding of anti-OMV antibodies (Fig. 2Dii, compare conditions 3 and 5). Adsorption of anti-O1 Ogawa OMV serum revealed that the magnitude of LPS-independent anti-OMV IgG1 response was 0.6 to 1.3% of the LPS response (Table 2). These data are consistent with a heat- and proteinase K-resistant component, namely, LPS, being the dominant OMV antigen, with only a small contribution from proteinase K-sensitive antigens.
Much is known about the antigenicity of V. cholerae O1 LPS. The 2-O-methyl group in the nonreducing terminal sugar of the Ogawa O antigen forms an Ogawa-specific B antigen around which antibodies can specifically bind (81), while an antigen dominant in the nonmethylated Inaba LPS, but also detectable for Ogawa, is termed the C antigen. Inaba and Ogawa also have a common LPS antigen formed by a combination of the core and O-antigen polysaccharides, termed the A antigen (80). To further explore the LPS dominance and potential cross-protection of OMV antigens, OMVs prepared from O1 Ogawa, O1 Inaba, or O139 were probed by ELISA for binding to anti-O1 Ogawa OMV antibodies from i.n. or orally immunized mice. We observed that all 9 i.n. immunized and 2 of 4 orally immunized mice had detectable O1 Ogawa and O1 Inaba OMV responses by ELISA, although the overall response to O1 Inaba OMVs was significantly lower than that to O1 Ogawa OMVs (Fig. 3A). Two orally immunized mice appear to have mounted a very strong O1 Ogawa-specific response, but the O1 Inaba responses were at levels below detection by ELISA, despite using a genetically homogenous inbred strain of mice. Only 2 out of 13 mice immunized with O1 Ogawa OMVs (9 i.n. and 4 orally) showed any anti-O139 OMV cross-reactive IgG1 response, the remainder being below the limit of detection by ELISA (Fig. 3A). These data are consistent with the Ogawa-specific LPS B antigen being dominant upon O1 Ogawa OMV immunization. The finding that O139 OMVs were very poorly recognized by anti-O1 Ogawa OMV antibodies by ELISA (Fig. 3A) lends further support to the notion that potentially cross-protective conserved protein antigens in OMVs are not immuno-dominant. Instead, the sum of the data so far indicates that LPS is the major O1 Ogawa OMV antigen recognized by i.n. or orally immunized mice.
FIG. 3.
Antibody responses after immunization with O1 Ogawa, O1 Inaba, or O139 OMVs. Terminal bleed serum samples from mice immunized with O1 Ogawa (A), O1 Inaba (B), a mixture of O1 Ogawa + Inaba (C), or O139 (D) OMVs by the i.n. or oral route, as indicated, were tested for binding to O1 Ogawa (Og), O1 Inaba (In), or O139 OMV-coated ELISA plates, as indicated, and anti-IgG1 secondary antibody. In panels A to C, *, P < 0.05, significant differences in binding between O1 Ogawa and Inaba OMVs (Kruskal-Wallis and posthoc Dunn's multiple comparison tests). n/s, not significant. (D) *, P < 0.05, binding of anti-O139 OMV serum was significantly greater for O139 OMVs than either O1 Inaba or O1 Ogawa OMVs (Kruskal-Wallis and post hoc Dunn's multiple comparison tests). Each symbol represents serum from one mouse. Bars, medians; dotted line, limit of detection.
Immunization with different types of V. cholerae OMVs induces LPS-dominant responses.
All previous V. cholerae OMV immunizations had been carried out using O1 Ogawa OMVs (71). In order to study protection from different epidemic cholera strains, we immunized mice i.n. or orally with O1 Ogawa, O1 Inaba, O1 Inaba, and O1 Ogawa mixed 1:1 or O139 OMVs (only the i.n. immunization was tested for O139 OMVs). The mixture of O1 Ogawa + Inaba OMVs was tested because O1 Ogawa OMV-immunized mice mounted an O1 Ogawa serogroup-biased response (Fig. 3A), and we hypothesized that an O1 Inaba + O1 Ogawa OMV mixture may generate more robust O1 cross-serotype responses.
ELISAs were used to determine the immunogenicity of each type of OMV by testing serum IgG1 titers against plates coated with the same OMVs immunized into mice or other types of OMVs to determine homologous and heterologous OMV antigen antibody responses. All OMVs were similarly immunogenic by ELISA against the same type of OMVs used for the immunization (Fig. 3A to D).
In general, oral immunization resulted in a less robust response compared to i.n.; in particular, 2 of 4 O1 Inaba OMV-orally immunized mice had undetectable or barely detectable IgG1 responses to O1 Inaba OMVs (Fig. 3B). A previous comparison, with three doses, found IgG1 titers (at day 78) after i.n. O1 Ogawa OMV immunization slightly higher than after oral O1 Ogawa OMV immunization, although neonatal protection from O1 Ogawa challenge (challenge doses from 1,000 to 10,000 CFU) was complete in both cases (70). The two-dose immunization and challenge data above strongly suggest that oral immunization is less robust than i.n. (Fig. 1) and also that higher doses of whole-cell killed V. cholerae are required for the doses to be immunogenic when delivered orally compared to i.n. (60).
As discussed above, immunization with O1 Ogawa OMVs induced antibodies that also bound to O1 Inaba OMVs in the majority of, but not all, individuals (Fig. 3A). Similar results were seen after O1 Inaba OMV immunization; antibodies from 6 of 7 i.n. immunized mice recognized both O1 Inaba and Ogawa OMVs by ELISA (Fig. 3B). In addition, as seen for O1 Ogawa OMV immunization (Fig. 3A), the IgG1 ELISA response to O1 Inaba OMVs, used for immunization, was significantly higher than the response to OMVs from the other O1 serotype (Fig. 3B). Immunization with a mixture of O1 Ogawa and O1 Inaba OMVs induced full and equal antibody responses to both O1 serotypes (Fig. 3C).
The ELISA response to O139 OMVs for serum from O1 OMV-immunized mice was below the limit of detection for 32 of 35 serum samples tested. In the 3 mice that had detectable binding, the titer was below 10 μg ml−1 IgG1 (Fig. 3A to C). In contrast, serum from O139 OMV-immunized mice showed strong IgG1 responses to O139 OMVs but poor responses to O1 OMVs, by ELISA (Fig. 3D). Anti-O139 OMV serum IgG1 also bound equally as well to OMVs derived from an acapsular O139 otnA mutant as to wild-type O139 OMVs (see Fig. S2A in the supplemental material). In addition, in a separate experiment, we found that serum from mice i.n. immunized with acapsular otnA mutant OMVs showed an IgG1 response to O139 and otnA mutant OMVs by ELISA that was not significantly different from that of mice immunized with wild-type O139 OMVs (see Fig. S2). These data indicate that the O139 capsule does not affect O139 OMV antigenicity.
In summary, after immunization with different OMV types, serum ELISA results with different OMV substrates suggest an LPS antigen-dominant response (Fig. 3), although responses to conserved proteins are detectable by Western blotting (Fig. 2B and data not shown). By mixing together O1 Inaba and Ogawa, a more consistent cross-serotype response was generated compared to those for OMVs from each serotype alone. There was little evidence by ELISA of significant cross-reacting antigens shared by O1 and O139 OMVs, although a higher proportion of O139 OMV-immunized mice produced antibodies with detectable O1 OMV binding than vice versa (Fig. 3).
LPS is the major protective antigen in OMVs.
To directly address the impact of LPS-dominant OMV antigen responses on the protection of mice from V. cholerae challenge, the mice immunized with either O1 Ogawa, O1 Inaba, O1 Ogawa + Inaba, or O139 OMVs were mated and their neonates were challenged with three different V. cholerae wild-type strains: O1 Ogawa AC53, O1 Inaba A1552, and O139 MO10. All challenge experiments were carried out with approximately 500× the ID50. We note that this is a higher challenge dose than was used in previous OMV vaccine studies (71). For AC53, the ID50 was previously determined to be ca. 200 CFU (71). Here we determined the ID50 for A1552 to be ca. 69 CFU (95% confidence interval, 8 to 146 CFU; coefficient of correlation [R2] = 0.83) and for MO10 to be ca. 1,340 CFU (95% confidence interval, 257 to 2,685; R2 = 0.87).
Significant protection from O1 Ogawa challenge was achieved by immunization via the i.n. route with O1 Ogawa, O1 Inaba, or a mixture of O1 Ogawa and Inaba OMVs (Fig. 4A). Oral immunization resulted in significant protection from O1 Ogawa challenge after immunization with O1 Ogawa OMVs or an O1 Ogawa + Inaba OMV mix and reduced burden after O1 Inaba OMV immunization, but this was not statistically significant compared to results for the orally sham-immunized control group (Fig. 4A). Analogous results were obtained with O1 Inaba challenge: i.n. immunization with O1 Inaba, O1 Ogawa, or an O1 Ogawa + Inaba mix of OMVs provided robust protection, as did oral immunization with Inaba OMVs or an O1 Inaba + Ogawa OMV mix, while oral immunization with O1 Ogawa OMVs reduced the O1 Inaba burden, but not to levels that were statistically significantly below that of the control group (Fig. 4B). Thus, homologous O1 serotype OMV immunization, alone or in a mixture, provided the most robust protection from challenge using the oral immunization route, while i.n. immunization with either O1 serotype of OMVs, alone or together, protected from O1 challenge from either O1 serotype challenge.
FIG. 4.
Homologous and heterologous V. cholerae challenge of neonates born to mice immunized with O1 or O139 OMVs. Mice were immunized by i.n. or oral route with either O1 Ogawa (Og), O1 Inaba (In), a mixture of O1 Ogawa + Inaba (Og + In), or O139 OMVs, as indicated. Control mice were sham-immunized with PBS by i.n. or oral route. An immunization protocol with three doses and 25 μg of OMVs per dose was used. Mice were mated, and their neonates were challenged at ca. 500× the ID50 with O1 Ogawa AC53 (A), O1 Inaba A1552 (B), or O139 MO10 (C). Exact input doses were as follows: O1 Ogawa, 6.7 ×104 to 2.1 ×105 CFU; O1 Inaba, 2.6 to 9.7 ×104 CFU; and O139, 3.5 to 8.1 ×105 CFU. Each symbol represents small-intestinal viable counts for one neonate 24 h postinfection. *, P < 0.05, compared to controls sham immunized via the same route (Kruskal-Wallis and post hoc Dunn's multiple comparison tests). Bars, medians; dotted line, limit of detection.
Intranasal immunization with O139 OMVs did not protect from O1 Ogawa (Fig. 4A) or O1 Inaba (Fig. 4B) challenge, despite some low, but detectable, O1 OMV binding from anti-O139 OMV sera by ELISA (Fig. 3D). Immunization with O139 OMVs did, however, significantly reduce colonization upon challenge with O139 (Fig. 4C). Intranasal immunization with O1 OMVs in any combination was unable to protect from O139 challenge (Fig. 4C), consistent with very poor anti-O139 OMV responses by ELISA from O1 OMV-immunized mice (Fig. 3A to C). In a separate experiment it was found that i.n. immunization with otnA mutant acapsular O139 OMVs was also protective against wild-type O139 challenge (see Fig. S2Bi in the supplemental material). Neither O139 nor otnA mutant OMVs protected from O1 Ogawa challenge (Fig. 4A and B; see also Fig. S2). Thus, the presence or absence of capsule on O139 OMVs does not significantly change the ability of O139 OMV immunization to protect from O139 challenge.
Overall, these data indicate that LPS dominates the response to OMVs in terms of antigenicity (Table 2 and Fig. 2), immunogenicity (Fig. 3), and protection (Fig. 4).
Heat-treated OMVs are immunogenic and protective.
Although the antigenicity of OMVs is not significantly compromised after prolonged heating (see Fig. S1B in the supplemental material), despite some protein loss (see Fig. S1A), the immunogenicity of heated OMVs could be compromised. We tested this possibility by using the samples described in the legend to Fig. 1A stored at −20°C or at 37°C for 60 days for i.n. immunization of mice. An IgG1 response to O1 Ogawa OMVs above that of sham-immunized controls was induced regardless of storage conditions (see Fig. S1C). The response to immunization with heated OMVs was slightly reduced compared to the response to the same OMV solution stored frozen, but the difference was not statistically significant (see Fig. S1C). We were surprised to observe low antibody titers regardless of whether the 60-day storage was at −20°C or at 37°C. This could be due to a reduction in the immunogenicity of the OMVs caused by extended storage after dilution to 2.5 μg ml−1 for the heat treatment and/or multiple freeze-thaw cycles for the running of gels and immunizations. Despite this relatively low response, significant protection was observed upon O1 Ogawa challenge regardless of whether or not the OMVs had been heated for 60 days prior to immunization (see Fig. S1Di). After prolonged storage of O1 Ogawa OMVs, regardless of the 60-day storage temperature, responses to O1 Inaba OMVs by ELISA were significantly lower than responses to O1 Ogawa (see Fig. S1C). Consequently, these mice were not significantly protected from O1 Inaba challenge (see Fig. S1Dii). These data are consistent with the significantly lower cross-serotype responses induced by O1 OMVs (Fig. 3A and B).
Heating, therefore, does not significantly reduce protection afforded by O1 Ogawa OMVs administered i.n. upon O1 Ogawa challenge (see Fig. S1Di in the supplemental material), despite loss of some proteins (see Fig. S1A), but we hypothesize that improper storage of OMVs may reduce their immunogenicity (see Fig. S1C), resulting in poor cross-serotype antibody responses and lack of cross-serotype protection (see Fig. S1C and Dii).
Cholera toxin is not required for OMV adjuvanticity and protection.
Cholera toxin (CTX) is composed of a B-pentamer that binds to GM1 gangliosides and a catalytic A subunit and is the cause of the life-threatening watery diarrhea that is characteristic of cholera (69). Cholera toxin, in native form and to a lesser degree the B subunit alone, is reported to have mucosal adjuvant properties, which could potentially play a role in OMV-mediated immunity (69). However, the OMVs used in this study are all made from LB-grown V. cholerae O1 El Tor or O139 strains, which do not make significant levels of CTX under these conditions (42), and unlike the closely related E. coli heat-labile enterotoxin (40), there is no evidence for CTX tethering to OMVs. Thus, it is unlikely that CTX is present in the OMVs used in this study to play any role in their adjuvanticity. Even so, in order to be certain whether there is any role for CTX in OMV-mediated protection, we prepared OMVs from an O1 Ogawa AC53-derived strain (Bah-2) from which the entire CTX phage has been deleted, thereby removing the cholera toxin genes, ctxAB, zonula occludens toxin, and accessory cholera enterotoxin (77). ΔCTXφ mutant OMVs were used for i.n. immunization of mice followed by neonatal challenge with wild-type AC53. Mice immunized with ΔCTXφ mutant OMVs raised an IgG1 response similar to those of mice immunized with wild-type OMVs (see Fig. S3A in the supplemental material). ΔCTXφ mutant OMV-immunized mice were also equally protected from wild-type O1 Ogawa or O1 Inaba challenge compared to mice immunized with wild-type OMVs (see Fig. S3B).
Immune protection of neonates occurs by blocking initial colonization without detectable bacterial killing.
Initial investigations into the efficacy of V. cholerae OMV immunization all involved harvesting of small intestines from infected neonates 24 h postinfection, at which time point very few, if any, viable bacteria were present (Fig. 4) (70, 71). Here we investigate early events by asking if, upon challenge of neonates suckled by OMV-immunized mice, the infecting bacteria get killed or remain viable but are unable to colonize the small intestine. The small intestine is the site of V. cholerae colonization in both humans and neonatal mice. Viable V. cholerae organisms in the large intestine of infected neonatal mice represent a population of bacteria that are in the process of being excreted; we note that collection of excreted fecal matter from neonates has not proved feasible.
We proceeded to test for viable counts in the small and large intestines of neonates born to sham- or O1 Ogawa OMV-immunized mice 2 or 4 h after infection. The spatial and temporal colonization dynamics of El Tor strain N16961 (hapR mutant) in CD-1 neonates led us to expect very few bacteria in the large intestine 2 h postinfection (7). However, for BALB/c mice infected with El Tor strain AC53, many bacteria had already reached the large intestine at this early time point (Fig. 5A). Small-intestinal viable counts were significantly lower for pups born to OMV-immunized dams than for controls as early as 2 h and 4 h postinfection (Fig. 5A), showing that prevention of colonization is occurring rapidly after infection. In contrast, viable counts in the large intestines were similar for neonates born to OMV- or sham-immunized mice (Fig. 5A). To test whether early killing of bacteria was occurring in challenged neonates born to OMV-immunized dams, we looked at viable counts 1 h postinfection in the stomach and small and large intestines. We noted that the number of bacteria in the large intestine 1 h and 2 h after infection was significantly lower for pups born to immunized mice than that for sham-immunized controls (Fig. 5A and B), suggesting a passage through the mouse at early time points in neonates from immunized dams slightly slower that that for controls. However, most importantly, the overall viable counts 1 h postinfection were no different between pups from OMV- and sham-immunized control dams (Fig. 5B), suggesting that bacterial killing did not play a significant role in protection from colonization in this model.
FIG. 5.
Spatial and temporal distribution of V. cholerae viable counts in neonates from OMV-immunized and control mice. (A) Viable counts for V. cholerae in the small intestine (SI) or large intestine (LI) 2 or 4 h postinfection as indicated for neonates born to PBS control-immunized (closed symbols) or O1 Ogawa OMV-immunized (open symbols) dams. (B) Viable V. cholerae counts in the stomach (ST), SI, or LI or total counts 1 h postinfection for neonates born to sham-immunized (Control, closed symbols) or O1 Ogawa OMV-immunized (OMV, open symbols) immunized dams. *, P < 0.05; +, P = 0.042, Mann-Whitney U tests, control compared with OMV-immunized mice. Each symbol represents one neonate. Bars, medians; dotted line, limit of detection.
In summary, although V. cholerae-infected neonates born to OMV-immunized mice are rapidly protected from colonization by antibodies in milk, viable counts at early times postinfection strongly suggest that bacterial killing is not a major protective mechanism and that live bacteria are excreted upon challenge of neonates born to OMV-immunized dams, i.e., the immunity is not sterilizing in this model. The impact of this finding upon the ability of the OMV vaccine to block V. cholerae transmission is currently being investigated (A. L. Bishop and A. Camilli, unpublished data).
Inhibition of V. cholerae motility in vitro by antibodies from OMV-immunized mice parallels in vivo protection.
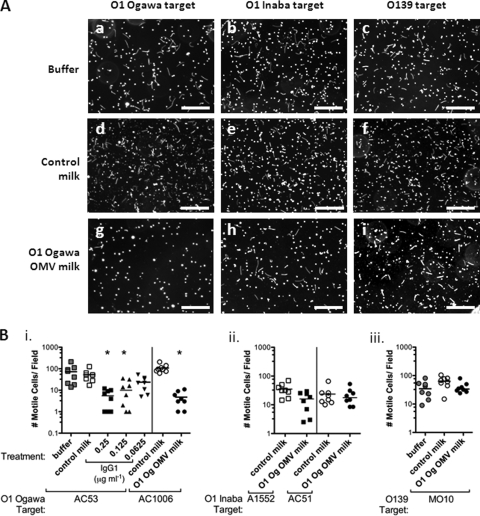
V. cholerae El Tor requires motility to facilitate colonization of the infant mouse small intestine, most definitively shown with competitions between wild-type V. cholerae and flagellate but nonmotile mot gene mutants (17, 46). We hypothesized that inhibition of motility and clumping/agglutination could be mechanisms by which antibodies in milk from OMV-immunized dams inhibit colonization of the small intestine. We tested this hypothesis in vitro using a motility assay in which milk, extracted from the stomachs of neonates suckled by i.n. OMV-immunized or sham-immunized control mice, was mixed 1:2 with in vitro-grown V. cholerae. Immune milk samples were diluted to contain the same amounts of anti-OMV IgG1, 0.25 μg ml−1 unless stated otherwise, while control milk was diluted by the lowest dilution factor required for the immune milk. After 10 min of exposure to milk extract, a sample was examined by dark-field microscopy using a long exposure (400 ms). Motile bacteria appear as lines and swirls, whereas stationary bacteria appear as spots (examples are shown in Fig. 6A). The number of motile bacteria per field in each image was quantified.
FIG. 6.
Milk from O1 Ogawa OMV-immunized mice specifically inhibits O1 Ogawa motility. Buffer alone or milk from neonates born to i.n. sham-immunized (control milk) or O1 Ogawa OMV-immunized mice (O1 Og OMV milk) was mixed 1:2 with V. cholerae (OD600 = 0.05) in LB. After 10 min, bacterial motility was observed by dark-field microscopy using a 400-ms exposure. The number of motile bacteria per field, which appear as lines or swirls, were counted. (A) Examples of images showing O1 Ogawa AC53 (a, d, and g), O1 Inaba A1552 (b, e, and h), or O139 MO10 (c, f, and i) targets mixed with buffer alone (a, b, and c), control milk (d, e, and f), or O1 Ogawa OMV-immunized mouse milk (g, h, and i). Scale bars equal 100 μm. (B) Quantification of motility in the presence of buffer, control milk, or O1 Ogawa OMV-immune milk, as indicated, for O1 Ogawa strains AC53 or AC1006 (i); O1 Inaba strains A1552 or AC51 (ii); O139 strain MO10 (iii). (Bi) For the O1 Ogawa AC53 target, different dilutions of immune milk were made to give 0.25, 0.125, or 0.0625 μg ml−1 of anti-O1 Ogawa (Og) OMV IgG1. In all other cases, immune milk was diluted to give 0.25 μg ml−1 IgG1, and control milk was diluted 1 in 1.6 (the lowest dilution used for immune milk). Each symbol represents milk from one mouse, or one buffer control, mixed with the indicated target bacterial suspension. *, P < 0.05, significant inhibition of motility compared to control milk (Kruskal-Wallis and Dunn's multiple comparison tests). Bars = medians.
Milk from control sham-immunized mice did not inhibit motility of O1 Ogawa, O1 Inaba, or O139 V. cholerae, compared to buffer alone (Fig. 6Bi and data not shown). Milk extract from O1 Ogawa OMV i.n. immunized mice inhibited O1 Ogawa motility, with two different O1 Ogawa target strains, in a dose-dependent manner (Fig. 6Bi). Using 10-min incubations, the major phenotype observed was retarded motility of individual bacteria (Fig. 6A). Longer exposure of V. cholerae O1 Ogawa to O1 Ogawa OMV immune milk extract (15 to 30 min) containing 0.25 μg ml−1 anti-O1 Ogawa IgG1 resulted in agglutination (data not shown). Milk extract from O1 Ogawa OMV-immunized mice did not significantly inhibit motility of two O1 Inaba target strains (Fig. 6Bii), O139 MO10 (Fig. 6Biii) and an O139 acapsular otnA mutant (data not shown). Lack of O139 motility inhibition is consistent with ELISA data showing little cross-reaction between O1 Ogawa serum and O139 OMVs (Fig. 3A). It was unexpected that motility of the O1 Inaba strains was not inhibited by anti-O1 Ogawa OMV milk antibodies (Fig. 6Bii), considering that the majority of serum samples from these same mice bound to O1 Inaba OMVs by ELISA, although at a lower level than O1 Ogawa OMVs (Fig. 3A), and provided protection from O1 Inaba challenge (Fig. 4B).
Milk extract from O1 Ogawa + Inaba OMV-immunized mice was also tested with immune samples diluted to 0.25 μg ml−1 anti-O1 Ogawa or O1 Inaba IgG1 (the ELISA result for the OMV type appropriate to the target was used to make dilutions), with O1 Ogawa (AC53), O1 Inaba (A1552), and O139 (MO10) motility targets. As for O1 Ogawa OMV immune milk, motility of O1 Ogawa was inhibited, but motility of O1 Inaba or O139 was not inhibited (data not shown). These results were surprising considering that milk from O1 Ogawa + Inaba OMV-immunized mice bound equally well to O1 Ogawa and O1 Inaba OMVs. A magnitude of IgG1 response similar to that seen for anti-O1 Inaba + O1 Ogawa OMV-immunized mouse milk against O1 OMVs by ELISA was also seen for O139 OMV-immunized mouse milk extract against O139 OMVs (data not shown), yet motility of O139 was not inhibited by 0.25 μg ml−1 anti-O139 IgG1 (data not shown).
As motility inhibition results did not follow the same pattern for O1 Inaba or O139 targets as protection observed in vivo (Fig. 4B and C), we hypothesized that either anti-OMV IgG1 is not a good marker for protection (motility assay dilutions were calculated based upon ELISAs for this dominant isotype), the concentration of antibodies in milk extract was not sufficient to inhibit motility, or motility was not in fact a significant contributor to protection. In relation to the first possibility, we considered that antibodies generated in response to OMVs may bind less well to whole bacteria than to the OMVs themselves. This would mean that anti-OMV ELISA responses were not as good a marker for protection as comparisons of ELISAs and protection data had suggested. Therefore, we tested whole-cell killed bacterium (a 1:1 mixture of formalin fixed and heat killed) binding by ELISA and saw levels of IgG1 binding slightly lower than those for OMVs, but the levels were similar between O1 Inaba and O1 Ogawa for O1 Inaba + O1 Ogawa OMV immune milk and were of a similar magnitude for anti-O139 WCK binding to O139 OMV immune milk (data not shown).
To address the second possibility, that antibody titers were too low in milk, we turned to the serum samples, which contain around 10-fold more of the same antibody isotypes found in milk (see Table 2, O1 Ogawa OMV serum and milk anti-O1 Ogawa ELISA data) (70). Immune sera from O1 Ogawa + Inaba OMV-i.n. immunized mice were diluted to 1 and 0.25 μg ml−1 anti-OMV IgG1 in PBS (using ELISA data to calculate each dilution as above) and tested with O1 Ogawa, O1 Inaba, and O139 motility targets. Sham-immunized control serum was diluted 4.4-fold, which was the lowest dilution needed for any of the immune serum samples to reach 1 μg ml−1. The least diluted solution of anti-O1 Ogawa + Inaba serum was also tested against O139, for which there was no significant binding by ELISA (Fig. 3C). O1 Ogawa + Inaba OMV-immunized serum samples significantly inhibited motility of O1 Ogawa and O1 Inaba, compared to control serum, although there was a large spread of results for the 11 serum samples tested (see Fig. S4 in the supplemental material). A total of 1 μg ml−1 of anti-OMV IgG1 significantly inhibited motility for two O1 Ogawa (see Fig. S4A and B) and two O1 Inaba (see Fig. S4C and D) targets, while at 0.25 μg ml−1, one O1 Ogawa strain (AC53; see Fig. S4A) and one O1 Inaba strain (AC51; see Fig. S4D) were significantly inhibited. These data suggest that the inhibition is more robust with 1 μg ml−1 than 0.25 μg ml−1 anti-OMV IgG1. A total of 0.25 μg ml−1 anti-OMV IgG1 was the maximum concentration that could be tested for milk samples in the motility assay, due to the low concentration of IgG1, which may explain the lack of motility inhibition for O1 Inaba strains with immune milk extracts from O1 Ogawa (Fig. 6Bii) or O1 Ogawa + Inaba (data not shown) OMV-immunized mice despite protection from O1 Inaba challenge (Fig. 4B).
O1 Ogawa or O1 Inaba OMV-immunized mouse serum ELISA responses to heterologous O1 serotype OMVs were 3.3- or 3.5-fold lower, respectively, than homologous responses (Fig. 3A and B). Consistent with the ELISA data, serum from O1 Ogawa OMV-immunized mice containing 1 or 4 μg ml−1 anti-O1 Ogawa IgG1 inhibited O1 Ogawa AC53 but not O1 Inaba A1552 motility (data not shown). Similarly, serum from O1 Inaba OMV-immunized mice inhibited O1 Inaba but not O1 Ogawa motility, with 0.25, 1, or 4 μg ml−1 anti-O1 Inaba OMV IgG1 (data not shown). O139 motility was not inhibited by serum containing 4 μg ml−1 anti-O1 Inaba or O1 Ogawa OMV IgG1 (data not shown).
Anti-O1 Ogawa + Inaba OMV serum did not inhibit motility of O139 with 1 μg ml−1 of anti-O1 OMV IgG1 (see Fig. S4E in the supplemental material), consistent with a lack of O139 cross-reacting antibodies (Fig. 3C). O139 OMV immune serum at 1 μg ml−1 was able to reduce O139 motility, although this was not quite statistically significant, but did not inhibit O139 motility at 0.25 μg ml−1 (Fig. 7A), consistent with the lack of inhibition of motility from immune milk samples also containing 0.25 μg ml−1 anti-O139 OMV IgG1 (data not shown). Increasing the amount of anti-O139 IgG1 in the motility assay to 4 μg ml−1 resulted in a statistically significant inhibition of O139 motility (Fig. 7A) but did not inhibit motility of O1 Ogawa (Fig. 7C).
FIG. 7.
O139 capsule increases the amount of antibody required to inhibit motility. Serum from control sham-immunized (diluted 1 in 1.1, the lowest dilution needed for the immune serum), O139 OMV-immunized, or O139 + O1 OMV-immunized mice at 0.25, 1, or 4 μg ml−1 of anti-OMV IgG1, as indicated, was mixed 1:2 with either wild-type O139 MO10 (A), acapsular O139 mutant MO10 otnA::pGP704 (B), or O1 Ogawa AC53 (C) targets each diluted in LB to an OD600 of 0.05. After 10 min, motility was observed by dark-field microscopy with a 400-ms exposure. Numbers of motile cells per field were counted. Each symbol represents motility of the indicated target bacterium in the presence of serum from one mouse. *, P < 0.05, significant inhibition of motility compared to control serum (Kruskal-Wallis and Dunn's multiple comparison tests). Bars, medians; dotted line, limit of detection.
With 0.25 to 1 μg ml−1 of anti-OMV IgG1, O139 seemed resistant to motility inhibition compared to O1. We considered the possibility that the O139 capsule, which is composed of the same sugars as O139 (20), is absorbing antibodies without inhibiting motility. Thus, the O139 capsule may increase the amount of antibody required to reach the LPS on the outer membrane and inhibit motility compared to O1. To test this hypothesis, we used the acapsular MO10 otnA mutant as a target in the motility assay. Motility of the acapsular strain was dramatically inhibited at 4 or 1 μg ml−1 of anti-O139 OMV IgG1, and even at 0.25 μg ml−1, motility was inhibited, although this did not reach statistical significance (Fig. 7B).
A drop in motility would occur if bacteria were being killed by immune milk or serum. Such killing would have to be specific antibody mediated, as control milk or serum did not inhibit motility compared to buffer alone (Fig. 6Bi and data not shown). Complement-mediated V. cholerae specific antibody-mediated killing by human serum has been used as a surrogate marker for protection from cholera for many years (13). The vibriocidal antibody assay requires the addition of guinea pig serum, as a source of exogenous complement, to a mixture of bacteria and test serum, which is diluted to gain the lowest titer at which killing occurs. After 1 h of incubation at 37°C, brain heart infusion (BHI) is added, which stops the complement reaction, and the bacteria are grown for a further 3 h to accentuate differences in the killed versus nonkilled populations (see, for instance, reference 63). It is unlikely that antibody-mediated killing is occurring in our assays because (i) no exogenous complement has been added, (ii) bacteria are mixed only with serum or milk for 10 min at room temperature, (iii) exposure to immune milk in vivo does not appear to kill the bacteria (Fig. 6), and (iv) human milk contains very little of the membrane attack complex components of the complement cascade needed to actually kill bacteria (61). Nevertheless, we addressed this possibility by examining bacterial viability in the presence of serum or milk antibodies. V. cholerae bacteria were mixed with milk or serum from immune or control mice at the maximal concentrations used for motility assays or buffer alone for 10 min (serum) or 30 min (milk) at room temperature. We then added BHI (serum) or LB broth (milk), diluted the bacteria further, and plated for viable counts. For serum samples the mixes were also incubated with shaking for 3 h at 37°C to further test for killing or for delayed growth using OD600 readings. No significant killing or reduction in growth was seen for V. cholerae in immune milk or serum compared to buffer alone, control milk, or control serum (data not shown).
In summary, our motility data suggest that inhibition of motility in vitro with 1 to 4 μg ml−1 of anti-OMV IgG1 antibody more closely follows patterns of in vivo protection than results obtained with lower antibody concentrations and implies that inhibition of motility contributes to protection in vivo. In addition, the capsule of O139 appears to be responsible for the higher antibody titer needed to inhibit O139 motility in this in vitro assay.
Combined O1 and O139 OMVs protect from both O1 and O139 challenge.
As we had not achieved cross protection between O1 and O139 using O1 or O139 OMVs alone, due to the LPS dominance of the anti-OMV response, we tested a 1:1:1 mixture of O1 Inaba, O1 Ogawa, and O139 OMVs i.n. for antibody responses to all three OMVs by ELISA and for protection from challenge with O1 and O139 strains. Immunizations were each performed with 25 μg of OMVs (8.3 μg of each LPS type), which previous data for O1 Ogawa OMVs suggest should be sufficient antigen for protection (70). Immunization i.n. was carried out with two different triple OMV mixes (containing different batches of each of the 3 types of OMVs), each with 5 mice. The pooled IgG1 antibody ELISA responses for the 10 mice are shown in Fig. 8A. Similar levels of IgG1 (Fig. 8Ai), IgA (Fig. 8Aii), and total Ig (Fig. 8Aiii) responses were seen with each type of OMV in the immunization. Vibriocidal antibody titers correlate, although not perfectly, with human protection from cholera; therefore, we determined vibriocidal antibody titers for our triple O1 Inaba + O1 Ogawa + O139 OMV- and sham-immunized mouse serum samples. Killing of O1 Ogawa, in the presence of guinea pig complement, was observed for all 10 immune serum samples (median reciprocal titer of 960), 9 of 10 also showed killing of O1 Inaba (median reciprocal titer of 640), and 8 of 10 immune serum samples killed O139 MO10 (median reciprocal titer of 300) (Fig. 8Aiv). Neither sera from sham-immunized mice nor preimmune sera were able to kill O1 V. cholerae (reciprocal titer <20) (data not shown). O139 MO10 was not killed by the lowest dilutions of anti-OMV immune serum (below 1 in 100) but was, in 8 of 10 cases, killed at 1 in 200 or higher dilutions, causing our effective limit of detection to be 100. The reason for this deviation from the linear range at the lowest dilutions is unknown but may be due to the presence of inhibitory factors in the serum.
FIG. 8.
Immunization with a mixture of O1 and O139 OMVs provides cross-serogroup protection. Mice were immunized i.n. with a 1:1:1 mixture of O1 Ogawa + O1 Inaba + O139 OMVs using 25 μg total OMVs per dose and a three-dose immunization. (A) Terminal bleeds from these mice show IgG1 (i), IgA (ii), and total Ig (iii) responses, measured by ELISA and vibriocidal antibody titers (iv) against O1 Ogawa (Og), O1 Inaba (In), or O139 OMVs (ELISAs) or live bacteria (vibriocidal assay), as indicated, that were similar for O1 Ogawa, Inaba, and O139. Each symbol represents serum from one immunized mouse. (B) Neonates born to these mice were protected from small intestinal colonization (viable counts), compared to control sham-immunized mice, upon challenge with O1 Ogawa AC53 (input 1.2 to 2.2 ×105) (i), O1 Inaba A1552 (input 5.9 to 6.9 ×104) (ii), or O139 (input 2.8 to 7.6 ×105) (iii). Each symbol represents one infected neonate born to control or O1 Ogawa + O1 Inaba + O139 OMV-immunized mice as indicated. *, P < 0.05, significantly lower viable counts compared to control (Mann-Whitney U test). Bars, medians; dotted line, limit of detection.
Neonates born to mice triple immunized with O1 Ogawa + O1 Inaba + O139 OMVs were significantly protected from challenge with O1 or O139 V. cholerae (Fig. 8B). These data suggest that the mixing of O1 and O139 OMVs provides multiple serotype protection.
Serum from mice immunized with a mixture of O1 Ogawa + O1 Inaba + O139 OMVs (excluding the two mice with the lowest antibody titers) (Fig. 8A) was also tested for inhibition of motility against O139 and O1 Ogawa targets. With wild-type O139 or acapsular O139 otnA mutant targets, anti-O1+ O139 OMV serum gave motility inhibition results very similar to those for anti-O139 serum (Fig. 7A and B). Serum from O1 + O139 OMV-immunized mice, unlike serum from mice immunized with O139 OMVs alone, dramatically inhibited O1 Ogawa motility (Fig. 7C).
DISCUSSION
The oral immunization route in mice appears to be less robust than the i.n. route; for instance, a 10-fold higher dose of WCK V. cholerae via the oral route is needed to gain a response similar to that for i.n. immunization (60). In previous experiments using a maternal protection cholera model, i.n. OMVs could be reduced to just 0.25 μg per dose and still remain immunogenic and protective (70). Also, initial comparisons between oral and i.n. delivery of OMVs using three 25-μg immunizations found the two delivery routes to be similarly immunogenic (slightly higher IgG1 titers for i.n. than oral at day 78) and protective (challenge doses of ca. 2,000 or 20,000 CFU) (71). However, when we tested oral and i.n. immunization with just two doses (25 μg per dose), we found that this provided no detectable antibody response and no protection after oral immunization but was both immunogenic and protective using i.n. delivery. A two-dose regimen, which is what is currently used for the Dukoral WCK-CTB cholera vaccine (for individuals over 6 years old), may therefore be sufficient for protection by OMVs, but the route of delivery may be crucial. It is also possible that a higher dose of OMVs administered orally may overcome the need for additional doses.
Although i.n. delivery of OMVs to mice seems to give a more robust protection than oral delivery, the safety of i.n. immunization was brought into question by human trials of an intranasal liposome-based influenza virus vaccine that resulted in a vaccine-related increased incidence of Bell's palsy, which is thought to have been caused by E. coli heat-labile toxin used as an adjuvant in the vaccine (56). In contrast, a live-attenuated intranasal influenza vaccine (FluMist) without any additional adjuvant is well tolerated and has no correlation with Bell's palsy (12). Experiments with mice have also brought the safety of any preparation containing cholera toxin delivered intranasally into question; GM1 binding can allow toxin and coadministered antigen access to olfactory nerves and cause signs of brain inflammation (79). Oral immunization has an excellent safety record for both WCK and live-attenuated V. cholerae vaccines, even in small children (see, for instance, references 1 and 62). Despite this, for oral immunization with WCK V. cholerae a hyper-cholera toxin-producing classical biotype strain was removed from the reformulated vaccine, and the WHO recommended monitoring of toxin levels (84). Inclusion of CTB in the WCK-CTB vaccine provides cross protection against enterotoxigenic E. coli (ETEC) strains expressing heat-labile toxin, whose B subunit is highly homologous, making it useful in preventing ETEC infections, particularly for travelers (25). However, CTB is not required for induction of immune responses to WCK V. cholerae in mice (unpublished data mentioned in reference 60) or for protection by WCK-CTB in humans (24). Consistent with this, we found that OMVs from a CTXφ mutant were just as immunogenic and protective as wild-type O1 Ogawa OMVs, via the i.n. route with a three-dose regimen, showing that CTX is not required as an adjuvant for successful V. cholerae OMV immunization. After the experiences with the influenza virus vaccine, including E. coli heat-labile toxin and the link to Bell's palsy, and based upon our observation that CTX is not needed for OMV immunogenicity, for safety purposes, CTX-negative strains will be pursued for future testing of V. cholerae OMV-based vaccines. Although CTX is not required for OMV adjuvanticity, delivery of antigens to generate an immune response should require an adjuvant. There are very few identified nonliving adjuvants that work on the mucosal surface by adequately providing the “I am foreign” and “I am dangerous” signals thought to be required to stimulate an immune response (32). It is intriguing that oral delivery of V. cholerae antigens in the form of OMVs (71), liposomes (protein and LPS antigens reconstituted into vesicles) (19), ghosts (bacteria from which the cytoplasm has been removed by a phage pore-forming toxin) (29), or WCK (24) appears to break mucosal oral tolerance and provides a natural mucosal adjuvanticity. The adjuvant properties of OMVs, and other mucosal delivery systems, such as those mentioned above, are the subject of many ongoing studies (51).
The OMV heating experiment presented here, which reduced protein content, suggests that the ability of OMVs to be delivered appropriately, their adjuvant properties, and their subsequent delivery to and activation of B cells to induce an antibody response are not significantly reduced by extended heating at 37°C. However, responses to both frozen and heated OMVs in this experiment were low compared to those for other immunizations, most likely because both solutions of OMVs were stored in PBS in diluted form and were freeze-thawed a number of times prior to immunization. Thus, some aspects of OMV integrity that were lost upon extended storage and repeated freeze-thaw, but not by heat treatment, do appear to be required for full immunogenicity, and such treatment of OMVs should be avoided. The heat stability of OMV antigens (administered i.n.) delivering protection from homologous challenge is encouraging for the possible use of OMVs as vaccines without the need for refrigeration.
Antibody responses to OMV protein antigens were detected by Western blotting. However, the following data are in support of LPS as the dominant protective antigen in V. cholerae OMVs. First, purified O1 Ogawa LPS is strongly recognized by anti-OMV serum. Second, depletion of proteins from OMVs with heat or proteinase K treatment did not significantly reduce their antigenicity in ELISAs, revealing that heat and proteinase K-stable components of OMVs, rather than proteins, are the major antigens. Third, for the majority of immunized mice there was a significant cross-reaction of anti-O1 Ogawa OMV serum with O1 Inaba OMVs, and vice versa, but very little binding to O139 OMVs. Similarly anti-O139 OMV sera did not bind well to O1 Inaba or Ogawa OMVs by ELISA. Finally, antibody inhibition of motility in vitro also followed serogroup-dependent patterns. These data together are consistent with LPS being the major antigen recognized by mice after i.n. or oral immunization with V. cholerae OMVs. Adsorption of anti-OMV serum with LPS confirmed by ELISA an LPS-independent response to OMVs around 100-fold lower than the LPS response.
The impact of the LPS-driven immune response upon maternal protection was tested directly by immunization with different OMVs and challenge of pups with homologous or heterologous serotype or serogroup V. cholerae. We found that the anti-OMV IgG1 titer was generally inversely related to the number of CFU, although attempts to plot median numbers of CFU for neonates from each dam versus IgG1 titer for that dam did not give a clear correlation. The most robust protection was from homologous LPS types for OMV immunization and V. cholerae challenge. O1 serotype cross-protection was significant after i.n. but not oral O1 OMV immunization. This cross-protection notwithstanding, our data show that O1 serotype-specific antigens are also immuno-dominant since the median IgG1 titers against the heterologous O1 serotype OMVs were in all cases lower. Disparities between O1 serotype homologous and heterologous responses have been observed for cholera patient and household contact vibriocidal antibody titers, with 11% O1 Ogawa seroconversion and only 2% O1 Inaba seroconversion (13). Titration of the amount of O1 Ogawa OMVs used for i.n. immunization showed significant protection to homologous challenge with as little as 0.025 μg of OMVs and an antibody titer of around 50 μg ml−1 serum IgG1 (70). From the data shown here, we hypothesize that this may not, however, be sufficient antigen to provide cross-protection against O1 Inaba challenge. Vaccination with a mixture of O1 Ogawa + Inaba OMVs gave more robust dual serotype antibody responses and protection. Consistent with LPS-driven responses, O1 OMV immunization could not protect from O139 challenge or vice versa.
The ideal cholera vaccine would protect from both O1 and O139 V. cholerae. Immunization of rabbits with a live attenuated O1 V. cholerae vaccine did not protect against O139 challenge or vice versa, further supporting LPS-dominant protection in response to vaccines (3, 4). Natural infection with O1 elicits antibodies that are vibriocidal against O1, but not O139, and vice versa for O139 infection (63), suggesting that this too is an LPS-dominant response. The LPS dominance of natural immune responses to cholera may be why O139 caused explosive outbreaks when it emerged within areas where O1 V. cholerae was endemic (5). An attempt to achieve O1 and O139 protection has been made by the production of the bivalent O1-and-O139-containing WCK vaccine that has proven immunogenic toward both serogroups, but with a stronger response to O1 than O139 (8). Here we show that the addition of O139 OMVs along with O1 OMVs provided full responses (including vibriocidal antibodies) to and robust protection from colonization upon challenge by both O1 and O139. Indeed protection from O139 challenge appeared more robust after triple O1 Ogawa + O1 Inaba + O139 OMV immunization than from single O139 OMV immunization. In contrast, levels of protection from O1 were similar regardless of whether O1 OMVs were alone (O1 Inaba + Ogawa) or combined with O139 OMVs. The basis for the effect of enhanced protection from O139 challenge is unknown.
Previous studies showed that antibodies in milk from OMV-vaccinated dams protect their suckling neonates from V. cholerae challenge, with very few bacteria recovered from small intestines 24 h postinfection (71). Here we show, with temporal analysis of viable counts, that protection occurs rapidly after challenge but does not involve detectable levels of bacterial killing. Instead, bacteria remain viable and are excreted. For immunized adults with a fully developed immune system, the killing of bacteria upon encountering V. cholerae may make a more significant contribution than that that we observed using a neonatal passive protection model. However, we note that for volunteers immunized with live attenuated or WCK cholera vaccines and then challenged with between 105 and 106 V. cholerae CFU, shedding of the challenge strain was observed with the majority of individuals that were protected from symptomatic cholera, although CFU were at lower levels, 500 to 3 × 106 CFU g−1 stool, compared to those for unimmunized controls with symptomatic cholera, 106 to 108 CFU g−1 stool (15, 48, 75).
Consistent with our previous report (70), milk extracted from the stomachs of pups born to OMV-immunized dams contained anti-OMV IgG1 and, at a lower level, IgA antibodies. Note that this is different from the situation in humans, in which IgA is dominant in breast milk (33). Milk from O1 Ogawa OMV-immunized mice inhibited O1 Ogawa V. cholerae motility in vitro and, with higher levels of antibodies (1 to 4 μg ml−1 anti-OMV IgG1) achieved by using serum in the assay rather than milk, also inhibited motility of O139 or O1 Inaba in a serogroup-dependent manner. These data suggest that protection of pups from V. cholerae infection mediated by antibodies in milk could occur through inhibition of motility. V. cholerae has a single polar flagellum that is covered by an outer membrane sheath (20), making it seem plausible that anti-LPS antibodies could provide a relatively simple physical inhibition of motility.
These data together suggest that inhibition of motility and possibly agglutination (observed in vitro with higher antibody levels or after longer exposure) could be responsible for neonatal protection by OMV-immunized dams. These data are consistent with previous reports that after immunization of dams with WCK or various V. cholerae fractions, including an OMV-like preparation, and challenge of pups, histology revealed low bacterial numbers that were confined to the lumen of the small intestine, whereas in unimmunized controls they were able to penetrate into the mucus layer and crypts (34). Guentzel et al. also observed that nonmotile mutant V. cholerae infecting naïve neonatal mice behaved similarly to wild-type bacteria infecting pups from immunized mice, i.e., the bacteria were unable to colonize the small intestine but appeared viable in the large intestine (34). These data are consistent with our in vitro observation of motility inhibition by milk antibodies and support a neonatal protection model in which the immunity is not sterilizing but colonization is prevented due to the inability of the bacteria to access the mucosa due to a block of motility and/or clumping of bacteria.
We see that a higher antibody titer was needed for inhibition of motility in vitro for O139 compared to O1 and that an acapsular O139 mutant is significantly less resistant to inhibition of motility than wild-type encapsulated O139. We propose that this may result in the need for higher antibody titers for in vivo protection from O139. An attempt was made to correlate antibody levels for each dam with the median number of CFU for neonates after challenge, but these analyses did not show any clear trends, possibly because there is too much variation in suckling habits within each litter. Therefore, this hypothesis remains untested. Our observation that the capsule changes the threshold of antibody required to inhibit motility are consistent with observations that the capsule inhibits antibody-dependent complement-mediated killing of O139 (9).
Immunization with either wild-type O139 or otnA mutant OMVs resulted in similar serum IgG1 titers, similar levels of protection from O139 challenge, and similar levels of inhibition of motility, suggesting that there is no gain to be had from immunization with an acapsular OMV strain rather than wild-type O139 OMVs. We note that a comparison of the immunogenicity of whole-cell killed O139 variants showed that the LPS, rather than the capsule, was the most important immunogen but that the presence of the capsule did not significantly inhibit antibody responses to the vaccine (11). The efficacy of protection was not tested in this study.
In summary, we have shown that OMVs from V. cholerae can be delivered most effectively in mice via the i.n. route; two doses are sufficient to provide protection via this route but not when administered orally. We found that, similar to WCK vaccines, V. cholerae OMVs induce LPS-dominant and serogroup- and, to a lesser extent, serotype-specific antibody responses, resulting in protection that is most robust upon homologous challenge. However, mixing of OMVs is an effective way to produce multiserotype and multiserogroup protection. As was found for WCK vaccines, cholera toxin is not required for V. cholerae OMV immunogenicity and protection. Finally, our results support a role for inhibition of motility and/or agglutination, but we do not find evidence for bacterial killing, in protection in the neonatal challenge model.
Further studies are needed to directly compare the effectiveness of OMV immunization with oral WCK vaccines using the maternal protection model. Data presented here suggest that, in their native form, OMVs appear to be largely an LPS delivery system, not dissimilar from WCK vaccines. The degree and duration of memory responses to OMVs (71) may be similar to those to WCK, though this remains to be tested. Also, the impact of intranasal compared to oral delivery of OMVs upon memory responses is unknown. It is possible, although not proven, that OMVs may deliver their antigens in a way subtly different than that of WCK. Direct fusion of OMVs from other Gram-negative bacteria with epithelial cells has been documented, for instance (31, 44), while the cellular nature of WCK vaccines may make them more likely to be phagocytosed by professional phagocytes and/or transcytosed by M cells at the mucosal surface (16). The impact of these potential differences upon antigen delivery and subsequent adaptive immune response and memory is unknown and warrants further investigation.
As OMVs require centrifugation and filtration to remove bacteria and ultracentrifugation to harvest and wash them, we cannot claim that OMVs are easier to produce than WCK vaccine preparations. Attempts to replace the ultracentrifugation step are under way. In terms of quality control and heat stability, there is probably little distinction to be made between the whole bacteria and the OMVs that they shed. There is currently no precedent for large-scale preparation of native OMVs; however, detergent-extracted OMVs from N. meningitidis type B have been mass produced (76). We would expect i.n. vaccine delivery in humans to be more efficient than oral with the same amount of antigen, as seen here for OMVs in a mouse model, as it avoids antigen destruction by stomach acid and digestive enzymes, which may facilitate a response with lower doses (51). There is currently little precedence for i.n. delivery of Gram-negative bacterial antigens in humans; however, small adult volunteer studies with either native N. meningitidis type B OMVs (wild-type levels of LPS) or WCK Bordetella pertussis delivered i.n. have found these preparations to be safe and immunogenic (35).
Supplementary Material
Acknowledgments
This research was supported by NIH grant AI055058 awarded to A.C., and A.C. is a Howard Hughes Medical Institute investigator.
We thank T. Pitta and R. Costello from Micro Video Instruments, Inc., for technical advice on dark-field imaging. We are grateful to L. Bourassa and F. Wallace-Gadsden for critical reading of the manuscript.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 2 August 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ahmed, T., A. M. Svennerholm, A. Al Tarique, G. N. Sultana, and F. Qadri. 2009. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine 27:1433-1439. [DOI] [PubMed] [Google Scholar]
- 2.Alaniz, R. C., B. L. Deatherage, J. C. Lara, and B. T. Cookson. 2007. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 179:7692-7701. [DOI] [PubMed] [Google Scholar]
- 3.Albert, M. J., K. Alam, M. Ansaruzzaman, F. Qadri, and R. B. Sack. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O139 (Bengal strain) after oral immunization of rabbits with V. cholerae O1 vaccine strain CVD103-HgR. J. Infect. Dis. 169:230-231. [DOI] [PubMed] [Google Scholar]
- 4.Albert, M. J., K. Alam, A. S. Rahman, S. Huda, and R. B. Sack. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J. Infect. Dis. 169:709-710. [DOI] [PubMed] [Google Scholar]
- 5.Albert, M. J., A. K. Siddique, M. S. Islam, A. S. Faruque, M. Ansaruzzaman, S. M. Faruque, and R. B. Sack. 1993. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet 341:704. [DOI] [PubMed] [Google Scholar]
- 6.Ali, M., M. Emch, L. Von Seidlein, M. Yunus, D. A. Sack, M. Rao, J. Holmgren, and J. Clemens. 2005. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet 366:44-49. [DOI] [PubMed] [Google Scholar]
- 7.Angelichio, M. J., J. Spector, M. K. Waldor, and A. Camilli. 1999. Vibrio cholerae intestinal population dynamics in the suckling mouse model of infection. Infect. Immun. 67:3733-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anh, D. D., D. G. Canh, A. Lopez, V. D. Thiem, P. T. Long, N. H. Son, J. Deen, L. Von Seidlein, R. Carbis, S. Han, S. Shin, S. Attridge, J. Holmgren, and J. Clemens. 2007. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine 25:1149-1155. [DOI] [PubMed] [Google Scholar]
- 9.Attridge, S. R., and J. Holmgren. 2009. Vibrio cholerae O139 capsular polysaccharide confers complement resistance in the absence or presence of antibody yet presents a productive target for cell lysis: implications for detection of bactericidal antibodies. Microb. Pathog. 47:314-320. [DOI] [PubMed] [Google Scholar]
- 10.Attridge, S. R., C. Johansson, D. D. Trach, F. Qadri, and A. M. Svennerholm. 2002. Sensitive microplate assay for detection of bactericidal antibodies to Vibrio cholerae O139. Clin. Diagn. Lab. Immunol. 9:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attridge, S. R., G. Wallerstrom, B. L. Li, R. Morona, and J. Holmgren. 2009. Differential immunogenicity of Vibrio cholerae O139 variants expressing different combinations of naturally occurring and atypical forms of the serogroup polysaccharide. Vaccine 27:1055-1061. [DOI] [PubMed] [Google Scholar]
- 12.Belshe, R. B., K. L. Nichol, S. B. Black, H. Shinefield, J. Cordova, R. Walker, C. Hessel, I. Cho, and P. M. Mendelman. 2004. Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5-49 years. Clin. Infect. Dis. 39:920-927. [DOI] [PubMed] [Google Scholar]
- 13.Benenson, A. S., A. Saad, and W. H. Mosley. 1968. Serological studies in cholera. 2. The vibriocidal antibody response of cholera patients determined by a microtechnique. Bull. World Health Organ. 38:277-285. [PMC free article] [PubMed] [Google Scholar]
- 14.Beveridge, T. J. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black, R. E., M. M. Levine, M. L. Clements, C. R. Young, A. M. Svennerholm, and J. Holmgren. 1987. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect. Immun. 55:1116-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramwell, V. W., and Y. Perrie. 2006. Particulate delivery systems for vaccines: what can we expect? J. Pharm. Pharmacol. 58:717-728. [DOI] [PubMed] [Google Scholar]
- 17.Butler, S. M., and A. Camilli. 2005. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nat. Rev. Microbiol. 3:611-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilli, A., S. Schild, and E. J. Nelson. 16 April 2009. Cholera Vaccines. International patent WO/2009/049013.
- 19.Chandrasekhar, U., S. Sinha, H. R. Bhagat, V. B. Sinha, and B. S. Srivastava. 1994. Comparative efficacy of biodegradable liposomes and microspheres as carriers for delivery of Vibrio cholerae antigens in the intestine. Vaccine 12:1384-1388. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee, S. N., and K. Chaudhuri. 2003. Lipopolysaccharides of Vibrio cholerae. I. Physical and chemical characterization. Biochim. Biophys. Acta 1639:65-79. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee, S. N., and J. Das. 1967. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J. Gen. Microbiol. 49:1-11. [DOI] [PubMed] [Google Scholar]
- 22.Chen, D. J., N. Osterrieder, S. M. Metzger, E. Buckles, A. M. Doody, M. P. DeLisa, and D. Putnam. 2010. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc. Natl. Acad. Sci. U. S. A. 107:3099-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholera Working Group, I. C. D. D. R., Bangladesh. 1993. Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Lancet 342:387-390. [PubMed] [Google Scholar]
- 24.Clemens, J. 1990. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 335:270-273. [DOI] [PubMed] [Google Scholar]
- 25.Clemens, J. D., D. A. Sack, J. R. Harris, J. Chakraborty, P. K. Neogy, B. Stanton, N. Huda, M. U. Khan, B. A. Kay, M. R. Khan, et al. 1988. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J. Infect. Dis. 158:372-377. [DOI] [PubMed] [Google Scholar]
- 26.Comstock, L. E., D. Maneval, P. Panigrahi, A. Joseph, M. M. Levine, J. B. Kaper, J. G. Morris, and J. A. Johnson. 1995. The capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in Vibrio cholerae O1. Infect. Immun. 63:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cumberland, S. 2009. An old enemy returns. Bull. World Health Organ. 87:85-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eko, F. O., T. Schukovskaya, E. Y. Lotzmanova, V. V. Firstova, N. V. Emalyanova, S. N. Klueva, A. L. Kravtzov, L. F. Livanova, V. V. Kutyrev, J. U. Igietseme, and W. Lubitz. 2003. Evaluation of the protective efficacy of Vibrio cholerae ghost (VCG) candidate vaccines in rabbits. Vaccine 21:3663-3674. [DOI] [PubMed] [Google Scholar]
- 30.Eubanks, E. R., M. N. Guentzel, and L. J. Berry. 1977. Evaluation of surface components of Vibrio cholerae as protective immunogens. Infect. Immun. 15:533-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiocca, R., V. Necchi, P. Sommi, V. Ricci, J. Telford, T. L. Cover, and E. Solcia. 1999. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188:220-226. [DOI] [PubMed] [Google Scholar]
- 32.Gallucci, S., and P. Matzinger. 2001. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 13:114-119. [DOI] [PubMed] [Google Scholar]
- 33.Goldman, A. S. 2007. The immune system in human milk and the developing infant. Breastfeed. Med. 2:195-204. [DOI] [PubMed] [Google Scholar]
- 34.Guentzel, M. N., L. H. Field, E. R. Eubanks, and L. J. Berry. 1977. Use of fluorescent antibody in studies of immunity to cholera in infant mice. Infect. Immun. 15:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haneberg, B., and J. Holst. 2002. Can nonliving nasal vaccines be made to work? Expert Rev. Vaccines 1:227-232. [DOI] [PubMed] [Google Scholar]
- 36.Harris, J., R. Larocque, F. Chowdhury, A. Khan, T. Logvinenko, A. Faruque, E. Ryan, F. Qadri, S. Calderwood, and A. Ko. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hisatsune, K., S. Kondo, Y. Isshiki, T. Iguchi, and Y. Haishima. 1993. Occurrence of 2-O-methyl-N-(3-deoxy-l-glycero-tetronyl)-d-perosamine (4-amino-4,6-dideoxy-d-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem. Biophys. Res. Commun. 190:302-307. [DOI] [PubMed] [Google Scholar]
- 38.Hisatsune, K., S. Kondo, Y. Isshiki, T. Iguchi, Y. Kawamata, and T. Shimada. 1993. O-antigenic lipopolysaccharide of Vibrio cholerae O139 Bengal, a new epidemic strain for recent cholera in the Indian subcontinent. Biochem. Biophys. Res. Commun. 196:1309-1315. [DOI] [PubMed] [Google Scholar]
- 39.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horstman, A. L., and M. J. Kuehn. 2000. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 275:12489-12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 42.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 43.Johnson, J. A., C. A. Salles, P. Panigrahi, M. J. Albert, A. C. Wright, R. J. Johnson, and J. G. Morris. 1994. Vibrio cholerae O139 synonym bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect. Immun. 62:2108-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadurugamuwa, J. L., and T. J. Beveridge. 1998. Delivery of the non-membrane-permeative antibiotic gentamicin into mammalian cells by using Shigella flexneri membrane vesicles. Antimicrob. Agents Chemother. 42:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keenan, J. I., S. G. Rijpkema, Z. Durrani, and J. A. Roake. 2003. Differences in immunogenicity and protection in mice and guinea pigs following intranasal immunization with Helicobacter pylori outer membrane antigens. FEMS Immunol. Med. Microbiol. 36:199-205. [DOI] [PubMed] [Google Scholar]
- 46.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. U. S. A. 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levine, M. M., R. E. Black, M. L. Clements, L. Cisneros, D. R. Nalin, and C. R. Young. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818-820. [DOI] [PubMed] [Google Scholar]
- 48.Levine, M. M., J. B. Kaper, D. Herrington, G. Losonsky, J. G. Morris, M. L. Clements, R. E. Black, B. Tall, and R. Hall. 1988. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect. Immun. 56:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longini, I. M., M. Yunus, K. Zaman, A. K. Siddique, R. B. Sack, and A. Nizam. 2002. Epidemic and endemic cholera trends over a 33-year period in Bangladesh. J. Infect. Dis. 186:246-251. [DOI] [PubMed] [Google Scholar]
- 50.Lopez, A., J. Clemens, J. Deen, and L. Jodar. 2008. Cholera vaccines for the developing world. Hum. Vaccin. 4:165-169. [DOI] [PubMed] [Google Scholar]
- 51.Mann, J. F., R. Acevedo, J. D. Campo, O. Perez, and V. A. Ferro. 2009. Delivery systems: a vaccine strategy for overcoming mucosal tolerance? Expert Rev. Vaccines 8:103-112. [DOI] [PubMed] [Google Scholar]
- 52.Miller, V. L., V. J. DiRita, and J. J. Mekalanos. 1989. Identification of toxS, a regulatory gene whose product enhances toxR-mediated activation of the cholera toxin promoter. J. Bacteriol. 171:1288-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moisi, M., C. Jenul, S. M. Butler, A. New, S. Tutz, J. Reidl, K. E. Klose, A. Camilli, and S. Schild. 2009. A novel regulatory protein involved in motility of Vibrio cholerae. J. Bacteriol. 191:7027-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mosley, W. H., S. Ahmad, A. S. Benenson, and A. Ahmed. 1968. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bull. World Health Organ. 38:777-785. [PMC free article] [PubMed] [Google Scholar]
- 56.Mutsch, M., W. Zhou, P. Rhodes, M. Bopp, R. T. Chen, T. Linder, C. Spyr, and R. Steffen. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350:896. [DOI] [PubMed] [Google Scholar]
- 57.Nelson, E., A. Chowdhury, J. Flynn, S. Schild, L. Bourassa, Y. Shao, R. Larocque, S. B. Calderwood, F. Qadri, and A. Camilli. 2008. Transmission of Vibrio cholerae is antagonized by lytic phage and entry into the aquatic environment. PLoS Pathog. 4:e1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nesper, J., A. Kraiss, S. Schild, J. Blass, K. E. Klose, J. Bockemühl, and J. Reidl. 2002. Comparative and genetic analyses of the putative Vibrio cholerae lipopolysaccharide core oligosaccharide biosynthesis (wav) gene cluster. Infect. Immun. 70:2419-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nesper, J., S. Schild, C. M. Lauriano, A. Kraiss, K. E. Klose, and J. Reidl. 2002. Role of Vibrio cholerae O139 surface polysaccharides in intestinal colonization. Infect. Immun. 70:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nygren, E., J. Holmgren, and S. R. Attridge. 2008. Murine antibody responses following systemic or mucosal immunization with viable or inactivated Vibrio cholerae. Vaccine 26:6784-6790. [DOI] [PubMed] [Google Scholar]
- 61.Ogundele, M. 2001. Role and significance of the complement system in mucosal immunity: particular reference to the human breast milk complement. Immunol. Cell Biol. 79:1-10. [DOI] [PubMed] [Google Scholar]
- 62.Qadri, F., M. I. Chowdhury, S. M. Faruque, M. A. Salam, T. Ahmed, Y. A. Begum, A. Saha, A. Al Tarique, L. V. Seidlein, E. Park, K. P. Killeen, J. Mekalanos, J. D. Clemens, D. A. Sack, and PXV Study Group. 2007. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine 25:231-238. [DOI] [PubMed] [Google Scholar]
- 63.Qadri, F., G. Mohi, J. Hossain, T. Azim, A. M. Khan, M. A. Salam, R. B. Sack, M. J. Albert, and A. M. Svennerholm. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramamurthy, T., S. Garg, R. Sharma, S. K. Bhattacharya, G. B. Nair, T. Shimada, T. Takeda, T. Karasawa, H. Kurazano, A. Pal, et al. 1993. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet 341:703-704. [DOI] [PubMed] [Google Scholar]
- 65.Roberts, R., G. Moreno, D. Bottero, M. E. Gaillard, M. Fingermann, A. Graieb, M. Rumbo, and D. Hozbor. 2008. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 26:4639-4646. [DOI] [PubMed] [Google Scholar]
- 66.Ryan, E., S. B. Calderwood, and F. Qadri. 2006. Live attenuated oral cholera vaccines. Expert Rev. Vaccines 5:483-494. [DOI] [PubMed] [Google Scholar]
- 67.Sack, D. A., R. B. Sack, and C. L. Chaignat. 2006. Getting serious about cholera. N. Engl. J. Med. 355:649-651. [DOI] [PubMed] [Google Scholar]
- 68.Saha, D., R. Larocque, A. Khan, J. Harris, Y. A. Begum, S. M. Akramuzzaman, A. S. Faruque, E. Ryan, F. Qadri, and S. B. Calderwood. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318-2322. [DOI] [PubMed] [Google Scholar]
- 69.Sánchez, J., and J. Holmgren. 2008. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell. Mol. Life Sci. 65:1347-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schild, S., E. J. Nelson, A. L. Bishop, and A. Camilli. 2009. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect. Immun. 77:472-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schild, S., E. J. Nelson, and A. Camilli. 2008. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect. Immun. 76:4554-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song, T., F. Mika, B. Lindmark, Z. Liu, S. Schild, A. Bishop, J. Zhu, A. Camilli, J. Johansson, J. Vogel, and S. N. Wai. 2008. A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Mol. Microbiol. 70:100-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stroeher, U. H., L. E. Karageorgos, M. H. Brown, R. Morona, and P. A. Manning. 1995. A putative pathway for perosamine biosynthesis is the first function encoded within the rfb region of Vibrio cholerae O1. Gene 166:33-42. [DOI] [PubMed] [Google Scholar]
- 74.Sur, D., A. Lopez, S. Kanungo, A. Paisley, B. Manna, M. Ali, S. K. Niyogi, J. K. Park, B. Sarkar, M. K. Puri, D. Kim, J. Deen, J. Holmgren, R. Carbis, R. Rao, T. V. Nguyen, A. Donner, N. K. Ganguly, G. B. Nair, S. Bhattacharya, and J. Clemens. 2009. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 374:1694-1702. [DOI] [PubMed] [Google Scholar]
- 75.Tacket, C. O., M. B. Cohen, S. S. Wasserman, G. Losonsky, S. Livio, K. Kotloff, R. Edelman, J. B. Kaper, S. J. Cryz, R. A. Giannella, G. Schiff, and M. M. Levine. 1999. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El Tor Inaba three months after vaccination. Infect. Immun. 67:6341-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan, L. K., G. M. Carlone, and R. Borrow. 2010. Advances in the development of vaccines against Neisseria meningitidis. N. Engl. J. Med. 362:1511-1520. [DOI] [PubMed] [Google Scholar]
- 77.Taylor, D. N., K. P. Killeen, D. C. Hack, J. R. Kenner, T. S. Coster, D. T. Beattie, J. Ezzell, T. Hyman, A. Trofa, M. H. Sjogren, et al. 1994. Development of a live, oral, attenuated vaccine against El Tor cholera. J. Infect. Dis. 170:1518-1523. [DOI] [PubMed] [Google Scholar]
- 78.Trach, D. D., J. D. Clemens, N. T. Ke, H. T. Thuy, N. D. Son, D. G. Canh, P. V. Hang, and M. R. Rao. 1997. Field trial of a locally produced, killed, oral cholera vaccine in Vietnam. Lancet 349:231-235. [DOI] [PubMed] [Google Scholar]
- 79.van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165:4778-4782. [DOI] [PubMed] [Google Scholar]
- 80.Villeneuve, S., A. Boutonnier, L. A. Mulard, and J. M. Fournier. 1999. Immunochemical characterization of an Ogawa-Inaba common antigenic determinant of Vibrio cholerae O1. Microbiology 145:2477-2484. [DOI] [PubMed] [Google Scholar]
- 81.Villeneuve, S., H. Souchon, M. M. Riottot, J. C. Mazie, P. Lei, C. P. Glaudemans, P. Kovác, J. M. Fournier, and P. M. Alzari. 2000. Crystal structure of an anti-carbohydrate antibody directed against Vibrio cholerae O1 in complex with antigen: molecular basis for serotype specificity. Proc. Natl. Acad. Sci. U. S. A. 97:8433-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wachsmuth, I. K., G. M. Evins, P. I. Fields, O. Olsvik, T. Popovic, C. A. Bopp, J. G. Wells, C. Carrillo, and P. A. Blake. 1993. The molecular epidemiology of cholera in Latin America. J. Infect. Dis. 167:621-626. [DOI] [PubMed] [Google Scholar]
- 83.Waldor, M. K., R. Colwell, and J. J. Mekalanos. 1994. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc. Natl. Acad. Sci. U. S. A. 91:11388-11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.WHO. 2004. Guidelines for the production and control of inactivated oral cholera vaccines. World Health Organization, Geneva, Switzerland.
- 85.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.