Abstract
Novel approaches targeting the host's immune response to treat Staphylococcus aureus infections have significant potential to improve clinical outcomes, in particular during infection with antibiotic-resistant strains. The hyaluronic acid-binding peptide (HABP) PEP35 was assessed for its ability to treat S. aureus infections using a clinically relevant murine model of surgical wound infection. PEP35 demonstrated no direct antimicrobial activity against a range of antibiotic-susceptible and antibiotic-resistant clinical isolates of Staphylococcus aureus. However, when this peptide was administered at the onset of infection and up to 4 h postchallenge with a methicillin-susceptible (MSSA) or a methicillin-resistant (MRSA) strain of S. aureus, it significantly reduced the bacterial burden at the wound infection site. PEP35 reduced the tissue bacterial burden by exclusively modulating the local neutrophil response. PEP35 administration resulted in a significant early increase in local CXCL1 and CXCL2 production, which resulted in a more rapid influx of neutrophils to the infection site. Importantly, neutrophil influx was not sustained after treatment with PEP35, and administration of PEP35 alone did not induce a local inflammatory response. The immunomodulatory effects of PEP35 on CXC chemokine production were TLR2 and NF-κB dependent. We propose a novel role for a HABP as an innate immunomodulator in the treatment of MSSA and MRSA surgical wound infection through enhancement of the local CXC chemokine-driven neutrophil response.
Since the dawn of antibiotic/antimicrobial use, these medicines have saved countless lives representing one of the greatest achievements of modern medicine. However, as a consequence of their widespread usage they are no longer as effective as they once were. In many cases their target organisms have undergone evolutionarily adaptations and have become resistant to the effects of these powerful drugs (1, 6). Significant efforts must be used to develop new alternative therapeutic approaches that can complement antibiotic treatment of bacterial infections.
Staphylococcus aureus is the most common organism isolated from surgical site infections (SSIs) accounting for ca. 30% of all SSIs (3, 28). The invasive nature of S. aureus means that initial infection at the wound site has the potential to result in severe life-threatening disease such as septicemia, particularly in immunocompromised patients. The situation is complicated further by the rampant spread of antibiotic-resistant strains of S. aureus in the hospital setting (HA-MRSA). An alternative and attractive strategy to increase the chances of success in eradicating this type of infection would be the use of combined therapies that target the organism directly (antibiotics) and indirectly (enhanced host response via immunomodulatory drugs). The goal for successful immunomodulatory therapy in the treatment of bacterial infection is to boost the immune response sufficiently so as to eradicate the invading microorganism without causing a prolonged associated inflammatory response that could result in host tissue damage.
Optimal clearance of S. aureus infection requires a tightly controlled neutrophil response whereby sufficient neutrophils are recruited to clear the invading organism but these neutrophils are in turn cleared from the site of infection in a timely manner (30, 46). Polymorphonuclear neutrophils (PMN) are typically recruited to the site of infection in response to the local production of CXC chemokines (26). Upon exposure to microbial components, resident cells at sites of infection such as tissue macrophages (9), dendritic cells (4), endothelial cells (8), and epithelial cells (20) can produce CXC chemokines. Pathogen recognition receptors (PRRs) such as Toll-like receptors (TLRs) expressed by these resident cell types recognize pathogen-associated molecular patterns (PAMPs) on the invading microorganism. This triggers activation of various signal transduction pathways in these cells, culminating in activation of (among others) a family of transcription factors known as the NF-κB family, which plays a key role in regulating the immune response to infection, including the induction of chemokine production (27, 45).
Natural host defense peptides (HDPs) are a critical component of the host's innate immune response (51). In addition to their documented direct antimicrobial effects, these peptides can trigger a range of immunomodulatory properties, such as activation of TLR signaling and the induction of chemokine production by host cells (14, 16). Recent studies have identified the therapeutic potential for naturally expressed or synthetically produced homologues of HDPs in the treatment of various infections. The direct antimicrobial activity of these peptides has been well studied, and several peptides have entered clinical trials as topical antimicrobials (11); however, the immunomodulatory therapeutic potential of HDPs has not been explored to the same extent. A recent study demonstrated an exclusive immunomodulatory role for a HDP known as IDR-1 in the treatment of infection with a number of organisms, including S. aureus. The proposed mechanism of action of this peptide relies upon the induction of monocyte/macrophage activation (42). In addition, generation of a 10-mer peptide from cystatin (a natural cysteine protease inhibitor) demonstrated therapeutic benefit against Leishmania infection in part through the induction of proinflammatory cytokine production by macrophages (36). To date, however, the potential for immunomodulatory peptides to impact the neutrophil response during infection remains to be established.
We characterized the immunomodulatory properties of a hyaluronic acid-binding peptide (HABP) PEP35 for the treatment of S. aureus surgical wound infection. Previously, we have shown that treatment with this peptide decreases tissue bacterial burden in a clinically relevant murine model of S. aureus surgical wound infection (52); however, the underlying mechanisms mediating these effects were not defined. We now demonstrate for the first time the ability of a synthetic HDP to exclusively promote CXC chemokine-driven neutrophil recruitment to the infection site in a controlled manner that results in efficient bacterial clearance in the absence of a sustained local proinflammatory response. These effects of PEP35 are mediated in part through TLR2-dependent activation of the transcription factor NF-κB.
MATERIALS AND METHODS
Peptides.
Peptide 35 (PEP35) is the lead peptide in a series of about four generations of sequences designed at Cangene Corp., based on two hyaluronic acid (HA)-binding domains of the HA-binding protein RHAMM (receptor hyaluronic acid-mediated motility) (50). HA interacts with its receptors via a 9- to 11-amino-acid binding motif of the form B-X7-B, with B being the basic amino acid lysine or arginine and X representing nonacidic amino acids. PEP35 was synthesized to contain four B-X7-B motifs. PEP35 has the sequence LKQKIKHVVKLKVVVKLRSQLVKRKQN. PEP35 was synthesized by Bachem, Ltd. (Torrence, CA) under GMP regulations using BOC chemistry and purified to >95% purity. Each synthetic peptide was analyzed by reversed-phase high-pressure liquid chromatography to assure purity. A control peptide based on a scrambled sequence of the 27 amino acids in PEP35 was also used (KKKKKLQLQLNLIKKKVQVSVVVVRRH).
Bacterial strains.
S. aureus strain PS80 (serotype 8) was obtained from the American Type Culture Collection (catalog no. 27700). S. aureus strain USA100 used in the in vivo models studies was obtained from the Network of Antimicrobial Resistance in S. aureus (NARSA) and is designated as a healthcare-associated MRSA strain (29). S. aureus strain UC-18 was provided by Innovotech, Inc., Edmonton, Alberta, Canada. Staphylococci were cultivated for 24 h at 37°C on Columbia agar (Difco Laboratories, Detroit, MI) supplemented with 2% NaCl prior to preparation of the inoculums in phosphate-buffered saline (PBS).
Bacterial isolates for MIC testing were obtained from the CANWARD 2007 study (54). The precise methods of isolate collection in the CANWARD study have been previously described (54). Resistance to methicillin was confirmed for all S. aureus strains at the coordinating laboratory (Health Sciences Centre, Winnipeg, Manitoba, Canada) as described previously (39). Community-associated (CA)-MRSA and healthcare-associated (HA)-MRSA were defined genotypically on the basis of their pulsed-field gel electrophoresis epidemic type. Vancomycin-intermediate S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA) isolates were obtained through the NARSA program (39).
Antimicrobial susceptibility testing.
A custom designed panel of antimicrobials was created containing PEP35, cefazolin, clindamycin, daptomycin, levofloxacin, linezolid, and vancomycin. Following two subcultures from frozen stock, the MICs of the antimicrobial agents for each isolate were determined by the Clinical and Laboratory Standards Institute (CLSI; 2005) approved broth macrodilution method (54). Briefly, test tubes containing doubling antibiotic dilutions (2 ml per tube) of cation-adjusted Mueller-Hinton broth were inoculated to achieve a final concentration of approximately 5 × 105 CFU/ml and incubated in ambient air for 24 h prior to reading. Colony counts were performed periodically to confirm inocula. PEP35 was solubilized in 10 mM sodium acetate. Susceptibility testing was expressed as the MIC, MIC50, MIC90, and MIC range (all as μg/ml) (39).
Animals.
Wild-type mice (C57BL/6, 6 to 8 weeks old) were obtained from Jackson Laboratory (Bar Harbor, ME), TLR-deficient mice (TLR2−/−) on a C57BL/6 background were bred in house or were from the Jackson Laboratory. All animal experiments were performed in accordance with the guidelines set forth by the Harvard Medical School Standing Committee on Animals.
Mouse model of S. aureus wound infection.
Surgical wound infection was established as previously described (30, 31). A total of 10 μl of S. aureus organisms (104 CFU/ml) suspended in PBS with or without PEP35 were introduced into the wound site. For most experiments, peptides were solubilized in the S. aureus-PBS suspension prior to being administered to the wound, which ensured a simultaneous introduction of S. aureus and peptides to the wound tissues. For therapeutic studies, peptides or PBS were administered via intramuscular injection (0.05-ml volume) at selected time points after surgery. The injection site represents an area within a 1-cm radius from the incision site. At specific time points after the induction of infection, wound tissue was excised and analyzed as described below.
For histological analysis, excised tissue was fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E) for microscopic examination of leukocyte infiltration. Individual tissue sections were examined and scored for relative neutrophil infiltration by using a Zeiss Axioskop 2 Plus microscope. Tissue sections were ranked in severity and assigned a score of between 1 and 4.
Neutrophil accumulation at the wound site was quantified by measuring tissue myeloperoxidase (MPO) levels as previously described (30). The MPO activity was calculated per gram of tissue weight.
For cytokine and chemokine analysis, wound tissue was homogenized in a lysis buffer, and CXCL1 (KC), CXCL2 (MIP-2), and CCL2 (MCP-1) levels were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Total tissue bacterial burden was established by homogenizing tissue in tryptic soy broth and plating serial dilutions on tryptic soy agar plates. The results were expressed as CFU/gram of tissue weight.
In vitro macrophage analysis.
Total peritoneal leukocytes were harvested from mice by peritoneal lavage using cRPMI (5 ml). Cells were then incubated in complete RMPI medium containing 10% fetal bovine serum (FBS) for 90 min at 37°C, to allow peritoneal macrophages adhere to 96-well plates. Nonadherent cells were then washed away. Adherent macrophages were then stimulated with a known concentration of heat-killed S. aureus (exposed to 70°C in a heating block for 30 min) in the presence or absence of PEP35 (0.5 to 100 μg/ml). After 24 h in culture, the supernatants were collected for CXC chemokine analysis by ELISA. For NF-κB inhibition assays, Bay 11-7082 (Sigma Aldrich) at the indicated concentrations was added to the cells for 1 h prior to stimulation with bacteria and peptide. Control wells were treated with medium containing dimethyl sulfoxide (DMSO) at a similar concentration to that present in the inhibitor.
In vitro human macrophage cell line analysis.
The human monocyte cell line 28SC was used to establish the effects of PEP35 on human cells. A total of 1.0 × 105 to 1.0 × 106 cells/ml cells were seeded into 96-well plates in 10% FBS plus Dulbecco modified Eagle medium. The cells were then stimulated with a known concentration of heat-killed S. aureus (106 CFU/ml) or Escherichia coli lipopolysaccharide (LPS) (6,250 endotoxin units [EU]/ml) in the presence or absence of PEP35 (100 μg/ml). After 24 h in culture, supernatants were collected for CXCL8 chemokine analysis by ELISA.
Statistical analysis.
All statistical analysis was performed by using a Mann-Whitney two-tailed t test (Prism 4 GraphPad software). P values of <0.05 were considered statistically significant.
RESULTS
PEP35 does not exhibit direct antimicrobial activity for S. aureus.
The direct antimicrobial activity of PEP35 against MSSA, CA-MRSA, HA-MRSA, VISA, and VRSA isolates was tested in comparison to known antibiotic compounds (Table 1). Against MSSA, PEP35 displayed poor activity, with an MIC50 and an MIC90 of 256 and 512 μg/ml, respectively (MIC range, 128 to 512 μg/ml). This poor activity of PEP35 was consistent across the various S. aureus genotypes, with an MIC50 and an MIC90 of 512 and 512 μg/ml, respectively, for HA-MRSA; an MIC50 and an MIC90 of 256 and 512 μg/ml, respectively, for CA-MRSA; an MIC50 and an MIC90 of 512 and 1,024 μg/ml, respectively, for VISA; and an MIC50 of 256 μg/ml, respectively, for VRSA. The poor antimicrobial activity of PEP35 against S. aureus genotypes was not associated with susceptibility or resistance to other chemically unrelated drug classes. These data predict that PEP35 will have little or no direct antimicrobial activity in vivo.
TABLE 1.
PEP35 has no direct antimicrobial activity against antibiotic-susceptible or -resistant S. aureus
| Phenotypea | No. of strains | PEP35 MIC (μg/ml) |
MIC90 (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC50/MIC90 | Range | Cefazolin | Clindamycin | Daptomycin | Levofloxacin | Linezolid | Vancomycin | ||
| MSSA | 20 | 256/512 | 128-512 | 1 | >8 | 0.25 | >32 | 4 | 1 |
| HA-MRSA | 20 | 512/512 | 256-512 | 64 | 0.25 | 0.25 | 8 | 2 | 1 |
| CA-MRSA | 20 | 256/512 | 128-1,024 | >128 | >8 | 0.25 | >32 | 2 | 1 |
| VISA | 11 | 512/1,024 | 128-1,024 | >128 | >8 | 2 | >32 | 2 | 1 |
| VRSA | 7 | 256b | 128-256 | >128 | >8 | >32 | >32 | 4 | 1 |
MSSA, methicillin-susceptible S. aureus; HA-MRSA, healthcare-associated MRSA; CA-MRSA, community-associated methicillin-resistant S. aureus; VISA, vancomycin-intermediate S. aureus; VRSA, vancomycin-resistant S. aureus.
MIC50.
PEP35 promotes early CXC chemokine-driven neutrophil recruitment locally at the S. aureus surgical wound infection site.
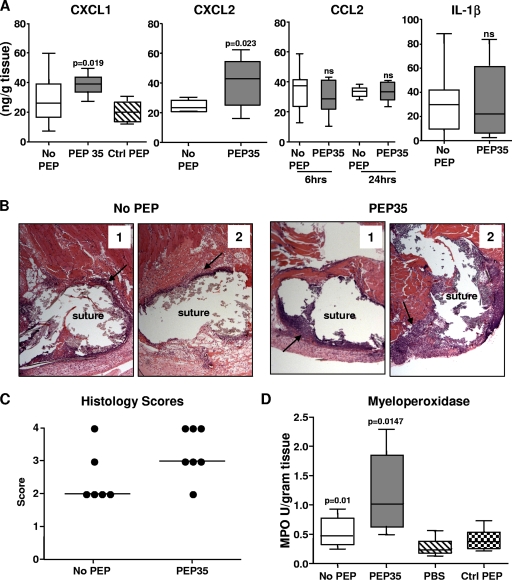
We hypothesized that PEP35 may be having some local immunomodulatory activity at the infection site. To investigate this concept, we established surgical wound infections in wild-type (WT) mice and assessed the local CXC chemokine response in the presence of PEP35. Administration of PEP35 (100 μg) in combination with S. aureus resulted in a significant increase in local CXCL1 and CXCL2 chemokine production at 6 and 24 h, respectively, postchallenge (Fig. 1 A). We have previously identified these time points as the peaks of CXCL1 and CXCL2 production in this model (30, 31). Administration of a scrambled control peptide did not have any appreciable effect on CXC chemokine production at the wound site. PEP35 exclusively enhanced CXC chemokine production because local production of the CC chemokine CCL2 and the proinflammatory cytokine interleukin-1β (IL-1β) remained unchanged in the presence of PEP35 (Fig. 1A).
FIG. 1.
PEP35 promotes early CXC chemokine production and associated neutrophil recruitment at the S. aureus wound infection site. (A) Surgical wounds were established in WT mice and infected with S. aureus strain PS80 (102 CFU) alone or in combination with PEP35 (100 μg) or a control peptide. The tissue levels of CXC chemokines CXCL1 and CXCL2 were measured at 6 and 24 h, respectively, postchallenge; the tissue levels of the CC chemokine CCL2 were also measured at 6 and 24 h postchallenge; and the tissue levels of proinflammatory cytokine IL-1β were measured at 6 h (n = 5 to 20 mice per group). (B) Wound tissue was excised at 24 h after induction of infection, formalin fixed, and embedded in paraffin. Tissue sections were H&E stained to visualize inflammatory infiltration around the wound suture site (two representative sections [1 and 2] of n = 6 to 7 individual mice per group are shown). (C) Tissue sections were scored, and the bar represents median values. (D) Wound tissue was also excised at 24 h and homogenized, and the tissue MPO levels were quantified (n = 10 to 20 mice per group). For all of the data presented the median is represented by the horizontal bar within the box. The upper and lower boundaries represent the 25th to 75th percentiles of the data, and whiskers represent the 10th and 90th percentiles of the data.
To establish if this enhanced CXC chemokine production translated to an increase in local PMN recruitment to the wound site, we scored H&E-stained wound tissue sections from mice challenged with S. aureus alone or S. aureus in combination with PEP35 (100 μg). Treatment with PEP35 resulted in an increase in PMN infiltration to the wound site at 24 h postchallenge (Fig. 1B and C). PMN infiltration was then quantitated by using a MPO assay (15, 41). Consistent with the histological analysis, mice that received PEP35 treatment demonstrated significantly higher MPO levels at 24 h postchallenge compared to untreated mice or mice that received control peptide. Uninfected mice challenged with PBS only had significantly lower levels of MPO compared to S. aureus-challenged mice (Fig. 1D).
PEP35 does not result in sustained neutrophil influx to the infection site.
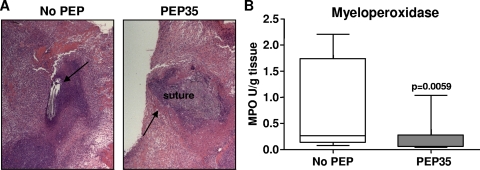
To ascertain whether PEP35 treatment resulted in sustained neutrophil influx at the infection site, we challenged mice with S. aureus in the presence or absence of PEP35 and then examined PMN infiltration to the wound site on day 5 postchallenge. Typically, in this infection model, maximal infection levels are seen by day 3 (31). On day 5 postchallenge, mice that had received PEP35 in combination with S. aureus demonstrated a reduced level of neutrophil influx at the wound site (Fig. 2 A) and had significantly reduced levels of tissue MPO compared to untreated mice (Fig. 2B). These data suggest that in the presence of PEP35 the bacterial infection is cleared more rapidly, and there does not appear to be sustained inflammatory infiltration as a consequence of PEP35 administration.
FIG. 2.
PEP35 does not result in sustained neutrophil influx at the S. aureus wound infection site. (A) Surgical wounds were established in WT mice and infected with S. aureus strain PS80 (102 CFU) alone or in combination with PEP35 (100 μg) or a control peptide. Wound tissue was excised on day 5 after the induction of infection, formalin fixed, and embedded in paraffin. Tissue sections were H&E stained to visualize inflammatory infiltration around the wound suture site (representative sections of n = 5 individual mice per group). (B) Wound tissue was also excised on day 5 and homogenized, and the tissue MPO levels were quantified (n = 12 to 14 mice per group). The median is represented by the horizontal bar within the box. The upper and lower boundaries represent the 25th to 75th percentiles of the data, and whiskers represent the 10th and 90th percentiles of the data.
PEP35 administration in the absence of infection had no impact on the host's immune response.
To investigate whether PEP35 alone induced any proinflammatory responses, we established a sterile wound in WT mice and then administered PEP35 or PBS directly into the surgical site in the absence of any bacterial challenge. Local production of the CXC chemokines CXCL1 and CXCL2 was measured over a 3-day time period. Administration of PEP35 (100 μg) did not result in any significant (P > 0.05 at all time points) CXC chemokine production locally at the wound site above that induced by surgical procedure itself (PBS challenge) (Table 2). In addition, all of the mice survived, and no adverse effects on the health of the mice or healing of the surgical site were noted as a consequence of PEP35 administration.
TABLE 2.
PEP35 alone does not induce CXC chemokine production at the surgical wound site
| Chemokine and treatment | Mean production (ng/g of tissue) ±SEM at: |
|||
|---|---|---|---|---|
| 6 h | 24 h | 48 h | 72 h | |
| CXCL1 | ||||
| PBS | 21.4 ± 2.2 | 8.1 ± 2.7 | 3.9 ± 0.4 | 3.6 ± 0.5 |
| PEP35 (100 μg) | 18.5 ± 1.0 | 5.4 ± 0.9 | 4.8 ± 0.5 | 5.6 ± 0.1 |
| CXCL2 | ||||
| PBS | 0.8 ± 0.1 | 3.7 ± 0.9 | 2.2 ± 0.5 | 1.9 ± 0.4 |
| PEP35 (100 μg) | 0.5 ± 0.07 | 3. 1 ± 0.9 | 2.3 ± 0.3 | 3.1 ± 0.6 |
Administration of PEP35 as a treatment up to 4 h postinfection promotes significant CXC chemokine-driven neutrophil recruitment and associated bacterial clearance.
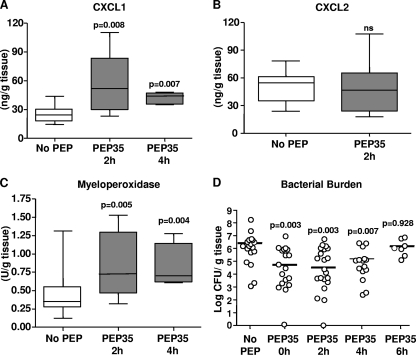
To establish the optimal therapeutic window for administration of PEP35, we established S. aureus surgical wound infections in WT mice and then administered PEP35 as an intramuscular injection directly into the wound site at various time points after bacterial challenge. Administration of PEP35 (100 μg) up to and including 4 h after bacterial challenge resulted in a comparable increase in CXCL1 production at 6 h (Fig. 3 A) to that seen when PEP35 was administered at the same time as the challenge inoculum (Fig. 1A). Interestingly, however, the stimulation of CXCL2 production at 24 h was not significantly enhanced when PEP35 was administered 2 h after bacterial challenge (Fig. 3B). Administration of PEP35 up to and including 4 h after bacterial challenge also significantly increased tissue MPO levels (Fig. 3C). Consistent with the observed immunomodulatory effects, administration of PEP35 at 2 and 4 h after bacterial challenge resulted in an ∼2-log decrease in median tissue bacterial burdens on day 3, an effect similar to that seen when PEP35 was administered at the same time as the bacterial challenge (0 h). When PEP35 was administered 6 h after the bacterial challenge the effect was no longer significant. We then went on to ascertain the optimal concentration of PEP35 required to promote bacterial clearance. Administration of 75 or 50 μg of PEP35 at 2 h after bacterial challenge efficiently reduced tissue bacterial burden at the wound site on day 3 (see Fig. S1 in the supplemental material).
FIG. 3.
Administration of PEP35 up to 4 h postinfection enhances the local neutrophil response and promotes bacterial clearance. Surgical wounds were established in WT mice and infected with S. aureus strain PS80 (102 CFU) alone. At the indicated time points postinfection, PEP35 (100 μg) or PBS was administered by intramuscular injection directly into the wound site. CXCL1 (A) and CXCL2 (B) were measured in the wound tissue at 6 and 24 h, respectively, after bacterial challenge. (C) To access neutrophil influx to the infection site, wound tissue was excised at 24 h and homogenized, and the tissue MPO levels were quantified. For data presented in panels A and B, the median is represented by the horizontal bar within the box. The upper and lower boundaries represent the 25th to 75th percentiles of the data, and whiskers represent the 10th and 90th percentiles of the data (n = 5 to 15 mice per group). (D) Wound tissue was also excised on day 3, and the total tissue bacterial burden was quantitated by plate counts. The results are expressed as the log CFU per gram of tissue; the median is indicated by a bar (n = 10 to 20 mice per group).
PEP35 promotes CXC chemokine-driven neutrophil recruitment and subsequent bacterial clearance during HA-MRSA infection.
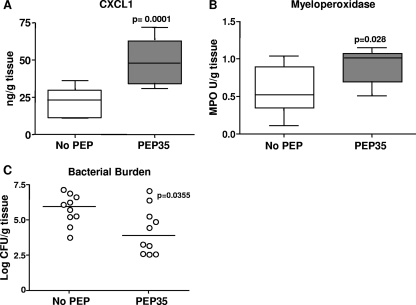
To confirm that PEP35 retained its immunomodulatory effects during infection with a common HA-MRSA strain, we established infection in WT mice using S. aureus strain USA 100 in the presence or absence of PEP35. CXCL1 levels at 6 h were significantly elevated in the presence of PEP35 compared to the levels induced by bacteria alone (Fig. 4 A). This increase in local CXCL1 production translated into a significant increase in neutrophil influx to the infection site, as assessed by the quantitation of tissue MPO levels at 24 h (Fig. 4B). In addition, we assessed tissue bacterial burden on day 3 postchallenge and demonstrated a significant ∼2-log reduction in median tissue bacterial burdens in the presence of PEP35 (Fig. 4C). Furthermore, PEP35 was capable of reducing tissue bacterial burden when a potent biofilm-forming HA-MRSA strain (UC-18 [24, 25]) was used (see Fig. S2 in the supplemental material).
FIG. 4.
PEP35 enhances the local neutrophil response and promotes bacterial clearance during HA-MRSA surgical wound infection. (A) Surgical wounds were established in WT mice and in mice infected with S. aureus strain USA100 (102 CFU) alone or in combination with PEP35 (100 μg). Tissue levels of CXCL1 were measured at 6 h postchallenge (n = 10 mice per group). (B) Wound tissue was excised at 24 h and homogenized, and the tissue MPO levels were quantified (n = 8 mice per group). For the data in panels A and B, the median is represented by a horizontal bar within the box. The upper and lower boundaries represent the 25th to 75th percentiles of the data, and whiskers represent the 10th and 90th percentiles of the data. (C) Wound tissue was also excised on day 3, and the total tissue bacterial burden was quantitated by plate counts. The results are expressed as the log CFU per gram of tissue, and the median is indicated by a bar (n = 10 mice per group).
PEP35 dose dependently modulates CXC chemokine production by primary murine macrophages.
To establish that monocyte/macrophages were present at the wound site immediately following S. aureus surgical wound infection, we isolated infiltrating leukocytes from infected wound tissue by collagenase digestion and analyzed the phenotypes of these cells using monoclonal antibodies against monocyte/macrophage surface markers, followed by fluorescence-activated cell sorting analysis. Of the infiltrating CD45+ leukocytes, ∼80.3% ± 5.99% were CD11b+ and ∼21.8% ± 5.98% were F4/80+ at 3 h postchallenge. These data indicate the presence of tissue macrophages at the wound site at early time points postchallenge and identify them as a potential cellular target for the actions of PEP35 at the wound site.
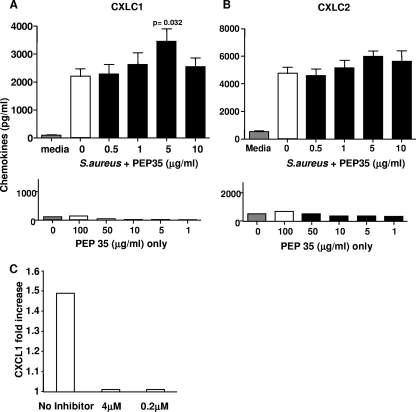
To delineate the mechanism of action of PEP35, we utilized an in vitro model system testing responses of naive murine peritoneal macrophages to PEP35. The very low numbers of viable macrophages obtained from uninfected surgical wounds precluded the use of wound tissue macrophages in these studies. Macrophages were stimulated with heat-killed S. aureus (105 or 104 CFU/ml) in the presence or absence of PEP35. Heat-killed S. aureus resulted in significant CXCL1 and CXCL2 production at 24 h. Previous time course experiments had established this as the optimal time point for S. aureus-induced CXC chemokine production by peritoneal macrophages in vitro, and dose-response studies demonstrated that 105 or 104 CFU of heat-killed bacteria/ml induced optimal (and similar) levels of CXC chemokine production (2,193 ± 288 versus 2,367 ± 503 pg of CXCL1/ml for 105 and 104 CFU/ml, respectively). The addition of PEP35 resulted in a dose-dependent increase in CXCL1 production (Fig. 5 A), with the maximum effect observed at a concentration of 5 μg/ml; thereafter, the effects diminished. A similar trend was observed for CXCL2 production by macrophages after PEP35 treatment, although this did not reach statistical significance (Fig. 5B). Treatment of the peritoneal macrophages with PEP35 alone did not induce CXCL1 or CXCL2 production above the levels of the medium control even at very high doses (Fig. 5A and B).
FIG. 5.
PEP35 has a dose-dependent differential effect on CXC chemokine production by primary peritoneal macrophages. In vitro cultures of purified WT murine peritoneal macrophages (105 cells) were stimulated with heat-killed S. aureus strain PS80 (105 CFU/ml) in the presence or absence of increasing concentrations of PEP35 for 24 h. CXCL1 (A) and CXCL2 (B) levels in the supernatant were measured by ELISA (upper panel). Macrophages were also stimulated with increasing concentrations of PEP35 alone in the absence of S. aureus. CXC chemokine levels were measured in the supernatant at 24 h (lower panel). The results represent the means ± the standard error of the mean (n = 10 individual experiments). In vitro cultures of purified WT murine peritoneal macrophages (105 cells) were incubated with the NF-κB inhibitor Bay 11-7082 (4 or 2 μM) for 1 h prior to stimulation with heat-killed S. aureus strain PS80 (104 CFU/ml) in the presence or absence of PEP35 (1 μg/ml) for 24 h. CXC chemokine levels in the supernatant were measured by ELISA (C). The results are expressed as the fold increase induced by PEP35 compared to that induced by the no-PEP35 treatment and represent the pooled data for five individual experiments.
To determine whether PEP35 remained functionally active in the context of human monocyte/macrophages, we demonstrated the ability of PEP35 to enhance heat-killed S. aureus and E. coli-LPS induced production of CXCL8 by the human monocyte cell line 28SC (see Fig. S3 in the supplemental material).
The immunomodulatory effects of PEP35 are dependent upon NF-κB activation.
Innate immune recognition of S. aureus potentially involves numerous distinct signaling pathways (10, 19). A common component of all of these signaling pathways involves activation of the transcription factor NF-κB (7, 47). In addition, NF-κB is critically involved in driving CXC chemokine gene transcription in a variety of different cell types in response to various inflammatory stimuli (18, 33, 35). Therefore, we investigated PEP35-mediated CXC chemokine production in the absence of NF-κB activation. Primary WT peritoneal macrophages were pretreated with Bay 11-7082, a compound that inhibits a critical stage in the NF-κB activation pathway (IκBα phosphorylation) or medium containing DMSO only for 1 h prior to stimulating cells with S. aureus in the presence of PEP35 (1 μg/ml). CXCL1 production was then quantified at 24 h, and the results demonstrate that PEP35 was incapable of enhancing S. aureus-induced CXCL1 production in the presence of the NF-κB inhibitor (Fig. 5C). The data in this figure are presented as the fold increase in CXC chemokine production compared to the no-peptide control due to the fact that DMSO (required to solubilize inhibitor) was added to all cells (PEP35 and untreated). These data suggest that PEP35 requires NF-κB signaling to mediate its immunomodulatory effects on CXC chemokine production at the infection site.
PEP35 immunomodulatory effects are partially TLR2 dependent.
To begin to identify a target receptor for the actions of PEP35 in vivo, we established S. aureus surgical wound infections in TLR2−/− mice in the presence or absence of PEP35 treatment. In the presence of PEP35, CXC chemokine production was marginally but not significantly increased compared to untreated mice (38.2 versus 45.6 ng/g of tissue for untreated versus PEP35-treated mice, respectively) (Fig. 6 A), but this did not translate to an increase in neutrophil recruitment to the wound infection site because MPO levels were unchanged in the presence of PEP35 (Fig. 6B). Total tissue bacterial burden was also not significantly reduced in the presence of PEP35 in the TLR2−/− mice (Fig. 6C). However, there was a partial effect of PEP35 in the absence of TLR2 signaling. The reduction in median tissue bacterial burden in the presence of PEP35 was 0.8 log in the TLR2−/− mice, which is a reduced effect compared to that seen in WT mice (the log reduction in the presence of PEP35 was ∼2 log (Fig. 3D).
FIG. 6.
The immunomodulatory effects of PEP35 in vivo are partially TLR2 dependent. (A) Surgical wounds were established in TLR2−/− mice and infected with S. aureus strain PS80 (102 CFU) alone or in combination with PEP35 (100 μg) or a control peptide. The tissue levels of CXCL1 were measured at 6 h postchallenge (n = 8 mice per group). (B) Wound tissue was also excised at 24 h and homogenized, and the tissue MPO levels were quantified (n = 8 mice per group). For all data presented, the median is represented by the horizontal bar within the box. The upper and lower boundaries represent the 25th to 75th percentiles of the data, whiskers represent the 10th and 90th percentiles of the data. (C) Wound tissue was excised on day 3 postchallenge, and the total tissue bacterial burden was quantitated by plate counts. The results are expressed as the log CFU per gram of tissue, and the median is indicated by a bar (n = 8 mice per group).
DISCUSSION
We characterized the immunomodulatory properties of a hyaluronic acid-binding peptide (HABP), PEP35, and propose this as a potentially novel, safe, and effective therapeutic for the prevention or treatment of S. aureus surgical wound infections with both MSSA and MRSA in the hospital setting. Our data demonstrate that PEP35 transiently enhances innate immune signaling pathways through NF-κB signaling, which depends (partially) upon TLR2 activation. This results exclusively in increased CXC chemokine production and promotes neutrophil recruitment to the infection site. Importantly, PEP35 treatment did not result in a sustained influx of neutrophils to the site of infection, highlighting it as an ideal immunomodulatory therapeutic. It enhances the innate immune response and promotes efficient bacterial clearance without a sustained inflammatory response that could cause local tissue damage.
An HABP similar to PEP35 was previously shown to inhibit contact hypersensitivity responses in mice by blocking skin directed trafficking of inflammatory leukocytes (38). Given the fact that PEP35 improved bacterial clearance during S. aureus surgical wound infection (52), we thought it unlikely that PEP35 was inhibiting leukocyte trafficking to the infection site. Initial studies therefore investigated the direct antimicrobial effects of PEP35 on S. aureus. An in-depth analysis was performed by using CLSI methodology to establish the MIC of PEP35 against a wide range of S. aureus clinical isolates including MSSA, HA-MRSA, CA-MRSA, VISA, and VRSA strains. The MIC range for PEP35 against these strains was 128 to 1,024 μg/ml, suggesting little to no direct activity and limited potential for development as an antimicrobial with direct antibacterial activity. Commonly used antimicrobials for the treatment MRSA infections typically display MICs in the range of 0.12 to 4 μg/ml (59).
Having determined that the primary mechanism for PEP35 activity was not as a direct antimicrobial, we now propose PEP35 as an immunomodulator that exclusively enhances early CXC chemokine production. Enhanced CXC chemokine production subsequently promotes a rapid recruitment of neutrophils to the S. aureus wound infection site. It is widely accepted that neutrophils are a critical determinant in the outcome of S. aureus infection (46); however, recent studies suggest that this neutrophil response must also be tightly controlled (13, 30, 31). The fact that a single dose of PEP35 induced a rapid but not sustained (inflammatory response at the infection site was reduced in PEP35-treated mice compared to control mice by day 5) influx of neutrophils to the infection site highlights its great potential as an immunomodulatory treatment for bacterial infections.
PEP35 is very fast acting at the infection site, and optimal immunomodulatory effects (increased CXC chemokine production and neutrophil recruitment) are achieved when PEP35 is administered within the first 4 h of bacterial challenge. These data suggest that PEP35 preferentially modulates early events in the host's immune response to infection. Consistent with this conclusion, our in vitro data demonstrate that PEP35 has a more pronounced effect on CXCL1 production over that of CXCL2 in macrophages. In addition, PEP35 demonstrated reduced efficacy in terms of CXCL2 production when administered at 2 h after bacterial challenge, supporting the notion that this may not be the primary target pathway for PEP35 in vivo. It has previously been established that during an inflammatory immune response CXCL1 is typically produced with a more rapid time course than other CXC chemokines (2, 12, 21, 30, 31). Therefore, at the S. aureus infection site the more rapid production of CXCL1 makes this a more likely target pathway for the “early” actions of PEP35. It is also possible that PEP35 may impact upon specific signaling pathways that preferentially regulate the production of CXCL1 as opposed to CXCL2, since previous studies have demonstrated that CXCL1 and CXCL2 may be under distinct regulatory controls in vivo (12). Further studies are required to extensively profile the effects of PEP35 on other inflammatory mediators produced at the S. aureus infection site; however, our data indicate that PEP35 may be exclusively enhancing CXC chemokine production since we did not observe any increase in CC chemokine or proinflammatory cytokine production at the wound site in the presence of PEP35.
The lack of a prolonged inflammatory response after PEP35 treatment may be attributed to the relatively short half-life of the peptide as is typical of cationic peptides in vivo (16). Preliminary studies indicate that the estimated half-life of PEP35 in the circulation is 20 min when administered intravenously and, after local administration, the peptide is detectable in the circulation for 60 min postdosing (L. Saward and X. Han, data not shown). These data imply that PEP35 has a very narrow window of opportunity to modulate or enhance only those aspects of the host immune response that are occurring within that time window. We therefore propose that the optimal treatment regimen for PEP35 would be repeated prophylactic administration following surgery. The presence of PEP35 at the wound site would ensure that immediately upon contamination of the wound with S. aureus, PEP35 would rapidly enhance neutrophil recruitment to the infection site, facilitating a rapid clearance of the invading organism and preventing the progression of fulminant wound infection. The feasibility of this approach is supported by the fact that PEP35 alone does not cause any associated inflammation at the site of administration. The benefits of repeated administration of PEP35 once wound infection has been diagnosed should also be considered. Preliminary studies have demonstrated that administration of PEP35 at the time of challenge and again at 24 h postchallenge resulted in a significant reduction in tissue bacterial burden at the wound site on day 3 (no PEP35 versus PEP35 at time zero and 24 h postchallenge = 5.5 ± 0.2 and 2.3 ± 0.94 log CFU/g of tissue, respectively).
Our previous data suggest that PEP35 is most effective in the presence of low numbers of bacteria (52). Therefore, we predict that it will be most effective therapeutically very early in the infectious process, before clinical symptoms may even become apparent. In the present study, therefore, we focused on establishing the mechanisms of action of PEP35 at very early time points in the infectious process. We have, however, generated some preliminary data on the effect of PEP35 on established infection at later time points in the presence of a higher bioburden. The administration of PEP35 at day 3 and again on day 5 after establishing S. aureus surgical wound infection resulted in 1.5-log reduction in tissue bacterial burden at the wound site on day 7 (tissue bacterial burden for no PEP versus repeated PEP35 treatment = 6.47 ± 0.3 and 5.1 ± 0.6 log CFU/g of tissue, respectively). The focus of the present study was, however, to establish the mechanism of action of PEP35 in vivo, and further work is required to optimize its clinical efficacy.
Although PEP35 shares some immunomodulatory properties with endogenous antimicrobial peptides such as the cathelicidin family of peptides, it lacks the direct antimicrobial activity of these host defense peptides. The human cathelicidin LL-37 has the ability to modulate the immune response through the recruitment of neutrophils, monocytes, T cells, and mast cells (53) and has been shown to promote CXC chemokine production (55). However, in the presence of bacterial stimulation, LL-37 has also been shown to downregulate NF-κB induced signaling in macrophages (32), a mechanism distinct from that described here for PEP35.
A recent study has already highlighted the therapeutic potential of an immunomodulatory host defense peptide (HDP) in the treatment of S. aureus infections. This HDP enhanced production of the CC chemokine CCL2, which is responsible for driving monocyte/macrophage recruitment and activation during the immune response. (42). We now propose PEP35 as an alternative immunomodulatory peptide treatment for S. aureus wound infections, which utilizes a distinct mechanism of action, i.e., promoting early recruitment of neutrophils to the infection site. In our model, PEP35 exclusively increased CXC chemokine production at the wound infection site and had no significant effect on the production of the CC chemokine CCL2. In line with this, PEP35 did not significantly increase the proportions of F4/80+ macrophages present at the wound site at 24 h (no PEP = 35.12%, PEP35 = 42%) or 72 h (no PEP = 38.3%, PEP35 = 43.5%) after bacterial challenge. As a consequence of the surgical trauma, monocytes are recruited rapidly to the wound site and differentiate into tissue macrophages. These tissue macrophages are therefore available as resident cells at the wound site and can rapidly respond to the bacteria and/or PEP35. Thus, macrophages represent an early effector cell target for PEP35 and can participate in the recruitment of neutrophils to the site of infection by secreting CXC chemokines (and proinflammatory cytokines). Preliminary studies using a murine skeletal muscle cell line (the other major resident cell type present at the surgical wound site in our model) demonstrated that these cells were not capable of producing significant CXC chemokines in response to S. aureus stimulation (R. M. McLoughlin, data not shown). It must be considered, however, that PEP35 may be capable of activating other effector cell types, e.g., dendritic cells and fibroblasts, which may also be present at the wound site.
Our in vitro and in vivo results indicate that in the absence of bacterial stimulation PEP35 is not effective, suggesting that instead PEP35 acts to modulate a signaling pathway that is initially activated by the bacteria. During S. aureus infection distinct innate signaling pathways are activated by the multitude of different PAMPs expressed by the bacteria. Significant controversy exists currently with regard to the specific signaling pathways engaged by individual S. aureus virulence factors, and the relative contribution of each of these innate signaling pathways to the host's immune response remains unclear (17, 37, 48). A common point of convergence, however, between multiple pathways is the family of NF-κB transcription factors. The NF-κB family of transcription factors consists of a group of proteins that are retained as homo- or heterodimers (45) in an inactive state in the cytoplasm bound to inhibitory proteins known as IκBs. Activation of a PPR or proinflammatory cytokine receptor activates a signal transduction cascade, which leads to degradation of the IκB inhibitor complex and the release of NF-κB dimers that can then translocate to the nucleus and turn on target gene transcription (5). Drugs that inhibit the IκB complex degradation can be used to inhibit the actions of NF-κB (34, 44). In the presence of the IκB inhibitor Bay 11-7082 (40), PEP35 was unable to induce any increase in CXCL1 production by the macrophages above that induced by S. aureus alone. These data suggest that activation of the NF-κB transcription complex is critically required to mediate the effects of PEP35 on CXC chemokine production.
In an attempt to establish a more precise mechanism of action for PEP35 in our system, we investigated the interaction between PEP35 and TLR2. Neutrophil recruitment and proinflammatory responses during S. aureus infection have previously been shown to be critically dependent upon TLR2 signaling in vivo (23, 43, 49). In TLR2−/− mice the actions of PEP35 were diminished, suggesting that TLR2 signaling is significantly involved in mediating the effects of PEP35 in vivo. It has recently been shown that the HA receptor CD44 can negatively regulate TLR2-mediated activation of NF-κB signaling through a direct association between CD44 and TLR2 on the cell surface (22). It is therefore tempting to speculate that PEP35 does not interact directly with TLR2 but may mediate its effects in vivo by somehow interfering with the regulatory effects of CD44 on TLR2-mediated signaling, thus indirectly and temporarily enhancing S. aureus activation of TLR2-mediated CXC chemokine production. The fact that PEP35 still retained some activity in the TLR2−/− mice (i.e., the bacterial burden was reduced by 0.8 log in the presence of PEP35) indicated that PEP35 may also modulate other PRRs that signal through NF-κB. In line with this, we observed the ability of PEP35 to enhance E. coli-LPS induced CXCL8 production by human monocytes, suggesting that PEP35 can also interact with TLR4 (see Fig. S3 in the supplemental material). A large-scale screen beyond the scope of the present study is ultimately required to absolutely establish the precise cellular receptors targeted by PEP35 in vivo, which results in NF-κB-mediated effects specifically on CXC chemokine production.
In conclusion, we propose PEP35 as a novel immunomodulatory treatment for S. aureus wound infection through its ability to enhance early CXC chemokine-driven neutrophil recruitment to the infection site. We demonstrate here for the first time the ability of a HABP to drive CXC chemokine production through a mechanism that is dependent upon activation of NF-κB signaling pathways and also appears to be TLR2 dependent. Novel immunomodulatory molecules such as this, which could be used to supplement or complement the effects of standard antibiotic therapy, represent an alternative and exciting approach for the future treatment of S. aureus and other bacterial infections, particularly in the face of rising levels of antibiotic resistance among bacterial strains. In the present study all experiments were carried out in the absence of antibiotic because it was necessary to first establish the mechanism of action of this immunomodulatory compound in vivo, which would have been difficult to achieve in the in the presence of antibiotics. Clearly, immunomodulatory therapies such as this represent the future of the treatment of S. aureus infections given that it is unlikely that bacterial resistance will develop toward these molecules since they are not directed against the microorganism.
Supplementary Material
Acknowledgments
This study was supported by Cangene Corp.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 19 July 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Appelbaum, P. C. 2006. MRSA: the tip of the iceberg. Clin. Microbiol. Infect. 12(Suppl. 2):3-10. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, D. A., J. A. Major, A. Chudyk, and T. A. Hamilton. 2004. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J. Leukoc. Biol. 75:641-648. [DOI] [PubMed] [Google Scholar]
- 3.Astagneau, P., C. Rioux, F. Golliot, and G. Brucker. 2001. Morbidity and mortality associated with surgical site infections: results from the 1997-1999 INCISO surveillance. J. Hosp. Infect. 48:267-274. [DOI] [PubMed] [Google Scholar]
- 4.Beaty, S. R., C. E. Rose, Jr., and S. S. Sung. 2007. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J. Immunol. 178:1882-1895. [DOI] [PubMed] [Google Scholar]
- 5.Bonizzi, G., and M. Karin. 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280-288. [DOI] [PubMed] [Google Scholar]
- 6.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. New Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 7.Chen, G., M. H. Shaw, Y. G. Kim, and G. Nunez. 2009. NOD-like receptors: role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 4:365-398. [DOI] [PubMed] [Google Scholar]
- 8.Chiang, L. Y., D. C. Sheppard, F. N. Gravelat, T. F. Patterson, and S. G. Filler. 2008. Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease. Infect. Immun. 76:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Filippo, K., R. B. Henderson, M. Laschinger, and N. Hogg. 2008. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J. Immunol. 180:4308-4315. [DOI] [PubMed] [Google Scholar]
- 10.Deshmukh, H. S., J. B. Hamburger, S. H. Ahn, D. G. McCafferty, S. R. Yang, and V. G. Fowler, Jr. 2009. Critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infect. Immun. 77:1376-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Easton, D. M., A. Nijnik, M. L. Mayer, and R. E. Hancock. 2009. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 27:582-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endlich, B., D. Armstrong, J. Brodsky, M. Novotny, and T. A. Hamilton. 2002. Distinct temporal patterns of macrophage-inflammatory protein-2 and KC chemokine gene expression in surgical injury. J. Immunol. 168:3586-3594. [DOI] [PubMed] [Google Scholar]
- 13.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 14.Hamill, P., K. Brown, H. Jenssen, and R. E. Hancock. 2008. Novel anti-infectives: is host defence the answer? Curr. Opin. Biotechnol. 19:628-636. [DOI] [PubMed] [Google Scholar]
- 15.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 16.Hancock, R. E., and H. G. Sahl. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551-1557. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto, M., K. Tawaratsumida, H. Kariya, A. Kiyohara, Y. Suda, F. Krikae, T. Kirikae, and F. Gotz. 2006. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 177:3162-3169. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann, E., O. Dittrich-Breiholz, H. Holtmann, and M. Kracht. 2002. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72:847-855. [PubMed] [Google Scholar]
- 19.Hruz, P., A. S. Zinkernagel, G. Jenikova, G. J. Botwin, J. P. Hugot, M. Karin, V. Nizet, and L. Eckmann. 2009. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc. Natl. Acad. Sci. U. S. A. 106:12873-12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung, C. C., C. T. Chang, K. H. Chen, Y. C. Tian, M. S. Wu, M. J. Pan, A. Vandewalle, and C. W. Yang. 2006. Upregulation of chemokine CXCL1/KC by leptospiral membrane lipoprotein preparation in renal tubule epithelial cells. Kidney Int. 69:1814-1822. [DOI] [PubMed] [Google Scholar]
- 21.Hurst, S. M., T. S. Wilkinson, R. M. McLoughlin, S. Jones, S. Horiuchi, N. Yamamoto, S. Rose-John, G. M. Fuller, N. Topley, and S. A. Jones. 2001. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 14:705-714. [DOI] [PubMed] [Google Scholar]
- 22.Kawana, H., H. Karaki, M. Higashi, M. Miyazaki, F. Hilberg, M. Kitagawa, and K. Harigaya. 2008. CD44 suppresses TLR-mediated inflammation. J. Immunol. 180:4235-4245. [DOI] [PubMed] [Google Scholar]
- 23.Kielian, T., A. Haney, P. M. Mayes, S. Garg, and N. Esen. 2005. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect. Immun. 73:7428-7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostenko, V., H. Ceri, and R. J. Martinuzzi. 2007. Increased tolerance of Staphylococcus aureus to vancomycin in viscous media. FEMS Immunol. Med. Microbiol. 51:277-288. [DOI] [PubMed] [Google Scholar]
- 25.Kundsin, R. B., C. W. Walter, and P. Morin. 1964. Staphylococcus aureus UC-18: agent of nosocomial infections. Science 145:1322-1323. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J., G. Cacalano, T. Camerato, K. Toy, M. W. Moore, and W. I. Wood. 1995. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 155:2158-2164. [PubMed] [Google Scholar]
- 27.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 28.Lilani, S. P., N. Jangale, A. Chowdhary, and G. B. Daver. 2005. Surgical site infection in clean and clean-contaminated cases. Indian J. Med. Microbiol. 23:249-252. [PubMed] [Google Scholar]
- 29.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLoughlin, R. M., J. C. Lee, D. L. Kasper, and A. O. Tzianabos. 2008. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 181:1323-1332. [DOI] [PubMed] [Google Scholar]
- 31.McLoughlin, R. M., R. M. Solinga, J. Rich, K. J. Zaleski, J. L. Cocchiaro, A. Risley, A. O. Tzianabos, and J. C. Lee. 2006. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc. Natl. Acad. Sci. U. S. A. 103:10408-10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mookherjee, N., H. L. Wilson, S. Doria, Y. Popowych, R. Falsafi, J. J. Yu, Y. Li, S. Veatch, F. M. Roche, K. L. Brown, F. S. Brinkman, K. Hokamp, A. Potter, L. A. Babiuk, P. J. Griebel, and R. E. Hancock. 2006. Bovine and human cathelicidin cationic host defense peptides similarly suppress transcriptional responses to bacterial lipopolysaccharide. J. Leukoc. Biol. 80:1563-1574. [DOI] [PubMed] [Google Scholar]
- 33.Morel, J. C., C. C. Park, P. Kumar, and A. E. Koch. 2001. Interleukin-18 induces rheumatoid arthritis synovial fibroblast CXC chemokine production through NFκB activation. Lab. Invest. 81:1371-1383. [DOI] [PubMed] [Google Scholar]
- 34.Mori, N., Y. Yamada, S. Ikeda, Y. Yamasaki, K. Tsukasaki, Y. Tanaka, M. Tomonaga, N. Yamamoto, and M. Fujii. 2002. Bay 11-7082 inhibits transcription factor NF-κB and induces apoptosis of HTLV-1-infected T-cell lines and primary adult T-cell leukemia cells. Blood 100:1828-1834. [DOI] [PubMed] [Google Scholar]
- 35.Mukaida, N., A. Hishinuma, C. O. Zachariae, J. J. Oppenheim, and K. Matsushima. 1991. Regulation of human interleukin 8 gene expression and binding of several other members of the intercrine family to receptors for interleukin-8. Adv. Exp. Med. Biol. 305:31-38. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee, S., A. Ukil, and P. K. Das. 2007. Immunomodulatory peptide from cystatin, a natural cysteine protease inhibitor, against leishmaniasis as a model macrophage disease. Antimicrob. Agents Chemother. 51:1700-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullaly, S. C., and P. Kubes. 2006. The role of TLR2 in vivo following challenge with Staphylococcus aureus and prototypic ligands. J. Immunol. 177:8154-8163. [DOI] [PubMed] [Google Scholar]
- 38.Mummert, M. E., M. Mohamadzadeh, D. I. Mummert, N. Mizumoto, and A. Takashima. 2000. Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J. Exp. Med. 192:769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichol, K. A., M. DeCorby, M. McCracken, M. R. Mulvey, J. A. Karlowsky, D. J. Hoban, and G. G. Zhanel. 2009. Comparison of community-associated and hospital-associated methicillin-resistant Staphylococcus aureus in Canada: results from CANWARD 2007. Can. J. Infect. Dis. Med. Microbiol. 20:31-36. [Google Scholar]
- 40.Pierce, J. W., R. Schoenleber, G. Jesmok, J. Best, S. A. Moore, T. Collins, and M. E. Gerritsen. 1997. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272:21096-21103. [DOI] [PubMed] [Google Scholar]
- 41.Rice, L., D. Orlow, K. Ceonzo, G. L. Stahl, A. O. Tzianabos, H. Wada, W. C. Aird, and J. A. Buras. 2005. CpG oligodeoxynucleotide protection in polymicrobial sepsis is dependent on interleukin-17. J. Infect. Dis. 191:1368-1376. [DOI] [PubMed] [Google Scholar]
- 42.Scott, M. G., E. Dullaghan, N. Mookherjee, N. Glavas, M. Waldbrook, A. Thompson, A. Wang, K. Lee, S. Doria, P. Hamill, J. J. Yu, Y. Li, O. Donini, M. M. Guarna, B. B. Finlay, J. R. North, and R. E. Hancock. 2007. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 25:465-472. [DOI] [PubMed] [Google Scholar]
- 43.Sun, Y., A. G. Hise, C. M. Kalsow, and E. Pearlman. 2006. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect. Immun. 74:5325-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutton, C. E., S. J. Lalor, C. M. Sweeney, C. F. Brereton, E. C. Lavelle, and K. H. Mills. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31:331-341. [DOI] [PubMed] [Google Scholar]
- 45.Vallabhapurapu, S., and M. Karin. 2009. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27:693-733. [DOI] [PubMed] [Google Scholar]
- 46.Verdrengh, M., and A. Tarkowski. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect. Immun. 65:2517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verstrepen, L., T. Bekaert, T. L. Chau, J. Tavernier, A. Chariot, and R. Beyaert. 2008. TLR-4, IL-1R, and TNF-R signaling to NF-κB: variations on a common theme. Cell Mol. Life Sci. 65:2964-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Aulock, S., T. Hartung, and C. Hermann. 2007. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 178:2610-2611. (Comment and Author Reply.) [DOI] [PubMed] [Google Scholar]
- 49.von Aulock, S., S. Morath, L. Hareng, S. Knapp, K. P. van Kessel, J. A. van Strijp, and T. Hartung. 2003. Lipoteichoic acid from Staphylococcus aureus is a potent stimulus for neutrophil recruitment. Immunobiology 208:413-422. [DOI] [PubMed] [Google Scholar]
- 50.Yang, B., B. L. Yang, R. C. Savani, and E. A. Turley. 1994. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 13:286-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yount, N. Y., A. S. Bayer, Y. Q. Xiong, and M. R. Yeaman. 2006. Advances in antimicrobial peptide immunobiology. Biopolymers 84:435-458. [DOI] [PubMed] [Google Scholar]
- 52.Zaleski, K. J., T. Kolodka, C. Cywes-Bentley, R. M. McLoughlin, M. L. Delaney, B. T. Charlton, W. Johnson, and A. O. Tzianabos. 2006. Hyaluronic acid binding peptides prevent experimental staphylococcal wound infection. Antimicrob. Agents Chemother. 50:3856-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39-48. [DOI] [PubMed] [Google Scholar]
- 54.Zhanel, G. G., J. A. Karlowsky, M. DeCorby, K. A. Nichol, A. Wierzbowski, P. J. Baudry, P. Lagace-Wiens, A. Walkty, F. Schweizer, M. McCracken, M. R. Mulvey, and D. J. Hoban. 2009. Antimicrobial resistant pathogens in Canadian hospitals: results of the Canadian ward surveillance study (CANWARD 2007). Can. J. Infect. Dis. Med. Microbiol. 20:9-19.20190888 [Google Scholar]
- 55.Zuyderduyn, S., D. K. Ninaber, P. S. Hiemstra, and K. F. Rabe. 2006. The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J. Allergy Clin. Immunol. 117:1328-1335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.