Abstract
Taenia ovis is a cestode parasite infecting primarily sheep as intermediate hosts and dogs as definitive hosts. The first highly effective, recombinant vaccine against a parasitic organism was developed against T. ovis infection in sheep. Three separate host-protective antigens (To16, To18, and To45W) have been cloned from the oncosphere of the parasite. We localize these antigens in the oncosphere by using quantitative immunogold labeling and transmission electron microscopy. The three antigens were uniquely associated with penetration gland cells. The cytoplasm and secretory granules of both penetration gland type 1 and type 2 cells exhibited statistically significant levels of staining for each of the three antigens. The intensity of labeling of the penetration gland type 1 cell was approximately three to five times greater (P < 0.01) compared to the level of staining intensity seen in the penetration gland type 2 cell. In activated oncospheres, secretory blebs were found to contain granules with a structure similar to those observed in the penetration gland cells. The granules within the secretory blebs were shown to stain specifically for the presence of each of the three host-protective antigens. The absence of surface location of the T. ovis antigens suggests that the parasite may not be susceptible to vaccine-induced antibody- and complement-mediated attack until some postoncospheral development has occurred after infection of the intermediate host.
Taenia ovis (phylum Platyhelminthes, class Cestoda, family Taeniidae) is a parasite primarily infecting dogs as definitive hosts and sheep or goats as intermediate hosts. T. ovis is an economically important species, causing cysticercosis in intermediate hosts, leading to economic losses in the sheep meat industry, particularly in Australia and New Zealand (1, 12, 17). T. ovis was the first parasite against which a highly effective recombinant vaccine (To45W) was developed (9). After discovery of the host-protective To45W antigen, two further host-protective recombinant antigens (To16 and To18) were also identified (4). T. ovis has provided a model for the development of vaccines against other taeniid species, and collectively these are the most-effective defined-antigen vaccines against any parasitic infection (15, 16). All of the host-protective antigens for the taeniid cestodes have been isolated from the oncosphere. These vaccines appear to target the parasites either at the time they infect the intermediate host, or shortly thereafter, because vaccinated animals show little, if any, evidence of the presence of parasites following a challenge infection. The principal immune mechanism is believed to be antibody and complement-mediated lysis of the oncosphere or early developing parasite (26).
There has been much speculation about the association of the host-protective immune response and the role of oncospheral penetration gland secretions as secretory blebs; however, this has never been confirmed. Miyazato et al. (20) identified secretory blebs arising from oncospheres of Hymenolepis nana in vivo while they were penetrating the intestinal mucosa, indicating that the blebs are not simply in vitro artifacts. Silverman and Hansen (30) proposed that resistance to reinfection in taeniids was most likely to be due to exogenous antigens released during cysticercus development. Rickard and Bell (23-25) showed that some host-protective antigens from oncospheres were excretory/secretory products. Silverman and Maneely (29), Heath (5), and Rajasekariah et al. (21, 22) proposed that the secretions contained in secretory blebs produced from the penetration glands of activated oncospheres may be a source of host-protective antigens. However, there has been little direct evidence to support the hypothesis. Benitez et al. (2) identified an antigen, referred to as HP6, as being associated with the penetration glands of Taenia saginata oncospheres. Rajasekariah et al. (21, 22) reported that host-protective antigens were present in oncospheres prior to their activation and were not simply produced de novo by metabolically active parasites.
Recently, three host-protective antigens in T. ovis oncospheres were localized by using immunohistochemical and light microscopic methods (7). These studies revealed that the antigens were present uniquely in two pairs of oncospheral cells, arranged bilaterally. The techniques used in the studies described by Jabbar et al. (7) did not, however, provide details about the type and characteristics of the cells which contained the antigens, or whether the T. ovis host-protective antigens were present in the secretions from activated oncospheres. Alternative methods for examination of the specimens that can provide much greater detail, while still allowing immunological localization of specific antigens, are immunogold labeling and transmission electron microscopy. A recent comprehensive reconstruction of the cellular structure of T. ovis oncosphere from serial sections provides a clear basis for an accurate localization of antigens in T. ovis oncospheres (8). We present quantitative data from immunogold labeling which localizes To16, To18, and To45W antigens in both nonactivated and activated oncospheres and secretory blebs.
MATERIALS AND METHODS
Collection of parasite material.
T. ovis was maintained in dogs and sheep as previously described (3, 10). All procedures were performed in accordance with the requirements of the University of Melbourne Animal Ethics Committee.
Collection, hatching, and activation of oncospheres.
Nonactivated oncospheres were obtained by treating mature eggs of T. ovis with sodium hypochlorite, while activation was achieved by incubating the eggs in artificial gastric and intestinal fluids as previously described (7). Activated oncospheres were processed for immunolabeling within approximately 1 to 2 h after activation.
Production of antigens.
Recombinant antigens (To16, To18, and To45W) were expressed in Escherichia coli as glutathione S-transferase (GST) and maltose-binding (MBP) fusion proteins and purified as previously described (4, 9, 32). Two control proteins (GST and MBP) were also prepared.
Production of polyclonal antisera.
A group of six BALB/c mice (6 to 8 weeks old) was used to raise antiserum against To16-MBP recombinant protein. On day 0, the animals were bled from the tail to collect preimmunization sera and injected subcutaneously with 100 μg of recombinant protein plus 100 μl of Freund incomplete adjuvant (Sigma-Aldrich, Australia) on days 0, 15, 30, 45, and 75. The mice were bled from the tail to check the titer, and on day 85 all of the animals were bled by cardiac puncture under anesthesia to collect the antiserum. The antisera to To18-MBP (rabbit) and To45W-MBP (mouse) and the control proteins (rabbit ant-MBP and mouse anti-MBP) used here were as previously described (7).
Western blotting.
Western blot analysis was used to determine the specificity of antibodies raised against the To16-MBP antigen. Electrophoresis of recombinant proteins was carried out in an SDS-13% PAGE gel (11), and electroblotting was performed (31) by using a Trans-Blot SD semidry electrophoretic cell (Bio-Rad, California) to a nitrocellulose membrane (Hybond ECL-Amersham Biosciences, United Kingdom). The membrane was blocked with 5% skim milk powder in phosphate-buffered saline plus 0.05% Tween 20 (PBST; Sigma-Aldrich) overnight at 4°C. After a washing step, the membrane was incubated with immune serum diluted 1:1,000 in PBST at an ambient temperature for 1 h. Preimmune serum at equivalent dilution was used as a negative control. The membrane was washed and incubated with secondary antibodies labeled with horseradish peroxidase (HRP; sheep anti-mouse; Sigma-Aldrich) and diluted 1:4,000 in 5% skim milk powder in PBST at an ambient temperature for 1 h. After a washing step, enhanced chemiluminescent substrate for the detection of HRP (Pierce ECL Western blotting substrate; Quantum Scientific, Australia) was added to the blot, followed by exposure to X-ray film. The developed film was scanned by using an SI-375 personal densitometer (Molecular Dynamics).
ELISA.
Animals immunized with To16-MBP were assessed for specific antibody titer against the respective immunizing antigen by using an enzyme-linked immunosorbent assay (ELISA) essentially as previously described (10). The secondary antibody used for ELISA was the same as that used for Western blotting. The optical density at 450 nm (OD450) for the antiserum was plotted, and the titer was read as the dilution at which the OD450 equaled 1.0.
Cryofixation of oncospheres.
Oncospheres (nonactivated and activated) were gently pelleted (centrifugation at 192 × g for 10 min), and the supernatant was discarded. Approximately two 3-μl droplets of the suspension were sandwiched between type A brass freezer hats (ProSciTech, Thuringowa, Australia). The enclosed oncosphere suspensions were frozen by using a Leica (Vienna, Austria) EM high-pressure freezer. The freezer hats enclosing the frozen oncosphere suspensions were split apart and stored in liquid N2 in cryovials prior to freeze-substitution in a Leica EM automated freeze substitution unit.
Freeze substitution.
Frozen oncosphere pellets were freeze substituted in 0.1% uranyl acetate in acetone at −90°C for 48 h, and the temperature was raised to −50°C at 6°C h−1. Pellets were scraped out of the freezer hats, rinsed in three changes of acetone (30 min each), and infiltrated with a graded series of Lowicryl HM20 low-temperature resins (Polysciences, Warrington, PA) in acetone consisting of 25% (8 h), 50% (overnight), 75% (8 h), and 100% resin (overnight). The infiltrated samples were placed in a fresh change of 100% resin in gelatin capsules, polymerized under UV light for 48 h at −50°C, and brought to room temperature at 6°C h−1. The soft sample blocks were then hardened under UV light for a further 24 h at room temperature.
Immunogold labeling and transmission electron microscopy.
The embedded oncospheres in blocks were sectioned with a diamond knife on a Leica Ultracut R microtome (Vienna), and ultrathin sections (90 nm) were collected onto pioloform-coated 100 mesh hexagonal gold grids (ProSciTech). The grids were blotted with the section side up on filter paper overnight prior to immunolabeling. The sections were blocked for nonspecific antibody binding sites by incubating the grids on 20-μl droplets of blocking buffer (1% bovine serum albumin [Sigma-Aldrich] in PBS containing 0.01% of each of Tween 20 and Triton X-100) for 30 min. The grids were then incubated on 20-μl droplets of optimally diluted primary antibodies against To16-MBP (1:20,000), To18-MBP (1:50,000), and To45W-MBP (1:25,000) in blocking buffer overnight at 4°C. Controls were reacted with anti-MBP antisera at the same dilution as that of the specific antisera. Grids were washed three times on 20-μl droplets of blocking buffer for 5 min each and then incubated on 20-μl droplets of diluted (1:100 with blocking agent) colloidal gold-conjugated secondary antibodies (12-nm colloidal gold-AffiniPure goat anti-rabbit and goat anti-mouse IgG; Jackson Immunoresearch, Pennsylvania) overnight at 4°C. Labeled grids were rinsed three times on the drops of blocking buffer and then three times on drops of PBS, followed by three 30-s immersions in distilled water before air drying. The immunolabeled sections on grids were sequentially stained with 2% uranyl acetate for 10 min and triple lead stain for 5 min (27) and viewed in a Phillips CM120 Biotwin transmission electron microscope at 120 kV. Images were captured with a Gatan Multiscan 600CW digital camera (Gatan, Inc., California) using the Gatan software. Photomicrographs of immunogold labeling were prepared at a magnification sufficiently low to provide an understanding of the ultrastructural features of the regions containing positive labeling, while allowing visual recognition of individual immunogold particles in the photomicrographs. At the magnifications chosen for the photomicrographs, it was difficult to clearly differentiate the immunogold particles from other ultrastructural features visually. For this reason, all immunogold particles in each photomicrograph were identified unequivocally at higher magnifications in the electron microscope, and every particle labeled in the figures is shown.
Quantification of immunogold labeling and statistical analyses.
A quantitative analysis of the distribution of immunogold particles was performed to determine the immunolabeling density for To16, To18, and To45W. Five oncospheres with intact ultrastructure and clear immunolabeling from the sections stained with specific as well as control (anti-MBP) antisera were randomly chosen for counting the total immunogold particles. Immunogold particles were counted on all cell types, muscles, hooks, and membranes of the oncospheres. The total area for all cell types was measured for each oncosphere by using the software ImageJ (National Institutes of Health), and the numbers of immunogold particles in each cell were counted manually. The labeling density was calculated by dividing the immunogold particle numbers by the area (i.e., the antigen number per square micrometer of cellular area). The distribution of immunogold particles was categorized according to different cell types to determine cells associated with the antigens. The unit of analysis was the oncosphere, and oncospheres with two cells of a particular type used the sum of the gold particles divided by the sum of their areas as the response variable. Statistical comparison of labeling density for To16, To18, To45W, and control antisera for each cell type was performed by using one-way analysis of variance and Dunnett's test. PASW 18 for Windows (SPSS, Inc., Chicago, IL) was used to perform the analysis. A paired t test compared the labeling density between the penetration gland type 1 and 2 cells within the oncospheres (nonactivated and activated) reacted with To16, To18, and To45W antisera. This test was performed by using software Stata 11.0 for Windows (StataCorp, College Station, TX). A P value of <0.01 was considered statistically significant.
RESULTS
Specificity and sensitivity of antisera.
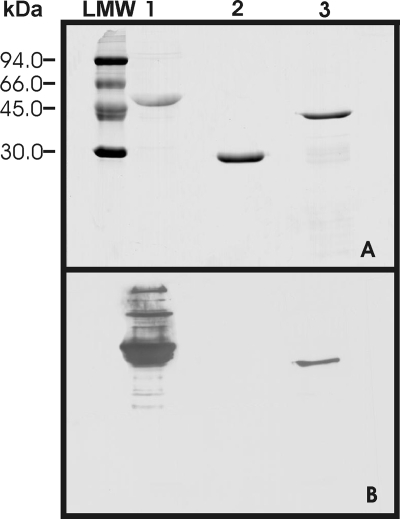
Details of the specificity and sensitivity of antisera against To18-MBP and To45W-MBP used in the present study have been described in a previous publication (7). The antiserum raised against To16-MBP was found to be specific to the antigen (Fig. 1). In ELISA, the titer of the antiserum was determined to be 50,000 (data not shown).
FIG. 1.
Western blot analysis of the To16 recombinant antigen of T. ovis. (A) Coomassie blue-stained SDS-PAGE gel containing the antigens. Lane 1, maltose-binding protein (MBP) (42.6 kDa); lane 2, glutathione S-transferase (GST) (26.9 kDa); lane 3, To16-GST (41.9 kDa). LMW, low-molecular-weight marker. The gel was transferred to a nitrocellulose membrane and probed with specific antisera raised against To16-MBP (B), followed by incubation with HRP-conjugated antibodies, and detected with a chemiluminescent substrate. The antiserum raised against To16 was found to have specific reactivity with To16 antigen and the control (MBP) fusion protein.
Immunogold labeling.
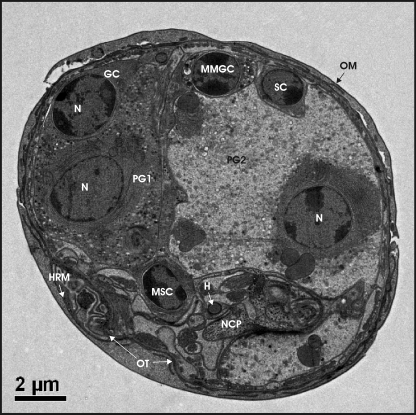
The general architecture of T. ovis oncospheres is depicted in Fig. 2. The penetration gland type 1 and type 2 cells are shown together with some of the other oncosphere features examined in the antigen localization studies. A full description of the ultrastructure of the oncosphere is given in Jabbar et al. (8).
FIG. 2.
Transmission electron micrograph showing cell types, membranes and hook(s) in a nonactivated oncosphere of Taenia ovis. Abbreviations: GC, germinative cell; H, hook; HRM, hook region membrane; MMGC, median mesophoric gland cell; MSC, median somatophoric cell; N, nucleus; NCP, nerve cell process; OM, oncospheral membrane; OT, oncospheral tegument; PG1, penetration gland cell type 1; PG2, penetration gland cell type 2; SC, somatic cell.
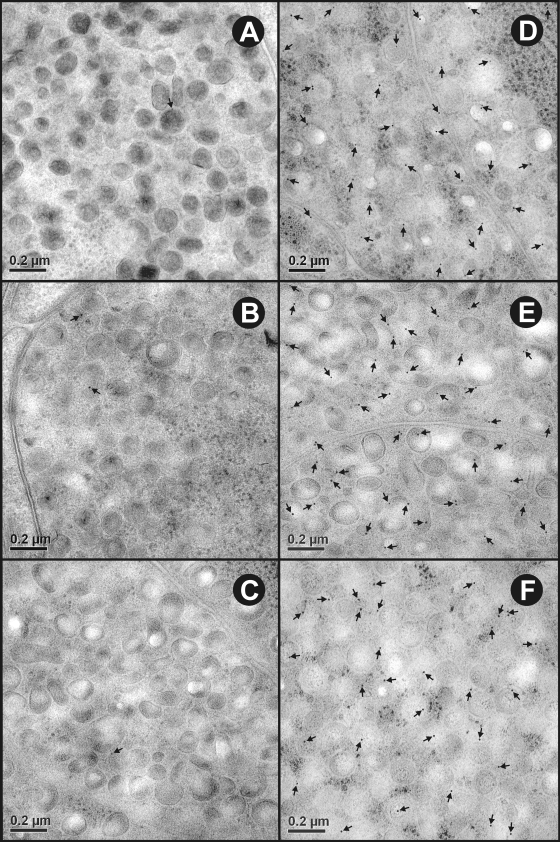
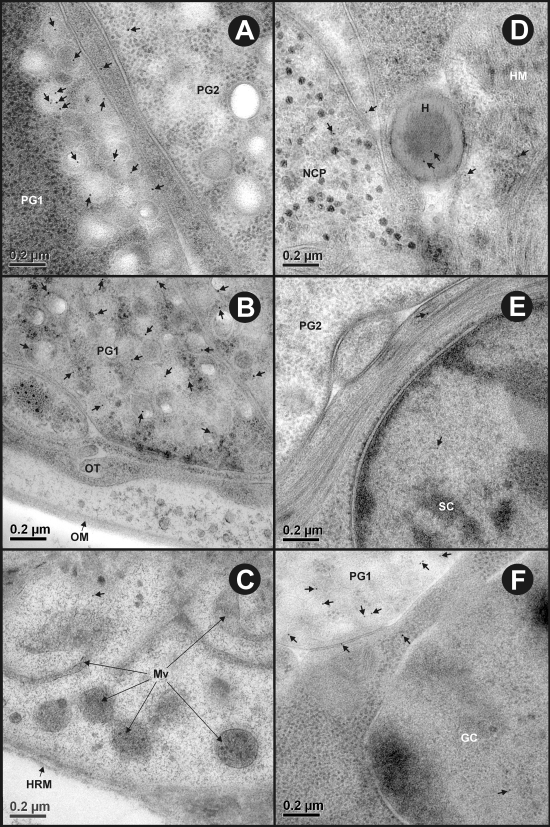
Maximum specific immunogold labeling density for all three T. ovis antigens (To16, To18, and To45W) was found in the cytoplasm and secretory granules of the penetration gland type 1 cell (Fig. 3, Table 1 ). Staining was also apparent for the three antigens in the penetration gland type 2 cell granules and cytoplasm (Fig. 4, Table 1). The specificity of the immunolabeling was confirmed by quantitation of the density of immunogold particles which was significantly (P < 0.01) greater in penetration gland type 1 and penetration gland type 2 cells compared to the same regions reacted with control antisera raised against MBP (Fig. 3, 4, and 5A; Table 1). A low density of gold particles was present in other oncospheral structures and cells and occasionally on the sections supporting film that were reacted with the specific antisera (To16, To18, and To45W). However, this was not statistically different from the background level seen with the control (anti-MBP) antisera (P > 0.01) (Fig. 5, Table 1). Large-sized selected images for one antigen to clearly show the immunogold particles in sections probed with test antisera may be found in the supplemental material.
FIG. 3.
Ultrastructural immunolocalization of To16, To18 and To45W in the penetration gland type 1 (PG1) cell of T. ovis nonactivated oncospheres. (A to C) Sections probed with control antisera raised against the MBP fusion protein had a negligible amount of nonspecific staining. (D to F) Sections probed with specific antisera raised against To16, To18, and To45W, respectively, revealed immunogold particles (arrows) distributed in the cytoplasm and secretory granules of the PG1 cell.
TABLE 1.
Localization of three host-protective antigens (To16, To18, and To45W) in nonactivated and activated oncospheres of T. ovisa
| Type of oncosphere | Specificity of antiserum | Structural feature (density of gold particles in mean counts μm−2 ± SD)b |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PG1 | PG2 | Blebs | MMGC | MSC | SC | GC | NCP | Hook | HM | HRM | SM | OT | OM | ||
| Nonactivated | MBP | 0.25 ± 0.08 (5) | 0.23 ± 0.04 (5) | 0 (1) | 0.11 ± 0.20 (3) | 0.18 ± 0.05 (3) | 0.23 ± 0.22 (5) | 2.37 ± 1.40 (3) | 0.32 ± 0.35 (5) | 0 ± 0 (3) | 0.66 ± 0.44 (5) | 0.43 ± 0.23 (5) | 0.68 ± 0.40 (5) | ||
| To16-MBP | 7.63 ± 1.42*a (5) | 2.59 ± 0.62*b (5) | 0.23 ± 0.32 (2) | 0.19 ± 0.14 (4) | 0.21 ± 0.19 (4) | 0.42 ± 0.16 (3) | 1.33 ± 1.30 (5) | 0.33 ± 0.26 (5) | 0.22 ± 0.26 (4) | 0.70 ± 0.11 (5) | 0.54 ± 0.14 (5) | 0.71 ± 0.20 (5) | |||
| To18-MBP | 9.84 ± 2.13*a (5) | 2.26 ± 0.55*b (5) | 0.18 ± 0.16 (3) | 0.21 ± 0.05 (2) | 0.25 ± 0.09 (2) | 0.13 ± 0.15 (4) | 1.65 ± 1.51 (5) | 0.69 ± 0.53 (5) | 0 ± 0 (2) | 0.55 ± 0.23 (4) | 0.37 ± 0.12 (5) | 0.49 ± 0.25 (5) | |||
| To45W-MBP | 6.83 ± 1.13*a (5) | 1.75 ± 0.27*b (5) | 1.42 (1) | 0.40 ± 0.28 (4) | 0.32 ± 0.23 (4) | 0.32 ± 0.17 (2) | 0.57 ± 0.62 (5) | 2.28 ± 0.62 (4) | 0.70 ± 0.32 (5) | 0.24 ± 0.24 (3) | 1.09 ± 0.25 (5) | 0.60 ± 0.29 (5) | 0.66 ± 0.30 (5) | ||
| Activated | MBP | 0.33 ± 0.07 (4) | 0.25 ± 0.12 (5) | 0.33 ± 0.29 (4) | 0.35 ± 0.29 (4) | 0.42 ± 0.43 (4) | 0.21 ± 0.29 (2) | 0 ± 0 (2) | 1.33 ± 1.09 (5) | 0.62 ± 0.44 (5) | 0.54 ± 0.38 (5) | 0.40 ± 0.37 (5) | 0.47 ± 0.24 (5) | ||
| To16-MBP | 6.76 ± 1.50*a (5) | 2.00 ± 0.64b (5) | 2.34 ± 1.51 (3) | 1.06 ± 0.63 (3) | 0.95 ± 0.28 (3) | 1.82 ± 0.49 (2) | 1.05 ± 0.49 (4) | 4.10 ± 3.45 (3) | 0.81 ± 0.10 (3) | 0.72 ± 0.10 (2) | 0.68 ± 0.39 (5) | 0.56 ± 0.20 (5) | |||
| To18-MBP | 11.30 ± 1.72*a (5) | 3.26 ± 1.11*b (5) | 8.93 ± 1.45* (3) | 1.40 (1) | 1.33 ± 0.31 (3) | 1.13 ± 0.20 (3) | 1.05 (1) | 1.52 ± 0.72 (4) | 6.39 ± 4.57 (4) | 1.00 ± 0.43 (4) | 0.29 ± 0.57 (4) | 1.00 ± 0.45 (5) | 0.82 ± 0.18 (5) | ||
| To45W-MBP | 8.83 ± 2.75*a (5) | 2.15 ± 1.24b (5) | 8.59 ± 4.80* (2) | 0.32 ± 0.30 (3) | 0.42 ± 0.38 (3) | 0.88 ± 0.12 (2) | 0.83 ± 0.33 (3) | 1.73 ± 1.18 (4) | 0.88 ± 0.24 (4) | 0.15 ± 0.31 (4) | 0.90 ± 0.16 (5) | 0.61 ± 0.15 (5) | |||
The densities of gold particles in various structures and cells revealed after reaction with test (To16-MBP, To18-MBP, and To45W-MBP) or control (anti-MBP) antisera are presented.
Abbreviations: PG1 and PG2, penetration gland cell types 1 and 2; MMGC, median mesophoric gland cell; MSC, median somatophoric cell; SC, somatic cell; GC, germinative cell; NCP, nerve cell process; HM, hook muscle; HRM, hook-region membrane; SM, somatic muscles; OT, oncospheral tegument; OM, oncospheral membrane. An asterisk (*) indicates a significant difference from anti-MBP antisera (P < 0.01). A superscript “a” or “b” within a row indicates P < 0.01 as determined by a paired t test. Significantly different results are indicated in boldface. Values in parentheses indicate the number of oncospheres counted for each cell/ultrastructural feature per type of antisera.
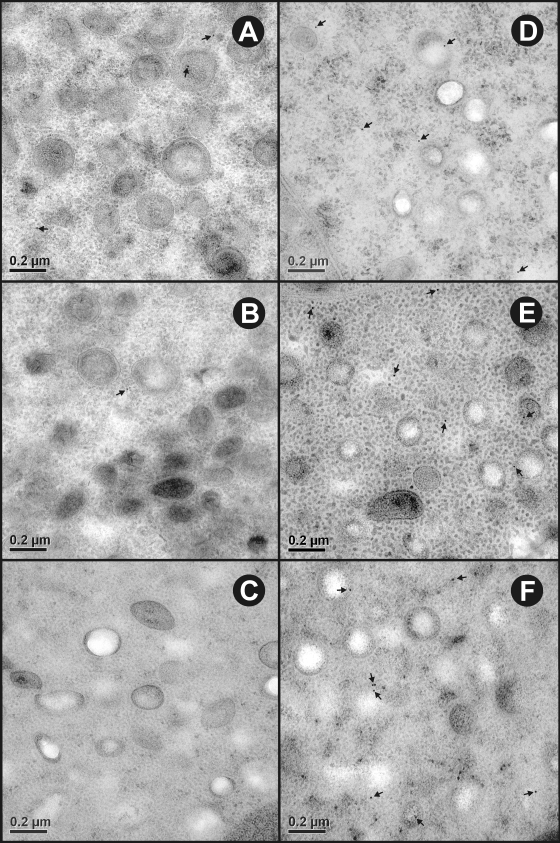
FIG. 4.
Ultrastructural immunolocalization of To16, To18, and To45W in the penetration gland type 2 (PG2) cell of T. ovis nonactivated oncospheres. (A to C) Sections probed with control antisera raised against the MBP fusion protein had a negligible amount of nonspecific staining. (D to F) Sections probed with specific antisera raised against To16, To18, and To45W, respectively, revealed immunogold particles (arrows) distributed sparsely in the cytoplasm and secretory granules of the PG2 cell.
FIG. 5.
Representative images showing immunolabeling density in various ultrastructural features and cells of T. ovis nonactivated oncospheres reacted with antisera against To18. (A) Oncospheral penetration gland cell types 1 (PG1) and 2 (PG2) showing the difference in their labeling density (arrows). (B) Immunogold particles (arrows) are restricted to the PG1 cell, showing no reactivity with the oncospheral membrane (OM) or tegument (OT). (C) The somatophoric pole of the oncosphere showing no labeling on the hook region membrane (HRM) and microvilli (Mv) of the oncospheral tegument (arrows). (D to F) A low background level of labeling (arrows) in Hook (H), hook muscles (HM), nerve cell process (NCP), somatic (SC), and germinative (GC) cells.
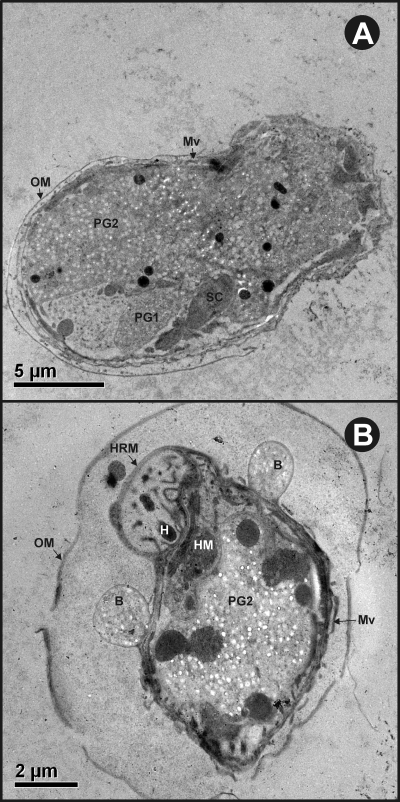
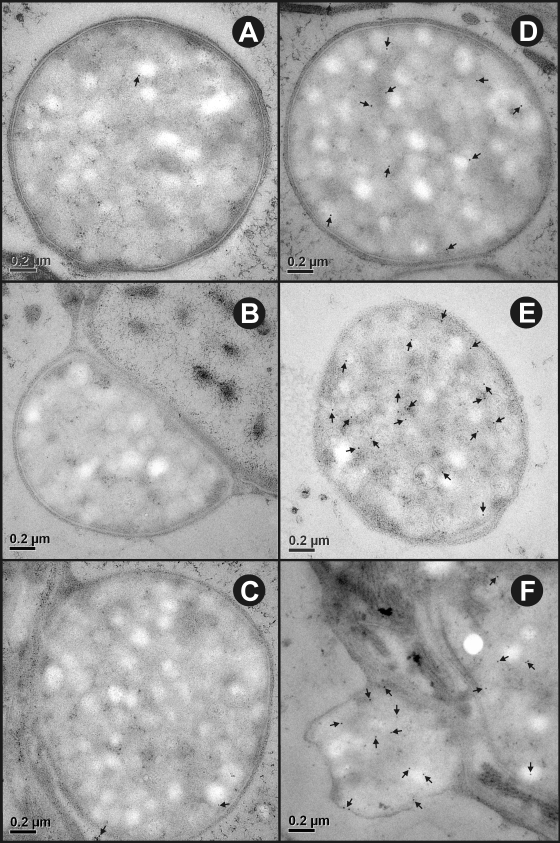
In activated oncospheres, the granules which had been restricted to penetration gland cells in nonactivated oncospheres appeared to spread throughout the oncospheral parenchyma (Fig. 6A). Some activated oncospheres were associated with structures that are known as secretory blebs (Fig. 6B). The blebs were surrounded by a membrane which appeared to have been derived from the oncospheral tegument (Fig. 6B and 7) and appeared to be filled with numerous secretory granules indistinguishable from those seen in the penetration gland cells. The characteristics of the granules in the blebs did not allow their origin to be identified as being associated with either of the two types of penetration gland cells. The contents of the blebs labeled specifically for the presence of each of the three antigens studied (Fig. 7, Table 1). The membrane surrounding the blebs did not appear to stain positively with the specific antisera. Statistical comparisons of the labeling density seen with antisera against To18 and To45W found the densities to be significantly greater than the density of labeling seen with control antisera (P < 0.01). Two of the three bleb samples that reacted with antisera against To16 appeared to show relatively high concentrations of the label (2.51 and 3.76 immunogold particles μm−2), whereas the third specimen showed little binding to this antiserum (0.75 immunogold particles μm−2). Hence, the comparison for the mean of the three specimens (reacted against To16 antisera) was not statistically different compared to the level of reactivity seen with control antisera. Control antisera (rabbit or mice antisera against MBP) showed only a low level of background reactivity in the various oncospheral structures (Table 1).
FIG. 6.
Transmission electron micrographs showing T. ovis activated oncosphere escaping from the oncospheral membrane (A) and an activated oncosphere with secretory blebs (B). Abbreviations: B, secretory bleb; H, hook; HM, hook muscles; HRM, hook region membrane; Mv, microvilli; OM, oncospheral membrane; PG1, penetration gland cell type 1; PG2, penetration gland cell type 2; SC, somatic cell.
FIG. 7.
Ultrastructural immunolocalization of To16, To18, and To45W in the secretory blebs of T. ovis activated oncospheres. (A to C) Sections probed with control antisera raised against MBP. (D to F) Sections probed with specific antisera raised against To16, To18, and To45W, respectively. In all cases, immunogold particles (arrows) are distributed in the granular contents of the secretory blebs.
Quantitative evaluation of the density of the immunogold particles associated with the various oncospheral structures and cells showed no difference between test and control antisera-treated oncospheres for the distribution of immunogold particles on all structures other than penetration gland type 1 and penetration gland type 2 cells (nonactivated and activated oncospheres) and the secretory blebs (activated oncospheres) (Table 1). All control values for immunogold density (MBP) were less than the values in the three noncontrol groups (To16-, To18-, and To45-MBP) for penetration gland type 1 and type 2 cells (nonactivated and activated oncospheres) and blebs (activated oncospheres).
DISCUSSION
In this study, immunogold labeling of To16, To18, and To45W in T. ovis nonactivated and activated oncospheres demonstrated that all three antigens were uniquely associated with the penetration gland cells of the oncospheres. All antigens were distributed predominantly in the cytoplasm and secretory granules of the penetration gland type 1 cell, with the penetration gland type 2 cell also staining positively but at a lower level (Fig. 3 and 4, Table 1). The bilaterally arranged pairs of cells are the cell bodies of a syncytial penetration gland type 1 cell (8). The findings are consistent with the observations made previously using light microscopy for localization of the To16, To18, and To45W antigens (7). Benitez et al. (2) identified the penetration gland as the location of an 18-kDa protein in T. saginata oncospheres, although no quantitative data were provided. Nevertheless, the data described here are consistent with the localization of the homologue of this antigen, To18, in T. ovis.
In both light microscopic studies (7) and the results presented here using immunogold labeling the distribution of the three host-protective antigens changed markedly after the oncospheres were activated. Prior to activation, the antigens were localized exclusively in the penetration gland cells. After activation, the penetration gland granules and antigens become distributed throughout the oncospheral extracellular matrix while still being present within the penetration gland cells. A similar situation was described by Mayta et al. (18), who found that following activation of T. solium oncospheres, the Tso31 protein was present throughout the oncosphere and even on the oncospheral tegument, although these authors did not provide details about antigen distribution in nonactivated oncospheres. The mechanism by which this change in antigen distribution occurs is unclear. Possibly one or both of the penetration gland cells rupture(s), releasing their contents into the oncospheral extracellular matrix. In the specimens examined here, the typical penetration gland type 1 and type 2 granules were apparent, but it could not be determined whether their distribution was restricted to the cytoplasm of intact penetration gland cells. In addition, activated oncospheres were examined by using transmission electron microscopy relatively soon after activation and any change(s) in penetration gland cell integrity over time was not assessed. These aspects of postactivation changes in antigen distribution and penetration cell integrity warrant further studies.
The data described here do not allow accurate quantitation of antigen between different specimens, for example, between the penetration gland cells in nonactivated and activated oncospheres. Using information obtained with light microscopy and the staining intensity of oncospheral penetration glands, Lethbridge and Gijsbers (13) described a decreased staining intensity after activation of Hymenolepis diminuta oncospheres. This has been interpreted as being due to the contents of the glands being secreted and is in keeping with our observation that the penetration gland cell contents were present in the secretory blebs. However, it may also be due to the penetration gland cell contents staining less intensively after the cell contents are released into the oncospheral extracellular matrix.
While studying the ultrastructure of E. granulosus oncospheres that had escaped from the oncospheral membrane, Holcman et al. (6) described the penetration glands as being almost devoid of granules. The amount of time that an individual oncosphere has spent outside the oncospheral membrane is difficult to determine. Not all oncospheres activate and those that activate do not do so simultaneously. After activation, taeniid oncospheres undergo rhythmic movements and the oncospheral membrane is discarded prior to the parasite penetrating the host's intestinal mucosa. Initially, activated oncospheres may be seen that have yet to shed the oncospheral membrane and an example can be seen in Fig. 6. As well as finding the penetration glands of activated E. granulosus oncospheres to be almost devoid of granules, Holcman et al. (6) reported the presence of granules having similar electron densities to penetration gland granules, within the oncospheral tegument. However, we did not observe the presence of secretory granules from the penetration glands within the oncospheral tegument. Holcman et al. (6) also described three main types of vesicles or granules in 2-day-old oncospheres of E. granulosus cultured in vitro and named them disc-like vesicles with dense granular contents (designated Vd); vesicles with spherical electron-dense granules (Vg1); and vesicles similar in shape and size but with an electron-dense, rod-shaped core (Vg2). These authors hypothesized that Vg1 vesicles might be involved in the evasion of host's immune response. However, the vesicles described in E. granulosus (6) were not observed in recently activated oncospheres of T. ovis. This discrepancy in the presence of vesicles could be due to species variation or differences in fixation methods between the two studies.
In the present study, the host-protective T. ovis antigens were found to be present in membrane-bound secretory granules within the secretory blebs. It is well known that after activation, oncospheres secrete/excrete some membrane-bound products. Lethbridge and Gijsbers (13) suggested that the membrane-bound secretory bodies were released from the penetration glands of activated H. diminuta oncospheres. These authors termed these secretory bodies “secretory blebs.” Several authors have speculated about the potential antigenic nature of penetration gland secretions from Taenia oncospheres (5, 21, 22, 28, 29), but there has been no direct evidence to support this hypothesis. Indirect experimental evidence supports the hypothesis that oncospheral secretions are antigenic. Rickard and Bell (23) achieved a high level of immunity against T. ovis in sheep, in which they had previously implanted activated oncospheres within diffusion chambers having a pore size too small to allow intact oncospheres to escape, but large enough to allow the release of excretory/secretory (ES) products. Subsequently, Rajasekariah et al. (21) achieved a high level of protection against the establishment of Taenia taeniaeformis in mice by vaccinating with antigens collected either as a pellet (gravity sedimented or centrifugation) or gravity sedimented supernatant. These researchers hypothesized that the centrifuged pellet (3,500 × g, 1 h) and gravity-sedimented supernatant contained oncospheral material, secretory blebs, and that membrane-bound secretory bodies were likely to have induced the high level of protection in mice against T. taeniaeformis challenge infection. However, prior to the studies described here, no data were available to support this hypothesis. The results provided here with T. ovis give unequivocal evidence that the secretory blebs from penetration glands contain the host-protective antigens.
The discovery that the host-protective antigens in the nonactivated and activated oncospheres of T. ovis are not located on the parasite tegument was unexpected. The principal immunological mechanism by which anti-oncospheral responses have their host-protective effect is through specific antibody and complement-mediated killing of the invading oncospheres (14, 26). This capacity can be demonstrated in vitro (10), as well as in vivo (19). Logically, this mechanism of attack may be expected to have its effects on the tegument. Jabbar et al. (7) demonstrated that the host-protective T. ovis antigens appeared to become associated with the surface of 7- to 9-day-old metacestodes. However, this observation has not been complemented with ultrastructural localization. In addition, another important question remains unsolved, namely, exactly when the host-protective antigens become associated with the parasite's surface. This can be answered by taking specimens at different time intervals following the culture of activated oncospheres. Future studies on developing metacestodes are required to determine precisely when host-protective antigens of taeniid cestodes become associated with the surface and when the parasite is killed by antibody- and complement-mediated attack.
Supplementary Material
Acknowledgments
We are grateful to Ian Beveridge for his comments to improve the quality of the manuscript.
The study was supported by the National Health and Medical Research Council, Australia grants 350279, 400109, and 628320.
Editor: J. H. Adams
Footnotes
Published ahead of print on 19 July 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Arundel, J. H. 1972. A review of cysticercoses of sheep and cattle in Australia. Aust. Vet. J. 48:140-155. [DOI] [PubMed] [Google Scholar]
- 2.Benitez, L., T. Garate, L. J. Harrison, P. Kirkham, S. M. Brookes, and R. M. Parkhouse. 1996. Cloning and sequencing of the gene encoding the principal 18-kDa secreted antigen of activated oncospheres of Taenia saginata. Mol. Biochem. Parasitol. 78:265-268. [DOI] [PubMed] [Google Scholar]
- 3.Coman, B. J., and M. D. Rickard. 1975. The location of Taenia pisiformis, Taenia ovis, and Taenia hydatigena in the gut of the dog and its effect on net environmental contamination with ova. Zeitsch. Parasit. 47:237-248. [DOI] [PubMed] [Google Scholar]
- 4.Harrison, G. B., D. D. Heath, R. P. Dempster, C. Gauci, S. E. Newton, W. G. Cameron, C. M. Robinson, S. B. Lawrence, M. W. Lightowlers, and M. D. Rickard. 1996. Identification and cDNA cloning of two novel low molecular weight host-protective antigens from Taenia ovis oncospheres. Int. J. Parasitol. 26:195-204. [DOI] [PubMed] [Google Scholar]
- 5.Heath, D. D. 1973. Resistance to Taenia pisiformis larvae in rabbits. II. Temporal relationships and the development phase affected. Int. J. Parasitol. 3:491-498. [DOI] [PubMed] [Google Scholar]
- 6.Holcman, B., D. D. Heath, and R. J. Shaw. 1994. Ultrastructure of oncosphere and early stages of metacestode development of Echinococcus granulosus. Int. J. Parasitol. 24:623-635. [DOI] [PubMed] [Google Scholar]
- 7.Jabbar, A., C. T. Kyngdon, C. G. Gauci, A. K. Walduck, C. McCowan, M. K. Jones, I. Beveridge, and M. W. Lightowlers. 2010. Localisation of three host-protective oncospheral antigens of Taenia ovis. Int. J. Parasitol. 40:579-589. [DOI] [PubMed] [Google Scholar]
- 8.Jabbar, A., S. Crawford, D. Młocicki, Z. P. Świderski, D. B. Conn, M. K. Jones, I. Beveridge, and M. W. Lightowlers. Ultrastructural reconstruction of the Taenia ovis oncospheres from serial sections. Int. J. Parasitol., in press. [DOI] [PubMed]
- 9.Johnson, K. S., G. B. Harrison, M. W. Lightowlers, K. L. O'Hoy, W. G. Cougle, R. P. Dempster, S. B. Lawrence, J. G. Vinton, D. D. Heath, and M. D. Rickard. 1989. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature 338:585-587. [DOI] [PubMed] [Google Scholar]
- 10.Kyngdon, C. T., C. G. Gauci, R. A. Rolfe, J. C. Velasquez Guzman, M. J. Farfan Salazar, M. R. Verastegui Pimentel, A. E. Gonzalez, H. H. Garcia, R. H. Gilman, R. A. Strugnell, and M. W. Lightowlers. 2006. In vitro oncosphere-killing assays to determine immunity to the larvae of Taenia pisiformis, Taenia ovis, Taenia saginata, and Taenia solium. J. Parasitol. 92:273-281. [DOI] [PubMed] [Google Scholar]
- 11.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 12.Lawson, J. R. 1994. Hydatid disease and sheep measles: the history of their control and the economics of a recent change of control policy. New Zeal. J. Zool. 21:83-89. [Google Scholar]
- 13.Lethbridge, R. C., and M. F. Gijsbers. 1974. Penetration gland secretion by hexacanths of Hymenolepis diminuta. Parasitology 68:303-311. [DOI] [PubMed] [Google Scholar]
- 14.Lightowlers, M. W., M. A. Gemmell, G. B. L. Harrison, D. D. Heath, M. D. Rickard, and M. G. Roberts. 1992. Control of tissue parasites. II. Cestodes, p. 171-198. In W. K. Yong (ed.), Animal parasite control utilizing biotechnology. CRC Press, Inc., Boca Raton, FL.
- 15.Lightowlers, M. W., A. Flisser, C. G. Gauci, D. D. Heath, O. Jensen, and R. Rolfe. 2000. Vaccination against cysticercosis and hydatid disease. Parasitol. Today 16:191-196. [DOI] [PubMed] [Google Scholar]
- 16.Lightowlers, M. W. 2006. Cestode vaccines: origins, current status and future prospects. Parasitology 133(Suppl.):27-42. [DOI] [PubMed] [Google Scholar]
- 17.Love, S. 2008. Sheep measles: another profit killer. Primefact 55 (January 2008). Department of Primary Industries, New South Wales, Australia.
- 18.Mayta, H., K. Hancock, M. Z. Levine, R. H. Gilman, M. J. Farfan, M. Verastegui, W. S. Lane, H. H. Garcia, A. E. Gonzalez, and V. C. Tsang. 2007. Characterization of a novel Taenia solium oncosphere antigen. Mol. Biochem. Parasitol. 156:154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell, G. F., J. W. Goding, and M. D. Rickard. 1977. Studies on immune responses to larval cestodes in mice: increased susceptibility of certain mouse strains and hypothymic mice to Taenia taeniaeformis and analysis of passive transfer of resistance with serum. Aust. J. Exp. Biol. Med. Sci. 55:165-186. [DOI] [PubMed] [Google Scholar]
- 20.Miyazato, T., T. Furukawa, M. Inoque, A. Niwa, T. Inoque, and K. Shimoda. 1977. Electron microscopic observations on the penetration of oncospheres of Hymenolepis nana into the intestine of the mouse. Acta Med. Kinki University 2:1-18. [Google Scholar]
- 21.Rajasekariah, G. R., G. F. Mitchell, and M. D. Rickard. 1980. Taenia taeniaeformis in mice: protective immunization with oncospheres and their products. Int. J. Parasitol. 10:155-160. [DOI] [PubMed] [Google Scholar]
- 22.Rajasekariah, G. R., M. D. Rickard, and G. F. Mitchell. 1980. Immunization of mice against infection with Taenia taeniaeformis using various antigens prepared from eggs, oncospheres, developing larvae, and strobilocerci. Int. J. Parasitol. 10:315-324. [DOI] [PubMed] [Google Scholar]
- 23.Rickard, M. D., and K. J. Bell. 1971. Immunity produced against Taenia ovis and T. taeniaeformis infection in lambs and rats following in vivo growth of their larvae in filtration membrane diffusion chambers. J. Parasitol. 57:571-575. [PubMed] [Google Scholar]
- 24.Rickard, M. D., and K. J. Bell. 1971. Successful vaccination of lambs against infection with Taenia ovis using antigens produced during in vitro cultivation of the larval stages. Res. Vet. Sci. 12:401-402. [PubMed] [Google Scholar]
- 25.Rickard, M. D., and K. J. Bell. 1971. Induction of immunity of lambs to a larval cestode by diffusible antigens. Nature 232:120. [DOI] [PubMed] [Google Scholar]
- 26.Rickard, M. D., and J. F. Williams. 1982. Hydatidosis/cysticercosis: immune mechanisms and immunization against infection. Adv. Parasitol. 21:229-296. [DOI] [PubMed] [Google Scholar]
- 27.Sato, T. 1968. A modified method for lead staining of thin sections. J. Electron Microsc. 17:158-159. [PubMed] [Google Scholar]
- 28.Silverman, P. H. 1955. A technique for studying the in vitro effect of serum on activated taeniid hexacanth embryos. Nature 176:598-599. [DOI] [PubMed] [Google Scholar]
- 29.Silverman, P. H., and R. B. Maneely. 1955. Studies on the biology of some tapeworms of the genus Taenia. III. The role of the secreting gland of the hexacanth embryo in the penetration of the intestinal mucosa of the intermediate host, and some of its histochemical reactions. Ann. Trop. Med. Parasitol. 49:326-330. [PubMed] [Google Scholar]
- 30.Silverman, P. H., and E. L. Hansen. 1971. In vitro cultivation procedures for parasitic helminths: recent advances. Adv. Parasitol. 9:227-258. [DOI] [PubMed] [Google Scholar]
- 31.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woollard, D. J., C. G. Gauci, D. D. Heath, and M. W. Lightowlers. 1998. Epitope specificities and antibody responses to the EG95 hydatid vaccine. Parasite Immun. 20:535-540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.