Abstract
Sublingual (SL) immunization has been described as an effective novel way to induce mucosal immune responses in the respiratory and genital tracts. We examined the potential of SL immunization against Helicobacter pylori to stimulate immune responses in the gastrointestinal mucosa and protect against H. pylori infection. Mice received two SL immunizations with H. pylori lysate antigens and cholera toxin as an adjuvant, and after challenge with live H. pylori bacteria, their immune responses and protection were evaluated, as were immune responses prior to challenge. SL immunization induced enhanced proliferative responses to H. pylori antigens in cervicomandibular lymph nodes and provided at least the same level of immune responses and protection as corresponding intragastric immunization. Protection in SL-immunized mice was associated with strong H. pylori-specific serum IgG and IgA antibody responses in the stomach and intestine, with strong proliferation and gamma interferon (IFN-γ) and interleukin-17 (IL-17) production by spleen and mesenteric lymph node T cells stimulated with H. pylori antigens in vitro, and with increased IFN-γ and IL-17 gene expression in the stomach compared to levels in infected unimmunized mice. Immunohistochemical studies showed enhanced infiltration of CD4+ T cells and CD19+ B cells into the H. pylori-infected stomach mucosa of SL-immunized but not unimmunized H. pylori-infected mice, which coincided with increased expression of the mucosal addressin cell adhesion molecule (MAdCAM-1) and T and B cell-attracting chemokines CXCL10 and CCL28. We conclude that, in mice, SL immunization can effectively induce protection against H. pylori infection in association with strong T and B cell infiltration into the stomach.
At least half of the world's population is infected with Helicobacter pylori, one of the few microorganisms known to be able to colonize the human stomach. In 10 to 15% of infected individuals, chronic H. pylori infection causes duodenal ulcers, and infection with H. pylori has been shown to be a strong risk factor for the development of gastric adenocarcinoma and malignant mucosa-associated lymphomas (3, 17, 20). Although treatment with a combination of antibiotics and a proton pump inhibitor is usually effective in individual cases, limited treatment compliance, rapidly emerging antibiotic resistance, and frequent reinfection with H. pylori in countries where it is highly endemic make vaccination an increasingly attractive alternative or complement to standard therapy.
Vaccination, given either preventively or therapeutically, is especially needed in countries with a high incidence of gastric cancer (20), reinfection (22), or antibiotic resistance. However, clinical trials of various oral or parenteral H. pylori vaccine candidates have not shown much promise to date, pointing to the need for identifying improved antigen-adjuvant formulations and/or alternative routes of immunization in the quest for an effective vaccine against H. pylori (33).
The importance of cell-mediated mucosal immunity in protection against experimental H. pylori infection after vaccination is well established (1, 9, 10, 23, 35). In most studies, intragastric (IG) immunization has been used to achieve efficient stimulation of the gastrointestinal immune response. However, this route usually requires large amounts of antigen for efficient immunization, and the environment in the stomach and intestine may have adverse effects on the antigens and adjuvants used. Intranasal immunization against H. pylori has also been used in mice, but studies in humans have indicated that the nasal route of immunization is ineffective in stimulating immune responses in the intestine or stomach (12). In addition, intranasal immunization is associated with a risk of translocation of some types of antigens or adjuvants to the olfactory bulb of the brain, restricting its applicability in humans (31, 34).
Sublingual (SL) immunization has recently emerged as an attractive novel approach for mucosal vaccination against pathogens (7, 8, 31). In a model of influenza virus infection, SL immunization with live or adjuvanted killed virus induced immune responses and protection against aerosol challenge with live virus. In contrast to intranasal immunization, SL immunization had no evidence of vaccine or adjuvant entering the brain (31). In another study, SL immunization was found to induce vaccine-specific antibody and T cell responses in the genital tract and, after SL immunization with human papillomavirus (HPV)-like particles, protection against genital HPV infection, indicating the potential of SL immunization to stimulate immune responses also in nonrespiratory mucosal tissues (7).
In the present study, we examined whether SL immunization in mice can induce a mucosal immune response in the gastrointestinal tract. More specifically, we addressed the potential of SL immunization with H. pylori antigen and cholera toxin (CT) adjuvant to stimulate T and B cell responses in the stomach and protect against H. pylori infection. Our findings demonstrate that SL immunization induces strong systemic and stomach mucosal antibody and T cell responses and a high level of protection against H. pylori challenge. After SL immunization and H. pylori challenge, the stomach mucosa showed infiltration of both CD4+ T cells and CD19+ B cells and increased expression of gamma interferon (IFN-γ) and interleukin-17 (IL-17) compared to unimmunized infected mice. This was associated with increased expression of both the mucosal addressin cell adhesion molecule (MAdCAM-1) integrin and chemokines CXCL10 (10-kDa IFN-γ-induced protein) and CCL28 (mucosa-associated epithelial chemokine) in the immunized mice, which probably facilitated the migration of immunization-induced CD4+ T cells and CD19+ B cells into the stomach mucosa. Our results indicate that SL immunization against H. pylori effectively induces a strong immune response in the gastrointestinal tract mucosa and protects against infection, providing an attractive novel way of vaccinating against H. pylori infection.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old, specific-pathogen-free, female C57BL/6 mice were purchased from Harlan Laboratories (Horst, Netherlands). The mice were housed in microisolators at the Laboratory for Experimental Biomedicine (EBM) for the duration of the study. All experiments were approved by the ethics committee for animal experiments (Gothenburg, Sweden).
Cultivation of H. pylori used for infection.
The mouse-adapted H. pylori SS1 strain stored at −70°C as aliquots in Luria Bertani medium containing 20% glycerol was used as the stock culture for all experiments. The bacteria were grown for 3 days on Columbia iso-agar plates and further cultured overnight in brucella broth (Becton Dickinson Biosciences [BD], San Diego, CA) under microaerophilic conditions. Before infection of mice, the optical density (OD) of the bacteria was adjusted to 1.5, corresponding to approximately 1 × 109 viable bacteria/ml (28).
Antigen preparation and adjuvant for immunization.
The lysate antigens of H. pylori bacteria strain Hel 305 (CagA+ VacA+), which was originally isolated from a patient with a duodenal ulcer, was prepared as previously described (23). The protein content of the lysate antigens was measured using the noninterfering protein assay kit (Calbiochem, San Diego, CA). The prepared antigen was stored in aliquots at −70°C until further use. Aliquots for use in SL immunizations were freeze-dried and reconstituted to a protein concentration of 50 mg/ml to reduce the volume used in immunization. Lyophilized CT from Vibrio cholerae (Sigma Aldrich, St. Louis, MO) was reconstituted in distilled water to a concentration of 1 mg/ml and stored in aliquots at −70°C until use.
Immunization and infection with H. pylori.
Mice were given two immunizations containing 500 μg H. pylori lysate antigens and 10 μg CT. Immunizations were administered at a 2-week interval under deep anesthesia (isoflurane; Abbott Scandinavia AB, Solna, Sweden) by either the SL route (n = 17 mice), depositing 10 μl of lyophilized H. pylori lysate antigens reconstituted in CT without bicarbonate buffer through a micropipette under the tongue, or the intragastric (IG) route (n = 13 mice), using a feeding needle for placing 300 μl of H. pylori lysate antigens and CT in 3% sodium bicarbonate buffer directly into the stomach. One week after the last immunization, the mice were challenged with 3 × 108 live H. pylori SS1 bacteria in 300 μl brucella broth administered IG using a feeding needle under anesthesia, corresponding to approximately 100 times the minimal effective dose of colonization. Animals were sacrificed at 4 weeks after challenge, and the numbers of H. pylori bacteria in the stomach were determined. A reduction in bacterial counts in immunized compared to unimmunized mice was used as a measure of protection (23). Protection factors were calculated as the ratio of the geometric mean number of bacteria in infected control mice to the number of bacteria in individual vaccinated mice (28). We have previously shown comparable protection against H. pylori SS1 infection when immunizing IG with CT-adjuvanted H. pylori lysate antigens from strain Hel 305 or SS1 (27).
FTY720 treatment.
To prevent the egress of T and B cells from lymph nodes (18), mice were injected intraperitoneally with 1 μg of FTY720 per gram of body weight (Cayman Chemicals, Michigan) in a final volume of 250 μl at 3 h prior to immunization and thereafter every day for 5 days. At the time of sacrifice, mononuclear cells were isolated from the blood, stained for CD3 and CD19 (BD Biosciences), and examined by flow cytometry to confirm block of egress of lymph node T and B cells by FTY720.
Quantitative culture of H. pylori SS1 from the stomach.
To evaluate bacterial colonization in the stomachs of the sacrificed animals, one half of each stomach was homogenized in brucella broth using a tissue homogenizer (Ultra Turrax; IKA Laboratory Technologies, Staufen, Germany). Serial dilutions of the homogenates were plated on Helicobacter-selective plates. After 7 days of incubation at 37°C under microaerophilic conditions, visible colonies with typical H. pylori morphology were counted, and the urease test was performed for any uncertain colonies. Plates with 10 to 100 colonies were used for calculating the number of bacteria per stomach by multiplying by the appropriate dilution factor (23).
Serum antibody responses.
Blood was collected from the axillary plexus immediately before the mice were killed. Serum antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) against a membrane antigen preparation of H. pylori strain Hel 305 (MP Hel 305), which was applied overnight at room temperature. Levels of IgG antibodies were measured by testing serial dilutions of 1:100 prediluted sera, followed by detection of bound specific IgG antibodies with horseradish peroxidase (HRP)-coupled goat anti-mouse IgG (Jackson Immuno Research, West Grove, PA) secondary antibody and subsequent reaction with substrate the o-phenylenediamine dihydrochloride (OPD) added together with H2O2 and incubated at room temperature for 30 min. The enzymatic reaction was then stopped by adding 25-μl 1 M sulfuric acid, and the absorbance at 490 nm was then measured in a spectrophotometer. The antibody titers are defined as the reciprocal serum dilution giving an absorbance of 0.4 above the background.
Mucosal IgA antibody responses.
Locally produced IgA antibodies in the stomach and intestinal tissues were determined using the Perfext method (36). Briefly, after sacrifice, mice were extensively perfused with heparinized phosphate-buffered saline (PBS) to remove blood from the organs. Tissues were extracted from the stomach and small intestines (a 5-cm intestinal segment approximately 2 to 7 cm below the pylorus) using a 2% saponin-PBS solution as previously described in detail (23). Anti-H. pylori IgA antibodies in the supernatant of the saponin extracts were determined by ELISA (23), using microtiter plates coated with MP Hel 305 and sequential incubations with (i) a 3-fold dilution of saponin extract supernatant at room temperature for 90 min, (ii) an appropriate concentration of HRP-conjugated goat anti-mouse IgA (Southern Technology) at 4°C overnight, and (iii) OPD and H2O2 at room temperature for 30 min. The reaction was stopped by adding 25-μl 1 M sulfuric acid, and the absorbance at 490 nm (A490) was measured in a spectrophotometer.
Cellular immune responses.
For the proliferation assay, lymphocyte suspensions were prepared from the cervicomandibulary lymph nodes (CMLN), spleen, and mesenteric lymph nodes (MLN). Cells were seeded (2 × 105 cells per well) in the presence or absence of H. pylori strain Hel 305 lysate antigens (10 μg/ml) and cultured for 96 h in Iscove's medium (Biochrome, Berlin, Germany) supplemented with 5% heat-inactivated fetal calf serum (Sigma), 50 μM 2-mercaptoethanol (Sigma), 1 mM l-glutamine (Biochrome), and 50 μg/ml gentamicin (Sigma) at 37°C in a 5% CO2 atmosphere. Supernatants were collected and stored at −70°C for subsequent cytokine analysis. To determine proliferation, the cells were pulsed with 1 μCi of [3H] thymidine (Amersham Bioscience, Buckinghamshire, United Kingdom) for the last 6 to 8 h of culture. The cellular DNA was collected with a cell harvester (Skatron) on glass fiber filters (Wallac) and assayed for 3H incorporation using a liquid scintillation counter (Beckman, LKB, Bromma, Sweden). IL-17 was measured in culture supernatants using Duoset cytokine ELISA (R&D Systems, Abingdon, United Kingdom). IFN-γ was measured using the mouse inflammation cytometric bead array kit (BD Biosciences) according to the manufacturer's instructions.
RNA isolation.
The stomach was excised and dissected along the greater curvature. Any loose stomach contents were removed by washing in PBS. Two longitudinal strips including the antrum and corpus were cut and placed directly into RNAlater (Qiagen, Hilden, Germany). The samples were kept at 4°C for 2 days and then stored at −70°C. For RNA isolation, the tissue was thawed and transferred to RLT lysis buffer (Qiagen) with 1% β-mercaptoethanol and homogenized for 2 min at 30 Hz using a Tissue Lyser II (Qiagen). Total RNA was extracted using the RNeasy mini kit (Qiagen) and stored at −70°C until further analysis.
RT-PCR.
RNA (2 μg) was reverse transcribed into cDNA using the Omniscript kit (Qiagen). All real-time PCRs (RT-PCRs) were run in 96-well plates using the standard amplification conditions described for the 7500 RT-PCR system and 9 μl cDNA, 10 μl 2× Power SYBR green master mix (Applied Biosystems, Foster City, CA), and 1 μl of gene-specific oligonucleotide primers (Eurofins MWG Operon, Ebersberg, Germany) (Table 1). The reactions were run in duplicate, and β-actin was used as the reference gene in all experiments. The difference between β-actin and the target gene (ΔCT) was determined, and the relative expression was calculated using the formula 2ΔCT. The values were adjusted so that the mean in the infection control group was set to 1. The negative control (lacking reverse transcriptase) giving the lowest CT value was used to determine the detection limit.
TABLE 1.
Primers used for RT-PCR
| Gene | Primer sequence (5′ →3′) |
|
|---|---|---|
| Forward | Reverse | |
| IL-17A | CCGCAATGAAGACCCTGATAGA | TCATGTGGTGGTCCAGCTTTC |
| IFN-γ | GCATAGATGTGGAAGAAAAGAGTCTCT | GGCTCTGCAGGATTTTCATGT |
| CCL28 | TGCTGTCATCCTTCATGTTAAACG | TCTCTGCCATTCTTCTTTACCTCTGA |
| β-Actin | CTGACAGGATGCAGAAGGAGATTA | GCCACCGATCCACACAGAGT |
| CCR10 | TTCAGTCTTCGTGTGGCTGTTGTC | ACAGCGTCGTTGGCCTTCAC |
| CCR9 | CACTCACCTCCGCCGTACATCT | ATGCCAGGAATAAGGCTTGTGAGTT |
| CCL25 | GCTAGGAATTATCACCAGCAGGAAGT | ATGTCCTCTGGATTCCCACACA |
Immunohistochemistry.
Six-micrometer thin sections of stomach tissue were fixed in acetone and stained using rat anti-mouse CD4 (clone L3T4) CD8 (clone Ly-3), MAdCAM-1 (clone MECA 367), CD19 (clone 1D3), or rat IgG2a isotype control antibodies (all from BD) as previously described (26). Sections were evaluated using the BioPix analysis software (BioPix AB, Gothenburg, Sweden) to calculate the percentage of tissue area staining positive for CD4, CD8, CD19, or MAdCAM-1 in three to five independent fields at a magnification of ×400 (CD4, CD8, and CD19) or ×200 (MAdCAM-1).
Statistical analysis.
Analysis of variance (ANOVA) with Dunnette's posttest was used to compare multiple groups, while Student's t test was used to compare two groups (GraphPad Prism software; GraphPad Software Inc., San Diego, CA). For all tests, a P value of <0.05 was considered to indicate statistical significance.
RESULTS
Sublingual immunization induces immune protection against H. pylori infection.
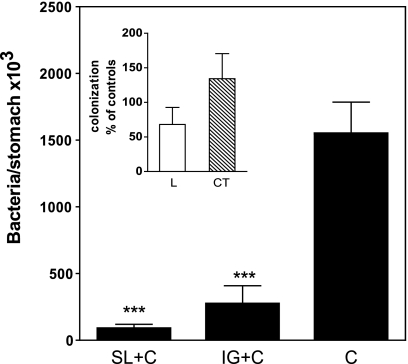
The primary objectives of the study were to determine whether the stomach and the small intestine are part of the mucosal effector sites that can be targeted by SL immunization and to evaluate the efficacy of SL immunization for inducing protection in the stomach against experimental H. pylori infection. To address these questions, we gave mice two SL immunizations 2 weeks apart with H. pylori lysate antigens and CT as an adjuvant. A group of mice immunized IG at the same time with the identical antigen-adjuvant mixture as for SL immunization (except given in bicarbonate to protect the CT adjuvant against degradation in the stomach) was also included in the study, as were groups of mice given two SL doses of the H. pylori lysate antigens alone or CT alone. One week after the last immunizations, the mice (and also unimmunized mice) were challenged intragastrically with a high dose of live H. pylori bacteria. Four weeks after challenge, animals were sacrificed and the numbers of H. pylori bacteria in the stomach were determined. SL immunizations with H. pylori lysate antigens and CT resulted in a significant reduction in the H. pylori load in the stomach (P < 0.001) (Fig. 1). SL immunization with H. pylori antigen and CT tended to be even more effective than corresponding immunization by the IG route, resulting in an approximately 20-fold reduction of bacteria, compared to a 6-fold reduction after IG immunization (P = 0.1475). Similar immunization with H. pylori lysate antigens alone or CT alone did not result in any detectable protection against H. pylori infection (Fig. 1, inset).
FIG. 1.
Sublingual immunization protects against H. pylori infection in mice. The data are mean H. pylori CFU + standard error of the mean (SEM) at 4 weeks after challenge in the stomachs of mice that were immunized twice with H. pylori lysate antigens and CT via the sublingual (SL) or intragastric (IG) route, followed 1 week later by challenge with live H. pylori (SL+C and IG+C, respectively) in comparison with concurrently challenged unimmunized mice serving as infection controls (C). Data are from two or three independent experiments with 5 to 10 mice per group in each experiment and show significant differences between both SL- and IG-immunized groups compared to infected controls. ***, P < 0.001. The inset shows colonization of H. pylori in mice that were similarly SL immunized with either lysate antigens alone (L) or CT alone (CT) compared to unimmunized concurrently challenged mice. Data are expressed as mean (+SEM) percentage of bacteria per stomach in individual immunized mice in relation to the mean number of bacteria in infection controls.
Sublingual immunization against H. pylori induces strong antibody responses in serum as well as in stomach and small intestinal mucosa.
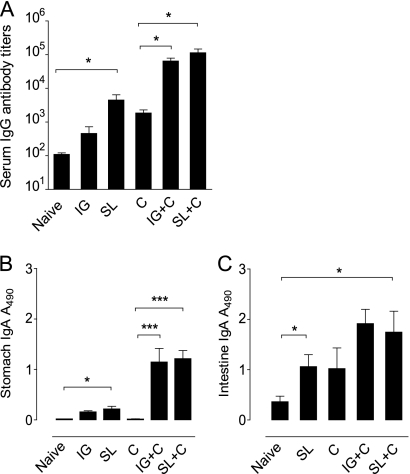
We determined IgG antibody responses to H. pylori in the serum and mucosal IgA antibody responses in the stomach and small intestines after SL or IG immunization both before and after challenge with live H. pylori bacteria. In serum, SL immunization induced a stronger H. pylori-specific IgG antibody response than IG immunization before challenge (P < 0.05) (Fig. 2 A). After challenge with H. pylori, the serum IgG antibody response was then further increased after either route of immunization. Compared to the titers measured after infection in unimmunized mice, an approximately 50-fold increase in H. pylori-specific IgG antibodies was measured in the SL-immunized mice (Fig. 2A).
FIG. 2.
Sublingual immunization induces H. pylori-specific serum antibody responses as well as a local H. pylori-specific IgA antibody response in the stomach mucosa both before and after challenge with live bacteria. Mice tested were as follows: age-matched unimmunized, uninfected C57BL/6 mice served as naïve controls; SL and IG are mice immunized by the SL or IG route and tested before challenge with H. pylori bacteria to study the effect of immunization alone on systemic and local antibody responses; C are unimmunized mice that were challenged with H. pylori and tested 4 weeks later; and SL+C and IG+C are mice that were immunized twice with H. pylori lysate antigens and CT via the sublingual (SL) or intragastric (IG) route, followed 1 week later by challenge with live H. pylori and tested 4 weeks after challenge. (A) Serum antibodies. H. pylori-specific serum IgG antibody titers in the groups of mice indicated were determined by ELISA. Data are representative of one of two independent experiments giving similar results. The bars represent mean + SEM antibody titers, *, P < 0.05. (B and C) IgA antibodies in stomachs and intestinal mucosae. Saponin extracts of stomach (B) and small intestinal (C) mucosa were analyzed for H. pylori-specific IgA antibodies by ELISA. Data represent mean (+SEM) absorbance values at 490 nm (A490) from one of two independent experiments giving similar results. *, P < 0.05; ***, P < 0.001.
Before immunization, mice had very low levels of H. pylori-reactive IgA antibodies in the mucosal tissues of the stomach (Fig. 2B) and small intestine (Fig. 2C). Both SL and IG immunizations gave rise to significantly increased levels of H. pylori-specific local IgA antibodies in the stomach and intestinal mucosa before challenge with H. pylori bacteria, and these responses increased further after challenge (Fig. 2B and C). The IgA antibody levels to H. pylori in the stomachs and intestines of immunized and infected mice were much higher than the undetectable (stomach) or low (intestine) IgA antibody responses induced by the H. pylori infection in unimmunized mice.
SL immunization induces H. pylori-specific Th1 and Th17 responses.
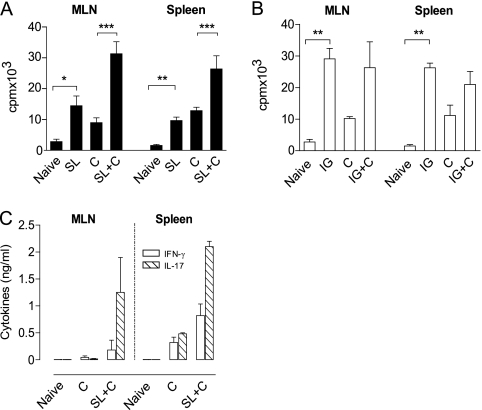
CD4 T cells have been shown to be indispensable for protection against H. pylori after vaccination (10, 25). We therefore investigated the T cell proliferative immune responses in MLN and spleen after SL and IG immunization and the effects of subsequent H. pylori challenge on those responses. When tested before infection, SL immunization induced a significant increase in the H. pylori-specific proliferative responses of both MLN (P < 0.05) and spleen cells (P < 0.01) compared to those in unimmunized mice, and these responses were substantially increased after challenge with H. pylori (Fig. 3 A); similar responses, although less influenced by the challenge, were induced by IG immunization (Fig. 3B). We also examined the production of IFN-γ and IL-17 from MLN and spleen cells stimulated with H. pylori antigens from SL-immunized mice and found that both of these cytokines, and especially IL-17, were increased after challenge, to levels much higher than in infected unimmunized mice (Fig. 3C). Our results indicate that SL immunization induces a mixed Th1/Th17 response, with a trend toward production of larger amounts of IL-17 than of IFN-γ after challenge.
FIG. 3.
Induction of cellular immune responses to H. pylori in sublingual (SL)-immunized and infected mice. (A and B) In vitro proliferative responses to H. pylori antigens by mesenteric lymph nodes (MLN) and spleen cells after SL (A) compared to IG (B) immunization. Age-matched unimmunized, uninfected C57BL/6 mice served as naïve controls. SL or IG indicate mice that were immunized but not challenged to study the effect of immunization on spleen and MLN proliferative responses. Group C are mice that were left unimmunized, challenged with live H. pylori, and tested 4 weeks later, serving as infection controls, while SL+C and IG+C are mice that were immunized twice SL or IG with H. pylori lysate antigens and CT, followed 1 week later by challenge, and tested 4 weeks after challenge. Data show the incorporation of radioactive thymidine added during the last 6 to 8 h of a 96-h culture, expressed as mean counts per minute (cpm) + SEM, *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) IFN-γ and IL-17 cytokine levels in cell culture supernatants of cells similar to those for panel A collected 96 h after stimulation, expressed as mean cytokine concentrations (ng per ml) + SEM. Data are from two independent experiments with a pool of five or six mice per group in each experiment.
Cervicomandibular lymph nodes are the inductive site for immune responses to sublingual immunization.
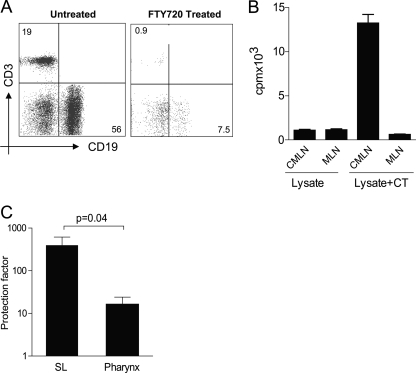
We wished to exclude the possibility that the induction of immune responses and protection against H. pylori infection seen after SL immunization was due to small amounts of antigen and CT being swallowed. Thus, we first studied the H. pylori-specific proliferative responses in CMLN and MLN after SL immunization with H. pylori antigens with or without CT (Fig. 4 B) in mice treated with FTY720 to prevent exit of activated cells from lymph nodes after immunization (18); the effect of the FTY720 treatment was monitored by flow cytometry, showing 90% reduced frequencies of T and B cells in circulation (Fig. 4A). Single-cell suspensions from CMLN and MLN were prepared at 5 days postimmunization and restimulated in vitro with H. pylori antigen. The results show that SL immunization with lysate antigens and CT but not that with lysate antigens alone resulted in a strong proliferative response to H. pylori antigens in the CMLN but not in the MLN (Fig. 4B), indicating that the CMLN is the site of priming of H. pylori-specific responses after SL immunization, with only later dissemination to other lymphoid organs (30).
FIG. 4.
CMLN are the primary inductive site after SL immunization. (A) FTY720 treatment prevents egress of T and B cells as evidenced by a reduced frequency of CD3 and CD19 cells in circulation as analyzed by flow cytometry. Numbers represent frequencies of CD3+ T cells and CD19+ B cells in the live lymphocyte gate. (B) Mice were immunized SL with lysate antigens alone or together with CT and treated with FTY720. Single-cell suspensions were prepared from the CMLN and MLN at 5 days after SL immunization and restimulated in vitro with H. pylori antigens. Bars represent mean cpm + SEM of incorporated radioactive thymidine. Data are representative of one of two independent experiments giving similar results. (C) Mice were immunized either SL or in the posterior pharynx and challenged intragastrically with H. pylori bacteria. The CFU recovered from the mouse stomach was used to calculate the protection factors as described in Materials and Methods.
Second, we directly compared the level of protection after SL immunization with that obtained by administering the same antigen-adjuvant mixture in the posterior pharynx. The results are shown in Fig. 4C and demonstrate that protection was much stronger (>20-fold) after SL immunization than after pharyngeal immunization. We conclude that swallowed antigen and adjuvant cannot explain the strong immune protection against H. pylori seen after SL immunization.
Infiltration of CD19+ B cells and CD4+ T cells and expression of Th1 and Th17 cytokines in the stomach mucosa of SL-immunized mice.
Since the results described above indicate that SL immunization against H. pylori infection effectively induces protection and immune responses to at least the same magnitude as corresponding immunization by the IG route, our subsequent experiments were specifically focused on further analyzing the immune responses in the stomach after SL immunization.
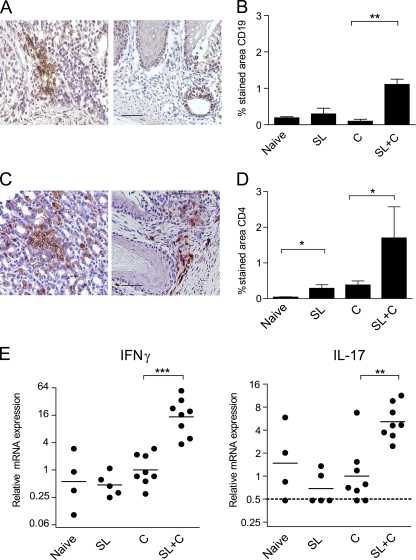
Immunohistochemical staining for CD19+ B cells and CD4+ or CD8+ T cells was carried out on stomach tissue sections in order to define the subsets of cells migrating to the site of infection after SL immunization and challenge with live bacteria. The results showed an increase in both CD19+ B cells (Fig. 5 A and B) and CD4+ T cells (Fig. 5C and D), but not CD8+ T cells (data not shown), in the gastric mucosa of SL-immunized and infected mice, whereas little or no cell infiltration was evident in mice that were SL immunized but not infected or in those that were infected but not immunized (Fig. 5A to D).
FIG. 5.
Increased expression of CD19+ B cells, CD4+ T cells, IFN-γ, and IL-17 in the stomach mucosa of SL-immunized mice. Age-matched unimmunized, uninfected C57BL/6 mice served as naïve controls. SL are mice that were immunized but not challenged to study the effect of immunization alone on the infiltration of T and B cells and cytokine gene expression in the stomach. Group C are mice that were left unimmunized and concurrently challenged and tested 4 weeks later, serving as infection controls, while SL+C are mice that were immunized twice SL with H. pylori lysate antigens and CT followed 1 week later by challenge with live H. pylori and tested 4 weeks after challenge. (A and C) Representative staining patterns for CD19+ B cells and CD4+ T cells in SL-immunized and challenged mice (left) or unimmunized challenged mice (right). Magnification, ×400; bar, 50 μm. (B and D) Cumulative results showing the percentage (mean + SEM) of stained stomach mucosa. Combined data from two experiments are shown, with three or four animals per group and experiment. **, P < 0.01; *, P < 0.05. (E) Expression of cytokine genes. Stomach tissue samples from the same mice as for panels B and D were immediately preserved in RNAlater. Isolated RNA samples were converted into cDNA and analyzed by RT-PCR for IFN-γ and IL-17 transcript levels. The dotted line represents the detection limit. Combined data from two experiments are shown, with n = 2 to 4 animals per group and experiment. ***, P < 0.001; **, P < 0.01.
RT-PCR analysis was carried out to study the expression of CD4+-derived cytokines IFN-γ and IL-17 in the gastric mucosa of immunized and infected mice compared to unimmunized infection controls at the time of sacrifice. Our results showed significantly elevated levels of both IFN-γ (P < 0.001) and IL-17 (P < 0.01) transcripts in SL-immunized mice compared to unimmunized infected mice, whereas the transcript levels in the stomach mucosa of mice that were SL immunized but not infected did not differ from the levels in naïve mice (Fig. 5E). Likewise, the expression of IFN-γ and IL-17 mRNA in the stomachs of mice immunized (ineffectively) with H. pylori lysate antigens without CT adjuvant or with CT without lysate antigens and then infected (Fig. 1, inset) did not differ from the expression in naïve mice (data not shown).
MAdCAM-1 expression is strongly increased in the stomachs of sublingually immunized and H. pylori-infected mice.
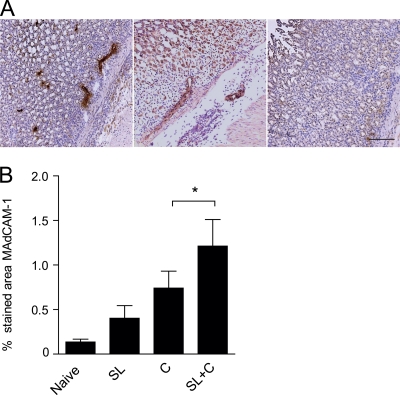
Since MAdCAM-1 is critical for the extravasation of gut-homing activated T and B cells into the gastrointestinal mucosa (5), we were interested in determining by immunohistochemistry whether SL immunization could induce expression of this integrin in the stomach.
Naïve mice had a very low level of MAdCAM-1 expression in the stomach endothelium, which was significantly increased after infection with H. pylori (Fig. 6). In the absence of infection, SL immunization tended to modestly stimulate MAdCAM-1 expression but not to the same extent as in mice that were first immunized SL and then infected, in which the highest levels of MAdCAM-1 were found (Fig. 6B).
FIG. 6.
Increased MAdCAM-1 expression in the stomachs of SL-immunized and H. pylori-infected mice. (A) MAdCAM-1 staining with characteristic endothelial localization in the stomach tissue of SL-immunized and infected mice (left) and infection control (middle) or isotype control staining (right). Magnification, ×200; bar, 100 μm. (B) Results showing the mean percentage of stomach mucosa specifically stained in the different treatment groups + SEM. Age-matched unimmunized, uninfected mice served as naïve controls. SL are mice that were immunized but not challenged to study the effect of immunization alone on MAdCAM-1 expression in the stomach mucosa. C are mice that were left unimmunized and concurrently challenged, serving as infection controls, while SL+C are mice that were immunized twice SL with H. pylori lysate antigens and CT followed 1 week later by challenge with live H. pylori. Combined data from two experiments are shown, with n = 2 or 3 animals per group and experiment, *, P < 0.05.
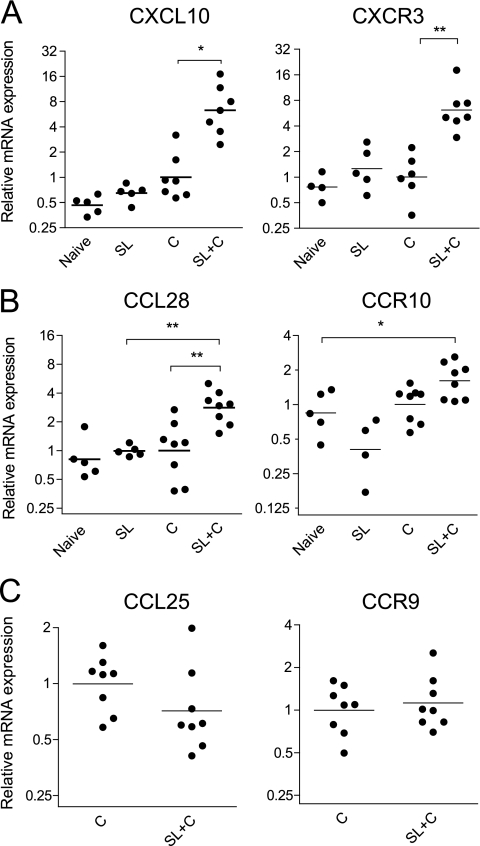
SL immunization and H. pylori infection stimulated strong chemokine responses and their corresponding receptors on B and T cells.
Locally produced chemokines can attract T and B cells with cognate chemokine receptors to inflamed tissues. We analyzed the expression of chemokines CXCL10 (IP-10) and CCL28 (MEC), which are known to attract Th1 cells and IgA plasma blasts, respectively, and their corresponding receptors on the cells, CXCR3 and CCR10 (13). In contrast to the less restricted expression of MAdCAM-1 in relation to infection and immunization, the expression of the CXCL10 and CCL28 genes in the stomach mucosa was elevated only in SL-immunized and infected mice, and the expression was closely matched by increased levels of their cognate receptors (Fig. 7 A and B). A similar analysis of the gene expression of CCL25 (TECK [thymus-expressed chemokine]), a small-intestine-specific chemokine known to attract CCR9+ B and T cells, and of CCR9, the chemokine receptor in the stomach, showed no difference between SL-immunized infected mice and unimmunized infection controls (Fig. 7C). Our findings indicate that SL immunization followed by H. pylori infection results in the specific upregulation of CXCL10 and CCL28 in the stomach, which together with enhanced MAdCAM-1 expression could explain the strong attraction of both CXCR3+ T cells and CCR10+ B cells to the stomach mucosa in SL-immunized and infected mice.
FIG. 7.
RT-PCR analysis shows increased expression of the CXCL10 (IP-10), CCL28 (MEC), CXCR3, and CCR10 genes but not the CCL25 and CCR9 genes in stomach mucosa of SL-immunized mice and H. pylori infected mice. (A) CXCL10 (left) and CXCR3 (right). (B) CCL28 (left) and CCR10 (right). (C) CCL25 (left) and CCR9 (right). Gene expression in the stomach mucosa of mice from different treatment groups was calculated as described in Materials and Methods. Age-matched unimmunized, uninfected C57BL/6 mice served as naïve controls. SL+C are mice that were immunized twice SL with H. pylori lysate antigens and CT, followed 1 week later by challenge with live H. pylori. Group C are mice that were left unimmunized and concurrently challenged, serving as infection controls, while SL and IG are mice that were immunized but not challenged to study the effect of immunization alone on chemokine and chemokine receptor gene expression in the stomach mucosa Combined data from two experiments are shown; n = 2 or 3 animals per group. *, P < 0.05; **, P < 0.01.
DISCUSSION
In this study, we show that the SL mucosa can be used as a novel site for effective vaccination against experimental H. pylori infection in mice. SL immunization with H. pylori antigens and CT as an adjuvant provided substantial protection against H. pylori infection in the stomach that was associated with strong H. pylori-specific T and B cell responses, both systemically and locally in the stomach. The mucosal immune response after SL immunization included H. pylori-specific IgA antibodies in the stomach and small intestine, production of IFN-γ and IL-17 cytokines by T cells in response to stimulation with H. pylori antigens, increased IFN-γ and IL-17 gene expression in the stomach mucosa, and enhanced local MAdCAM-1 protein expression and CCL28 (MEC) and CXCL10 (IP-10) gene expression in the stomach tissue. Together with the concomitantly increased gene expression of chemokine receptors CCR10 and CXCR3 in the stomach mucosa of SL-immunized and infected mice, these findings indicate that infection with H. pylori facilitated the recruitment to the stomach of mucosa-homing lymphocytes induced by SL immunization. Our results expand upon previous studies that used SL immunization to induce immune responses in the respiratory and genital tracts (7, 8, 31) by showing that this novel route of mucosal immunization can also be used to induce strong immune responses and protection against infection in the mucosa of the gastrointestinal tract.
Previous studies using the mouse-adapted H. pylori SS1 strain as the challenge strain for testing the protection afforded by vaccine candidates and adjuvant formulations via the IG route usually achieved a 5- to 10-fold reduction in bacterial load (1, 23, 32). This reduction is in near agreement with the 6-fold reduction measured in this study after IG immunization with H. pylori lysate antigens and CT adjuvant in bicarbonate buffer. The 20-fold reduction in bacterial load found after SL immunization with the same antigen and adjuvant combination, administered to fully anesthetized mice in a 10-microliter volume without any bicarbonate buffer, which is critical for protection after IG immunization (6), argues against the possibility that immune responses and protection against H. pylori infection after SL immunization were to any significant extent induced by swallowed antigen and adjuvant. This conclusion was further supported by directly comparing the levels of protection after SL immunization and corresponding administration of antigen and adjuvant in the posterior pharynx. The latter administration should increase the potential for swallowing, and still SL immunization induced a much stronger protection than the pharyngeal immunization.
Our studies have also indicated that after SL administration, only 2% of radioactively labeled protein could be recovered from the stomach and intestine over the first 2 h (unpublished results). Further, our results demonstrating that blocking lymphocyte egress from the lymph nodes results in a clear antigen-specific T cell response in the CMLN with no response in the MLN at 6 days after initial SL immunization with H. pylori lysate antigens and CT confirm previous findings by Song et al. (30) that the CMLN draining the SL mucosa is the primary inductive site for the immune responses after SL immunization. Those authors also showed that the activation of immune cells in the CMLN after SL immunization appears to specifically take place by major histocompatibility complex class II (MHCII)-expressing dendritic cells, which upregulate their expression of CCR7 after capturing antigen and adjuvant in the SL mucosa and migrate toward CCL19 and CCL21 chemokines produced in the CMLN. Further dissemination of primed B and T cells then takes place from the CMLN into the circulation and to other lymphoid and mucosal tissues (8, 30).
Sublingual immunization with H. pylori lysate antigens and CT gave rise to consistently strong B cell responses, both systemically and in the gastrointestinal mucosa. The role of antibodies in protecting against H. pylori infection is not clear. Studies in knockout mice have shown that B cells and antibodies are dispensable for protection against H. pylori infection, in contrast to CD4+ T cells (2, 10). On the other hand, it was shown in a recent human birth cohort study in Bangladesh that infants feeding on breast milk containing high titers of H. pylori-specific IgA antibodies have a significant delay in acquisition of H. pylori infection compared to infants feeding on low-titer breast milk, suggesting that IgA antibodies may play a role in the immune exclusion of bacteria at the site of infection (2a).
Strong H. pylori-specific T cell responses associated with IFN-γ and IL-17 production were seen in both MLN and the stomach mucosa of SL-immunized and infected mice. Although Th1 cells secreting IFN-γ have long been regarded as the primary mediators of protection, our findings concur with recent reports indicating that Th17 cells also contribute to immune protection against H. pylori infection, possibly through IL-17-induced production of neutrophil chemoattractants (9, 35). Interestingly, we detected much stronger IFN-γ and IL-17 expression and a stronger local IgA antibody response in the stomachs of SL-immunized mice after H. pylori challenge than before H. pylori, suggesting that local inflammation induced by H. pylori infection promotes the infiltration of effector T and B cells into the gastric mucosa, and possibly also their antigen-driven local expansion, as previously indicated in oral vaccination studies in humans (19).
The dissemination of T and B cells to the gastrointestinal tract following oral or intestinal vaccination is thought to be specifically mediated through interactions between α4β7 integrin and its ligand MAdCAM-1, which is expressed on normal and inflamed intestinal endothelium (4). In the stomachs of SL-immunized and infected mice, significant increases in MAdCAM-1, CD4+ T cells, and CD19+ B cells were measured. Increased MAdCAM-1 expression and the accumulation of vaccine-specific IgA antibody-secreting cells were previously reported in the gastric mucosa of H. pylori-infected individuals given oral cholera vaccine (16). Therefore, H. pylori-specific α4β7+ T cells, as well as B cells, induced by SL immunization likely enter the stomach mucosa during infection by interacting with the elevated levels of MAdCAM-1 in SL-immunized and infected mice. Once in the gastric tissue, these cells may play a role in controlling bacterial multiplication and pathogenicity (21).
In addition to α4β7-MAdCAM-1 interactions, the specificity of lymphocyte homing to gastrointestinal mucosa is governed by selective chemokine expression by epithelial cells lining the stomach, small intestine, and colon and the expression of their corresponding receptors on the lymphocytes (13). Recent studies in mice and humans have identified a role for the interaction between the chemokine CXCL10 (IP-10) and chemokine receptor CXCR3 in the specific migration of polarized Th1 CD4+ T cells to inflamed intestinal tissue (24, 29) and a similar role for the interaction between CCL28 (MEC) and CCR10 in attracting IgA+ plasmablasts to the bronchi, colon, salivary glands, and mammary glands (15). In SL-immunized mice infected with H. pylori, we measured using RT-PCR an increase in the expression of the CXCL10, CXCR3, CCL28, and CCR10 genes in the stomach, whereas expression of the small intestine-specific B and T cell chemoattractant CCL25 (TECK) and its receptor CCR9 (14) was not changed. Thus, our results suggest that in SL-immunized and H. pylori-infected mice, CXCR3-expressing Th1 cells and CCR10-expressing IgA+ plasmablasts enter the stomach in response to local production of CXCL10 and CCL28, respectively, in the stomach mucosa. In humans, increased expression of CCL28, but not CCL25, in H. pylori-infected individuals compared to uninfected individuals was recently reported, which correlated significantly with total IgA antibody levels in the stomach (11). Thus, in the stomachs of both H. pylori-infected humans and SL-immunized and infected mice, infection-induced inflammation appears to induce specific upregulation of CXCL10 and CCL28 (but not CCL25), and these chemokines contribute to the migration of lymphocytes into the stomach and protect against H. pylori infection.
The present study adds to recent reports that the SL mucosa is an attractive site for the induction of systemic and mucosal immune responses in the respiratory and genital tracts, and it also shows the potential of SL immunization for inducing mucosal T and B cell immune responses and protection against infection in the gastrointestinal tract. Our results indicate that SL immunization with H. pylori antigens and adjuvant, when given prophylactically, can provide immune responses and protection in mice against H. pylori in the stomach that are at least comparable to those obtained with IG immunization.
The encouraging results obtained in the present study hold promise for use of SL mucosal immunization against H. pylori infection in humans, especially when taking into account that humans lack the partly keratinized SL epithelium present in mice, which may further facilitate antigen uptake across the human SL mucosa. Indeed, although immune responses in mice may not accurately predict immune responsiveness to vaccines in humans, a first study of SL immunization of humans with a model mucosal vaccine antigen (CT B subunit) has given promising results indicating the feasibility of inducing gastrointestinal immune responses also in humans by SL immunization using a nonreplicating antigen (C. Czerkinsky et al., unpublished data). The present results indicate that SL immunization against H. pylori warrants further testing in humans once a suitable vaccine formulation for clinical testing is available. In conclusion, our findings suggest that SL immunization represents a promising novel approach to vaccination against H. pylori infection.
Acknowledgments
We acknowledge the excellent technical assistance with immunohistochemistry provided by Bin-Ling Li.
This work was supported by grants from the Swedish Research Council (Medicine) and the Marianne and Marcus Wallenberg Foundation and also by the Swedish Foundation for Strategic Research through its support of the Mucosal Immunology and Vaccine Program (MIVAC) at The University of Gothenburg. The International Vaccine Institute is supported, in part, by the Governments of the Republic of Korea, Sweden, Kuwait, and the Netherlands.
We declare no conflicts of interest.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. [DOI] [PubMed] [Google Scholar]
- 2.Akhiani, A. A., K. Schon, L. E. Franzen, J. Pappo, and N. Lycke. 2004. Helicobacter pylori-specific antibodies impair the development of gastritis, facilitate bacterial colonization, and counteract resistance against infection. J. Immunol. 172:5024-5033. [DOI] [PubMed] [Google Scholar]
- 2a.Bhuiyan, T. R., A. Saha, A. Lundgren, F. Qadri, and A. M. Svennerholm. Immune responses to Helicobacter pylori infection in Bangladeshi children during their first two years of life and relation between maternal antibodies and onset of infection. J. Infect. Dis., in press. [DOI] [PubMed]
- 3.Blaser, M. J., and J. C. Atherton. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Invest. 113:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher, E. C., M. Williams, K. Youngman, L. Rott, and M. Briskin. 1999. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72:209-253. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, D. J., and E. C. Butcher. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195:135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens, J. D., M. Jertborn, D. Sack, B. Stanton, J. Holmgren, M. R. Khan, and S. Huda. 1986. Effect of neutralization of gastric acid on immune responses to an oral B subunit, killed whole-cell cholera vaccine. J. Infect. Dis. 154:175-178. [DOI] [PubMed] [Google Scholar]
- 7.Cuburu, N., M.-N. Kweon, C. Hervouet, H.-R. Cha, Y.-Y. S. Pang, J. Holmgren, K. Stadler, J. T. Schiller, F. Anjuere, and C. Czerkinsky. 2009. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J. Immunol. 183:7851-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuburu, N., M. N. Kweon, J. H. Song, C. Hervouet, C. Luci, J. B. Sun, P. Hofman, J. Holmgren, F. Anjuere, and C. Czerkinsky. 2007. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 25:8598-8610. [DOI] [PubMed] [Google Scholar]
- 9.DeLyria, E. S., R. W. Redline, and T. G. Blanchard. 2009. Vaccination of mice against H. pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology 136:247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson, M., M. Hermansson, H. Svensson, A. Elfvin, L.-E. Hansson, E. Johnsson, A. Sjoling, and M. Quiding-Jarbrink. 2008. CCL28 is increased in human Helicobacter pylori-induced gastritis and mediates recruitment of gastric immunoglobulin A-secreting cells. Infect. Immun. 76:3304-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson, E. L., C. Bergquist, A. Edebo, C. Johansson, and A. M. Svennerholm. 2004. Comparison of different routes of vaccination for eliciting antibody responses in the human stomach. Vaccine 22:984-990. [DOI] [PubMed] [Google Scholar]
- 13.Kunkel, E. J., and E. C. Butcher. 2002. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16:1-4. [DOI] [PubMed] [Google Scholar]
- 14.Kunkel, E. J., J. J. Campbell, G. Haraldsen, J. Pan, J. Boisvert, A. I. Roberts, E. C. Ebert, M. A. Vierra, S. B. Goodman, M. C. Genovese, A. J. Wardlaw, H. B. Greenberg, C. M. Parker, E. C. Butcher, D. P. Andrew, and W. W. Agace. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazarus, N. H., E. J. Kunkel, B. Johnston, E. Wilson, K. R. Youngman, and E. C. Butcher. 2003. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J. Immunol. 170:3799-3805. [DOI] [PubMed] [Google Scholar]
- 16.Lindholm, C., A. Naylor, E. L. Johansson, and M. Quiding-Jarbrink. 2004. Mucosal vaccination increases endothelial expression of mucosal addressin cell adhesion molecule 1 in the human gastrointestinal tract. Infect. Immun. 72:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 18.Matloubian, M., C. G. Lo, G. Cinamon, M. J. Lesneski, Y. Xu, V. Brinkmann, M. L. Allende, R. L. Proia, and J. G. Cyster. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427:355-360. [DOI] [PubMed] [Google Scholar]
- 19.Mattsson, A., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, I. Ahlstedt, and A. Svennerholm. 1998. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Infect. Immun. 66:2705-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNamara, D., and E. El-Omar. 2008. Helicobacter pylori infection and the pathogenesis of gastric cancer: a paradigm for host-bacterial interactions. Dig. Liver Dis. 40:504-509. [DOI] [PubMed] [Google Scholar]
- 21.Michetti, M., C. P. Kelly, J. P. Kraehenbuhl, H. Bouzourene, and P. Michetti. 2000. Gastric mucosal alpha(4)beta(7)-integrin-positive CD4 T lymphocytes and immune protection against Helicobacter infection in mice. Gastroenterology 119:109-118. [DOI] [PubMed] [Google Scholar]
- 22.Niv, Y., and R. Hazazi. 2008. Helicobacter pylori recurrence in developed and developing countries: meta-analysis of 13C-urea breath test follow-up after eradication. Helicobacter 13:56-61. [DOI] [PubMed] [Google Scholar]
- 23.Nystrom, J., S. Raghavan, and A. M. Svennerholm. 2006. Mucosal immune responses are related to reduction of bacterial colonization in the stomach after therapeutic Helicobacter pylori immunization in mice. Microbes Infect. 8:442-449. [DOI] [PubMed] [Google Scholar]
- 24.Papadakis, K. A., J. Prehn, D. Zhu, C. Landers, J. Gaiennie, P. R. Fleshner, and S. R. Targan. 2004. Expression and regulation of the chemokine receptor CXCR3 on lymphocytes from normal and inflammatory bowel disease mucosa. Inflamm. Bowel Dis. 10:778-788. [DOI] [PubMed] [Google Scholar]
- 25.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raghavan, S., M. Fredriksson, A. M. Svennerholm, J. Holmgren, and E. Suri-Payer. 2003. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 132:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghavan, S., M. Hjulstrom, J. Holmgren, and A. M. Svennerholm. 2002. Protection against experimental Helicobacter pylori infection after immunization with inactivated H. pylori whole-cell vaccines. Infect. Immun. 70:6383-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghavan, S., A. M. Svennerholm, and J. Holmgren. 2002. Effects of oral vaccination and immunomodulation by cholera toxin on experimental Helicobacter pylori infection, reinfection, and gastritis. Infect. Immun. 70:4621-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki, S., H. Yoneyama, K. Suzuki, H. Suriki, T. Aiba, S. Watanabe, Y. Kawauchi, H. Kawachi, F. Shimizu, K. Matsushima, H. Asakura, and S. Narumi. 2002. Blockade of CXCL10 protects mice from acute colitis and enhances crypt cell survival. Eur. J. Immunol. 32:3197-3205. [DOI] [PubMed] [Google Scholar]
- 30.Song, J. H., J. I. Kim, H. J. Kwon, D. H. Shim, N. Parajuli, N. Cuburu, C. Czerkinsky, and M. N. Kweon. 2009. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. J. Immunol. 182:6851-6860. [DOI] [PubMed] [Google Scholar]
- 31.Song, J. H., H. H. Nguyen, N. Cuburu, T. Horimoto, S. Y. Ko, S. H. Park, C. Czerkinsky, and M. N. Kweon. 2008. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. U. S. A. 105:1644-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutton, P., S. J. Danon, M. Walker, L. J. Thompson, J. Wilson, T. Kosaka, and A. Lee. 2001. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut 49:467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svennerholm, A. M., and A. Lundgren. 2007. Progress in vaccine development against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 50:146-156. [DOI] [PubMed] [Google Scholar]
- 34.van Ginkel, F. W., R. J. Jackson, N. Yoshino, Y. Hagiwara, D. J. Metzger, T. D. Connell, H. L. Vu, M. Martin, K. Fujihashi, and J. R. McGhee. 2005. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect. Immun. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velin, D., L. Favre, E. Bernasconi, D. Bachmann, C. Pythoud, E. Saiji, H. Bouzourene, and P. Michetti. 2009. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology 136:2237-2246 e2231. [DOI] [PubMed] [Google Scholar]
- 36.Villavedra, M., H. Carol, M. Hjulstrom, J. Holmgren, and C. Czerkinsky. 1997. “PERFEXT”: a direct method for quantitative assessment of cytokine production in vivo at the local level. Res. Immunol. 148:257-266. [DOI] [PubMed] [Google Scholar]