Abstract
Despite effective antimicrobial chemotherapy, control of Chlamydia trachomatis urogenital infection will likely require a vaccine. We have assessed the protective effect of an outer membrane protein-based vaccine by using a murine model of chlamydial genital infection. Female mice were first vaccinated with Chlamydia muridarum major outer membrane protein (MOMP) plus the adjuvants CpG-1826 and Montanide ISA 720; then they were challenged with C. muridarum. Vaccinated mice shed 2 log10 to 3 log10 fewer inclusion-forming units (IFU) than ovalbumin-vaccinated or naïve animals, resolved infection sooner, and had a lower incidence of hydrosalpinx. To determine the relative contribution of T cells to vaccine-induced protection, mice were vaccinated, depleted of CD4+ or CD8+ T cells, and then challenged vaginally with C. muridarum. Depletion of CD4+ T cells, but not depletion of CD8+ T cells, diminished vaccine-induced protection, with CD4-depleted mice shedding 2 log10 to 4 log10 more IFU than CD8-depleted or nondepleted mice. The contribution of antibodies to vaccine-induced protection was demonstrated by the absence of protective immunity in vaccinated B-cell-deficient mice and by a 2 log10 to 3 log10 decrease in bacterial shedding by mice passively administered an anti-MOMP serum. Thus, optimal protective immunity in this model of vaccine-induced protection depends on contributions from both CD4+ T cells and antibody.
New cases of sexually transmitted diseases number more than 340 million worldwide annually and pose a formidable health risk to infected individuals (67-69). It is estimated that Chlamydia trachomatis, the causative agent of chlamydia, is responsible for more than 92 million of these cases. In the United States, where C. trachomatis infections are the infections most commonly reported to the Centers for Disease Control and Prevention, there are more than 4 million new cases each year (14, 67). As a bacterial agent of infection, C. trachomatis can be eradicated efficiently with appropriate antibiotic treatment, but more than 50% of infected individuals are asymptomatic and therefore lack the impetus to seek treatment (14). When left untreated, infection in women can lead to pelvic inflammatory disease, ectopic pregnancy, and tubal factor infertility and can cause severe and sometimes irreparable damage to the reproductive organs (14, 67). To combat the high rate of infection and disease, the development of an efficacious vaccine is critical.
Trachoma vaccine trials using whole organisms in the 1950s and 1960s had mixed results, with some studies inducing only partial, serovar-specific, short-lived immunity (4). In one study, a subset of vaccine trial participants experienced an increased incidence of disease and exacerbated pathology relative to that of their unvaccinated counterparts upon reexposure to chlamydiae, which led many researchers to abandon the use of whole organisms in immunizations (7, 8). Since then, no other human vaccine trials targeting ocular or urogenital C. trachomatis infections have been published. Instead, researchers have focused their efforts on animal models of ocular and genital infection.
To this end, the murine model of chlamydial genital infection, which closely mimics acute genital infection in women, has been employed extensively for the study of the immunological parameters of infection and for vaccine development. Mice infected with C. muridarum naturally resolve infection in approximately 4 weeks and develop long-lived adaptive immunity that markedly protects against reinfection (3, 37). Infection elicits Chlamydia-specific CD4+ T cells, CD8+ T cells, and antibody, but only CD4+ T cells are necessary for resolution of the primary infection (42). In contrast, immunity to reinfection is dominated by both protective CD4+ T cells and antibody (38). Clearance and immunity are highly dependent on a Th1-type response, specifically gamma interferon (IFN-γ)-secreting CD4 cells (9). On the other hand, Th2 responses are associated with scarring and immunopathology (62). For example, antibody responses dominated by IgG1 are not protective and may be associated with an increase in pathology, whereas anti-chlamydial antibodies of the IgG2a and IgG2b isotypes are associated with a protective response (51).
Using knowledge of the protective response gleaned from the murine model of infection-induced immunity, investigators have made modest strides toward the development of an efficacious vaccine. Studies utilizing whole elementary body (EB) immunization have induced significant protection, though most of these studies have limited real-world application. One notable example that induced almost sterilizing immunity involved the passive transfer of dendritic cells pulsed ex vivo with nonviable chlamydiae (65). Subunit antigen vaccines represent the bulk of vaccine studies, and vaccines based on combinations of a number of chlamydial antigens, adjuvants, and delivery systems have had various degrees of success in preventing infection (8, 22, 62). Chlamydial antigens, including secreted proteins, such as chlamydial protease-like activity factor (CPAF) (16, 33, 43-46), and membrane associated proteins, such as PorB (26, 30) and IncA (33), have also been used in subunit vaccines; however, the vast majority of studies have focused on the major outer membrane protein (MOMP), an immunodominant antigen in both human and animal studies (22, 62). Novel delivery systems, including Vibrio cholerae ghosts and cationic liposomes, have been introduced into chlamydial vaccine research, and while initial studies have shown incomplete protection, these systems may have the potential to elicit protective responses against chlamydial genital infection when used in conjunction with appropriate antigens (2, 19, 20, 23).
Despite substantial effort, no vaccine licensed for human use is currently available. Recently, a MOMP-based vaccine utilizing the adjuvants CpG-1826 and Montanide ISA 720, which together drive a strong Th1-type response, has been shown to confer considerable protection when mice are challenged directly in the upper genital tract with C. muridarum (51). In our current study, we sought to determine if this vaccine protected against vaginal challenge (the natural route of infection) and to evaluate the contributions of T cells and antibody to the vaccine-induced protective response. We found that the MOMP vaccine conferred significant protection against vaginal challenge and protected against infection-induced pathology (hydrosalpinx). Furthermore, optimal protection was dependent on both CD4+ T cells and antibody.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice and B6.129S2-Igh-6tm/Cgn/J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were maintained in the animal facilities at the University of Alabama at Birmingham or the University of Arkansas for Medical Sciences (Little Rock, AR). B6.129S2-Igh-6tm/Cgn/J mice, referred to below as B-cell-deficient mice, have a disruption in the membrane exon of the immunoglobulin μ chain gene, rendering them unable to produce mature B cells; they are, therefore, antibody negative (32). Five- to 15-week-old mice were used throughout these studies. All animal procedures were approved by the institutional animal care and use committees.
Bacterial growth, purification, and enumeration.
Chlamydia muridarum (strain Weiss) was grown in HeLa 229 cells and was purified by discontinuous density gradient centrifugation (10). The infectivity titer was determined on HeLa cell monolayers as previously described (10).
Preparation of C. muridarum MOMP.
MOMP was purified as previously described (51). Briefly, HeLa 229 cells were infected with C. muridarum at a multiplicity of infection (MOI) of 1.25, and 39 h later, they were washed with Hanks balanced salt solution, sonicated, and then centrifuged at 30,000 × g for 1 h. The pellet was resuspended in phosphate-buffered saline (PBS) and was then treated with 25 μg/ml DNase 1 for 1 h at 4°C. Following centrifugation for 1 h at 75,600 × g, the pellet was extracted twice in 0.2 M phosphate buffer (pH 5.5) containing 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 100 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM EDTA at 37°C and was centrifuged for 1 h at 75,600 × g between extractions. The pellet was then extracted in the phosphate buffer described above, with 2% Anzergent 3-14 (Z3-14) (Anatrace, Maumee, OH) in place of CHAPS at 37°C, and was then centrifuged for 1 h at 75,600 × g. The supernatant was applied to a 20-cm by 1.5-cm hydroxyapatite column (Bio-Gel HTP; Bio-Rad, Hercules, CA) that had been equilibrated with 0.02 M phosphate buffer (pH 5.5) with 0.1% Z3-14 and was eluted in a linear gradient from 0.02 M to 0.6 M the same phosphate buffer. Fractions exhibiting an increase in absorbance at 280 nm were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and those containing a major band at 40 kDa were pooled. The purified MOMP preparation reacted with an anti-MOMP monoclonal antibody (Mo33b) (17) and with convalescent serum by immunoblotting and exhibited a banding pattern, as shown previously (51).
Purified MOMP was refolded by dialysis in 0.1 M phosphate buffer with 0.05% Z3-14, 2 mM reduced glutathione, 1 mM oxidized glutathione, and 1 mM EDTA. The protein was fixed with 2% glutaraldehyde for 2 min at room temperature, and then the fixation was stopped with 2 M glycine. The protein was concentrated with a Centriplus filter (Millipore, Billerica, MA) and was dialyzed against 0.02 M phosphate buffer (pH 7.4) with 0.15 M NaCl and 0.05% Z3-14 prior to immunization.
Immunization and challenge of mice.
Animals were immunized with C. muridarum MOMP by the subcutaneous (s.c.) (10 μg/mouse) and intramuscular (i.m.) (10 μg/mouse) routes, boosted twice with the same immunization at 2-week intervals, and then rested for 4 to 6 weeks. The vaccine consisted of MOMP plus CpG oligodeoxynucleotide 1826 (5′TCCATGACGTTCCGACGTT-3′) (Invivogen, San Diego, CA) and Montanide ISA 720 (Seppic, Inc., Fairfield, NJ) at a 30:70 (vol/vol) ratio of MOMP plus CpG to Montanide, with a final CpG concentration of 10 μg/mouse per injection site. The preparation was emulsified with a syringe. A control group was immunized as described above with 10 μg/mouse ovalbumin in place of MOMP to mimic the control groups of other studies (29, 51). Mice that had resolved a prior genital infection with C. muridarum served as immune positive controls (infection-immune group). A group of naïve animals was challenged as a negative control. Five days prior to infectious challenge, mice were injected s.c. with 2.5 mg medroxyprogesterone acetate (SICOR Pharmaceuticals, Irvine, CA). On the day of challenge, the vaginal vault was gently swabbed with a calcium alginate-tipped swab (Fisher Scientific, Waltham, MA) to remove excess mucus. Mice were then inoculated vaginally with 5 μl containing 5 × 104 inclusion-forming units (IFU) (equivalent to 100 50% infectious doses [ID50]) of C. muridarum. The infection was monitored by enumerating the number of IFU recovered from cervicovaginal swabs using indirect immunofluorescence as previously described (37).
In vivo T-cell subpopulation depletion.
Mice were depleted of CD4+ or CD8+ T cells as previously described (40). Briefly, mice were injected intraperitoneally (i.p.) with 400 μg of an anti-CD4 (clone GK 1.5) or anti-CD8 (clone 2.43) monoclonal antibody on the following schedule, where day 0 is the day of infectious challenge: days −6, −5, −4, −1, 2, 5, 8, 11, 14, 17, 20, and 23. This depletion regimen effectively depleted either CD4+ or CD8+ T cells for the entire 4-week test period (42).
Passive transfer of serum.
Two sources of sera, designated convalescent and anti-MOMP sera, were used for passive immunization studies. Convalescent serum is defined as serum collected from mice that had resolved a primary chlamydial genital infection (infection immune). Anti-MOMP serum is defined as serum collected from mice that had been immunized with the MOMP vaccine as described above. Mice were injected i.p. with 0.5 ml of convalescent or anti-MOMP serum on the following schedule, where day 0 is the day of secondary infectious challenge: days −1, 0, 3, 6, 10, and 14. Normal, nonimmune serum had been tested previously in passive transfer experiments and was shown not to confer any level of protection (38). Therefore, those studies were not repeated here.
Chlamydia ELISA.
Antibody titers were determined by enzyme-linked immunosorbent assays (ELISA) using C. muridarum EBs as the antigen (37). Serum used in passive transfer experiments was evaluated prior to transfer. Mice were also bled 10 days after challenge in order to assess antibody levels in mice receiving passively administered serum. Vaginal wash specimens were collected 2 weeks after final vaccination or 43 days postinfection and were analyzed as previously described (37).
Chlamydia-specific cytokine responses of splenocytes.
Four weeks after the final vaccine booster or infection with C. muridarum, animals were sacrificed, and spleens were collected. Splenocytes from each mouse were mixed with UV-inactivated C. muridarum EBs at a ratio of 1:4 (cells to EBs), and the mixtures were incubated at 37°C for 1 h with tumbling. The splenocyte-EB mixture was then added to a 96-well flat-bottom tissue culture plate at 5 × 105 cells/well and was incubated for 72 h at 37°C. Supernatants were collected, clarified by centrifugation, and analyzed for interleukin-2 (IL-2), IL-4, IL-5, IL-10, IL-12(p70), granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor alpha (TNF-α), and IFN-γ by using a Bio-Plex Pro Th1/Th2 assay (Bio-Rad) according to the manufacturer's protocol.
Statistical analysis.
Two-way analysis of variance (ANOVA) with a Bonferroni posttest was used to analyze the difference between IFU counts of control and experimental groups. Differences in hydrosalpinx incidence were analyzed by a chi-square test.
RESULTS
MOMP vaccination provides significant protection against vaginal challenge and lowers the incidence of hydrosalpinx.
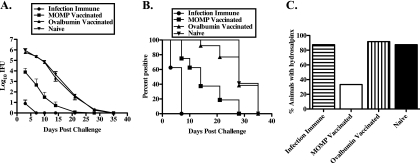
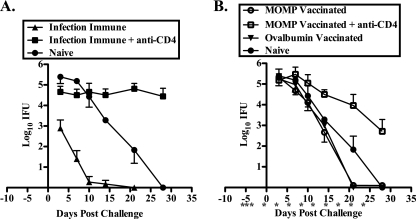
To determine if MOMP vaccination protected against vaginal challenge, mice were vaccinated with MOMP plus CpG-1826 and Montanide ISA 720 and were then challenged intravaginally with C. muridarum. MOMP-vaccinated animals shed 2 log10 to 3 log10 fewer IFU throughout the course of infection than either ovalbumin-vaccinated negative-control or naïve mice (P, <0.001 at days 3, 7, 10, 14, and 21) (Fig. 1 A). MOMP vaccination resulted in a shortened duration of infection, with 79% of mice clearing infection by 21 days postchallenge versus 24% of ovalbumin-vaccinated and none of the naïve mice (Fig. 1B). MOMP vaccination did not protect as well as prior infection, which induced immune responses that dramatically reduced the shedding of infectious bacteria and shortened the course of infection (P, <0.001 at days 3, 7, and 10; P, <0.05 at day 14) (Fig. 1A and B). Additionally, 40% of infection-immune animals were solidly protected against reinfection, whereas all MOMP-vaccinated animals were infected (Fig. 1B, day 3).
FIG. 1.
The MOMP vaccine protects in a vaginal model of genital infection. Female naïve, infection-immune, MOMP-vaccinated, and ovalbumin-vaccinated C57BL/6 mice were challenged intravaginally with 100 ID50 of C. muridarum. (A) Course of infection, represented as log10 chlamydial IFU recovered from cervicovaginal swabs ± standard errors of the means. (B) Percentage of culture-positive mice per group at each culture time point. (C) Percentage of mice with unilateral or bilateral hydrosalpinx at 70 days postinfection. The data represent duplicate determinations for groups of at least 5 mice each.
Hydrosalpinx, the accumulation of fluid in the oviduct due to the occlusion of the oviduct by scar tissue, is a measurement of infection-induced pathology that results from chlamydial genital tract infection. To determine if the MOMP vaccine protected against the hydrosalpinx that develops subsequent to lower genital tract infection, animals were sacrificed 70 days post-infectious challenge, and their genital tracts were assessed for the presence of hydrosalpinx. Significantly fewer MOMP-vaccinated animals than animals in the control groups developed either unilateral or bilateral hydrosalpinx (P, <0.0001) (Fig. 1C). Although the infection-immune group was protected against rechallenge, the high incidence of hydrosalpinx was expected, because the pathology is a result of the primary infection with C. muridarum, and therefore, the rate should be similar to that of the naïve group. Collectively, MOMP vaccination conferred significant protection against C. muridarum vaginal challenge, as demonstrated by reduced bacterial shedding, a shortened duration of infection, and a reduced incidence of hydrosalpinx.
MOMP vaccine-induced immune responses.
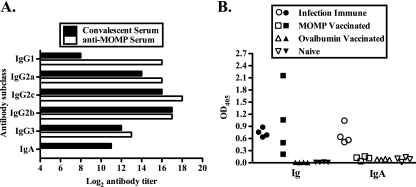
To assess the humoral immune response elicited following MOMP vaccination, sera from MOMP-vaccinated animals were analyzed by ELISA and immunoblotting, and the results were compared to those for convalescent serum. MOMP-vaccinated animals developed high-titer anti-chlamydial IgG1, IgG2b, IgG2c, and IgG3. The responses were similar to those of convalescent serum except for the IgG1 response, which was substantially higher in the anti-MOMP serum, and the IgA response, which was not detected in anti-MOMP serum (Fig. 2 A). C57BL/6 mice have IgG2c rather than IgG2a (36), but both were included in the analysis, because the reagents used to detect IgG2a and IgG2c cross-react, and historically IgG2a has been measured rather than IgG2c. Immunoblotting confirmed that sera from vaccinated animals reacted with the MOMP used in the vaccine as well as with the MOMP in whole C. muridarum EBs, and convalescent serum recognized the MOMP used in the vaccine (51) (data not shown). Local anti-chlamydial Ig and IgA were detected in secretions from infection-immune mice, whereas MOMP-vaccinated mice had anti-chlamydial Ig but no IgA (Fig. 2B).
FIG. 2.
Vaccination elicited a Chlamydia-specific antibody response. (A) Anti-chlamydial antibody titers of pooled anti-MOMP sera and pooled convalescent sera as determined by ELISA. (B) Anti-chlamydial antibody responses of vaginal wash fluids as determined by ELISA. Data are presented as optical densities (OD) for vaginal wash fluids from individual mice. Filled symbols represent the Ig response, and open symbols represent the IgA response.
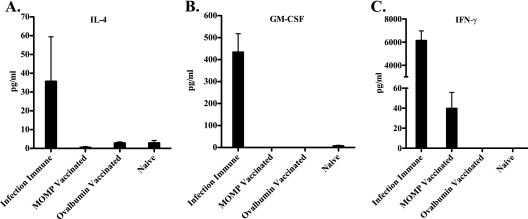
Cytokine responses of splenocytes from MOMP-vaccinated, infection-immune, ovalbumin-vaccinated, and naïve mice were measured using a multiplex Th1/Th2 cytokine assay. Splenocytes from infection-immune animals produced IL-4, GM-CSF, and IFN-γ when stimulated with chlamydiae (Fig. 3), while specific responses from vaccinated animals were limited to IFN-γ (Fig. 3C). No other antigen-specific cytokine responses were detected (data not shown).
FIG. 3.
Cytokine responses of splenocytes stimulated with UV-inactivated C. muridarum EBs. Splenocytes were collected from mice 4 weeks after infection or final vaccination and were then stimulated with C. muridarum EBs for 72 h. Supernatants were assessed for cytokines using a BioPlex Pro Th1/Th2 cytokine assay. Concentrations represent the cytokine concentration minus the background and are expressed as the average concentration for each group in pg/ml.
CD4+ T cells, but not CD8+ T cells, contribute to the MOMP vaccine-induced protective response.
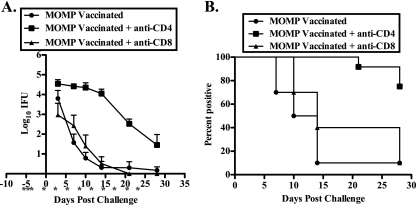
In an effort to elucidate the roles of CD4+ and CD8+ T cells in the vaccine-induced protective response, we used monoclonal antibodies to deplete CD4+ and CD8+ cells in vaccinated animals. As seen in Fig. 4 A, MOMP-vaccinated mice depleted of CD8+ T cells prior to challenge had a course of infection similar to that of nondepleted MOMP-vaccinated animals. In contrast, depletion of CD4+ T cells resulted in significantly greater bacterial shedding and infections of longer duration than those of either nondepleted or CD8+ T-cell-depleted vaccinated mice (P, <0.001 at days 7, 10, 14, and 21; P, <0.01 at day 28) (Fig. 4A and B). The incidence of hydrosalpinx in CD4-depleted MOMP-vaccinated animals (63.6%) was more than twice that in nondepleted animals (30%), while no increase was observed in CD8-depleted animals (40%). Thus, CD4+ T cells, but not CD8+ T cells, contribute importantly to the vaccine-induced protective response.
FIG. 4.
CD4+, but not CD8+, T cells are involved in the MOMP vaccine-induced protective response. Female C57BL/6 MOMP-vaccinated mice were either left untreated or depleted of CD4+ or CD8+ T cells prior to intravaginal infection with 100 ID50 of C. muridarum. Depletion was maintained throughout the course of infection as indicated (*). (A) Course of infection, expressed as log10 IFU ± standard error of the mean. (B) Percentage of culture-positive mice at each time point. The data represent duplicate determinations for groups of least 5 mice.
Antibody and CD4+ T cells are required for optimal MOMP vaccine-induced immunity.
Depletion of CD4+ T cells in vaccinated animals diminished protective immunity but did not completely ablate the animals' ability to control the infection (Fig. 4). Therefore, since CD8+ T cells do not apparently contribute to the protective response (Fig. 4), we sought to determine if antibody contributed significantly to vaccine-induced immunity. To test the effect of antibody deficiency on vaccine-induced protection, B-cell-deficient mice were vaccinated with MOMP or ovalbumin and were then challenged vaginally with C. muridarum. Naïve B-cell-deficient mice, which lack mature B cells and are therefore antibody negative, resolve C. muridarum genital infection similarly to wild-type immunocompetent animals (Fig. 1A) and develop significant immunity to reinfection (Fig. 5 A) (42). The immunity that develops in antibody-deficient mice following C. muridarum genital infection is dependent on CD4+ T cells, since mice depleted of these cells are unable to resolve the infection (Fig. 5A). The course of infection in MOMP-vaccinated B-cell-deficient animals was similar to those for both naïve and ovalbumin-vaccinated mice, indicating that antibody plays an important role in the vaccine-induced protective response (P, <0.05 at all days assessed) (Fig. 5B). MOMP-vaccinated antibody-deficient mice also were not protected against the development of hydrosalpinx; more than 80% of these animals developed bilateral or unilateral hydrosalpinx.
FIG. 5.
CD4+ T cells and antibody contribute to the MOMP vaccine-induced protective response. Female B-cell-deficient mice were separated into naïve, infection-immune, MOMP-vaccinated, and ovalbumin-vaccinated groups and were then either left untreated or treated with anti-CD4 prior to challenge with 100 ID50 of C. muridarum. Panels A and B represent results for animals from the same experiment, but the graphs are separated for clarity. Also, the course of infection in naïve animals is depicted on both graphs for comparison. (A) Courses of infection of naïve mice, infection-immune mice, and infection-immune mice treated with anti-CD4. (B) Courses of infection of naïve mice, MOMP-vaccinated mice, MOMP-vaccinated mice treated with anti-CD4, and ovalbumin-vaccinated mice. Data are presented as log10 IFU ± standard errors of the means of duplicate determinations for at least 5 mice.
Having found that both CD4+ T cells and antibody contribute to the vaccine-induced protective response (Fig. 4 and 5), we next wanted to assess the state of the protective response in the absence of both of these components. Thus, MOMP-vaccinated B-cell-deficient mice were depleted of CD4+ T cells and challenged. The initial course of infection in these mice was similar to that of infection-immune B-cell-deficient mice depleted of CD4+ T cells; mice in both groups shed high numbers of IFU for the first 2 weeks postinfection (Fig. 5A and B). However, bacterial shedding decreased in the MOMP-vaccinated group beginning 2 weeks postinfection and continued to decrease throughout the observation period (Fig. 5B). Bacterial shedding remained significantly higher in this group than in the naïve and nondepleted groups of vaccinated animals (Fig. 5B). Collectively, these data indicate that both CD4+ T cells and antibody are necessary for optimal MOMP vaccine-induced protection.
Anti-MOMP serum offers significant protection against reinfection.
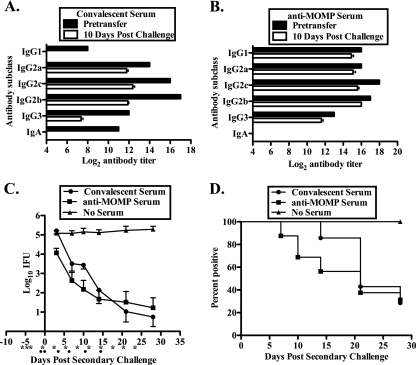
The B-cell-deficient mice not only are deficient in antibody but also lack other, antibody-independent B-cell responses (e.g., antigen presentation) (32). Therefore, we do not know if the diminished protective response elicited by MOMP vaccination of B-cell-deficient mice was due to the lack of specific antibody or to another B-cell function. To specifically assess the contribution of antibody to the vaccine-induced protective response, we used an established infection model of B-cell-deficient mice, which has proven ideal for assessing the protective efficacy of passively transferred serum (38). Infection-immune B-cell-deficient mice were depleted of CD4+ T cells, passively administered convalescent or anti-MOMP serum, and then challenged intravaginally with C. muridarum. The titers and Ig class/subclass characteristics of convalescent and anti-MOMP sera were determined by ELISA both prior to passive transfer and 10 days postchallenge (Fig. 6 A and B). Because these studies were performed using B-cell-deficient mice, the antibody detected 10 days post-infectious challenge reflects the passively transferred antibody and is not due to a response to infection. Notable differences between the convalescent and anti-MOMP sera include the lack of IgA in the anti-MOMP serum and the low IgG1 titer in the convalescent serum (Fig. 2 and 6). Following passive transfer, antibody titers diminished somewhat, and anti-chlamydial IgA was undetectable in the convalescent serum, as noted previously (38).
FIG. 6.
Passive transfer of protective immunity with anti-MOMP serum. Convalescent and anti-MOMP sera were evaluated by ELISA prior to passive transfer and 10 days post-secondary infection. (A and B) Anti-chlamydial titers for convalescent serum (A) and anti-MOMP serum (B), presented as log2 titers, prior to passive transfer and 10 days after reinfection. Pretransfer anti-chlamydial titers represent duplicate determinations for pooled convalescent or anti-MOMP sera, and day 10 postreinfection data represent the mean titer for at least 10 mice per group. (C and D) B-cell-deficient mice that had resolved a primary C. muridarum genital infection were first treated with anti-CD4 and were then either injected i.p. with convalescent or anti-MOMP serum or left untreated, as described in Materials and Methods. All mice were infected intravaginally with 100 ID50 of C. muridarum, and the course of infection was monitored. (C) Course of infection, expressed as log10 IFU ± standard error of the mean of duplicate determinations for at least 5 mice. (D) Percentage of culture-positive animals at each time point tested.
Infection-immune B-cell-deficient mice depleted of CD4+ T cells were unable to resolve infection and shed 105 chlamydiae for the duration of the experiment (Fig. 6C). In contrast, mice receiving either convalescent or anti-MOMP serum shed far fewer chlamydiae (2 log10 to 4 log10 fewer) at most time points tested (P, <0.05 at days 7 and 10 and <0.001 at days 14, 21, and 28 for convalescent serum; P, <0.05 at day 7 and <0.001 at days 10, 14, 21, and 28 for anti-MOMP serum). Furthermore, 50% of the anti-MOMP serum-treated mice and 70% of the convalescent serum-treated mice completely resolved the infection (Fig. 6D). These data clearly demonstrate the important role of antibody in MOMP vaccine-induced immunity.
DISCUSSION
The protective efficacy of an anti-chlamydial vaccine is judged by its ability to prevent both infection and infection-related sequelae. Parenteral vaccination with MOMP provides partial protection against vaginal challenge, as evidenced by a decrease in bacterial shedding and a lowered incidence of hydrosalpinx (Fig. 1) (51). Historically, two models of genital tract infection have been used to assess vaccines: upper genital tract challenge via the ovarian bursa and lower genital tract challenge via the vagina. In women, the natural route of infection with C. trachomatis initiates at the cervix, thus favoring the model of lower genital tract challenge for vaccine research. Vaginal challenge allows researchers to assess the ability of a vaccine to prevent infection and, in turn, the immunopathological damage to the genital tract resulting from infection. Although the upper genital tract infection model does not reproduce the natural infection route, it is quite useful for assessing the potential of a chlamydial vaccine to prevent infertility. Direct inoculation of chlamydiae into the upper genital tract increases the incidence of infertility from a primary infection. We and others have shown that parenteral vaccination with MOMP is protective in both upper and lower genital tract challenge models. MOMP vaccination shortens the course of infection, decreases bacterial shedding, and decreases the incidence of hydrosalpinx and/or infertility (Fig. 1) (51).
The importance of T cells in natural immunity to chlamydial genital infections has long been established (58, 59). Gene knockout mice, passive transfer of immune T cells, and in vivo depletion of T-cell subsets have demonstrated that CD4+ T cells are the predominant protective T-cell subset (37, 42, 64). In vivo cytokine depletion has been used to demonstrate that cytokines produced by CD4+ T cells of the Th1 lineage, such as IFN-γ and IL-12, are important for protection, while those produced by Th2 lineage cells, such as IL-4, are not protective and may lead to an exacerbation of the infection and an increase in pathology (61). In our current study, we used the adjuvants CpG-1826 and Montanide ISA 720 along with native MOMP to drive a strong Th1-type response, and we found that protection was dependent on CD4+ T cells but not on CD8+ T cells (Fig. 4). Similarly, protection following vaccination with recombinant MOMP was found to be CD4+ T-cell dependent (23). Although not all chlamydial vaccines eliciting Th1-type responses are protective, those that decidedly elicit Th1-type responses confer the most significant protection (7, 22). Studies utilizing adjuvants that induce Th2-type responses have not been as successful (22, 50, 51, 53).
Research from the past 20 years has produced data that challenge the paradigm of immunological duality, which posits that immunity to intracellular pathogens is dependent on cell-mediated responses, while antibody-mediated responses confer immunity to extracellular pathogens (11, 12). Several studies clearly show that antibody does provide a level of protective immunity to intracellular pathogens (6, 13, 38). Monoclonal antibodies raised to surface-exposed chlamydial antigens, such as MOMP, and convalescent serum have been shown to neutralize the infectivity of C. trachomatis and C. muridarum in in vitro cell infection assays (34, 37, 54, 55, 57, 72). Investigators have also noted an initial decrease in infectivity in vivo when EBs are coated with anti-MOMP monoclonal antibodies prior to vaginal challenge in mice and ocular infection in monkeys, and this limited protection is thought to be a result of direct neutralization of the pathogen (57, 73). Similar results have been reported for Mycobacterium bovis when bacteria are coated with monoclonal antibodies before intranasal inoculation (1). In contrast to the protection seen in studies with Mycobacterium tuberculosis (1), passive transfer of anti-chlamydial monoclonal antibodies prior to and after infectious challenge does not provide significant protection against infection, and this is particularly obvious in the absence of CD4+ T cells (17, 38, 57). The lack of protection against chlamydial genital infection conferred by passive transfer of convalescent serum and monoclonal antibodies has been attributed to the magnitude and composition of the local response. Earlier studies suggested that passive transfer may not result in the localization of sufficient antibody to the genital tract (57). However, more-recent work has shown that the passive transfer of monoclonal antibodies or convalescent serum does confer protection against reinfection and that antibody from the passively administered serum can be found in the vaginal secretions (38). It should be noted, however, that antibody-mediated protection is entirely dependent on CD4+ T-cell-mediated adaptive changes in the genital tract tissues but is not dependent on direct interaction with CD4+ T cells (38). We do not yet fully understand what comprises the adaptive changes that are needed for antibody to confer protection against genital Chlamydia infection. However, our studies indicate that protection appears not to be mediated by antibody directly neutralizing chlamydiae or simply through the interaction of antibody with chlamydiae and acute inflammatory cells. The adaptive changes that we believe are necessary for antibody to confer protection appear to be due either to recruitment and/or to activation of an effector cell population by CD4+ T cells. One possible mechanism would include the recruitment and activation of NK cells, which could confer protection through antibody-dependent cellular cytotoxicity. At this time, however, the precise mechanism by which antibody protects is unknown.
Using the model of genital tract reinfection in B-cell-deficient mice, we found that antibody contributed to the MOMP vaccine-induced protective response (Fig. 5). Vaccinated B-cell-deficient mice were not protected, and the course of infection in those mice was similar to those in naïve and ovalbumin-vaccinated animals. B cells have functions in addition to antibody production (e.g., antigen presentation); thus, it was important to determine whether the failure of the MOMP vaccine to protect B-cell-deficient mice was due to the absence of mature B cells or to the lack of antibody (21, 31). The passive transfer of anti-MOMP serum conferred a level of protective immunity on B-cell-deficient mice, indicating that antibody contributes to protective immunity in this vaccine model (Fig. 6). Collectively, our results provide further evidence that an efficacious vaccine against chlamydial genital infection should elicit both protective cell-mediated CD4+ T-cell and humoral immune responses.
MOMP vaccination resulted in significant protection against chlamydial vaginal challenge compared to nonvaccinated or ovalbumin-vaccinated negative controls, but it did not protect as well as infection-based immunity (Fig. 1A). This is particularly obvious in early infection (days 3 and 7), when infection-immune mice shed 3 log10 fewer IFU than MOMP-vaccinated mice. This result is in contrast to the protection observed in a cynomolgus monkey model of ocular infection. Monkeys immunized with C. trachomatis MOMP prepared similarly to the MOMP used in the current study displayed immunity superior to that elicited following infection (29). The difference between the levels of protective immunity elicited by MOMP vaccination in the two models is not understood but could be due to the model (mouse versus monkey or genital versus ocular infection), the pathogen (C. muridarum versus C. trachomatis), or the immune responses. One key difference, however, is that in mice, C. muridarum causes robust infection with marked inflammation (39). This marked inflammation, and specifically that mediated by CD4+ T cells, is thought to be essential for optimal immunity (38). During genital infection, epithelial cells infected with chlamydiae respond by secreting proinflammatory and immunoregulatory cytokines, which are important in driving protective Th1 immunity (8, 60). IL-18, for instance, is secreted by the infected epithelial cells and, in turn, interacts with IL-12, promoting the differentiation of the Th1 subset of helper T cells and inducing the production of IFN-γ, which is important for the clearance of, and immunity to, genital tract infection (8, 18, 27, 28, 56).
Although optimal immunity is conferred by a prior C. muridarum genital infection, infection and vaccination of distant mucosal sites have been shown to protect against genital challenge. Immunizing mice intranasally with inactivated C. muridarum results in long-lasting protection against genital challenge (48, 49). Mucosal vaccination with MOMP encapsulated with vault nanoparticles, which appear to target dendritic cells, induced protective immunity against genital challenge, with significant decreases in bacterial shedding at all time points tested, including points as early as day 3 postchallenge (15). In the current study, the MOMP vaccine was administered parenterally rather than mucosally, which likely explains the delayed protection observed in vaccinated mice compared to infection-immune mice (Fig. 1A). Although mucosal vaccination appears to elicit strong protective responses against genital challenge, at present no mucosal adjuvants have been approved for use in humans. Another notable example of a vaccine that induced remarkable protective immunity without resulting in inflammation in the genital tract was the passive transfer of dendritic cells pulsed ex vivo with inactivated C. muridarum (65). While this type of personalized medicine will not likely come to fruition as a prophylactic treatment for a nonlethal infection, it does demonstrate the usefulness of dendritic cells in vaccine strategies targeting Chlamydia and other pathogens (15, 24, 35, 70, 71).
The type and magnitude of the immune response are important factors that determine the quality of the protective response. We found that MOMP vaccination elicited cellular and humoral responses that differed from those elicited following infection. Splenocytes from MOMP-vaccinated mice produced significantly less IFN-γ than splenocytes from infection-immune animals (Fig. 3C). In addition to the differences in the magnitude of the response, the type of response elicited by vaccination also differed from that elicited by infection. A weak IFN-γ response was the only antigen-specific response detected from EB-stimulated splenocytes from MOMP-vaccinated animals, while infected animals produced IL-4 and GM-CSF in addition to IFN-γ (Fig. 3). Antibody responses also differed in that vaccinated animals produced substantially more IgG1 than infection-immune animals, and they did not produce C. muridarum-reactive IgA (Fig. 2). Although those differences could explain the disparity in protection observed following MOMP vaccination versus infection, they did not impact the ability of passively transferred antiserum to protect (Fig. 6). Furthermore, deficiency in IgA does not impact the course of C. muridarum infection in mice (41).
Other MOMP-based vaccines (5, 19, 20, 25, 63) that induce strong cellular and humoral Th1 type responses have not conferred the same level of protection as we, and others, have observed (7, 8, 29, 51), and the structural conformation of the MOMP antigen may explain the difference in the level of protection. Although the crystal structure of MOMP has yet to be resolved, Pal et al. have shown that a preparation of refolded native MOMP is more protective than recombinant MOMP (47, 50-53). A comparison of the refolded MOMP vaccine, as was used in the current study, to a preparation containing recombinant MOMP with the same adjuvants supports this notion (66). Likewise, dendritic cells pulsed ex vivo with recombinant MOMP are not protective, whereas dendritic cells pulsed with nonviable EBs are markedly protective (63).
In summary, we have shown that parenteral MOMP vaccination confers a significant level of protective immunity to genital challenge. Protection is dependent on both CD4+ T cells and antibody, and anti-MOMP serum protects as well as convalescent serum. Vaccination against genital infection with C. trachomatis remains a significant challenge. However, future studies using this model of infection to precisely define immune effector mechanisms and to determine how those mechanisms can be elicited locally through vaccination will likely provide essential information for the development of an efficacious vaccine.
Acknowledgments
We thank Luis de la Maza of the University of California at Irvine for assistance with antigen preparation.
This work was supported by National Institutes of Health grant AI-038991 and by the Arkansas Bioscience Institute.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.Abebe, F., and G. Bjune. 2009. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clin. Exp. Immunol. 157:235-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agger, E. M., I. Rosenkrands, J. Hansen, K. Brahimi, B. S. Vandahl, C. Aagaard, K. Werninghaus, C. Kirschning, R. Lang, D. Christensen, M. Theisen, F. Follmann, and P. Andersen. 2008. Cationic liposomes formulated with synthetic mycobacterial cord factor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One 3:e3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barron, A. L., H. J. White, R. G. Rank, B. L. Soloff, and E. B. Moses. 1981. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J. Infect. Dis. 143:63-66. [DOI] [PubMed] [Google Scholar]
- 4.Bell, S. D., Jr., R. L. Nichols, and N. A. Haddad. 1963. The immunology of the trachoma agent with a preliminary report on field trials on vaccine. Invest. Ophthalmol. 2:471-481. [PubMed] [Google Scholar]
- 5.Berry, L. J., D. K. Hickey, K. A. Skelding, S. Bao, A. M. Rendina, P. M. Hansbro, C. M. Gockel, and K. W. Beagley. 2004. Transcutaneous immunization with combined cholera toxin and CpG adjuvant protects against Chlamydia muridarum genital tract infection. Infect. Immun. 72:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady, L. J. 2005. Antibody-mediated immunomodulation: a strategy to improve host responses against microbial antigens. Infect. Immun. 73:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149-161. [DOI] [PubMed] [Google Scholar]
- 8.Brunham, R. C., D. J. Zhang, X. Yang, and G. McClarty. 2000. The potential for vaccine development against chlamydial infection and disease. J. Infect. Dis. 181(Suppl. 3):S538-S543. [DOI] [PubMed] [Google Scholar]
- 9.Cain, T. K., and R. G. Rank. 1995. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 63:1784-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall, A. 2003. Antibody-mediated immunity against intracellular pathogens: two-dimensional thinking comes full circle. Infect. Immun. 71:4225-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadevall, A. 1998. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 6:102-107. [DOI] [PubMed] [Google Scholar]
- 13.Casadevall, A., and L. A. Pirofski. 2004. New concepts in antibody-mediated immunity. Infect. Immun. 72:6191-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC. 2009. Sexually transmitted disease surveillance, 2008. Centers for Disease Control and Prevention, Atlanta, GA.
- 15.Champion, C. I., V. A. Kickhoefer, G. Liu, R. J. Moniz, A. S. Freed, L. L. Bergmann, D. Vaccari, S. Raval-Fernandes, A. M. Chan, L. H. Rome, and K. A. Kelly. 2009. A vault nanoparticle vaccine induces protective mucosal immunity. PLoS One 4:e5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cong, Y., M. Jupelli, M. N. Guentzel, G. Zhong, A. K. Murthy, and B. P. Arulanandam. 2007. Intranasal immunization with chlamydial protease-like activity factor and CpG deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine 25:3773-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotter, T. W., Q. Meng, Z.-L. Shen, Y.-X. Zhang, H. Su, and H. D. Caldwell. 1995. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4704-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 65:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eko, F. O., W. Lubitz, L. McMillan, K. Ramey, T. T. Moore, G. A. Ananaba, D. Lyn, C. M. Black, and J. U. Igietseme. 2003. Recombinant Vibrio cholerae ghosts as a delivery vehicle for vaccinating against Chlamydia trachomatis. Vaccine 21:1694-1703. [DOI] [PubMed] [Google Scholar]
- 20.Ekong, E. E., D. N. Okenu, J. Mania-Pramanik, Q. He, J. U. Igietseme, G. A. Ananaba, D. Lyn, C. Black, and F. O. Eko. 2009. A Vibrio cholerae ghost-based subunit vaccine induces cross-protective chlamydial immunity that is enhanced by CTA2B, the nontoxic derivative of cholera toxin. FEMS Immunol. Med. Microbiol. 55:280-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2007. Innate and adaptive immunity to Francisella. Ann. N. Y. Acad. Sci. 1105:284-324. [DOI] [PubMed] [Google Scholar]
- 22.Hafner, L., K. Beagley, and P. Timms. 2008. Chlamydia trachomatis infection: host immune responses and potential vaccines. Mucosal Immunol. 1:116-130. [DOI] [PubMed] [Google Scholar]
- 23.Hansen, J., K. T. Jensen, F. Follmann, E. M. Agger, M. Theisen, and P. Andersen. 2008. Liposome delivery of Chlamydia muridarum major outer membrane protein primes a Th1 response that protects against genital chlamydial infection in a mouse model. J. Infect. Dis. 198:758-767. [DOI] [PubMed] [Google Scholar]
- 24.Heijnen, I. A., M. J. van Vugt, N. A. Fanger, R. F. Graziano, T. P. de Wit, F. M. Hofhuis, P. M. Guyre, P. J. Capel, J. S. Verbeek, and J. G. van de Winkel. 1996. Antigen targeting to myeloid-specific human Fc gamma RI/CD64 triggers enhanced antibody responses in transgenic mice. J. Clin. Invest. 97:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey, D. K., R. C. Jones, S. Bao, A. E. Blake, K. A. Skelding, L. J. Berry, and K. W. Beagley. 2004. Intranasal immunization with C. muridarum major outer membrane protein (MOMP) and cholera toxin elicits local production of neutralizing IgA in the prostate. Vaccine 22:4306-4315. [DOI] [PubMed] [Google Scholar]
- 26.Ifere, G. O., Q. He, J. U. Igietseme, G. A. Ananaba, D. Lyn, W. Lubitz, K. L. Kellar, C. M. Black, and F. O. Eko. 2007. Immunogenicity and protection against genital Chlamydia infection and its complications by a multisubunit candidate vaccine. J. Microbiol. Immunol. Infect. 40:188-200. [PubMed] [Google Scholar]
- 27.Ito, J. I., and J. M. Lyons. 1999. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infect. Immun. 67:5518-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson, M., K. Schon, M. Ward, and N. Lycke. 1997. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect. Immun. 65:1032-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kari, L., W. M. Whitmire, D. D. Crane, N. Reveneau, J. H. Carlson, M. M. Goheen, E. M. Peterson, S. Pal, L. M. de la Maza, and H. D. Caldwell. 2009. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J. Immunol. 182:8063-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawa, D. E., J. Schachter, and R. S. Stephens. 2004. Immune response to the Chlamydia trachomatis outer membrane protein PorB. Vaccine 22:4282-4286. [DOI] [PubMed] [Google Scholar]
- 31.Kirimanjeswara, G. S., S. Olmos, C. S. Bakshi, and D. W. Metzger. 2008. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol. Rev. 225:244-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 33.Li, W., M. N. Guentzel, J. Seshu, G. Zhong, A. K. Murthy, and B. P. Arulanandam. 2007. Induction of cross-serovar protection against genital chlamydial infection by a targeted multisubunit vaccination approach. Clin. Vaccine Immunol. 14:1537-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucero, M. E., and C.-C. Kuo. 1985. Neutralization of Chlamydia trachomatis cell culture infection by serovar-specific monoclonal antibodies. Infect. Immun. 50:595-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore, T., C. O. Ekworomadu, F. O. Eko, L. MacMillan, K. Ramey, G. A. Ananaba, J. W. Patrickson, P. R. Nagappan, D. Lyn, C. M. Black, and J. U. Igietseme. 2003. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J. Infect. Dis. 188:617-624. [DOI] [PubMed] [Google Scholar]
- 36.Morgado, M. G., P. Cam, C. Gris-Liebe, P. A. Cazenave, and E. Jouvin-Marche. 1989. Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J. 8:3245-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison, S. G., and R. P. Morrison. 2005. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 175:7536-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison, S. G., and R. P. Morrison. 2000. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect. Immun. 68:2870-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison, S. G., and R. P. Morrison. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect. Immun. 69:2643-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison, S. G., and R. P. Morrison. 2005. The protective effect of antibody in immunity to murine chlamydial genital tract reinfection is independent of immunoglobulin A. Infect. Immun. 73:6183-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphey, C., A. K. Murthy, P. A. Meier, M. N. Guentzel, G. Zhong, and B. P. Arulanandam. 2006. The protective efficacy of chlamydial protease-like activity factor vaccination is dependent upon CD4+ T cells. Cell. Immunol. 242:110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murthy, A. K., B. K. Chaganty, W. Li, M. N. Guentzel, J. P. Chambers, J. Seshu, G. Zhong, and B. P. Arulanandam. 2009. A limited role for antibody in protective immunity induced by rCPAF and CpG vaccination against primary genital Chlamydia muridarum challenge. FEMS Immunol. Med. Microbiol. 55:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murthy, A. K., J. P. Chambers, P. A. Meier, G. Zhong, and B. P. Arulanandam. 2007. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect. Immun. 75:666-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murthy, A. K., Y. Cong, C. Murphey, M. N. Guentzel, T. G. Forsthuber, G. Zhong, and B. P. Arulanandam. 2006. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect. Immun. 74:6722-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pal, S., H. L. Davis, E. M. Peterson, and L. M. de la Maza. 2002. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect. Immun. 70:4812-4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal, S., T. J. Fielder, E. M. Peterson, and L. M. de la Maza. 1994. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 62:3354-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal, S., E. M. Peterson, and L. M. de la Maza. 1996. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect. Immun. 64:5341-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination of newborn mice induces a strong protective immune response against respiratory and genital challenges with Chlamydia trachomatis. Vaccine 23:5351-5358. [DOI] [PubMed] [Google Scholar]
- 51.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 73:8153-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pal, S., E. M. Peterson, R. Rappuoli, G. Ratti, and L. M. de la Maza. 2006. Immunization with the Chlamydia trachomatis major outer membrane protein, using adjuvants developed for human vaccines, can induce partial protection in a mouse model against a genital challenge. Vaccine 24:766-775. [DOI] [PubMed] [Google Scholar]
- 53.Pal, S., I. Theodor, E. Peterson, and L. M. de la Maza. 2001. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 69:6240-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pal, S., I. Theodor, E. M. Peterson, and L. de la Maza. 1997. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine 15:575-582. [DOI] [PubMed] [Google Scholar]
- 55.Peeling, R., I. W. Maclean, and R. C. Brunham. 1984. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect. Immun. 46:484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 57.Peterson, E. M., X. Cheng, V. L. Motin, and L. M. de la Maza. 1997. Effect of immunoglobulin G isotype on the infectivity of Chlamydia trachomatis in a mouse model of intravaginal infection. Infect. Immun. 65:2693-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramsey, K. H., and R. G. Rank. 1991. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect. Immun. 59:925-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rank, R. G., L. S. Soderberg, and A. L. Barron. 1985. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect. Immun. 48:847-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y.-X. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Invest. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roan, N. R., and M. N. Starnbach. 2008. Immune-mediated control of Chlamydia infection. Cell. Microbiol. 10:9-19. [DOI] [PubMed] [Google Scholar]
- 62.Rockey, D. D., J. Wang, L. Lei, and G. Zhong. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev. Vaccines 8:1365-1377. [DOI] [PubMed] [Google Scholar]
- 63.Shaw, J., V. Grund, L. Durling, D. Crane, and H. D. Caldwell. 2002. Dendritic cells pulsed with a recombinant chlamydial major outer membrane protein antigen elicit a CD4+ type 2 rather than type 1 immune response that is not protective. Infect. Immun. 70:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 63:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su, H., R. Messer, W. Whitmire, E. Fischer, J. C. Portis, and H. D. Caldwell. 1998. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable chlamydiae. J. Exp. Med. 188:809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun, G., S. Pal, J. Weiland, E. M. Peterson, and L. M. de la Maza. 2009. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine 27:5020-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.WHO. 1996. Global prevalence and incidence of selected curable sexually transmitted diseases: overviews and estimates. World Health Organization, Geneva, Switzerland.
- 68.WHO. 2001. Global prevalence and incidence of selected curable sexually transmitted infections: overviews and estimates. World Health Organization, Geneva, Switzerland.
- 69.WHO. 2007. Global strategy for prevention and control of sexually transmitted infections: 2006-2015. World Health Organization, Geneva, Switzerland.
- 70.Yu, H., X. Jiang, C. Shen, K. P. Karunakaran, and R. C. Brunham. 2009. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. J. Immunol. 182:1602-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu, H., X. Jiang, C. Shen, K. P. Karunakaran, J. Jiang, N. L. Rosin, and R. C. Brunham. 2010. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-γ)/tumor necrosis factor alpha and IFN-γ/interleukin-17 double-positive CD4+ T cells. Infect. Immun. 78:2272-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, Y.-X., S. J. Stewart, and H. D. Caldwell. 1989. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect. Immun. 57:636-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, Y. X., S. Stewart, T. Joseph, H. R. Taylor, and H. D. Caldwell. 1987. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J. Immunol. 138:575-581. [PubMed] [Google Scholar]