Abstract
CD4− CD8− (double-negative [DN]) T cells have recently been shown to display important immunological functions in human diseases. They express γδ or αβ T-cell receptors that recognize lipid/glycolipid antigens presented via the nonclassical major histocompatibility complex molecules of the CD1 family. We recently demonstrated that while αβ DN T cells serve primarily to express inflammatory cytokines, γδ DN T cells express mainly interleukin-10 (IL-10) in patients with cutaneous leishmaniasis. We also demonstrated a correlation between DN T cells and the expression of gamma interferon in the acute phase of Trypanosoma cruzi experimental infection. In this work, we sought to investigate whether αβ or γδ DN T cells display distinct immunoregulatory potentials in patients with polar forms of human Chagas' disease. Our data showed that in vitro infection with T. cruzi leads to expansion of DN T cells in patients with the indeterminate and severe cardiac clinical forms of the disease. However, while αβ DN T cells primarily produce inflammatory cytokines in both forms of the disease, γδ DN T cells display a marked, significant increase in antigen-specific IL-10 expression in indeterminate patients relative to cardiac patients. Finally, higher frequencies of the IL-10-producing γδ DN T cells were correlated with improved clinical measures of cardiac function in the patients, suggesting a protective role for these cells in Chagas' disease. Taken together, these data show distinct functional characteristics for αβ and γδ DN T cells associated with distinct morbidity rates and clinical forms in human Chagas' disease.
T-cell activation is a key event in the establishment of immune responses directed toward intracellular pathogens. Depending on the functional capacity of the activated T cells, the fate of the infection may take different paths either toward a protective or a pathogenic outcome. While it is important that a strong, activated immune response is elicited early on in the infection in order to eliminate (or control) the pathogen, the further control of this activation is necessary to reestablish homeostasis, avoiding tissue damage (17, 25).
One hallmark of most parasitic infections is that the great majority of individuals are able to trigger innate immunity and elicit an activated T-cell response during the acute infection, leading to the control of the parasite and establishment of a chronic infection. Interestingly, while many individuals develop severe forms of parasitic diseases once infection progresses to the chronic phase, most patients develop relatively mild forms, allowing for a host-parasite coexistence. One such example is observed upon human infection with the protozoan parasite Trypanosoma cruzi, which leads to Chagas' disease. As a result of thousands of years of coevolution between human host and the parasite (6), most infected individuals develop an asymptomatic, or “indeterminate” (I), form of Chagas' disease. This form is characterized by a lack of clinical signs and symptoms and has been associated predominantly with a modulatory cellular immune response based on cytokine profiles and downregulatory molecule expression (5, 20, 48, 49, 51). Chronic patients may also develop symptomatic clinical forms, mainly with digestive or cardiac alterations. Differential geographical prevalence of Chagas' disease clinical forms has been reported. In Brazil, 15 to 30% of Chagas' patients display the cardiac form, which is present in 20 states, while the digestive cases, observed in about 10% of infected individuals, have been reported in four states in the central region of the country (53). The digestive form is frequently found in Chile but is practically absent in Central America (42). These geographical differences might be related, in part, to host genetics and immune responses of local human populations, but it is believed that they are also related to the genetic diversity of T. cruzi strains (11). Different strains of parasite display tropism for different tissues, and, thus, an important factor determining the clinical course of disease might be the specific pool of infecting clones and their specific tropisms (29). However, a possible role for environmental, nutritional, and immunological aspects of the host cannot be discounted. While digestive and cardiac forms present significant morbidity, the cardiac form is the one associated with highest mortality. It is caused by neuronal and cardiomyocyte damage, ultimately resulting in ventricular dilation and subsequent functional heart failure, which can lead to death (44). Cardiac patients display a T-cell-mediated inflammatory response in situ (13, 24, 41), which is responsible for the pathology; this inflammatory profile is also observed in circulating activated T cells found at high frequencies in these patients (2, 16, 19, 32). Although it is clear that a plethora of parasite and host factors influences the clinical outcome of Chagas' disease, recent studies have suggested that activation of functionally distinct T-cell populations in T. cruzi-infected individuals may be responsible for the establishment of different clinical forms (17, 20). Thus, identifying these populations and the factors responsible for their activation will be critical for driving immune-based interventions to prevent pathology.
While the great majority of T cells express either the CD4 or the CD8 molecules, which are important for stabilizing the peptide-major histocompatibility complex (MHC) complex and which favor T-cell activation, a minority population of T cells that do not express CD4 or CD8 molecules has been identified in humans (8, 10, 27, 37). These double-negative (DN) T cells have been shown to be important sources of immunoregulatory cytokines in human leishmaniasis (4), to display modulatory functions (38), but also, under different circumstances, to display cytolytic activity (10, 36). A subpopulation of DN T cells is activated through the engagement of αβ or γδ T-cell receptors (TCRs) in the recognition of nonclassical MHC molecules of the CD1 family, presenting lipid or glycolipid antigens (36). This particular lipid/glycolipid antigenic recognition, as well as the immunoregulatory potential and susceptibility to chronic stimulation of these cells, highlights the important role these cells play in parasitic infections.
In our work with Bottrel et al., we determined that DN lymphocytes were the second most prevalent cell type producing gamma interferon (IFN-γ) in human cutaneous leishmaniasis and that this IFN-γ production was seen after short-term cultures with medium alone, as well as after stimulation with soluble Leishmania antigen (SLA) (9). The novel work of Antonelli et al. went on to demonstrate that DN T cells composed of two different cell populations are present in the blood of individuals infected with Leishmania braziliensis and that DN T cells expressing the αβ TCR displayed a profile consistent with activation of leishmanicidal and inflammatory activities (higher IFN-γ and tumor necrosis factor alpha [TNF-α]) while the DN subpopulation expressing γδ TCR had a modulatory potential via higher production of interleukin-10 (IL-10) (4). Interestingly, IFN-γ production has been associated with pathogenic responses in human leishmaniasis in more than one clinical form (3, 7, 22). We recently demonstrated that rats infected with the CL-Brenner clone of T. cruzi displayed a marked increase in the frequency of circulating DN T cells during the acute phase of infection (33). Taken together, these data led to the question of the role that DN T-cell subpopulations play in the clinical dichotomy of chronic human Chagas' disease.
To answer these questions, we investigated the immunoregulatory potential of DN T cells in patients with the two polar forms of Chagas' disease: indeterminate (I) and dilated cardiac (DC). Our data demonstrated that although no quantitative differences were seen with regard to the nonstimulated frequency of DN αβ and γδ T-cell subpopulations between patients and nonchagasic individuals, in vitro infection with trypomastigote forms of T. cruzi induced a marked increase in the frequency of these cells from chagasic patients. Moreover, the expanded αβ DN T cells displayed a greater inflammatory potential from cardiac patients than from indeterminate patients. This was accompanied by a greater down-modulatory ratio of IL-10 to inflammatory cytokine frequencies by γδ DN T cells from individuals with indeterminate disease, suggesting distinct roles for these cells in modulating the response in chronic Chagas' disease. Finally, we observed a correlation between higher frequencies of IL-10-producing γδ DN T cells and improved clinical measures of cardiac function, suggesting a protective role for these cells in human Chagas' disease. These data indicate that functionally distinct DN T cells are present in Chagas' disease patients and that they are associated with the resulting morbidity of the disease.
MATERIALS AND METHODS
Patients.
This study employed a cross-sectional design involving patients from areas of endemicity within Minas Gerais, Brazil, under the medical care of Manoel O. D. C. Rocha. A total of 12 patients with positive specific serology for T. cruzi, within the chronic phase of the disease, and with well-defined clinical forms were enrolled in this study. Detailed evaluations, including physical examinations, electrocardiograms, chest X rays, and echocardiograms were performed in order to classify patients into different groups as previously defined by us (43). Clinical groups were assigned as follows: the I group (n = 7), consisting of patients who did not present with any clinical manifestations or alterations upon clinical, radiological, and echocardiographic examination; the DC group (n = 5), consisting patients who presented with right and/or left ventricular dilation, global left ventricular dysfunction, and alterations in the cardiac electric impulse generation and conduction. In the latter group, the alterations were evident in electrocardiograms, chest X rays, and echocardiography, which showed the occurrence of heart enlargement in all cardiac patients analyzed. Left ventricular ejection fraction (LVEF) and left ventricular diastolic diameter (LVDD) were used as clinical parameters of ventricular function for the Chagas' patients (44). We also included in our analysis individuals without Chagas' disease (nonchagasic group [N]; n = 7), as determined by negative specific serological tests for Chagas' disease. Individuals with the digestive form of Chagas' disease were not included in this study due to low incidence of well-documented cases in our geographical location in Brazil. Characteristics of the study groups are summarized in Table 1. We excluded from our study individuals with any other chronic inflammatory diseases, valvular heart disease, coronary artery disease, arterial hypertension, diabetes mellitus, alcoholism, and bacterial infections. All individuals included in this work were volunteers, and treatment and clinical care were offered to all patients, as needed, despite their enrollment in this research project. This study is part of an extended project evaluating risk factors for cardiac damage/involvement in Chagas' disease, which has the approval of the Research Ethics Committee of the Federal University of Minas Gerais (COEP-UFMG-ETIC006/05) and is in accordance with the Helsinki Declaration. Peripheral blood was collected by venipuncture, and informed consent was obtained from all individuals.
TABLE 1.
Individuals analyzed in the study and their clinical status
| Patient no. | Serology for Chagas' disease | Clinical form | Age (yr) | Sex | LVEF (%) | LVDD (mm) |
|---|---|---|---|---|---|---|
| N1 | Negative | 43 | Female | |||
| N2 | Negative | 27 | Female | |||
| N3 | Negative | 23 | Female | |||
| N4 | Negative | 31 | Female | |||
| N5 | Negative | 21 | Male | |||
| N6 | Negative | 19 | Male | |||
| N7 | Negative | 20 | Male | |||
| I1 | Positive | Indeterminate | 55 | Male | 60 | 55 |
| I2 | Positive | Indeterminate | 38 | Female | 70 | 52 |
| I3 | Positive | Indeterminate | 50 | Female | 68 | 50 |
| I4 | Positive | Indeterminate | 34 | Female | 67 | 46 |
| I5 | Positive | Indeterminate | 68 | Female | NDa | ND |
| I6 | Positive | Indeterminate | 39 | Female | 69 | 46 |
| I7 | Positive | Indeterminate | 38 | Female | 66 | 49 |
| DC1 | Positive | Cardiac | 59 | Female | 34 | 70 |
| DC2 | Positive | Cardiac | 53 | Male | 22 | 69 |
| DC3 | Positive | Cardiac | ND | Male | ND | ND |
| DC4 | Positive | Cardiac | 50 | Male | 51 | 64 |
| DC5 | Positive | Cardiac | 63 | Male | 37 | 65 |
ND, not determined.
Parasites.
Trypomastigotes of the Y strain of T. cruzi were grown in Vero or L929 cell lines, as previously performed by us (49). Briefly, cells were infected with 10 trypomastigotes/cell and, after free trypomastigotes were removed by washing with culture medium, were maintained in RPMI medium enriched with 5% fetal calf serum and antibiotics (penicillin, 500 U/ml; streptomycin, 0.5 mg/ml) for approximately 5 days. After this period, trypomastigotes ruptured the cells and were collected from the supernatant. The contamination with amastigote forms was always below 3%. Parasites obtained in such a manner were used for infecting blood cells from patients and nonchagasic individuals.
Infection of blood cells from patients and nonchagasic individuals with T. cruzi trypomastigotes.
Infection of peripheral blood cells was performed using 10 trypomastigotes/cell, as previously described (49). Briefly, cells and parasites were incubated at 37°C in 5% CO2 for a period of 3 h. After this time, cells were washed by centrifugation with phosphate-buffered saline (PBS) for removal of free trypomastigotes. After centrifugation, the supernatant was removed, and a volume of RPMI medium supplemented with antibiotic/antimycotic (amphotericin B, 0.25 μg/ml; penicillin, 200 U/ml; and streptomycin, 0.1 mg/ml) and l-glutamine (1 mM) equal to the amount of blood initially incubated was added to the tubes. Infected cells were incubated at 37°C in 5% CO2 for a period of 14 h. After this period, brefeldin A (1 μg/ml) was added to prevent protein secretion, and cultures were reincubated for an additional 4 h. For all individuals, we carried out cultures of blood submitted to the same procedures described above, but in the absence of parasites, as nonstimulated controls.
Determination of the frequencies of DN T cells and expression of cytokines by αβ and γδ DN T cells.
Frequencies of αβ and γδ DN T cells, as well as expression of IFN-γ, TNF-α, IL-17, and IL-10 by these DN T-cell subpopulations, were determined by flow cytometry. Infected cells (treated as described above) or noninfected blood cells were harvested after the final 18 h of culture and submitted to specific staining for the above-mentioned molecules. We used a combination of CyChrome-labeled anti-CD4 and -CD8 to detect DN T cells, as previously done by us (4). Fluorescein isothiocyanate (FITC)-labeled anti-αβ or anti-γδ T cells were also used in the staining to identify the specific subpopulations. Cells were harvested and plated at a concentration of 200,000 cells/well and incubated with a 20-μl mixture of the surface antibodies (anti-CD4+ anti-CD8+ labeled with CyChrome and anti-αβ or anti-γδ labeled with FITC) for 15 min at 4°C. Samples were washed three times in phosphate-buffered saline (PBS)-1% bovine serum albumin (BSA) and fixed by a 20-min incubation with a 2% formaldehyde solution. After the fixing solution was removed by centrifugation and cells were washed with PBS, we permeabilized the cells by incubation for 10 min with a 0.5% saponin solution and proceeded with intracellular cytokine labeling. Samples were incubated with phycoerythrin (PE)-labeled anticytokine monoclonal antibodies for 20 min at room temperature, washed twice with 0.5% saponin solution, resuspended in PBS, and read in a flow cytometer. A minimum of 40,000 gated events from each sample were collected and analyzed using the FlowJo program. Analysis was performed by gating on the lymphocyte population and further gating on the CD4− CD8− αβ- or γδ-producing T cells to determine the expression of the different molecules, as previously done by us (4).
Statistical analysis.
The means of the different groups were compared using Tukey-Kramer all-pair comparison analysis of variance contained within the JMP software from SAS. A paired t test was used to ascertain differences among noninfected versus infected cultures within the same group of patients. Correlation analysis was done using Pearson's correlation coefficient. Differences that returned P values of less than or equal to 0.05 were considered statistically significant from one another.
RESULTS
In vitro infection with trypomastigote forms of T. cruzi induces an expansion of αβ and γδ DN T cells from chronic Chagas' disease patients.
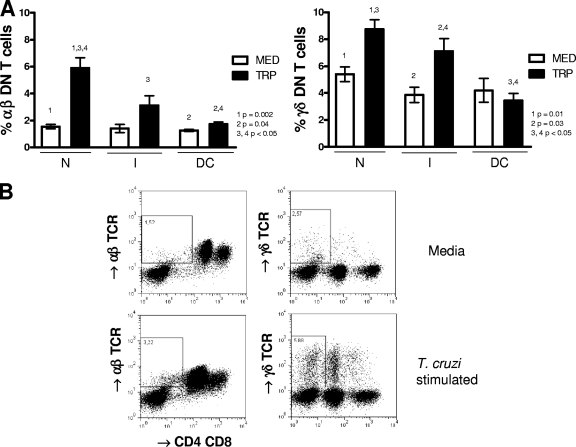
To determine the frequency of DN T-cell subsets in chronic Chagas' patients and nonchagasic individuals, we performed flow cytometric analysis of peripheral blood cells from these individuals, as described above. The analyses were carried out using nonstimulated cells to provide information about the frequency of these cells ex vivo from the patients, as well as after in vitro infection with trypomastigote forms of T. cruzi, to determine whether contact with the parasite led to the expansion of these cells and, if so, to what extent in the different groups. Our analysis showed that the frequencies of αβ and γδ DN T cells in nonstimulated cultures were similar among groups (Fig. 1 A, white bars). Moreover, we observed that within the total DN T-cell population, the frequencies of αβ and γδ TCR-expressing T-cell subpopulations were similar among groups (means ± standard deviations for αβ TCR subpopulations were 23% ± 8% [N], 30% ± 11% [I], and 25% ± 7% [DC], while those for γδ TCR subpopulations were 77% ± 8% [N], 70% ± 11% [I], and 75% ± 7% [DC]). Exposure of the cells from indeterminate and cardiac patients, as well as nonchagasic individuals to trypomastigote forms of T. cruzi led to an expansion of αβ and γδ DN T cells in all groups (Fig. 1A, dark bars; representative fluorescence-activated cell sorting [FACS] plots are shown in B).
FIG. 1.
T. cruzi activation of peripheral blood cells induces expansion of αβ and γδ double-negative (CD4− CD8−) T cells. Whole blood cells from noninfected controls (N), indeterminate chagasic patients (I), and dilated cardiac chagasic patients (DC) were incubated overnight as described in Materials and Methods with either medium alone (MED) or with live T. cruzi parasites (TRP) and then analyzed for the frequency of αβ and γδ CD4− CD8−T cells using flow cytometry. Panel A shows the average frequencies for each group ± standard deviations. The numbers of individuals in each group were as follows: N, seven; I, seven; and DC, five. Statistical significance is indicated in each graph, with differences between groups indicated by common numbers. Comparisons between groups were performed using a Tukey-Kramer comparison of all pairs, and comparisons within groups (MED versus TRP) were performed using a paired t test, as described in Materials and Methods. All patients were meticulously classified based on clinical criteria as described in Materials and Methods. Panel B shows representative dot plots from an indeterminate patient and gating for analysis of αβ and γδ CD4− CD8−T cells. Both anti-CD4 and anti-CD8 antibodies were conjugated with CyChrome, allowing identification of the DN T cells using specific antibodies against both αβ and γδ T-cell receptors conjugated with FITC. The gates used for determining the percentage of DN T cells and further analysis of cytokine expression in the DN T-cell populations are shown.
αβ and γδ DN T cells from chagasic patients display distinct immunoregulatory profiles.
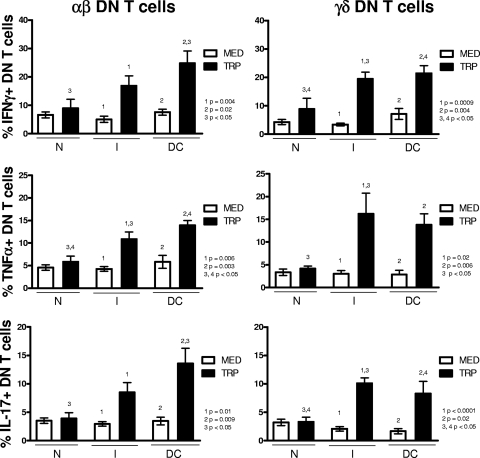
In order to determine the functional characteristics of αβ and γδ DN T cells from patients with polar clinical forms of Chagas' disease, we investigated the expression of inflammatory (IFN-γ, TNF-α, and IL-17) and anti-inflammatory (IL-10) cytokines by these cells in nonexposed cultures and cultures exposed to the parasite. We observed that, whereas there was no difference in the frequencies of αβ and γδ DN T cells expressing any of the cytokines in nonstimulated cultures of the different groups (Fig. 2 and 3, white bars), exposure to the parasite revealed dramatic differences among them. In contrast, stimulation of peripheral blood cells with T. cruzi led to a significant increase in the frequency of αβ DN T cells expressing IFN-γ, TNF-α, and IL-17 from chagasic patients but not from noninfected individuals (Fig. 2, left panels). The induction of inflammatory cytokines was more evident in cells from DC patients exposed to the parasite than from I patients, as demonstrated by the significantly higher frequency of cytokine-producing cells in the DC group than in the N group for all cytokines (Fig. 2, left panels). When the expression of inflammatory cytokines within the γδ DN T-cell population was analyzed, T. cruzi-induced increases in cells expressing IFN-γ, TNF-α, and IL-17 were seen for both clinical forms but not for nonchagasic individuals (Fig. 2, right panels). In this subpopulation, the induction of inflammatory cytokines was significantly higher in cells from the I and DC groups than from the N group after exposure to the parasite (Fig. 2, right panels).
FIG. 2.
T. cruzi activation of peripheral blood cells induces specific inflammatory cytokine production by αβ and γδ DN (CD4− CD8−) T cells from both indeterminate and dilated cardiac chagasic patients. Whole blood cells from noninfected controls (N), indeterminate chagasic patients (I), and dilated cardiac chagasic patients (DC) were incubated overnight with either medium alone (MED) or with live T. cruzi parasites (TRP) and then analyzed for the frequency of αβ or γδ DN T cells producing specific cytokines using flow cytometry, as described in Materials and Methods. The data represent the average for each group ± standard deviations. The numbers of individuals in each group were as follows: N, seven; I, seven; and DC, five. The top panel shows the average percentage of IFN-γ-producing cells within αβ or γδ DN T cells from individual cultures without (MED) or with (TRP) stimulus. The middle panel shows the same for TNF-α-producing cells within αβ or γδ DN T cells, and the bottom panel shows the values for IL-17-producing cells within αβ or γδ DN T cells. Statistical significance is indicated in each graph, with differences between groups indicated by common numbers. Comparisons between groups were performed using a Tukey-Kramer comparison of all pairs, and comparisons within groups (MED versus TRP) were performed using a paired t test, as described in Materials and Methods. All patients were meticulously classified based on clinical criteria, as described in Materials and Methods.
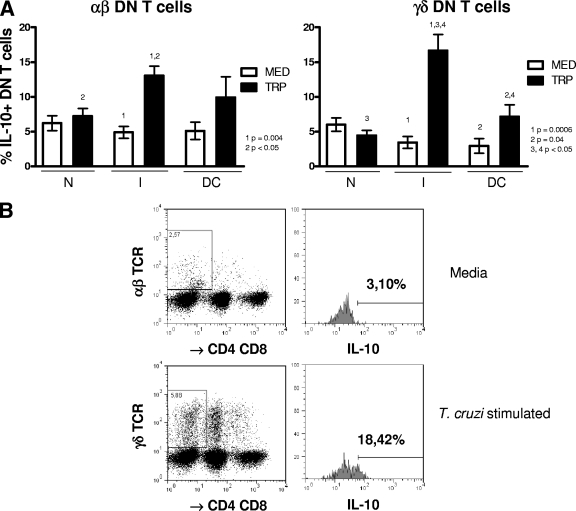
FIG. 3.
γδ DN (CD4− CD8−) T cells from indeterminate chagasic patients display a biased down-modulatory profile following stimulation with T. cruzi. Whole blood cells from noninfected controls (N), indeterminate chagasic patients (I), and dilated cardiac chagasic patients (DC) were incubated overnight with either medium alone (MED) or with live T. cruzi (TRP) and then analyzed using flow cytometry for the frequency of αβ or γδ DN T cells producing IL-10, as described in Materials and Methods. The data represent the average for each group ± standard deviations. The numbers of individuals in each group were as follows: N, seven; I, seven; and DC, five. Panel A shows the average percentage of IL-10-producing cells within the αβ or γδ DN T-cell population from individual cultures without (MED) or with (TRP) stimulus for each group. Statistical significance is indicated in each graph, with differences between groups indicated by common numbers. Comparisons between groups were performed using Tukey-Kramer comparison of all pairs, and comparisons within groups (MED versus TRP) were performed using a paired t test, as described in Materials and Methods. Panel B shows representative dot plots and gating for analysis of αβ and γδ CD4− CD8− T cells producing IL-10. Both anti-CD4 and anti-CD8 antibodies were conjugated with CyChrome, allowing identification of the DN T cells using specific antibodies against both αβ and γδ T-cell receptors conjugated with FITC. The gates used for determining the percentage of DN T cells producing IL-10 were then determined in a histogram using anti-IL-10 conjugated with PE. The percentages of cells producing IL-10 from cultures either with medium alone or with T. cruzi stimulation were determined, as described in Materials and Methods. All patients were meticulously classified based on clinical criteria, as described in Materials and Methods.
Analysis of expression of the down-modulatory cytokine IL-10 within the αβ and γδ DN T-cell populations showed that this cytokine was dramatically induced in T. cruzi cultures with cells from indeterminate patients (Fig. 3A; a representative FACS plot is shown in B). This increase in the frequency of IL-10-producing cells from indeterminate patients was seen when unstimulated and T. cruzi-stimulated cultures were compared, as well as in comparisons of T. cruzi-stimulated cultures of the I group versus the N and DC groups within the γδ DN T-cell subpopulation (Fig. 3). To determine the regulatory potentials of each subpopulation of αβ and γδ DN T cells from I and DC patients, we calculated regulatory ratios by dividing the frequency of cells producing IL-10 by the frequency of cells producing either IFN-γ, TNF-α, or IL-17. These results show that γδ DN T cells from group I have a much greater down-modulatory profile than γδ DN T cells from DC patients (Table 2). An analysis of the relative contribution by DN and other T cells to the overall frequency of IL-10-producing lymphocytes demonstrates that, on average, DN T cells account for at least 32% of the total IL-10 expression. This is a striking contribution, given that DN T cells represent a minority population of T cells. Interestingly, while the contribution of DN T cells to the overall IL-10 expression by lymphocytes in nonstimulated cultures from nonchagasic individuals was lower than that of CD4+ CD8+ T cells (33% ± 8% versus 51% ± 13%; P < 0.05), it was higher in nonstimulated cultures from indeterminate patients (49% ± 22% versus 27% ± 12%; P < 0.05). No statistically significant changes were observed in comparisons of the DN T cells and CD4+ CD8+ T cells from cardiac patients.
TABLE 2.
Indeterminate patients maintain a regulatory ratio of IL-10-producing cells
| Patient group | Regulatory ratioa |
||
|---|---|---|---|
| IL-10/IFN-γ | IL-10/TNF-α | IL-10/IL-17 | |
| Indeterminate | 1.01 ± 0.26 | 1.26 ± 0.21 | 1.66 ± 0.21 |
| Cardiac | 0.32 ± 0.07b | 0.55 ± 0.14b | 0.89 ± 0.09b |
The frequency of γδ DN T cells expressing the cytokines of interest following in vitro stimulation with T. cruzi was determined as described in Materials and Methods for each patient and then used to calculate regulatory ratios by dividing the frequency of cells producing IL-10 (a downregulatory cytokine) by the frequency of cells producing IFN-γ, TNF-α, or IL-17.
The values represent the average of ratios for IL-10/given cytokine ± the standard error for seven indeterminate and five cardiac patients. In all cases the comparison between the ratios for the indeterminate versus the cardiac groups returned a P value of <0.01.
Higher frequencies of IL-10-producing γδ DN T cells are correlated with improved clinical parameters of heart function in human Chagas' disease.
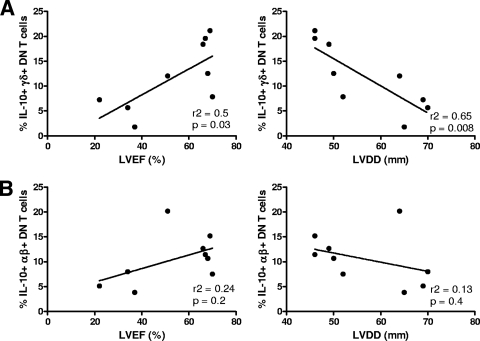
Previous studies performed by our group have suggested that IL-10 has a protective role in human Chagas' disease through an association between the indeterminate clinical form of Chagas' disease and high-producing genotypes for IL-10 (12). In order to further determine if the IL-10-producing γδ DN T-cell subpopulation is correlated with improved cardiac function, and thus with a possible protective role in human Chagas' disease, we performed a correlative analysis between the frequency of these cells and two clinical parameters of cardiac function: left ventricular ejection fraction (LVEF) and left ventricular diastolic diameter (LVDD). These distinct clinical parameters are directly and inversely correlated with better cardiac function, respectively (44). Strikingly, a significant positive correlation was seen between higher LVEF and higher frequencies of IL-10-producing γδ DN T cells (Fig. 4 A). Moreover, a highly significant negative correlation between lower LVDD and higher frequencies of IL-10-producing γδ DN T cells was also seen (Fig. 4 A). Interestingly, although αβ DN T cells also express IL-10 upon stimulation with the parasite, we did not observe correlations between the frequency of these cell subpopulations and the clinical parameters analyzed (Fig. 4 B). These data suggest that IL-10-producing γδ DN T cells display an important immunoregulatory role that leads to maintenance of better cardiac function in chagasic patients.
FIG. 4.
Higher frequencies of IL-10-producing γδ DN (CD4− CD8−) T cells are correlated with better heart function in chagasic patients. The frequency of γδ DN T cells producing IL-10 following stimulation with T. cruzi was calculated from a group of chagasic patients who had associated detailed clinical data measuring ventricular function. These measurements were the left ventricular ejection fraction (LVEF) and left ventricular diastolic diameter (LVDD). The higher the LVEF, the better the ventricular function, and the lower the LVDD, the better the ventricular function. Panel A shows Pearson's correlation plots between the frequency of IL-10-producing γδ DN T cells and LVEF or LVDD. Both plots demonstrate a highly significant correlation between higher frequencies of IL-10-producing γδ DN T cells and better ventricular function. In contrast, in panel B, no correlation is seen between IL-10-producing αβ DN T cells and measurements of ventricular function. Clinical data for a total of nine chagasic patients were used in this analysis. Statistical significance (P value) is indicated in each graph together with the r2 value.
DISCUSSION
Human infection with T. cruzi is the cause of Chagas' disease, an illness that currently affects approximately 18 million people in Latin America, where it is considered endemic. In addition, it is estimated that 100 million people are at risk of infection with T. cruzi. Although treatment is available and relatively effective (40, 47), toxicity and lack of widely distributed pediatric formulations are still major problems in human Chagas' disease. While vector transmission was controlled in certain areas of South America, disease transmission via blood transfusion and organ transplant has brought the disease to the attention of health professionals in Latin America and other countries where the disease is not endemic, such as the United States and other countries (28). Moreover, cases of acute Chagas' disease have been described in areas where acute cases were not reported for over 15 years (50). Despite the fact that most Chagas' patients display a relatively mild, asymptomatic, clinical form of the disease, about 30% of the patients develop severe disease, leading to cardiac involvement and, often, death (44). Thus, the social and economic burdens caused by Chagas' disease place it among the most morbid of all parasitic diseases.
The mechanisms behind the development of the severe cardiac form of Chagas' disease have not been completely elucidated. However, it is well accepted that T cells are key players in mounting an immune response during the chronic phase of the disease (17). Thus, T-cell activation and function are critical in determining the clinical outcome of Chagas' disease. Cardiac patients display a highly activated, inflammatory T-cell response both in situ (13, 24, 41) and in the peripheral blood (2, 16, 19, 32). Interestingly, however, patients who do not develop pathology and remain asymptomatic also display a high frequency of activated T cells in their bloodstream (18). This apparent contradiction has been better understood more recently, mainly due to the use of two important approaches: (i) clear definition of patient clinical forms by performing refined clinical analysis and (ii) identification and characterization of T-cell subpopulations that display distinct functional activities. Thus, recent studies using patients with well-defined clinical forms have shown that although T-cell activation is observed in severe and asymptomatic Chagas' patients, these cells have distinct functional potentials (17). Most studies have focused on the analysis of expression of factors that control the establishment of inflammatory responses in Chagas' disease, such as inflammatory cytokines and chemokines (20, 21). Studies performed by us and other groups have shown that major T-cell populations, defined by the expression of CD4 and CD8, display phenotypic and functional differences in individuals with different clinical forms of Chagas' disease. To this end, the frequencies of memory cells, as well as senescent cells, have been associated with the chronic cardiac form of Chagas' disease (1, 2, 23). While these studies have provided critical information, the determination of the contribution of distinct subpopulations to the immunoregulation and functional activities, as well as the antigens that lead to their activation, is critical for the understanding the mechanisms of generation of pathogenic versus protective responses in Chagas' disease.
A quantitatively small subpopulation of T cells that does not express CD4 or CD8 molecules has been identified, and because of the ability of these cells to tolerate chronic stimulation due to the lack of the stabilizing CD4 or CD8 molecules, they have been shown to be critical in chronic immune diseases, especially auto-immune processes (8, 30). Furthermore, a large portion of these cells are activated by recognizing lipid/glycolipid antigens presented via CD1 molecules (36). Glycolipid determinants from T. cruzi have been shown to be important in the activation of cellular immune responses in experimental infection (34). Although previous studies of murine infection with T. cruzi suggested that CD1 molecules were not critical in eliciting cellular responses to parasite components (34, 39), others have shown that CD1 presentation is important for natural killer T (NKT)-cell activation (14, 15, 31).
The role of DN T cells in T. cruzi infection has not yet been clarified. It has been shown that mice infected with the parasite display a 40- to 100-fold increase in the frequency of liver γδ CD4− CD8− lymphocytes, associated with expression of IFN-γ (45). Interestingly, the same group later showed that the liver is an important organ for parasite clearance in chronic infection (46). An increase in the DN T-cell frequency in the liver of animals infected with Plasmodium was also associated with parasite inhibition (35). Infection of rats with the highly virulent CL-Brenner clone of T. cruzi was associated with an expansion of CD4− CD8− T cells and IFN-γ production (33).
Recent studies have also pointed to important roles of DN T cells in human parasitic diseases. We have shown that αβ and γδ DN T cells display distinct immunoregulatory profiles in human cutaneous leishmaniasis (4, 25). Moreover, a high frequency of DN T cells was observed in the peripheral blood of individuals with Plasmodium falciparum malaria (52). In this work, we performed an analysis of the frequency of DN T cell αβ and γδ subpopulations in individuals with polar clinical forms of Chagas' disease. Our results showed that although there were no quantitative differences in the frequencies of these cells freshly isolated from chagasic patients and noninfected individuals, T. cruzi infection led to an expansion of DN T cells in vitro, and these cells were quite different in their immunoregulatory potentials. Although a parasite-induced expansion of DN T cells was observed in cultures of cells from patients as well as from noninfected individuals, the DN T cells from noninfected individuals did not express parasite-induced cytokines, compatible with a primary response. On the other hand, expanded cells from patients produced high levels of cytokines, indicative of an antigen-specific recall response, and also showed different cytokine expression profiles in indeterminate and cardiac patients. We observed that αβ DN T cells from individuals of the cardiac clinical form of Chagas' disease display higher expression of inflammatory cytokines upon in vitro stimulation with T. cruzi. Interestingly, γδ DN T cells from indeterminate patients displayed a markedly high expression of IL-10 following T. cruzi stimulation, which was not observed in cardiac patients. Analysis of the ratio IL-10/inflammatory cytokines revealed a clear down-modulatory environment associated with γδ DN T cells in indeterminate patients and not in cardiac patients. Given that we do not know the exact nature of the antigen responsible for the activation of these cells, we have not yet focused on any specific DN T-cell subpopulation, such as the DN NKT cells. Further studies are being carried out in our laboratory to clarify these questions. However, the observed functional differences presented here are clearly associated with important clinical features of the patients and continue to support earlier findings by our group and others defining key differences in the immunoregulatory environments between indeterminate and cardiac chagasic patients (20).
Monitoring cardiac function is an important procedure that permits one to follow the course of pathology development and worsening of human Chagas' disease. Unfortunately, due to the high costs of several of the required exams, it is not always possible to perform these procedures. We evaluated a group of clinically well-defined Chagas' patients in which two measures of cardiac function were performed: left ventricular ejection fraction and left ventricular diastolic diameter. These clinical characteristics, although physiologically related, reflect different levels of cardiac lesion. The greater the LVEF and the smaller the LVDD, the better the cardiac function. A positive correlation between a higher frequency of IL-10-producing γδ DN T cells and improved cardiac function as measured by LVEF was seen. Moreover, the higher the frequency of IL-10-producing γδ DN T cells, the lower the LVDD, which again indicates the association of IL-10-producing γδ DN T cells with better cardiac function. Previous studies performed by us showed that a down-modulatory profile, as accessed mainly by IL-10 and CTLA-4 expression, was predominant in indeterminate patients (48, 49). Moreover, we demonstrated that IL-10 promoter gene polymorphism, which leads to high IL-10 expression, is associated with the occurrence of the indeterminate clinical form. Here, we suggest that IL-10 derived from γδ DN T cells may also be involved in protection. This is an important finding since these cells are likely activated via distinct mechanisms compared to the other cell populations studied to date. This could aid in the development of novel antigen-based prophylactic or therapeutic interventions.
An important question still unanswered is why these cell populations display distinct functional capabilities in patients with indeterminate and cardiac clinical forms. This is particularly intriguing when we remember that indeterminate patients, who apparently display a modulated response that may be important for avoiding tissue inflammation, may develop cardiac disease in the future. The hypothesis is that these individuals undergo cellular functional changes, which would lead to pathology establishment. Assuming that these changes are a cause and not a consequence of pathology, then identifying such differences and determining their causes will provide critical information for preventing cardiac damage and a worsening clinical pathology.
Acknowledgments
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Disease, CNPq Universal grant, FAPEMIG, FINEP CT-Infra, and CNPq/Ministério da Saúde INCT-DT. W.O.D., M.O.D.C.R., L.R.D.V.A., L.M.D.M., and K.J.G. are CNPq fellows; F.N.A.V. and J.S.C.D.S. are CAPES fellows.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Albareda, M. C., S. A. Laucella, M. G. Alvarez, A. H. Armenti, G. Bertochi, R. L. Tarleton, and M. Postan. 2006. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas' disease patients. Int. Immunol. 18:465-471. [DOI] [PubMed] [Google Scholar]
- 2.Albareda, M. C., G. C. Olivera, S. A. Laucella, M. G. Alvarez, E. R. Fernandez, B. Lococo, R. Viotti, R. L. Tarleton, and M. Postan. 2009. Chronic human infection with Trypanosoma cruzi drives CD4+ T cells to immune senescence. J. Immunol. 183:4103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonelli, L. R., W. O. Dutra, R. P. Almeida, O. Bacellar, E. M. Carvalho, and K. J. Gollob. 2005. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol. Lett. 101:226-230. [DOI] [PubMed] [Google Scholar]
- 4.Antonelli, L. R., W. O. Dutra, R. R. Oliveira, K. C. Torres, L. H. Guimaraes, O. Bacellar, and K. J. Gollob. 2006. Disparate immunoregulatory potentials for double-negative (CD4− CD8−) αβ and γδ T cells from human patients with cutaneous leishmaniasis. Infect. Immun. 74:6317-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araujo, F. F., J. A. Gomes, M. O. Rocha, S. Williams-Blangero, V. M. Pinheiro, M. J. Morato, and R. Correa-Oliveira. 2007. Potential role of CD4+ CD25HIGH regulatory T cells in morbidity in Chagas disease. Front Biosci. 12:2797-2806. [DOI] [PubMed] [Google Scholar]
- 6.Aufderheide, A. C., W. Salo, M. Madden, J. Streitz, J. Buikstra, F. Guhl, B. Arriaza, C. Renier, L. E. Wittmers, Jr., G. Fornaciari, and M. Allison. 2004. A 9,000-year record of Chagas' disease. Proc. Natl. Acad. Sci. U. S. A. 101:2034-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacellar, O., H. Lessa, A. Schriefer, P. Machado, A. Ribeiro de Jesus, W. O. Dutra, K. J. Gollob, and E. M. Carvalho. 2002. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect. Immun. 70:6734-6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleesing, J. J., M. R. Brown, J. K. Dale, S. E. Straus, M. J. Lenardo, J. M. Puck, T. P. Atkinson, and T. A. Fleisher. 2001. TcR-alpha/beta+ CD4− CD8− T cells in humans with the autoimmune lymphoproliferative syndrome express a novel CD45 isoform that is analogous to murine B220 and represents a marker of altered O-glycan biosynthesis. Clin. Immunol. 100:314-324. [DOI] [PubMed] [Google Scholar]
- 9.Bottrel, R. L., W. O. Dutra, F. A. Martins, B. Gontijo, E. Carvalho, M. Barral-Netto, A. Barral, R. P. Almeida, W. Mayrink, R. Locksley, and K. J. Gollob. 2001. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect. Immun. 69:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks, E. G., S. P. Balk, K. Aupeix, M. Colonna, J. L. Strominger, and V. Groh-Spies. 1993. Human T-cell receptor (TCR) α/β+ CD4− CD8− T cells express oligoclonal TCRs, share junctional motifs across TCR Vβ-gene families, and phenotypically resemble memory T cells. Proc. Natl. Acad. Sci. U. S. A. 90:11787-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buscaglia, C. A., and J. M. Di Noia. 2003. Trypanosoma cruzi clonal diversity and the epidemiology of Chagas' disease. Microbes Infect. 5:419-427. [DOI] [PubMed] [Google Scholar]
- 12.Costa, G. C., M. O. da Costa Rocha, P. R. Moreira, C. A. Menezes, M. R. Silva, K. J. Gollob, and W. O. Dutra. 2009. Functional IL-10 gene polymorphism is associated with Chagas disease cardiomyopathy. J. Infect. Dis. 199:451-454. [DOI] [PubMed] [Google Scholar]
- 13.Cunha-Neto, E., V. J. Dzau, P. D. Allen, D. Stamatiou, L. Benvenutti, M. L. Higuchi, N. S. Koyama, J. S. Silva, J. Kalil, and C. C. Liew. 2005. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas' disease cardiomyopathy. Am. J. Pathol. 167:305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duthie, M. S., M. Kahn, M. White, R. P. Kapur, and S. J. Kahn. 2005. Both CD1d antigen presentation and interleukin-12 are required to activate natural killer T cells during Trypanosoma cruzi infection. Infect. Immun. 73:1890-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duthie, M. S., M. Kahn, M. White, R. P. Kapur, and S. J. Kahn. 2005. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect. Immun. 73:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutra, W. O., Z. M. da Luz, J. R. Cancado, M. E. Pereira, R. M. Brigido-Nunes, L. M. Galvao, D. G. Colley, Z. Brener, G. Gazzinelli, and J. F. Carvalho-Parra. 1996. Influence of parasite presence on the immunologic profile of peripheral blood mononuclear cells from chagasic patients after specific drug therapy. Parasite Immunol. 18:579-585. [DOI] [PubMed] [Google Scholar]
- 17.Dutra, W. O., and K. J. Gollob. 2008. Current concepts in immunoregulation and pathology of human Chagas disease. Curr. Opin. Infect. Dis. 21:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutra, W. O., O. A. Martins-Filho, J. R. Cancado, J. C. Pinto-Dias, Z. Brener, G. L. Freeman Junior, D. G. Colley, G. Gazzinelli, and J. C. Parra. 1994. Activated T and B lymphocytes in peripheral blood of patients with Chagas' disease. Int. Immunol. 6:499-506. [DOI] [PubMed] [Google Scholar]
- 19.Dutra, W. O., O. A. Martins-Filho, J. R. Cancado, J. C. Pinto-Dias, Z. Brener, G. Gazzinelli, J. F. Carvalho, and D. G. Colley. 1996. Chagasic patients lack CD28 expression on many of their circulating T lymphocytes. Scand. J. Immunol. 43:88-93. [DOI] [PubMed] [Google Scholar]
- 20.Dutra, W. O., C. A. Menezes, F. N. Villani, G. C. da Costa, A. B. da Silveira, D. Reis, and K. J. Gollob. 2009. Cellular and genetic mechanisms involved in the generation of protective and pathogenic immune responses in human Chagas disease. Mem. Inst. Oswaldo Cruz 104(Suppl 1):208-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutra, W. O., M. O. Rocha, and M. M. Teixeira. 2005. The clinical immunology of human Chagas disease. Trends Parasitol. 21:581-587. [DOI] [PubMed] [Google Scholar]
- 22.Faria, D. R., K. J. Gollob, J. Barbosa, Jr., A. Schriefer, P. R. Machado, H. Lessa, L. P. Carvalho, M. A. Romano-Silva, A. R. de Jesus, E. M. Carvalho, and W. O. Dutra. 2005. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect. Immun. 73:7853-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiuza, J. A., R. T. Fujiwara, J. A. Gomes, M. O. Rocha, A. T. Chaves, F. F. de Araujo, R. C. Fares, A. Teixeira-Carvalho, A. Martins-Filho Ode, G. G. Cancado, and R. Correa-Oliveira. 2009. Profile of central and effector memory T cells in the progression of chronic human Chagas disease. PLoS Negl. Trop. Dis. 3:e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonseca, S. G., M. M. Reis, V. Coelho, L. G. Nogueira, S. M. Monteiro, E. C. Mairena, F. Bacal, E. Bocchi, L. Guilherme, X. X. Zheng, F. Y. Liew, M. L. Higuchi, J. Kalil, and E. Cunha-Neto. 2007. Locally produced survival cytokines IL-15 and IL-7 may be associated to the predominance of CD8+ T cells at heart lesions of human chronic Chagas disease cardiomyopathy. Scand. J. Immunol. 66:362-371. [DOI] [PubMed] [Google Scholar]
- 25.Gollob, K. J., L. R. Antonelli, D. R. Faria, T. S. Keesen, and W. O. Dutra. 2008. Immunoregulatory mechanisms and CD4− CD8− (double negative) T cell subpopulations in human cutaneous leishmaniasis: a balancing act between protection and pathology. Int. Immunopharmacol. 8:1338-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27.Illum, N., E. Ralfkiaer, G. Pallesen, and C. Geisler. 1991. Phenotypical and functional characterization of double-negative (CD4− CD8−) alpha beta T-cell receptor positive cells from an immunodeficient patient. Scand. J. Immunol. 34:635-645. [DOI] [PubMed] [Google Scholar]
- 28.Leiby, D. A., R. M. Herron, Jr., E. J. Read, B. A. Lenes, and R. J. Stumpf. 2002. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion 42:549-555. [DOI] [PubMed] [Google Scholar]
- 29.Macedo, A. M., and S. D. J. Pena. 1998. Genetic variability of Trypanosoma cruzi: implications for the pathogenesis of Chagas disease. Parasitol. Today 14:119-124. [DOI] [PubMed] [Google Scholar]
- 30.Magerus-Chatinet, A., M. C. Stolzenberg, M. S. Loffredo, B. Neven, C. Schaffner, N. Ducrot, P. D. Arkwright, B. Bader-Meunier, J. Barbot, S. Blanche, J. L. Casanova, M. Debre, A. Ferster, C. Fieschi, B. Florkin, C. Galambrun, O. Hermine, O. Lambotte, E. Solary, C. Thomas, F. Le Deist, C. Picard, A. Fischer, and F. Rieux-Laucat. 2009. FAS-L, IL-10, and double-negative CD4− CD8− TCR α/β+ T cells are reliable markers of autoimmune lymphoproliferative syndrome (ALPS) associated with FAS loss of function. Blood 113:3027-3030. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros, M. M., J. R. Peixoto, A. C. Oliveira, L. Cardilo-Reis, V. L. Koatz, L. Van Kaer, J. O. Previato, L. Mendonca-Previato, A. Nobrega, and M. Bellio. 2007. Toll-like receptor 4 (TLR4)-dependent proinflammatory and immunomodulatory properties of the glycoinositolphospholipid (GIPL) from Trypanosoma cruzi. J. Leukoc. Biol. 82:488-496. [DOI] [PubMed] [Google Scholar]
- 32.Menezes, C. A., M. O. Rocha, P. E. Souza, A. C. Chaves, K. J. Gollob, and W. O. Dutra. 2004. Phenotypic and functional characteristics of CD28+ and CD28− cells from chagasic patients: distinct repertoire and cytokine expression. Clin. Exp. Immunol. 137:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagib, P. R., W. O. Dutra, E. Chiari, and C. R. Machado. 2007. Trypanosoma cruzi: populations bearing opposite virulence induce differential expansion of circulating CD3+ CD4− CD8− T cells and cytokine serum levels in young and adult rats. Exp. Parasitol. 116:366-374. [DOI] [PubMed] [Google Scholar]
- 34.Nakayasu, E. S., D. V. Yashunsky, L. L. Nohara, A. C. Torrecilhas, A. V. Nikolaev, and I. C. Almeida. 2009. GPIomics: global analysis of glycosylphosphatidylinositol-anchored molecules of Trypanosoma cruzi. Mol. Syst. Biol. 5:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pied, S., J. Roland, A. Louise, D. Voegtle, V. Soulard, D. Mazier, and P. A. Cazenave. 2000. Liver CD4− CD8− NK1.1+ TCRαβ intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J. Immunol. 164:1463-1469. [DOI] [PubMed] [Google Scholar]
- 36.Porcelli, S., C. T. Morita, and M. B. Brenner. 1992. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature 360:593-597. [DOI] [PubMed] [Google Scholar]
- 37.Porcelli, S., C. E. Yockey, M. B. Brenner, and S. P. Balk. 1993. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8− α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J. Exp. Med. 178:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Priatel, J. J., O. Utting, and H. S. Teh. 2001. TCR/self-antigen interactions drive double-negative T cell peripheral expansion and differentiation into suppressor cells. J. Immunol. 167:6188-6194. [DOI] [PubMed] [Google Scholar]
- 39.Procopio, D. O., I. C. Almeida, A. C. Torrecilhas, J. E. Cardoso, L. Teyton, L. R. Travassos, A. Bendelac, and R. T. Gazzinelli. 2002. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J. Immunol. 169:3926-3933. [DOI] [PubMed] [Google Scholar]
- 40.Rassi, A., A. O. Luquetti, A. Rassi, Jr., G. G. Rassi, S. G. Rassi, D. A. S. IG, and A. G. Rassi. 2007. Specific treatment for Trypanosoma cruzi: lack of efficacy of allopurinol in the human chronic phase of Chagas disease. Am. J. Trop. Med. Hyg. 76:58-61. [PubMed] [Google Scholar]
- 41.Reis, D. D., E. M. Jones, S. Tostes, E. R. Lopes, E. Chapadeiro, G. Gazzinelli, D. G. Colley, and T. L. McCurley. 1993. Expression of major histocompatibility complex antigens and adhesion molecules in hearts of patients with chronic Chagas' disease. Am. J. Trop. Med. Hyg. 49:192-200. [DOI] [PubMed] [Google Scholar]
- 42.Rezende, J. M., and A. O. Luquetti. 1994. Chagasic megavisceras. PAHO Sci. Publ. 547:149-171. [Google Scholar]
- 43.Rocha, M. O., A. L. Ribeiro, and M. M. Teixeira. 2003. Clinical management of chronic Chagas cardiomyopathy. Front. Biosci. 8:e44-54. [DOI] [PubMed] [Google Scholar]
- 44.Rocha, M. O., M. M. Teixeira, and A. L. Ribeiro. 2007. An update on the management of Chagas cardiomyopathy. Expert Rev. Anti Infect. Ther. 5:727-743. [DOI] [PubMed] [Google Scholar]
- 45.Sardinha, L. R., R. M. Elias, T. Mosca, K. R. Bastos, C. R. Marinho, M. R. D'Imperio Lima, and J. M. Alvarez. 2006. Contribution of NK, NK T, γδ T, and αβ T cells to the gamma interferon response required for liver protection against Trypanosoma cruzi. Infect. Immun. 74:2031-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sardinha, L. R., T. Mosca, R. M. Elias, R. S. do Nascimento, L. A. Goncalves, D. Z. Bucci, C. R. Marinho, C. Penha-Goncalves, M. R. Lima, and J. M. Alvarez. 2010. The liver plays a major role in clearance and destruction of blood trypomastigotes in Trypanosoma cruzi chronically infected mice. PLoS Negl. Trop. Dis. 4:e578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sosa-Estani, S., and E. L. Segura. 2006. Etiological treatment in patients infected by Trypanosoma cruzi: experiences in Argentina. Curr. Opin. Infect. Dis. 19:583-587. [DOI] [PubMed] [Google Scholar]
- 48.Souza, P. E., M. O. Rocha, C. A. Menezes, J. S. Coelho, A. C. Chaves, K. J. Gollob, and W. O. Dutra. 2007. Trypanosoma cruzi infection induces differential modulation of costimulatory molecules and cytokines by monocytes and T cells from patients with indeterminate and cardiac Chagas' disease. Infect. Immun. 75:1886-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souza, P. E., M. O. Rocha, E. Rocha-Vieira, C. A. Menezes, A. C. Chaves, K. J. Gollob, and W. O. Dutra. 2004. Monocytes from patients with indeterminate and cardiac forms of Chagas' disease display distinct phenotypic and functional characteristics associated with morbidity. Infect. Immun. 72:5283-5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steindel, M., L. Kramer Pacheco, D. Scholl, M. Soares, M. H. de Moraes, I. Eger, C. Kosmann, T. C. Sincero, P. H. Stoco, S. M. Murta, C. J. de Carvalho-Pinto, and E. C. Grisard. 2008. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn. Microbiol. Infect. Dis. 60:25-32. [DOI] [PubMed] [Google Scholar]
- 51.Vitelli-Avelar, D. M., R. Sathler-Avelar, R. L. Massara, J. D. Borges, P. S. Lage, M. Lana, A. Teixeira-Carvalho, J. C. Dias, S. M. Eloi-Santos, and O. A. Martins-Filho. 2006. Are increased frequency of macrophage-like and natural killer (NK) cells, together with high levels of NKT and CD4+ CD25high T cells balancing activated CD8+ T cells, the key to control Chagas' disease morbidity? Clin. Exp. Immunol. 145:81-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winkler, S., M. Willheim, K. Baier, W. Graninger, and P. G. Kremsner. 1999. Frequency of cytokine-producing CD4− CD8− peripheral blood mononuclear cells in patients with Plasmodium falciparum malaria. Eur. Cytokine Netw. 10:155-160. [PubMed] [Google Scholar]
- 53.World Health Organization. 2002. WHO Technical Report Series. Control of Chagas disease. World Health Organization, Geneva, Switzerland. [PubMed]