Abstract
The Gram-negative pathogen Francisella tularensis secretes a siderophore to obtain essential iron by a TonB-independent mechanism. The fslABCDE locus, encoding siderophore-related functions, is conserved among different Francisella strains. In the virulent strain Schu S4, fslE is essential for siderophore utilization and for growth under conditions of iron limitation. In contrast, we found that deletion of fslE did not affect siderophore utilization by the attenuated live vaccine strain (LVS). We found that one of the fslE paralogs encoded in the LVS genome, FTL_0439 (fupA/B), was able to partially complement a Schu S4 ΔfslE mutant for siderophore utilization. We generated a deletion of fupA/B in LVS and in the LVS ΔfslE background. The ΔfupA/B mutant showed reduced growth under conditions of iron limitation. It was able to secrete but was unable to utilize siderophore. Mutation of both fupA/B and fslE resulted in a growth defect of greater severity. The ΔfupA/B mutants showed a replication defect in J774.1A cells and decreased virulence following intraperitoneal infection in mice. Complementation of the ΔfupA/B mutation in cis restored the ability to utilize siderophore and concomitantly restored virulence. Our results indicate that fupA/B plays a significant role in the siderophore-mediated iron uptake mechanism of LVS whereas fslE appears to play a secondary role. Variation in iron acquisition mechanisms may contribute to virulence differences between the strains.
Francisella tularensis is the etiological agent of tularemia, a potentially life-threatening zoonosis prevalent in the Northern hemisphere (15, 22). Two subspecies, F. tularensis and F. holarctica, are responsible for the type A and the less severe type B forms of the disease, respectively. The highly infectious bacteria are transmissible by aerosol, by direct contact of skin with infected material, or by the gastroinestinal route and can infect a variety of mammalian and nonmammalian hosts. The closely related F. novicida species is avirulent for humans but retains many characteristics of the virulent species. An attenuated live vaccine strain (LVS) derived from an F. holarctica (type B) isolate has proved to be a useful model system for understanding many of the mechanisms relating to host immunity (4, 5). It has also been useful in understanding various aspects of Francisella-specific physiology and pathogenicity that extend to virulent strains as well.
F. tularensis is a Gram-negative bacterium with a relatively small genome (10). It is an intracellular pathogen infecting a number of different cell types, including primary macrophages and macrophage-like cell lines. Studies performed with these systems have provided most of our current understanding of the intracellular lifestyle of the bacterium (15, 21). Following uptake by macrophages, the bacteria initially reside in membrane-bound phagosomes but then break out into the cytoplasm, where they proceed to replicate to high levels. Survival and propagation in the host are dependent on both the ability to evade immune responses and the ability to acquire nutrients from the host environment.
The availability of iron, an element that is a limiting factor in the host environment (17), is critical for the survival of most pathogens. Although essential for mediating critical redox reactions in various metabolic pathways, iron in excess can lead to production of destructive hydroxyl radicals; free-iron levels are therefore closely regulated in cells. Practically all the iron in the host is sequestered in protein complexes, including enzymes and scavenging molecules like transferrin, or stored within ferritin. Pathogens consequently need to utilize special mechanisms such as siderophore production to acquire iron in an iron-scarce environment (13).
Strains of F. tularensis as well as F. novicida secrete a siderophore that resembles rhizoferrin and whose biosynthesis is dependent on the genes of the fsl (also called fig) operon (3, 23). The first five genes, namely, fslABCDE, are conserved across different strains, and the fslA, fslB, and fslC genes have been shown to be necessary for siderophore production (8). A unique feature of the F. tularensis genome is the absence of tonB, exbB, and exbD genes, which are essential for siderophore uptake in all Gram-negative systems characterized to date. We have shown that fslE is essential for siderophore utilization in the type A strain Schu S4, and similar findings for F. novicida have been previously reported (8, 16). FslE is predicted to form a beta-barrel in the outer membrane and thus may represent a new type of siderophore receptor (16).
FslE belongs to a family of proteins unique to Francisella strains (10). One of these paralogs in Schu S4, fupA (FTT0918), has been shown to be important for virulence and also to play a role in iron acquisition, including siderophore utilization (24). In LVS, the corresponding locus FTL_0439 is a hybrid gene (here termed fupA/B) resulting from recombination of fupA with the adjacent paralogous gene (here termed fupB); this recombination event has been shown to be a key cause for attenuation of LVS in mice following infection by the subcutaneous route (19).
We show here that fupA/B is primarily responsible for siderophore-mediated iron acquisition in LVS and that fslE plays a secondary role in aiding survival under conditions of iron limitation. Thus, although the process of siderophore production is conserved in LVS and Schu S4, siderophore utilization in the two strains is facilitated by different mechanisms. We also demonstrate the associated role of fupA/B in virulence following intraperitoneal (i.p.) infection of mice.
MATERIALS AND METHODS
Bacterial strains and culture.
The Francisella tularensis strains used in this study and listed in Table 1 were grown in various media as previously described (23). Strains were maintained on Mueller-Hinton agar supplemented with cysteine (MHA). Chamberlain's defined medium (CDM) and tryptic soy broth supplemented with 0.1% cysteine (TSB/C) were used for routine liquid culture. CDM prepared without addition of FeSO4 (CDM-Fe) was used as an iron-limiting medium for agar plates. Chelex-treated CDM (che-CDM) was supplemented with MgSO4 and CaCl2, with ferric pyrophosphate added as required. Kanamycin was used at a 15 μg/ml concentration.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| F. tularensis strains | ||
| LVS | F. tularensis subsp. holarctica live vaccine strain | K. Elkins |
| Schu S4 | F. tularensis subsp. tularensis | CDC |
| GR13 | LVS ΔfslE | This study |
| GR16 | LVS ΔfupA/B | This study |
| GR17 | LVS ΔfslE ΔfupA/B | This study |
| GR20 | GR16 (pGIR459); vector integrant | This study |
| GR21 | GR17 (pGIR459); vector integrant | This study |
| GR23 | GR17 (pGIR474); fslE plasmid integrant | This study |
| GR24 | GR16 (pGIR479); fupA/B plasmid integrant | This study |
| GR25 | GR17 (pGIR479); fupA/B plasmid integrant | This study |
| GR211 | Schu S4 ΔfslE | 15 |
| GR212 | GR211 (pGIR474); fslE plasmid integrant | This study |
| GR220 | GR211 (pGIR459); vector integrant | This study |
| GR222 | GR211 (pGIR479); fupA/B plasmid integrant | This study |
| GR223 | GR211 (pGIR480); FTL_0147 plasmid integrant | This study |
| Plasmids | ||
| pGIR459 | Suicide plasmid vector for targeting to region downstream of FTL_0133 | This study |
| pGIR474 | fslE under fslA promoter control cloned in pGIR459 | This study |
| pGIR467 | Suicide plasmid for generation of ΔfslE mutant | This study |
| pGIR479 | fupA/B under fslA promoter control cloned in pGIR459 | This study |
| pGIR480 | FTL_0147 under fslA promoter control cloned in pGIR459 | This study |
| pBAS1B | Suicide vector for generation of ΔfupA/B mutant | This study |
Escherichia coli strain MC1061.1 [Δ(ara-leu)7696 araD139 Δ(lac)X74 galKl6 galE15 mcrA mcrB1 rpsL(Strr) hsdR2 λ− F− recA] was used for routine cloning and was grown in Luria broth. Ampicillin was used at a concentration of 50 μg/ml (for liquid culture) or at 100 μg/ml (agar plates).
Construction of ΔfslE and ΔFTL_0439 (fupA/B) deletion mutants and complementing plasmids.
Deletion mutants of fslE and FTL_0439 were generated in a two-step process using the sacB suicide vector pGIR463 to construct the deletion constructs as previously described (16). GR13, the ΔfslE mutant in LVS, was obtained using the deletion construct pGIR467, which carried a 1.05-kb region at the 5′ end of fslE and a 1.13-kb 3′ flanking region obtained by PCR amplification using LVS DNA and Pfu DNA polymerase (Stratagene). To generate the FTL_0439 (fupA/B) mutant, a 1.883-kb region 70 bp upstream of the start codon of fupA/B was PCR amplified using primers 263 and 272 and FastStart Taq (Roche) (Table 2). A 2.0-kb region starting 2.081 kb downstream of fupA/B was amplified with primers 266 and 267. The 5′ and 3′ flanking sequences were cloned in pGIR463 to generate plasmid pBAS1B.
TABLE 2.
Primers used in this work
| Primer | Sequencea |
|---|---|
| 85 | 5′-ctactggcggccgcGTAGCTATGGCATACATCATC-3′ |
| 89 | 5′-ctactggagctcGTAGCATGAAAAGCTTACC-3′ |
| 152 | 5′-ctactggctagcTTGTATGGATTAGTCGAGC-3′ |
| 153 | 5′-ctactgagatctGACGAATGTTCATAACAATCTTAC-3′ |
| 197 | 5′-ctactggctagcAACAGATATACTGGTTAATC-3′ |
| 198 | 5′-ctactgtccggaAAAGGGGGTAATTGATAATAC-3′ |
| 200 | 5′-CAAACTGTTTTAAGAGCTCG-3′ |
| 213 | 5′-ctactggctagcAATAATCACTATCCAGAAG-3′ |
| 214 | 5′-ctactgtccggaCATGATAATGATAACGAATATC-3′ |
| 231 | 5′-ctactgtccggaTGGTTTTTCTGTATTAAAAGAG-3′ |
| 232 | 5′-ctactggcggccGCTTAAAGATATACAGCCATATCT-3′ |
| 242 | 5′-TTATAAGTGGTTTGAGTCATGG3′ |
| 256 | 5′-ctactgtccggaTTTGGTTTGCCAATTTTTTATTAG-3′ |
| 260 | 5′-ctactgtccggaTAATCTCTAGGGGTTGTTGTG-3′ |
| 261 | 5′-ctactggcggccGCCTAAACTATATATATACAGATAGG-3′ |
| 263 | 5′-ctactgtccggaCTTAGTTTCAATAAATTTCATAC-3′ |
| 266 | 5′-ctactgtccggaTAACTATTCAAATGTCTAGTAAAATCG-3′ |
| 267 | 5′-ctactgctcgagAAAAATGAAATTTGGAATGTCTG-3′ |
| 272 | 5′-ctactggctagcAAGAAAATTAAGAAATAAAAGCGG-3′ |
| 273 | 5′-ctactggcggccGCCTAAATATGTCTAGATATAAACTG-3′ |
| 281 | 5′-GTCTGCTTACAAACCTGAAG-3′ |
Uppercase letters correspond to Francisella genomic sequences; lowercase letters indicate nucleotides added to the primers.
The deletion plasmid constructs were introduced into the F. tularensis strains by electroporation. Kanamycin-resistant colonies were tested for the presence of plasmid integrated in the genome by the use of PCR amplification of genomic DNA and sets of primers such that one primer lay within the integrative plasmid and the other was outside the sequences cloned in the plasmid. Sucrose-resistant colonies arising from integrants were scored for loss of plasmid sequences with loss of accompanying kanamycin resistance, and isolates with deletions were identified using PCR and genomic DNA. The PCR products from deletion mutants GR13 (LVS ΔfslE), GR16 (LVS ΔfupA/B), and GR17 (LVS ΔfslE ΔfupA/B) were sequenced to verify the deletions. The mutation in GR13 resulted in deletion of the central 474-amino-acid coding region of fslE and placed the amino-terminal 18 codons in frame with the carboxy-terminal 8 codons. The ΔfupA/B deletions included the entire fupA/B locus as well as the insertion elements ISftu1 and ISftu2 immediately downstream of fupA/B.
A suicide plasmid targeting the sequences 3′ of FTL_0133 (feoB) was used for complementation of mutants in cis. This region includes a stretch of ∼830 bp that does not appear to encode a gene, and we have previously determined that integration in this region does not affect growth of LVS or of Schu S4 in response to iron levels. pGIR459, the integrative plasmid, was similar to pGIR463 and carried between NotI and SacI sites a 2-kb fragment corresponding to the 3′ flank of FTL_0133, which had been amplified using primers 85 and 89. A 230-bp fragment corresponding to the promoter of fslA was amplified using primers 213 and 214 and cloned in pGIR459 as an NheI-BspEI fragment to drive expression of genes for complementation. The genes were amplified by PCR (fslE from Schu S4 by the use of primers 231 and 232, fupA/B by the use of primers 256 and 273, and FTL_0147 by the use of primers 260 and 261) and cloned as NheI-NotI fragments to generate plasmids pGIR474, pGIR479, and pGIR480, respectively. These plasmids were introduced into the relevant bacterial strains by electroporation, and kanamycin-resistant colonies were confirmed to be the desired integrants by PCR of genomic DNA with sets of primers corresponding to the plasmid and chromosomal sequences. PCR analysis was carried out using either HotstarTaq (Qiagen) or Phusion High-Fidelity DNA polymerase (New England Biolabs).
Growth in iron-limiting media.
Growth of F. tularensis under conditions of iron limitation was essentially as previously described except that, in place of ferric chloride, ferric pyrophosphate was used at 2.5 μg/ml and 0.125 μg/ml for iron-replete and iron-limiting growth, respectively (23). Briefly, cells grown in CDM were washed and inoculated into the iron replete- and iron-limiting media to an optical density at 600 nm (OD600) of 0.01 (∼3 × 107 CFU/ml). Growth was monitored as changes in the OD600 of the liquid cultures over a period of 48 to 52 h. All experiments included three replicates and were performed at least two times with similar results. Growth differences between the strains at specific time points were statistically compared using the t test function of the Excel 2003 (Microsoft) program. For assessment of growth on iron-limiting agar, washed cells at an OD600 of 1.0 were serially diluted in che-CDM, and 5-μl volumes of the dilutions were spotted on a CDM-Fe plate and, in parallel, on an iron-rich MHA plate. These assays were repeated at least two times for each strain.
Bioassay for siderophore utilization.
Cells were tested for siderophore utilization as previously described (16, 23). For LVS and mutants, cells were washed and ∼3 × 106 CFU were spread on the CDM-Fe plates. For assays with GR211, ∼3 × 103 CFU were seeded on plates. Cells to be used for siderophore production were similarly washed and resuspended to an OD600 of 1.0 (∼3 × 109 CFU/ml). These cell suspensions (2.5 μl each) were spotted on the seeded plates and the plates incubated at 37°C for 2 to 4 days. A growth halo of colonies around a spot demonstrated the ability of the indicator strain (seeded on the plate) to grow on the iron-limiting plate by utilizing the siderophore secreted by the donor strain (spotted on the plate). Experiments were repeated at least two times and produced similar results.
Siderophore detection.
Cultures were grown in iron-replete or iron-limiting CDM. Production of the siderophore in supernatants of cultures was detected by a chromazurol-S (CAS) assay as previously described (23). Culture supernatants (100 μl) were mixed with 100 μl of the CAS reagent and 2 μl of the shuttle solution (20). The absorbance at 630 nm was read in a plate reader after 30 min at room temperature. The CAS activity was calculated as follows: (OD630 of blank − OD630 of sample)/OD630 of blank. The CAS activity was normalized to cell density (OD600) to obtain a specific CAS activity. The results determined with the different strains were analyzed using the t test function of Excel 2003 (Microsoft). Experiments were repeated with triplicate samples at least two separate times.
Western blotting.
Cell pellets from F. tularensis cultures were normalized with respect to cell density, and lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% gels and transferred to polyvinylidene difluoride (PVDF). The primary antibody used was rabbit antiserum raised to the FslE peptide NPSLSRFLQSQGHPTDTIGS conjugated to keyhole-limpet hemocyanin and subjected to affinity purification with the same peptide (Covance, Denver, PA). This anti-FslE antibody was used at a 1:2,500 dilution. The blots were developed by chemiluminescence after incubation with secondary goat anti-mouse-peroxidase conjugate (Sigma).
Intracellular invasion and replication.
F. tularensis strains were cultured to the mid-exponential phase in CDM and stored in aliquots at −80°C. The titers of the aliquots were determined, and the aliquots served as stock for use in intracellular growth experiments. Mouse macrophage-like J774.1 cells (ATCC TIB-67) were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco) (DMEM + FBS) at 37°C with 5% CO2. The cells were seeded in 24-well plates at a concentration of 2 × 105 cells/well in 1 ml of DMEM + FBS 1 day prior to the experiment. The J774A.1 cells were infected with LVS and the mutants at a multiplicity of infection (MOI) ranging from 10 to 30 in 0.5 ml of DMEM + FBS/well. The number of bacteria present in each inoculum was verified by plating on MHA. The plates were centrifuged at 950 × g for 10 min and incubated for 1 h at 37°C to allow bacterial attachment and entry. The medium was then removed by aspiration, and extracellular bacteria were killed by incubation of macrophages with gentamicin (100 μg/ml) in 500 μl of DMEM + FBS for 1 h. The medium was then removed, the cells were washed with DMEM lacking FBS, and the cells in one set of wells were lysed for the 2-h time point. Fresh DMEM + FBS was added into the remaining wells, and the plates were returned to the incubator. Sets of wells were treated with 100 μl of 1% saponin at 37°C for 10 min to lyse cells and release intracellular bacteria at 2, 8, and 25 h postinfection. The lysates were serially diluted with phosphate-buffered saline (PBS), and 50-μl volumes of the dilutions were spotted onto MHA plates. The MHA plates were incubated at 37°C for 2 days to determine the number of colony-forming units in the lysates. The t test function of Excel 2003 (Microsoft) was used for statistical analysis of the intracellular replication of the different strains. Experiments were performed with quadruplicate samples at least two separate times and gave similar results.
Analysis of virulence in mouse model.
Six- to 8-week-old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were infected with LVS and the mutants by using the frozen stocks described above. The bacteria were diluted in PBS and injected by the intraperitoneal route in a volume of 100 μl. The actual numbers of bacteria delivered were verified by plating on MHA. Animals were monitored daily for a period of 14 days. Animals demonstrating signs of irreversible morbidity were euthanized. In some experiments, mice surviving the initial challenge were intraperitoneally rechallenged with LVS after 3 weeks at the doses stated above. All mouse infection experiments were conducted with the approval of the Animal Care and Use Committee of the University of Virginia. Values for the 50% lethal dose (LD50) of the mutant strains were derived by probit regression analysis using the PASW Statistics 18 program licensed through the University of Virginia.
RESULTS
The LVS ΔfslE mutant is not defective for growth under conditions of iron limitation.
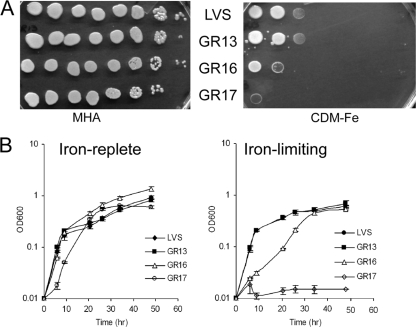
fslE is essential for siderophore utilization in Schu S4 (16). Since the fslABCDE locus is completely conserved in LVS and Schu S4, we expected that fslE would play a similar role in LVS. We tested LVS ΔfslE mutant strain GR13 for the ability to grow under conditions of iron limitation. We spotted serial dilutions of LVS and GR13 on iron-replete agar (MHA supplemented with iron) and on iron-limiting agar (CDM-Fe) and compared their growth characteristics. The two strains grew equally well on the iron-replete plate, and surprisingly, equally well (up to the third dilution) on the iron-limiting plate (Fig. 1 A). We also examined growth of LVS and GR13 in liquid che-CDM supplemented with high or limiting levels of ferric pyrophosphate. The two strains grew similarly in both media (Fig. 1B).
FIG. 1.
Growth deficiency of ΔfupA/B mutants under conditions of iron limitation. (A) Growth on iron-replete (MHA) and iron-limiting (CDM-Fe) agar plates. LVS and the mutants were grown in CDM, and serially diluted cultures were spotted on iron-replete and iron-limiting plates in parallel. (B) Growth in liquid medium. Washed cells were inoculated in iron-replete and iron-limiting che-CDM, and growth was followed over a period of 48 h. Cultures were grown in triplicate, and means and standard deviations of data representing the results of one representative experiment are shown in the growth plot. The strains used were LVS, GR13 (LVS ΔfslE), GR16 (LVS ΔfupA/B), and GR17 (LVS ΔfslE ΔfupA/B).
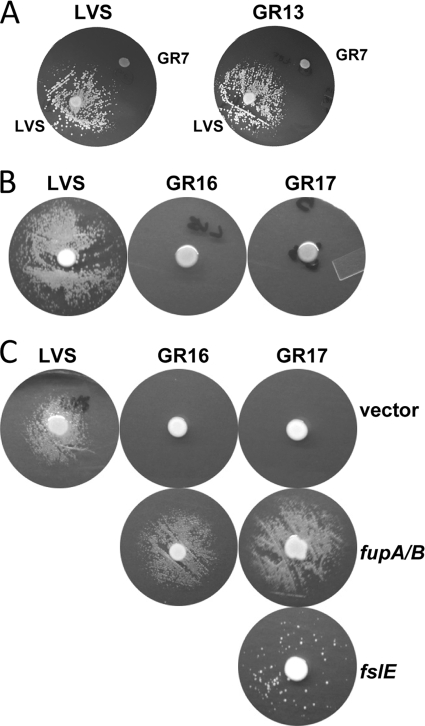
To determine whether the ΔfslE mutation had any effect on siderophore utilization, LVS and GR13 were tested for siderophore-dependent growth on iron-limiting agar. In this assay, LVS cells seeded on the plate were able to form a growth halo around a spot of siderophore-secreting LVS but not around the siderophore-deficient ΔfslA mutant. GR13 behaved similarly to LVS, demonstrating the same level of growth as that seen around LVS and an absence of growth around the siderophore-deficient strain (Fig. 2 A).
FIG. 2.
Siderophore utilization by mutants and complemented strains. Plates were seeded with strains as noted. (A) Siderophore-dependent growth of LVS and GR13 (LVS ΔfslE), showing growth around the siderophore-secreting LVS spot and lack of growth around siderophore-deficient ΔfslA strain GR7. (B) Deficiency of GR16 (LVS ΔfupA/B) and GR17 (LVS ΔfslE ΔfupA/B) mutants in utilization of siderophore secreted by LVS in the central spot. (C) The siderophore utilization defect is fully or partially restored in complemented strains. Siderophore-dependent growth of the ΔfupA/B strains was fully restored by reintroduction of fupA/B in cis, whereas reintroduction of fslE in GR17 partially complemented the growth defect. The siderophore source in this set of assays was LVS (vector).
These results indicated that siderophore utilization in LVS is not primarily mediated by fslE as it is in Schu S4 and that an alternate gene is responsible for this function.
FTL_0439 (fupA/B) partially complements an Schu ΔfslE mutant.
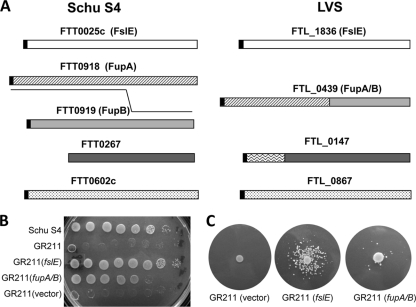
fslE is one member of a sequence-related family of genes unique to F. tularensis (10). Two of the paralogs in LVS, FTL_0439 and FTL_0147, differ in structure from their Schu S4 counterparts (Fig. 3 A). We reasoned that one of these LVS-specific paralogs might conceivably function in siderophore utilization in place of fslE. FTL_0439 appears to be a product of recombination between adjacent paralogs corresponding to FTT0918 (fupA) and FTT0919 (herein designated fupB) of Schu S4 (11, 19). This hybrid gene carries an in-frame fusion of the amino-terminal part of fupA to the carboxy-terminal part of fupB. We here refer to FTL_0439 as fupA/B. By analysis of the sequence database in GenBank, we determined that the second LVS-specific paralog FTL_0147 encodes a protein with a 140-amino-acid extension at the amino terminus relative to the Schu S4 gene FTT_0267 (Fig. 3A).
FIG. 3.
Partial complementation of GR211 (Schu ΔfslE) by LVS homolog FTL_0439. (A) The FslE protein family in F. tularensis strain Schu S4 and LVS. The paralogs within Schu S4 share sequence similarity of 52 to 68% and identity of 45 to 62%. Orthologs between SchuS4 and LVS share 98 to 99% identity. FTL_0439 and FTL_0147 show variations from their orthologs in Schu S4. FTL_0439 (FupA/B) is a hybrid consisting of the amino-terminal half of FupA fused to the carboxy-terminal half of FupB. The amino-terminal end of FTL_0147 from LVS is deleted in the ortholog FTT0267 in Schu S4. The black boxes represent potential signal peptides. (B) Growth on iron-limiting CDM agar of serially diluted cultures of Schu S4, Schu S4 ΔfslE (GR211), and complemented strains. (C) Siderophore utilization by Schu ΔfslE partially complemented by fupA/B. Iron-limiting plates were seeded with GR211 harboring the vector or with the fslE or fupA/B complement as noted. SchuS4 cells were spotted as a source of siderophore, and growth halos around the spots were recorded.
In order to determine the functionality of these LVS-specific paralogs in siderophore utilization, we tested them for the ability to complement the Schu ΔfslE mutant, GR211, for iron acquisition. We introduced the genes into the GR211 chromosome under the control of the fslA promoter on a suicide plasmid. Empty vector and vector bearing fslE served as negative and positive controls, respectively, for the complementation in cis. As shown in Fig. 3B, when serial dilutions were spotted on an iron-limiting plate, GR211 and the vector control transformant barely grew, even at the highest cell concentration. Complementation with the parental Schu S4-derived fslE predictably reversed the growth defect. Introduction of fupA/B permitted cells to grow at higher dilutions, although not as well as with the homologous complement (Fig. 3B). Complementation was also observed in liquid growth (data not shown). The paralog FTL_0147 introduced into GR211 did not complement the growth-deficient phenotype in any way (data not shown). This could have been either because FTL_0147 is not functional in iron uptake or because an adequate level of the gene product was not attained in the transformed cells.
The ability of fupA/B to complement the siderophore utilization defect of GR211 was assessed in the plate-based growth assay. As shown in Fig. 3C, GR211 with the vector complement was not able to form growth halos around the source of siderophore. Homologous complementation with fslE restored the ability to utilize siderophore for growth. When fupA/B was introduced, growth of a few colonies was consistently observed, suggesting that the paralog could partially complement the siderophore utilization defect.
These results suggested that fupA/B could function in siderophore-mediated iron acquisition in LVS.
Generation of the LVS ΔfupA/B and LVS ΔfslE ΔfupA/B strains.
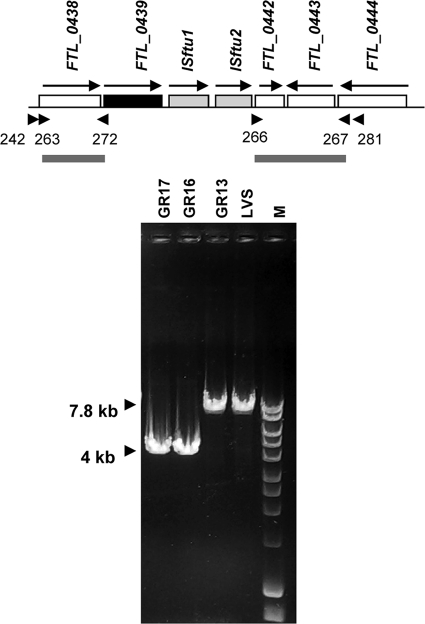
The fupA/B locus is flanked on the 5′ side by the gene for malate dehydrogenase, maeA (FTL_0438). On the 3′ side are copies of each of the insertion elements ISftu1 and ISftu2 (encoding transposases represented by open reading frames [ORFs] FTL_0440 and FTL_0441, respectively) followed by locus FTL_0442, encoding a hypothetical protein (Fig. 4). We deleted a ∼3.8-kb chromosomal fragment spanning these insertion elements along with fupA/B to generate strain GR16. We took this approach because the strategy for deletion mutagenesis requires homologous recombination at the 5′ and 3′ flanking regions cloned on the deletion plasmid construct, and we were concerned that the presence of multiple copies of ISftu1 and ISftu2 elsewhere in the genome (61 and 44 copies, respectively) (9) could create problems and interfere with specific recombination if the cloned 3′ flanking sequence included these elements. We created a similar deletion in the GR13 (LVS ΔfslE) background to generate the LVS ΔfslE ΔfupA/B strain GR17. We confirmed the presence of the deletions using PCR of genomic DNA and primers flanking the locus as depicted in Fig. 4. LVS and GR13 gave a product of ∼7.8 kb, while GR16 and GR17 gave a smaller product of ∼4 kb. We also sequenced the PCR product in the region corresponding to the deletion to confirm the mutations.
FIG. 4.
Chromosomal organization of the FTL_0439 (fupA/B) region in F. tularensis LVS and PCR analysis of deletion. Arrows indicate the direction of transcription. The arrowheads represent primers 263-272 and 266-267 used in amplifying the flanking regions for generation of the pBAS1B deletion construct depicted underneath. The deletion includes the two IS elements (gray boxes) in addition to fupA/B (black box). Primer 242 located upstream of the fupA/B gene and primer 281 located downstream of ISftu2 were used for analyzing genomic DNA from LVS and the mutants. Lane M shows a 1-kb DNA ladder (Bioline).
The ΔfupA/B and ΔfslE ΔfupA/B mutants are defective for growth under conditions of iron limitation.
We spotted serial dilutions of LVS, GR16, and GR17 on an iron-replete agar plate (MHA supplemented with iron) and on an iron-limiting agar plate (CDM-Fe). In comparison to LVS and GR13 (LVS ΔfslE), both strains carrying the ΔfupA/B mutation showed growth defects under conditions of iron limitation (Fig. 1A). GR16 (LVS ΔfupA/B) showed good growth only at the highest density, while growth of the ΔfslE ΔfupA/B GR17 double mutant was further restricted to the margins of the spot at the highest density.
We compared the growth characteristics of the mutants in liquid che-CDM supplemented with high and low levels of ferric iron (Fig. 1B). LVS and GR13 grew similarly under both sets of conditions. GR16 also grew similarly to LVS in iron-replete medium. When iron levels were limiting, however, GR16 showed a slower growth rate (P < 0.001 for time points up to 26 h). GR17 grew slowly even at high iron levels, reaching the stationary phase later (P < 0.001 during exponential growth). It was more severely affected by iron limitation and did not show any growth under these conditions.
The results of growth on agar and in liquid media indicated that fupA/B plays a significant role in growth of LVS under conditions of iron limitation. They further suggested that fslE also had a role in conjunction with fupA/B.
fupA/B is necessary for siderophore utilization.
In order to determine whether the growth defect of the ΔfupA/B mutants (GR16 and GR17) under conditions of iron limitation was related to siderophore-mediated iron uptake, we tested the ability of the strains to produce and to utilize siderophore. Both mutant strains were capable of siderophore production (see below). In the siderophore utilization assay with LVS as the source of siderophore, we observed halos of growth around the siderophore source on the LVS-seeded plate (Fig. 2B). There was, however, no siderophore-promoted growth on the plates seeded with GR16 and GR17. These results indicated that the ΔfupA/B mutants were unable to utilize siderophore to support growth on the iron-limiting plate.
Complementation of mutants in cis for iron-deficient growth.
We complemented the strains with fupA/B introduced on a chromosomally integrated plasmid. We tested the ability of GR16 (LVS ΔfupA/B) and GR17 (LVS ΔfslE ΔfupA/B) harboring the control plasmid vector, fslE+ plasmid, or fupA/B+ plasmid to grow under conditions of iron limitation (Fig. 5). We used LVS with the plasmid vector as a control for the transformants. As shown in Fig. 5A, the growth defect of GR16 transformed with the vector on iron-limiting agar was reversed in the fupA/B transformant. In iron-replete liquid medium, all the strains grew similarly to LVS (vector) (data not shown). Under iron-limiting conditions, GR16 with the control vector displayed the parental growth deficiency, but the defect was fully reversed upon reintroduction of fupA/B (Fig. 5C).
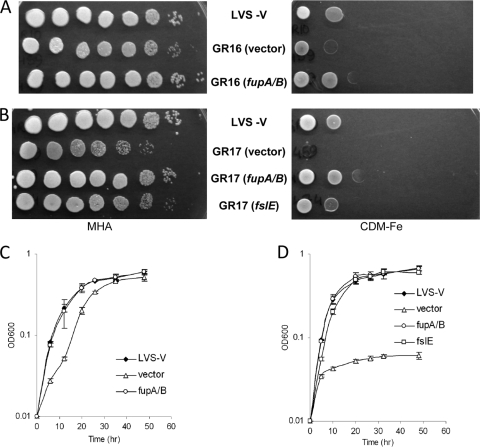
FIG. 5.
Complementation of ΔfupA/B mutants for growth under conditions of iron limitation. (A) Complementation of GR16 (LVS ΔfupA/B). Growth of serially diluted cultures of GR16 transformed with vector or fupA/B plasmid was compared to that of LVS with vector (LVS-V) on iron-rich MHA and iron-limiting CDM-Fe agar. (B) Complementation of GR17 (LVS ΔfslE ΔfupA/B). Growth of serially diluted cultures of GR17 transformed with vector, fupA/B, or fslE plasmid was compared to that of LVS with vector on iron-rich and iron-limiting agar. (C) Complementation of GR16 in iron-limiting liquid media. Growth of GR16 harboring vector or the fupA/B plasmid was compared to that of LVS with vector (LVS-V). (D) Complementation of GR17 in iron-limiting liquid media. Growth of GR17 with control vector or the fupA/B or fslE plasmid was compared to that of LVS with vector (LVS-V).
We tested complementation of the double mutant GR17 individually with fslE and fupA/B. As shown in Fig. 5B, GR17 transformed with vector did not grow on iron-limiting agar. Growth was completely restored by the introduction of fupA/B. Introduction of fslE, interestingly, restored substantial growth but did not completely complement the growth defect. Complementation was also tested under liquid growth conditions. All the strains grew similarly to LVS with vector in iron-replete CDM except GR17 harboring the vector, whose growth resembled that of the parental GR17 strain (data not shown and Fig. 1B). In iron-limiting medium, the severe growth defect of vector-transformed GR17 was completely reversed by expression of fupA/B (Fig. 5D). Introduction of fslE also significantly improved growth, and although growth was slower than that of the fupA/B-transformed strain, the cells eventually reached a similar density.
The strains were also tested for siderophore utilization. As shown in Fig. 2C, LVS (vector) utilized siderophore secreted by the same cells to form a growth halo similar to that seen with the parental LVS, while the vector-transformed ΔfupA/B mutant strains could not. Introduction of fupA/B in cis was sufficient to restore the siderophore-dependent growth of the mutants. Introduction of fslE in the GR17 double mutant allowed a few colonies to develop around the siderophore source, which is consistent with a partial complementation of the siderophore utilization defect.
These complementation results indicated that the reduced-growth phenotypes of GR16 and GR17 under conditions of iron limitation were specifically due to mutation of fupA/B. Furthermore, we found that expression of FslE following introduction of the gene at an alternate site on the chromosome could partially compensate for the absence of FupA/B, although why this was so is not clear.
The ΔfupA/B mutation results in deregulation of siderophore production and FslE protein expression.
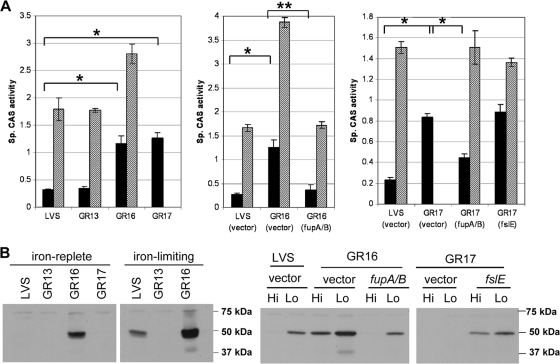
Having determined that mutation of fupA/B resulted in loss of the ability to utilize siderophore, we examined siderophore production in the different mutant strains. The strains were grown under iron-replete or iron-limiting conditions, and the levels of siderophore in culture supernatants were assayed using the CAS reagent. As shown in Fig. 6 A, LVS exhibited normal iron-regulated siderophore levels: it had a low specific CAS activity when grown in iron-replete medium, whereas the activity increased ∼7-fold in iron-limiting medium. GR13 (LVS ΔfslE) displayed levels of siderophore production similar to those seen with LVS. GR16 (LVS ΔfupA/B) and GR17 (LVS ΔfslE ΔfupA/B) showed significantly increased siderophore production even under iron-replete conditions, with levels ∼4- to 5-fold greater than those seen with LVS. Iron limitation elicited a further increase in siderophore production in GR16. Since GR17 was unable to grow in iron-limiting medium, no value was obtained for this growth condition.
FIG. 6.
Deregulation of siderophore and FslE levels in ΔfupA/B mutants. (A) Specific levels of CAS activity representing siderophore levels in culture supernatants of LVS and the mutants were determined after growth in iron-replete CDM (black bars) or iron-limiting CDM (hatched bars). The strains harboring complements were similarly assayed. Cultures were grown in triplicate, and the means and standard deviations of data representing the results of representative experiments are shown. *, P < 0.001; **, P < 0.002. (B) Western blotting with FslE antibodies. Cultures were grown in iron-replete (Hi) and iron-limiting (Lo) media. Whole-cell lysates normalized for cell density were subjected to SDS-PAGE, transferred to a PVDF membrane, and probed with polyclonal antiserum raised against an FslE peptide. The locations of the prestained standards run on the gel are indicated. The strains used were LVS, GR13 (LVS ΔfslE), GR16 (LVS ΔfupA/B), and GR17 (LVS ΔfslE ΔfupA/B).
Expression of fslE in LVS and the fupA/B mutant strains after growth under iron-replete and iron-limiting conditions was evaluated by Western blotting. As seen with Schu S4, FslE in LVS was detectable only under iron-limiting conditions of growth whereas GR13, which carried a defined in-frame deletion of fslE, showed concomitant loss of the 50-kDa FslE protein (Fig. 6B). Unlike LVS, GR16 (LVS ΔfupA/B) produced detectable levels of FslE even under iron-replete conditions. In low-iron media, GR16 produced levels of FslE even higher than those seen with LVS. Thus, FslE production mirrored the pattern of increased siderophore levels seen with GR16. GR17, while having elevated siderophore levels similar to those of GR16, showed no FslE production in iron-replete medium, as it was additionally a ΔfslE mutant.
To determine whether the deregulation was specifically due to fupA/B mutation, we examined siderophore production as well as FslE levels in the complemented strains. As shown in Fig. 6A, vector-transformed LVS, GR16, and GR17 behaved similarly to the parental strains with regard to siderophore production. Reintroduction of fupA/B in GR16 and in GR17 restored the iron-regulated pattern seen with LVS. Reintroduction of fslE in GR17 restored FslE production but did not rectify the deregulated expression. It should be noted here that since GR17 (vector) does not grow in iron-limiting media, a large aliquot of cells grown in iron-replete medium was inoculated in iron-limiting medium at 37°C for a period of 20 h in order to prepare the low-iron lysates for this blot.
These results indicated that expression of the fsl genes encoding siderophore-related functions is in some way regulated by fupA/B. Deletion of fupA/B caused a deregulation of expression not seen with the ΔfslE mutant.
Invasion and replication assay in J774A.1 macrophages.
To determine whether mutation in fupA/B impairs the ability of the strains to invade and grow intracellularly, we infected murine macrophage-like J774A.1 cells with LVS and the fslE and fupA/B mutants. We evaluated invasion at 2 h and intracellular replication at 8 and 25 h postinfection. At 2 h, all four strains had similar intracellular numbers, indicating similar invasive capabilities (Fig. 7). No discernible differences were observed at 8 h, but by 24 h, there was a significant difference in the intracellular titers of the strains. LVS and GR13 replicated to similar intracellular levels, but the GR16 and GR17 strains showed significantly lower levels (P < 0.02) (Fig. 7). Thus, fupA/B deletion resulted in a reduced capacity for intracellular growth.
FIG. 7.
Intracellular replication of LVS and the mutants. J774A.1 macrophages in a 24-well plate were infected with bacteria at an MOI of 10 to 30 in quadruplicate. Bacterial entry and replication were determined over a 25-h period; the results are expressed as mean log numbers of colony-forming units per milliliter. The error bars indicate standard deviations. Mutants found to be significantly defective in intracellular growth are indicated by asterisks (P < 0.02). The strains used were LVS, GR13 (LVS ΔfslE), GR16 (LVS ΔfupA/B), and GR17 (LVS ΔfslE ΔfupA/B).
Virulence in mice.
Mice are tolerant to the LVS by the intradermal route of infection (LD50 > 106 CFU) but are highly susceptible to infection by the intraperitoneal (i.p.) route, where the LD50 is ∼10 CFU (6). We tested the virulence of the fslE and fupA/B mutants in mice following i.p. infection. Groups of 4 to 5 C57BL/6 mice were challenged with various doses of bacteria, and the outcome was evaluated over a period of 14 days. Mice typically succumb between days 5 and 7 postinfection. Results of different trials are summarized in Table 3. The ΔfslE mutant was similar in virulence to LVS. The ΔfupA/B mutant showed reduced virulence, which is consistent with the reported attenuation of a transposon insertion mutant of the gene (12). Virulence of the strain was restored in the complement, indicating that the virulence defect was associated with the fupA/B mutation. Using Probit analysis, we determined that the ΔfupA/B mutant had an LD50 of 3 × 104 CFU whereas the ΔfslE ΔfupA/B mutant had an LD50 of 2.7 × 105 CFU by the i.p. route. These studies showed that fupA/B is important for the virulence of LVS and that fslE also contributes to a small extent, as demonstrated by the difference in the LD50 values of the single and double fupA/B mutants.
TABLE 3.
Virulence of strains following i.p. infection of C57BL/6 mice
| Expt | Challenge strain | Dosea (CFU) | No. of survivors/total no. of mice |
|---|---|---|---|
| 1b | LVS | 11 | 2/5 |
| GR13 | 13 | 3/5 | |
| GR16 | 19 | 5/5 | |
| GR17 | 32 | 5/5 | |
| 2c | LVS | 820 | 1/5 |
| GR13 | 840 | 0/5 | |
| GR16 | 1,450 | 5/5 | |
| GR17 | 1,890 | 5/5 | |
| 3 | LVS | 2,080 | 0/4 |
| GR16 (vector) | 2,270 | 4/4 | |
| GR16 (fupA/B) | 1,945 | 0/4 | |
| 4 | GR16 | 4.5 × 103 | 4/4 |
| 4.5 × 104 | 2/4 | ||
| 4.5 × 105 | 1/4 | ||
| GR17 | 8.7 × 103 | 4/4 | |
| 8.7 × 104 | 3/4 | ||
| 8.7 × 105 | 1/4 |
C57BL/6 mice were infected with various amounts of CFU by the i.p. route.
The survivors were subsequently challenged with 820 CFU of LVS by the i.p. route, and all mice survived the LVS challenge.
The survivors were subsequently challenged with 1.2 × 105 CFU of LVS by the i.p. route, and all except one in the GR17 mouse group survived the LVS challenge.
The survivors from experiments 1 and 2 were further challenged with a higher dose of LVS (820 CFU in the first set and 1.2 × 105 CFU in the second set) to determine whether they had developed protective immunity. The mice were all protected from the second challenge, except for one mouse in the second set that had been initially challenged with the double mutant. Overall, these experiments suggested that exposure to the ΔfupA/B strains could induce protective immunity to i.p. challenge with LVS.
DISCUSSION
The fur-fslABCDE locus is highly conserved between Schu S4 and LVS, and the siderophore produced by either strain promotes growth of both strains under conditions of iron limitation (23). This would suggest that the biosynthetic and uptake machinery is conserved between the two strains. In previous studies, we have shown that fslE is essential for siderophore-mediated iron acquisition in Schu S4 and that an Schu ΔfslE mutant is considerably attenuated for growth under conditions of iron limitation (16). The predicted FslE ORF in LVS differs at only 5 residues from that in Schu S4, and the substitutions are largely conservative. As demonstrated here, FslE in LVS is expressed during iron-limited growth. Our finding that the LVSΔfslE strain showed no deficiency for siderophore utilization relative to parent LVS was, therefore, surprising.
Within the family of five sequence-related proteins initially identified in Schu S4, FslE is one of the three paralogs predicted to have signal peptides and coiled-coil domains (10). We subsequently used the PRED-TMBB program to predict that FslE could likely form a beta-barrel in the outer membrane and assume a conformation reminiscent of TonB-dependent receptors (1, 16). The Schu S4 paralogs fupA and the adjacent fupB are also predicted to encode potential beta-barrel proteins by the same program. fupA has been shown to be important for virulence in Schu S4 and to also contribute to siderophore utilization and iron acquisition, while fupB disruption was shown to not affect virulence (11, 24). The LVS genome encodes only four paralogs of this family. The LVS locus FTL_0439 represents an fupA/B hybrid gene, with the sequence encoding the amino-terminal 306-amino-acid residues of fupA fused to the sequence encoding the 254 carboxy-terminal residues of fupB at a 27-nucleotide repeat sequence. fupA/B and FTL_0147 have been predicted to encode signal peptides by use of the SignalP 3.0 program (2), and the PRED-TMBB program predicts that they could both form beta-barrels in the outer membrane. We found that fupA/B could complement the ΔfslE mutation whereas FTL_0147 could not. It appears, therefore, that while several of the paralogs may assemble as beta-barrels in the outer membrane, not all of them are likely to play a role in siderophore-mediated iron acquisition.
Our studies demonstrated that FupA/B plays a major role in siderophore-mediated iron acquisition in LVS. We found that FslE also contributes in a minor way to iron acquisition in LVS, a finding in contrast to the results seen with Schu S4, where it plays a significant role. Schu S4 grows to a higher dilution than LVS on iron-limiting plates, indicating that it is better able to grow under conditions of iron limitation. This likely reflects differences in iron acquisition processes between the two strains that may be partly accounted for by differences in functions of FslE and the FupA-FupA/B proteins with respect to siderophore utilization.
The fsl operon was found to be deregulated in the Schu ΔfupA mutant even under iron-replete conditions (11). We made similar observations with the ΔfupA/B mutant in the LVS. FupA was proposed to regulate the intracellular free-iron pool and overall iron homeostasis in Schu S4, as the Schu ΔfupA mutant was found to be iron deficient. Our studies suggest that the FupA/B hybrid protein retains at least some of this function of FupA.
LVS exhibited an extremely high (>106 CFU) LD50 by the subcutaneous route in comparison to clinical strains, which have an LD50 of <5 CFU. This is reflected in the reduced dissemination of LVS from an intradermal site to the liver and spleen compared to that seen with the more virulent Schu S4 (24). Virulent strains possess intact fupA and fupB genes, and the deletion event leading to the formation of the fupA/B recombinant gene was predicted to be one potential cause for the attenuation of the LVS (18). This idea was supported by the finding that introduction of fupA into LVS greatly enhanced its virulence by the subcutaneous route of infection (19). In contrast to the attenuation seen following subcutaneous infection, LVS remained as virulent as clinical strains when administered by the intraperitoneal route of infection in mice, with an LD50 of <5 CFU (6). Our studies clearly demonstrate that the fupA/B function is important for virulence of strains administered by the intraperitoneal route. fupA/B was initially identified in a screening of Himar1-based transposon mutants for defective replication in macrophages (12). The mutant had reduced cytotoxicity for infected J774A.1 cells and reduced virulence following intraperitoneal mouse infection. Our results confirmed these findings and further demonstrated that the virulence defect in mice could be reversed by reintroducing the fupA/B gene into the mutant. Although avirulent for mice, the ΔfupA/B mutants were able to protect immunized mice against subsequent challenge with LVS. Whether the virulence function of FupA/B is directly connected to its role in iron acquisition is not yet clear.
In the well-characterized siderophore-mediated iron uptake systems of other Gram-negative bacteria, the receptor acquires the energy for moving the ferrisiderophore across the outer membrane by interaction with TonB (7, 14). The mechanism by which FslE functions in acquisition of iron by Schu S4 in the absence of TonB is not understood. There is no ready explanation for why FslE plays a limited role in the LVS siderophore acquisition although it is expressed and regulated similarly to what was seen with Schu S4. While it is conceivable that the few amino acid changes in the LVS protein make it ineffective, we favor the possibility that an accessory function is missing or altered in LVS, with FupA being one such candidate. Furthermore, since the fslE mutant of Schu S4 has no appreciable siderophore utilization ability despite having an intact fupA gene, it would appear that the FupA/B hybrid protein has acquired a capability for siderophore utilization that is lacking in FupA. Future studies need to determine whether a common mechanism underlies FupA/B and FslE function in iron acquisition.
Acknowledgments
We thank Barbara J. Mann for critical reading of the manuscript.
This work was supported by grant AI067823 from the NIAID and by intramural funding from the University of Virginia School of Medicine.
Editor: S. M. Payne
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Bagos, P. G., T. D. Liakopoulos, I. C. Spyropoulos, and S. J. Hamodrakas. 2004. PRED-TMBB: a web server for predicting the topology of beta-barrel outer membrane proteins. Nucleic Acids Res. 32:W400-W404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 3.Deng, K., R. J. Blick, W. Liu, and E. J. Hansen. 2006. Identification of Francisella tularensis genes affected by iron limitation. Infect. Immun. 74:4224-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eigelsbach, H. T., and C. M. Downs. 1961. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J. Immunol. 87:415-425. [PubMed] [Google Scholar]
- 5.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5:135-142. [DOI] [PubMed] [Google Scholar]
- 6.Elkins, K. L., R. K. Winegar, C. A. Nacy, and A. H. Fortier. 1992. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb. Pathog. 13:417-421. [DOI] [PubMed] [Google Scholar]
- 7.Faraldo-Gomez, J. D., and M. S. P. Sansom. 2003. Acquisition of siderophores in Gram-negative bacteria. Nat. Rev. Mol. Cell Biol. 4:105-116. [DOI] [PubMed] [Google Scholar]
- 8.Kiss, K., W. Liu, J. F. Huntley, M. V. Norgard, and E. J. Hansen. 2008. Characterization of fig operon mutants of Francisella novicida U112. FEMS Microbiol. Lett. 285:270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson, P., D. Elfsmark, K. Svensson, P. Wikstrom, M. Forsman, T. Brettin, P. Keim, and A. Johansson. 2009. Molecular evolutionary consequences of niche restriction in Francisella tularensis, a facultative intracellular pathogen. PLoS Pathog. 5:e1000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson, P., P. C. Oyston, P. Chain, M. C. Chu, M. Duffield, H. H. Fuxelius, E. Garcia, G. Halltorp, D. Johansson, K. E. Isherwood, P. D. Karp, E. Larsson, Y. Liu, S. Michell, J. Prior, R. Prior, S. Malfatti, A. Sjostedt, K. Svensson, N. Thompson, L. Vergez, J. K. Wagg, B. W. Wren, L. E. Lindler, S. G. Andersson, M. Forsman, and R. W. Titball. 2005. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat. Genet. 37:153-159. [DOI] [PubMed] [Google Scholar]
- 11.Lindgren, H., M. Honn, I. Golovlev, K. Kadzhaev, W. Conlan, and A. Sjostedt. 2009. The 58-kilodalton major virulence factor of Francisella tularensis is required for efficient utilization of iron. Infect. Immun. 77:4429-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maier, T. M., M. S. Casey, R. H. Becker, C. W. Dorsey, E. M. Glass, N. Maltsev, T. C. Zahrt, and D. W. Frank. 2007. Identification of Francisella tularensis Himar1-based transposon mutants defective for replication in macrophages. Infect. Immun. 75:5376-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miethke, M., and M. A. Marahiel. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71:413-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noinaj, N., M. Guillier, T. J. Barnard, and S. K. Buchanan. 26 April 2010, posting date. TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 15.Pechous, R. D., T. R. McCarthy, and T. C. Zahrt. 2009. Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol. Mol. Biol. Rev. 73:684-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramakrishnan, G., A. Meeker, and B. Dragulev. 2008. fslE is necessary for siderophore-mediated iron acquisition in Francisella tularensis Schu S4. J. Bacteriol. 190:5353-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 18.Rohmer, L., M. Brittnacher, K. Svensson, D. Buckley, E. Haugen, Y. Zhou, J. Chang, R. Levy, H. Hayden, M. Forsman, M. Olson, A. Johansson, R. Kaul, and S. I. Miller. 2006. Potential source of Francisella tularensis live vaccine strain attenuation determined by genome comparison. Infect. Immun. 74:6895-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomonsson, E., K. Kuoppa, A. L. Forslund, C. Zingmark, I. Golovliov, A. Sjostedt, L. Noppa, and A. Forsberg. 2009. Reintroduction of two deleted virulence loci restores full virulence to the live vaccine strain of Francisella tularensis. Infect. Immun. 77:3424-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 21.Sjöstedt, A. 2006. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect. 8:561-567. [DOI] [PubMed] [Google Scholar]
- 22.Sjöstedt, A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105:1-29. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan, J. T., E. F. Jeffery, J. D. Shannon, and G. Ramakrishnan. 2006. Characterization of the siderophore of Francisella tularensis and role of fslA in siderophore production. J. Bacteriol. 188:3785-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twine, S., M. Bystrom, W. Chen, M. Forsman, I. Golovliov, A. Johansson, J. Kelly, H. Lindgren, K. Svensson, C. Zingmark, W. Conlan, and A. Sjostedt. 2005. A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect. Immun. 73:8345-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]