Abstract
Patients with paracoccidioidomycosis (PCM) exhibit a suppression of the cellular immune response characterized by negative delayed-type hypersensitivity (DTH) to Paracoccidioides brasiliensis antigens, the apoptosis of lymphocytes, and high levels of expression of cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4), interleukin-10 (IL-10), and transforming growth factor β (TGF-β). The aim of this study was to investigate whether and how regulatory T cells (Treg cells) are involved in this immunosuppression by analyzing the number, phenotype, and activity of these cells in patients with active disease (AD group) and patients who had received treatment (TD group). Our results showed that the AD patients had more Treg cells than the TD patients or controls (C group) and also had elevated levels of expression of regulatory markers (glucocorticoid-induced tumor necrosis factor [TNF] receptor-related protein [GITR], CTLA-4, CD95L, LAP-1, and CD38). An analysis of regulatory activity showed that Treg cells from the AD group had greater activity than did cells from the other groups and that cell-cell contact is mandatory for this activity in the C group but was only partially involved in the regulatory activity of cells from AD patients. The addition of anti-IL-10 and anti-TGF-β neutralizing antibodies to the cultures showed that the production of cytokines may be another mechanism used by Treg cells. In conclusion, the elevated numbers of these cells with an increased regulatory phenotype and strong suppressive activity suggest a potential role for them in the immunosuppression characteristic of paracoccidioidomycosis. In addition, our results indicate that while Treg cells act by cell-cell contact, cytokine production also plays an important role.
Infections caused by fungi are currently among the most life-threatening diseases. Paracoccidioidomycosis (PCM), a disease caused by the dimorphic fungus Paracoccidioides brasiliensis, is the major cause of systemic mycosis in Latin America (8). Skin test surveys using antigens of P. brasiliensis showed that the majority of infected individuals in areas where the disease is endemic do not manifest any clinical symptoms of the disease (15). The natural route of infection is almost certainly the inhalation of fungal conidia, which usually leads to asymptomatic infection (15). The disease presents with a wide spectrum of clinical and immunological manifestations, varying from benign and localized forms to severe and disseminated forms. According to current classifications, PCM may be divided into three major groups: the asymptomatic infection observed for healthy individuals who live in areas of endemicity and exhibit a positive delayed-type hypersensitivity (DTH) test (positive PCM infection [PI]) and two clinical forms of the disease, known as the acute or juvenile form (JF) and the chronic or adult form (AF) (8, 15). The JF affects young patients of both sexes equally. It is characterized by systemic lymph node involvement, hepatosplenomegaly, and bone marrow dysfunction and resembles a lymphoproliferative disease. The AF almost always affects adult males, and its clinical presentation is very heterogeneous, ranging from isolated lesions in the respiratory tract (mild or benign forms) to widely disseminated forms (8, 15).
The pattern of the immune response to P. brasiliensis is believed to determine disease progression and clinical outcome. Effective defense against P. brasiliensis depends mainly upon Th1 cells, and acquired resistance is governed by cytokines that activate T cells and macrophages. Among these, tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) play a particularly prominent role (9, 10). However, paracoccidioidomycosis patients, particularly those with the most severe forms of the disease, show an impaired cellular immune response characterized by the production of large amounts of suppressive cytokines such as interleukin-10 (IL-10) and transforming growth factor β (TGF-β) as well as reduced levels of production of IFN-γ and TNF-α (22-24, 31). Peripheral blood cells of paracoccidioidomycosis patients also exhibit high levels of expression of Fas ligand (Fas-L, or CD95L) and cytotoxic-T-lymphocyte antigen 4 (CTLA-4), which are involved in increased apoptosis-induced cell death (11). Altogether, these characteristics may be indicative of the involvement of regulatory T cells (Treg cells) in the immunosuppression observed for these patients, as the suppressive effects associated with these cells are known to be exerted through mechanisms involving these molecules (40).
Treg cells were initially described as a unique population of CD4+ T cells that prevent the proliferation of self-reactive lymphocytes and subsequent autoimmune disease. They are classically defined by their constitutive expression of CD25 (also known as the IL-2 receptor α-chain) (40) and also express CTLA-4, the tumor necrosis factor family member GITR (glucocorticoid-induced TNF receptor-related protein), CD38, CD103, and membrane-bound TGF-β1 (40). However, none of these markers are specific to natural Treg cells, as they can also be expressed by activated T cells. The expression of the transcription factor Foxp3 is the most definitive signature of natural Treg cells in mice, but its expression can also be transiently upregulated by activated human T cells (44). Treg cells can suppress cell proliferation and immune responses by means of several mechanisms, including cytokine production, cell-cell contact, and the synthesis of immunosuppressive metabolites. The regulation of the cell-mediated immune response has been demonstrated for both infectious and autoimmune diseases, and it has been shown that Treg activity can dampen host immunopathology and benefit pathogens, allowing disease chronification (40).
A recent study of human paracoccidioidomycosis showed that although patients and healthy individuals have the same numbers of circulating Treg cells, the cells in the former group exhibit greater suppressive activity (13). These findings indicate that these cells may play a role in the immunosuppression observed during the course of the disease. To our knowledge, there are, with the exception of the study mentioned above, no data in the literature about the mechanisms through which Treg cells exert their suppressive function in PCM or the effect of antifungal treatment on the number and activity of these cells. The objective of this study was therefore to further explore these questions.
MATERIALS AND METHODS
Samples.
Peripheral venous blood was collected from healthy donors (control [C] group) and PCM patients, the latter being divided into two groups: patients with active disease (AD group) selected before or within the first month of treatment and patients who had received treatment, had negative serological tests for P. brasiliensis, and had no clinical signs of the disease (TD group). All PCM patients were presenting (AD), or presented before the treatment (TD), the multifocal or disseminated adult form or the juvenile form of the disease. Diagnosis was confirmed by the detection of the fungus in clinical specimens and serological tests, which were carried out at the Hospital de Clínicas, State University of Campinas (UNICAMP). In accordance with the rules of the Medical Research Ethics Committee at the Faculty of Medical Sciences, UNICAMP, all individuals signed a voluntary informed-consent form agreeing to participate in the study.
Immunohistochemical analysis.
Biopsy specimens were taken from 10 patients with paracoccidioidomycosis who were being cared for at the Hospital de Clínicas, State University of Campinas, São Paulo, Brazil. The biopsy specimens were obtained for diagnostic purposes before the treatment began. Paraffin-embedded sections (5 μm) were deparaffinized, rehydrated in a graded alcohol series, placed into Tris-buffered saline (TBS), and then boiled at 95°C in citrate buffer (pH 6.0), for 30 min for antigen retrieval. Nonspecific protein binding was blocked by incubating the material with a serum-free protein block (Dako Corporation, Carpinteria, CA). The slides were then incubated with the primary goat anti-human Foxp3 monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) for 1 h at 37°C in a humidifying chamber. The signal was visualized with the Envision amplification system (Dako Corporation, Carpinteria, CA) according to the manufacturer's instructions. The slides were counterstained with Mayer's hemalum (Merck, Darmstadt, Germany).
Flow cytometry.
To reduce the effect of the in vitro activation of T cells, phenotypic analysis of Treg cells was carried out in total ex vivo blood samples after red cell lysis. The leukocytes were incubated for 20 min at 4°C with either antibodies against surface molecules (CD4, CD25, CD62L, CD38, CD103, CD95, CD95L, GITR, CD152, and CCR5; all from BD Biosciences, San Jose, CA) conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), or PE-Cy5 or biotinylated anti-surface TGF-β1 (LAP-1) and anti-Toll-like receptor 2 (TLR2) (both from R&D Systems, Minneapolis, MN). After incubation the cells were washed and, in the case of TGF-β1 and TLR2, incubated with streptavidin-PE conjugate (Invitrogen-Caltag, Carlsbad, CA) for an additional 15 min. After an additional wash, the cells were fixed with 2% paraformaldehyde, permeabilized with saponin (0.5% in phosphate-buffered saline [PBS]) for 10 min at 4°C, and incubated with anti-Foxp3 antibody (Biolegend, San Diego, CA) for 30 min at 4°C, followed by another wash and fixation step.
To analyze cell proliferation, peripheral blood mononuclear cells (PBMCs) or CD4+ T cells (isolated as described below) were labeled with carboxyfluorescein deacetate succinimidyl ester (CFSE; final concentration of 1.5 μM) and cultured for 5 days (see below). After this period, the cells were harvested and further labeled with anti-CD4 antibodies (PE-Cy5) and fixed, as described above. The cells were acquired with a FACScalibur flow cytometer, and the analysis was performed by using FCS Express software (De Novo Software).
Purification of CD4+ CD25+ and CD4+ CD25− T cells.
PBMCs were isolated by using Ficoll-Hypaque (Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation and then washed, counted, and used to isolate T-cell subpopulations. CD4+ CD25+ (Treg cells) and CD4+ CD25− T cells were isolated by using the CD4+ CD25+ regulatory T-cell isolation kit (MACs; Miltenyi Biotec, Bergisch, Gladbach, Germany) according to the manufacturer's instructions.
Media.
All cells were grown in RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% heat-inactivated AB normal human serum, 100 U/ml gentamicin, 2 mM l-glutamine, and 1 mM sodium pyruvate (all from Sigma-Aldrich, St. Louis, MO).
Cocultures and proliferation assays.
To investigate the regulatory function of CD4+ CD25+ and CD4+ CD25− T cells isolated from patients and healthy controls, the cells were cultured with PBMCs (1 × 105 cells/well) from allogeneic healthy donors or autologous CD4+ CD25− T cells in different ratios (1:2; 1:4, and 1:10) in 96-well round-bottomed plates in the presence of concanavalin A (ConA) (2.5 μg/ml) at 37°C and 5% CO2. The results were expressed as the percent inhibition of proliferation based on the ConA-induced proliferation of allogeneic T cells cultured without CD4+ CD25+ T cells. To investigate the mechanism by which Treg cells exert their suppressive effect, the cocultures were performed in a Transwell system by using Nunc Transwell plates; CD4+ CD25+ cells were cultured in the top chamber, and PBMCs or CD4+ CD25− T cells were cultured in the bottom chamber.
To evaluate the possible role of suppressive cytokines produced by CD4+ CD25+ T cells on the proliferative response of PBMCs or CD4+ CD25− T cells, the culture media were supplemented with recombinant human TGF-β (0.2 ng/ml) and/or IL-10 (2 ng/ml) or with neutralizing antibodies against IL-10 (1 ng/ml) and/or TGF-β (0.25 ng/ml). To investigate whether the suppressive effect of CD4+ CD25+ T cells on proliferation was a result of the consumption of growth factors (particularly IL-2), we supplemented the coculture medium with recombinant IL-2 (0.5 ng/ml) or neutralizing anti-IL-2 antibody (100 ng/ml) or treated CD4+ CD25+ T cells with anti-CD25 blocking antibody (1 μg/ml) prior to coculturing. After the culture period, the cells were harvested and analyzed by flow cytometry as described above. All recombinant cytokines and neutralizing/blocking antibodies were obtained from R&D Systems (Minneapolis, MN).
ELISA for cytokines.
To measure the production of IL-10 and TGF-β in the cocultures, 1 × 106 CD4+ CD25− T cells were cultured as described above in 1 ml of culture medium at 37°C in a humidified atmosphere containing 5% CO2 in 24-well plates (Costar, Cambridge, MA). Culture supernatants were removed after 48 h and assayed for cytokine production. Cytokine concentrations were determined by a specific enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Real-time reverse transcription (RT)-PCR.
PBMCs from patients and healthy donors were obtained by centrifugation on a Ficoll-Hypaque density gradient. Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA), quantified by spectrophotometry (NanoDrop; Thermo Scientific, Waltham, MA), and treated with human DNase I to eliminate genomic DNA contamination. After DNase treatment, 1 μg of total RNA was used for cDNA synthesis, and the cDNA was amplified by using Sybr green PCR master mix and real-time PCR equipment (StepOne system; Applied Biosystems, Foster City, CA). The results were expressed as relative expression (2−ΔΔCT), as described previously by Livak and Schmittgen (19), normalized to the ubiquitin gene. The following primers were used: forward primer 5′-ATT TGG GTC GCG GTT CTT G-3′ and reverse primer 5′-TGC CTT GAC ATT CTC GAT GGT-3′ for ubiquitin, forward primer 5′-GAA ACA GCA CAT TCC CAG AGT TC-3′ and reverse primer 5′-ATG GCC CAG CGG ATG AG-3′ for Foxp3, forward primer 5′-GGC CAG GGC ACC CAG TCT-3′ and reverse primer 5′-TCG AAG CAT GTT AGG CAG GTT-3′ for IL-10, and forward primer 5′-TGA GGG CTT TCG CCT TAG C-3′ and reverse primer 5′-CGG TAG TGA ACC CGT TGA TGT-3′ for TGF-β.
Statistical analysis.
The results for the different groups were analyzed by using one-way analysis of variance (ANOVA), followed by the Bonferroni multiple-comparison test. To compare the effects of the different treatments in each group, we used the ANOVA test for repeated measures or a paired Student's t test. A P value of ≤0.05 was considered statistically significant. All statistical analyses were carried out with SigmaStat v1.0 (Jandel Corporation).
RESULTS
Numbers and phenotypes of peripheral CD4+ CD25+ and CD4+ CD25+ Foxp3+ T cells.
As mentioned above, PCM is characterized by a wide range of clinical and immunological manifestations, which reflect in the clinical forms of the disease. In order to eliminate these intrinsic differences, we analyzed only patients presenting the multifocal (disseminated) AF or the JF of PCM. These patients, despite their differences in clinical manifestations, present similar immunological responses, characterized by the immunosuppression of cellular immune mechanisms, as demonstrated by previous studies (7, 24, 31).
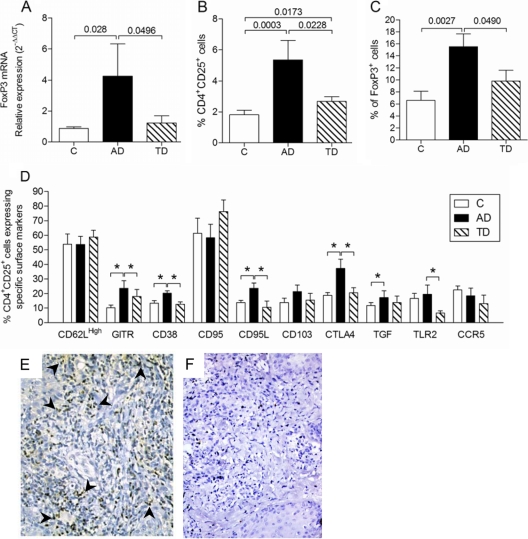
The transcription factor Foxp3, although not exclusive, is the most specific marker for Treg cells (44). For this reason we first analyzed Foxp3 mRNA expression in PBMCs from patients with active disease (AD group), patients who had been treated and clinically cured (TD group), and healthy controls (C group). Foxp3 mRNA levels were measured by the relative ΔΔCT quantification method, and the ubiquitin gene was used as an endogenous control. Our results showed that the expression of Foxp3 mRNA is four times higher in PBMCs from the AD group than in those from the control or TD group (Fig. 1A), indicating a possible increase in number of Treg cells in the circulation of PCM patients. To confirm these data, we evaluated the phenotype of CD4+ T cells in the peripheral blood of PCM patients and healthy controls by flow cytometry. The results showed a higher number of CD4+ CD25+ cells in the circulation of patients with active PCM than in patients who had been treated or controls (Fig. 1B). In addition, Foxp3+ cells were more frequent in CD4+ CD25+ lymphocytes from the AD group than in those from the other groups analyzed (Fig. 1C).
FIG. 1.
Number and phenotype of peripheral blood CD4+ T cells. (A) Expression of Foxp3 mRNA in ex vivo PBMCs from healthy individuals (C group) (n = 10) and patients with active (AD group) (n = 10) or treated (TD group) (n = 10) paracoccidioidomycosis. Values were normalized by using ubiquitin mRNA expression as an endogenous control, and the results are expressed as relative expression (2−ΔΔCT), as described in Materials and Methods. (B) Percentages of CD4+ CD25+ T cells in peripheral blood from healthy individuals (C group) (n = 13), patients with paracoccidioidomycosis presenting with active disease (AD group) (n = 14), or patients who had received treatment (TD group) (n = 12). (C) Percentages of CD4+ CD25+ cells expressing Foxp3 in the C, AD, and TD groups. (D) Expression of surface markers on CD4+ CD25+ cells from all the groups analyzed. The data were analyzed as described in Materials and Methods. The P values are shown above the brackets in A, B, and C. *, P ≤ 0.05 (ANOVA test with the Bonferroni posttest). (E) Foxp3+ cells (dark brown [arrowheads point to some examples]) in a representative biopsy specimen of a lesion from a patient with PCM. Magnification, ×400. (F) Representative result of a control slide (without the primary antibody).
The higher levels of expression of CD25 and Foxp3 found in lymphocytes from patients with active PCM may be indicative of an elevated number of Treg cells, but as human activated T cells also express these molecules transiently, we used flow cytometry to investigate another activation marker, the CD69 molecule, as well as the expression of the following markers associated with the regulatory activity of Treg cells: CTLA-4, GITR, CD38, CD95L, LAP-1 (membrane-bound TGF-β), CD62L, CD103, and CD95. Using different gates, we analyzed these markers in the population of CD4+ T cells that expressed or did not express the CD25 molecule (CD4+ CD25+ and CD4+ CD25−, respectively). The activation marker CD69 was not differentially expressed among the groups (data not shown). However, analysis of the regulatory markers showed higher surface expression levels of GITR, CTLA-4, CD95L, LAP-1, and CD38 in CD4+ CD25+ cells from patients with active disease (AD group) than in the same cells from individuals who had received treatment (TD group) or controls (C group) (Fig. 1D). CD4+ CD25− T cells from all groups expressed low or undetectable levels of these regulatory markers (data not shown). These findings indicate that there is an increased number of Treg cells in the peripheral blood of PCM patients with active disease and that this number drops to levels similar to those found in healthy individuals after effective antifungal treatment.
In addition to the presence of an augmented number of peripheral CD4+ CD25+ T cells, the analysis of all lesions from patients with PCM showed numerous Foxp3-positive cells in the inflammatory infiltrate surrounding the granulomatous reaction (Fig. 1E). The controls, done in the absence of a specific antibody, were always negative (Fig. 1F).
Evaluation of the suppressive activity of CD4+ CD25+ T cells.
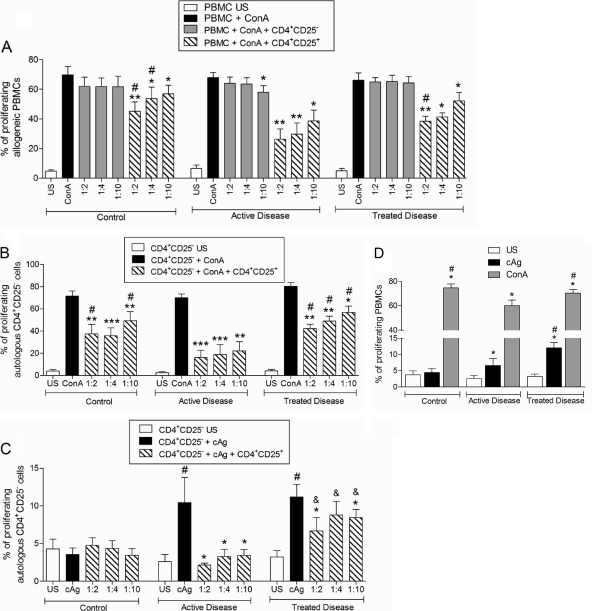
To evaluate the suppressive activity of CD4+ CD25+ and CD4+ CD25− T cells, we cocultured allogeneic PBMCs (from healthy controls) stimulated with ConA and CD4+ CD25+ or CD4+ CD25− T cells from patients with active PCM (AD group), patients who had received treatment (TD group), or healthy individuals (C group). The PBMCs were labeled with CFSE and stimulated for 96 h, after which the cells were labeled with anti-CD4 antibodies and analyzed by using flow cytometry. The results were expressed as the percentage of proliferating cells, as determined by the decrease in the intensity of CFSE fluorescence.
In all three groups, CD4+ CD25+ T cells, but not CD4+ CD25− T cells, were able to inhibit the proliferation of allogeneic PBMCs, and the suppressive effect was number dependent (Fig. 2A). CD4+ CD25+ T cells were also found to exert a suppressive activity on autologous CD4+ CD25− T cells, as the results showed that the proliferation of these cells in the presence of Treg cells was also inhibited (Fig. 2B).
FIG. 2.
(A) Proliferative response of unstimulated (US) or ConA (2.5 μg/ml)-stimulated allogeneic PBMCs cocultured with different numbers of CD4+ CD25− cells or CD4+ CD25+ T cells from healthy individuals (control group) (n = 10) and patients with active (n = 10) or treated (n = 10) paracoccidioidomycosis. (B) Proliferative response of unstimulated or ConA (2.5 μg/ml)-stimulated autologous CD4+ CD25− T cells cocultured with different numbers of CD4+ CD25+ T cells from healthy individuals (control group) (n = 10) and patients with active (n = 10) or treated (n = 10) paracoccidioidomycosis. (C) Proliferative response of unstimulated or cAg (cellular P. brasiliensis antigen) (10 μg/ml)-stimulated autologous CD4+ CD25− T cells cocultured with different numbers of CD4+ CD25+ T cells from healthy individuals (control group) (n = 10) and patients with active (n = 10) or treated (n = 10) paracoccidioidomycosis. The ratios of CD4+ CD25+ cells to PBMCs or CD4+ CD25− cells were 1:2, 1:4, and 1:10. (D) Proliferative response of unstimulated or cAg (10 μg/ml)- or ConA (2.5 μg/ml)-stimulated PBMCs from control individuals (n = 10) and patients with active (n = 10) or treated (n = 10) paracoccidioidomycosis before the removal of CD4+ CD25+ T cells. The results were analyzed as described in Materials and Methods. ***, P ≤ 0.0001; **, P ≤ 0.001; *, P ≤ 0.05 (in relation to the ConA-stimulated cells, determined by a paired Student's t test); #, P ≤ 0.05 in relation to cells from AD patients submitted to the same conditions (A and B). #, P ≤ 0.0001 in relation to unstimulated cells; *, P ≤ 0.05 in relation to cAg-stimulated cells (paired t test); &, P ≤ 0.05 in relation to cells from AD patients submitted to the same conditions (C). *, P ≤ 0.05 in relation to unstimulated cells from the same group; #, P ≤ 0.05 in relation to cells from AD patients submitted to the same stimulus (D).
Next, we examined the effect of autologous CD4+ CD25+ T cells in cocultures stimulated with P. brasiliensis cellular antigen (cAg). As expected, only cells from patients (AD and TD groups) were able to respond to the antigenic stimulus (Fig. 2C). In addition, the ability of CD4+ CD25+ T cells to inhibit the proliferative response to P. brasiliensis antigen was number dependent (most notably in the AD group). As can be observed in Fig. 2A to C, the suppressive effect of CD4+ CD25+ T cells from patients with active disease was greater than that induced by CD4+ CD25+ T cells from controls and patients who had received treatment.
Interestingly, purified CD4+ CD25− T cells from patients with active disease showed a response to ConA or cAg stimuli similar to that observed for patients who had received treatment (Fig. 2B and C). Before purification, T cells from patients with active disease exhibited a diminished response to both unspecific and specific stimuli compared with T cells from patients who had been treated (Fig. 2D).
Evaluation of cell-cell contact requirement for the suppressive activity of CD4+ CD25+ T cells.
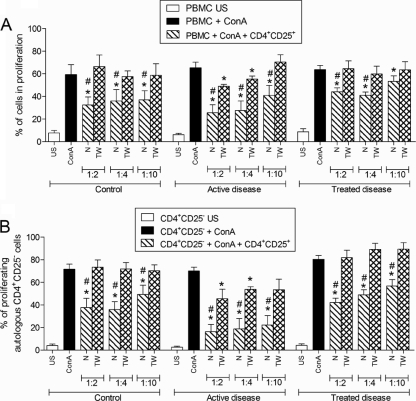
To investigate the requirement for cell-cell contact for CD4+ CD25+ T cells to exert suppressive activity, the experiments described above were repeated by using a Transwell system (0.4-mm membrane). As can be observed in Fig. 3A, when the CD4+ CD25+ T cells from healthy individuals or patients who had received treatment were separated from PBMCs, the suppressive effect on proliferation was eliminated. However, the separation of CD4+ CD25+ T cells from patients with active disease only partially reversed the suppressive effect of Treg cells on allogeneic PBMCs.
FIG. 3.
(A) Proliferative response of unstimulated (US) or ConA (5 μg/ml)-stimulated allogeneic PBMCs cocultured with different numbers of CD4+ CD25− T cells or CD4+ CD25+ T cells from healthy individuals (control group) (n = 10) and patients with active (n = 10) or treated (n = 10) paracoccidioidomycosis under normal conditions (N) or in the Transwell system (TW). (B) Proliferative response of unstimulated or ConA-stimulated autologous CD4+ CD25− T cells cocultured with different numbers of CD4+ CD25+ T cells from healthy individuals (control group) (n = 10) and patients with active (n = 10) or treated (n = 10) paracoccidioidomycosis under normal conditions or in the Transwell system. Ratios of CD4+ CD25+ cells to PBMCs or CD4+ CD25− cells were 1:2; 1:4, and 1:10. The data were analyzed as described in Materials and Methods. *, P ≤ 0.05 in relation to ConA-stimulated cells; #, P ≤ 0.05 in relation to the Transwell culture (paired t test).
The results for the suppressive activity of CD4+ CD25+ T cells on the proliferation of autologous CD4+ CD25− T cells stimulated with ConA were similar to those obtained with PBMCs. In the cocultures using the Transwell system, the inhibition of proliferation in the cells from healthy individuals and patients who had been treated was completely reversed, whereas in cells from the patients with active disease, it was only partially reversed (Fig. 3B).
These data indicate that although the CD4+ CD25+ Treg cells from all the groups were able to suppress the proliferation of allogeneic PBMCs, the underlying regulatory mechanisms in the cells from each group were at least partially different.
Role of IL-10 and TGF-β in the suppressive activity of CD4+ CD25+ T cells from patients with paracoccidioidomycosis.
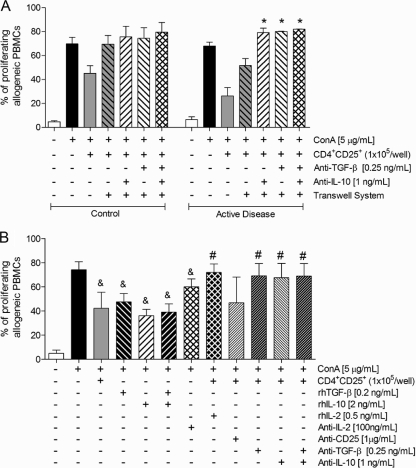
As demonstrated above, the suppressive activity of CD4+ CD25+ Treg cells from patients with active disease is only partially dependent on cell-cell contact. To investigate whether this function was also mediated by soluble factors such as the suppressor cytokines IL-10 and TGF-β, cocultures were set up in Transwell systems, as described above, in the presence of neutralizing antibodies. As shown in Fig. 4A, the coculture of allogeneic PBMCs with CD4+ CD25+ T cells from patients with active disease (ratio of 1:2) inhibited cell proliferation by approximately 50%, and the separation of CD4+ CD25+ T cells from PBMCs using the Transwell system partially reversed this suppressive effect. In contrast, the addition of anti-IL-10 and anti-TGF-β antibodies (alone or in combination) was able to abolish the suppressive effect of Treg cells (Fig. 4A). The addition of antibodies in cocultures of PBMCs and CD4+ CD25+ T cells from healthy individuals did not enhance the effect of the physical separation of cells by the Transwell system.
FIG. 4.
(A) Proliferative responses of unstimulated allogeneic PBMCs and allogeneic PBMCs stimulated with ConA and cocultured with CD4+ CD25+ T cells from healthy individuals (control group) (n = 10) or patients with active disease (n = 10) under normal conditions or in the Transwell system in the presence of neutralizing antibodies (anti-TGF-β and anti-IL-10, either alone or combined). (B) Proliferative responses of unstimulated allogeneic PBMCs and allogeneic PBMCs stimulated with ConA and cocultured with CD4+ CD25+ T cells from patients with active disease (n = 10) under normal conditions in the presence of recombinant cytokines (IL-10, TGF-β, and IL-2) or neutralizing antibodies (anti-IL-10, anti-TGF-β, anti-IL-2, or anti-CD25). The conditions used in the coculture are shown. The populations were analyzed as described in Materials and Methods. *, P ≤ 0.05 for PBMCs cocultured with CD4+ CD25+ T cells in a Transwell system; &, P ≤ 0.05 for cells stimulated with ConA; #, P ≤ 0.05 for PBMCs cocultured in the presence of CD4+ CD25+ T cells (paired t test).
To further confirm the participation of suppressive cytokines in the inhibitory effect of CD4+ CD25+ T cells from patients with active disease, we next examined the effect of the addition of IL-10 and TGF-β (alone or combined). As can be observed in Fig. 4B, in cultures of PBMCs stimulated with ConA without CD4+ CD25+ T cells, the addition of cytokines (alone or combined) resulted in an inhibitory effect similar to that observed for cocultures with CD4+ CD25+ T cells. Furthermore, the addition of neutralizing antibodies against these two cytokines (alone or combined) was able to completely eliminate the suppressive effect exerted by CD4+ CD25+ T cells.
Another mechanism used by Treg cells is the consumption of growth factors, notably IL-2, a cytokine essential to the survival and proliferation of conventional CD4+ T cells. In our system, the addition of recombinant IL-2 in cocultures of PBMCs and CD4+ CD25+ T cells resulted in an increased proliferation rate. However, the effect of the treatment of CD4+ CD25+ T cells with anti-CD25 (IL-2 receptor-blocking antibody) was not conclusive, as patients' responses varied greatly (Fig. 4B). Nevertheless, the results indicate that the consumption of IL-2 may be an additional mechanism used by CD4+ CD25+ T cells to exert their suppressive effect on allogeneic PBMCs.
Analysis of IL-10 and TGF-β mRNA expression by PBMCs from patients with active disease, patients who had received treatment, and healthy individuals.
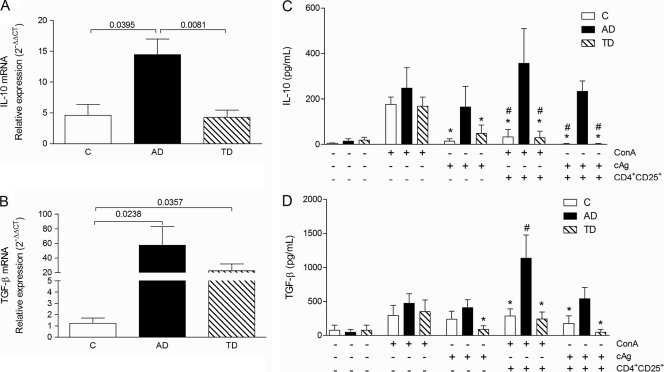
As demonstrated above, the suppressive effect of Treg cells from patients with active disease may be at least partially a result of the production of suppressor cytokines. We therefore decided to determine the relative levels of mRNA expression for IL-10 and TGF-β in PBMCs from patients and controls. Figure 5A shows that IL-10 mRNA expression is approximately three times higher in patients with active disease than in healthy individuals or patients who had received treatment, whereas the level of expression of TGF-β mRNA was higher in both groups of patients than in healthy individuals, with the level of expression in the AD group being the highest (Fig. 5B).
FIG. 5.
Expression of mRNA for IL-10 (A) and TGF-β (B) in ex vivo PBMCs from healthy individuals (C group) (n = 10) and patients presenting with active paracoccidioidomycosis (AD group) (n = 10) or treated paracoccidioidomycosis (TD group) (n = 10). The values were normalized by using ubiquitin mRNA expression, and the results are expressed as relative expression (2−ΔΔCT), as described in Materials and Methods. (C and D) Production of suppressive cytokines IL-10 (C) and TGF-β (D) in coculture supernatants stimulated with ConA or P. brasiliensis antigen (cAg) in the presence or absence of Treg cells from controls (C group) (n = 10), patients with active paracoccidioidomycosis (AD group) (n = 10), or patients with treated paracoccidioidomycosis (TD group) (n = 10). P values are shown above the brackets (ANOVA test with the Bonferroni posttest) (A and B). *, P ≤ 0.05 in relation to AD group under the same conditions; #, P ≤ 0.05 in relation to the same conditions in the absence of CD4+ CD25+ cells (C and D).
Production of IL-10 and TGF-β in supernatants of cocultures.
To confirm the production of suppressive cytokines in cocultures of CD4+ CD25− T cells and CD4+ CD25+ Treg cells, we used ELISA to analyze the supernatants. As described above, CD4+ CD25− T cells were stimulated with ConA or the cellular antigen from P. brasiliensis (cAg) for 48 h in the presence or absence of autologous CD4+ CD25+ Treg cells from patients with active disease (AD group), patients who had been treated (TD group), or healthy individuals. As shown in Fig. 5C, when stimulated with ConA, the cells from the three groups produced large quantities of IL-10 in similar amounts. However, only cells from PCM patients (with active disease or who had been treated) produced this cytokine when stimulated with the specific antigen. While the presence of CD4+ CD25+ Treg cells inhibited the production of IL-10 by cells from the control patients or patients who had been treated, it led to a slight increase in the level of production of this cytokine by cells from patients with active disease (Fig. 5C).
We observed that the presence of CD4+ CD25+ T cells did not have any effect on cocultures of cells from controls or patients who had been treated but that there was an increase in the level of production of TGF-β in cocultures of cells from the AD group (Fig. 5D).
DISCUSSION
A shared characteristic of patients with both the adult and juvenile forms of paracoccidioidomycosis is the suppression of the cellular immune response, most notably in those with the more-severe forms of the disease (5-7, 26, 27, 31, 32). However, the mechanisms involved in this immunosuppression are still not completely understood, although some studies pointed to an imbalance in the cytokine production profile (5-7, 18, 22-24, 31).
After the “rediscovery” and characterization of a population of CD4+ T cells with regulatory activity (34-36), numerous studies have shown the participation of these cells in the regulation of the immune response in several infectious diseases (3, 4, 12, 13, 16, 29, 33), including human paracoccidioidomycosis (13).
We have shown here that patients with active paracoccidioidomycosis have a higher percentage of CD4+ CD25+ Foxp3+ T cells than healthy controls. We also observed that after effective antifungal treatment, the number of Treg cells in the circulation decreases concomitantly with patient recovery.
In a recent study, Cavassani et al. (13) failed to detect any difference between the percentages of circulating regulatory cells in paracoccidioidomycosis patients and healthy controls. These discrepant results may be attributable to the patients in the two studies being at different stages in their treatment, as we observed that the number of Treg cells decreases as the disease improves. However, corroborating our data, Cavassani et al. also found higher levels of expression of regulatory T-cell markers such as CTLA-4 and GITR in cells from patients than in those from controls (13). In our study, in addition to the elevated numbers of circulating regulatory cells, patients with active disease exhibited higher levels of expression of regulatory cell markers such as CTLA-4 (CD152), surface-bound TGF-β (LAP-1), GITR, CD95L, and TLR-2.
CTLA-4 (CD152) plays an important role in the immunosuppression observed for paracoccidioidomycosis, as patients' PBMCs stimulated in vitro in the presence of neutralizing antibodies against this molecule recover their ability to proliferate and produce IFN-γ (11). Our results showed that there were increased levels of expression of CTLA-4 in CD4+ CD25+ T cells from patients with active disease and that they decreased after treatment. The interaction of CTLA-4 with its ligands (CD80 and CD86) expressed by dendritic cells (DCs) induces the production of IDO (indoleamine-2,3-dioxygenase), a potent inhibitory molecule that induces the production of proapoptotic mediators, which in turn suppress effector T cells (14).
Patients with the active form of PCM also exhibit a higher frequency of circulating CD95L+ cells than controls and treated patients. CD95L and its ligand CD95 play a major role in the induction of apoptosis (17, 42). It has been demonstrated that the CD95-CD95L interaction in human paracoccidioidomycosis induces the apoptosis of activated lymphocytes and that blocking this interaction in vitro results in a reduction in the level of apoptosis (11).
In addition to elevated expression levels of CTLA-4 and CD95L, cells from the AD group also had elevated expression levels of GITR, which is expressed constitutively by regulatory T cells. The interaction of GITR with its ligand (GITRL), expressed in antigen-presenting cells (APCs), induces the proliferation of lymphocytes after T-cell receptor (TCR) engagement (38). Thus, increased GITR expression levels in CD4+ CD25+ Treg cells in patients with active disease may contribute to the expansion of this population of cells in the circulation. In experimental Candida albicans infection, the knockout of the GITR gene increases the survival of infected mice as a result of the increase in the level of production of Th1 cytokines (1). GITR has other functions besides its role in the expansion and maintenance of the regulatory T-cell population. In a recently published work, it was demonstrated that the interaction between the GITR expressed in regulatory T cells and the GITRL expressed in B lymphocytes induces the production of immunoglobulins, particularly IgG4 (37). Interestingly, paracoccidioidomycosis patients with severe forms of the disease typically produce elevated quantities of IgG4, which decrease with effective treatment (24).
Recently, Sutmuller et al. (39) showed that Toll-like receptor 2 (TLR2) plays a major role in the expansion and control of Treg cell functions. TLR2 knockout mice are resistant to infection with Candida albicans, a fact that has been associated with the reduction in levels of Treg cells and the diminished production of IL-10 (29). In an experimental model of paracoccidioidomycosis, Loures et al. (20, 21) demonstrated that TLR2 knockout mice present an uncontrolled inflammatory response due to the diminished expansion of Treg cells, associated with an increased Th17 immune response (22); on the other hand, TLR4 knockout mice were characterized by a diminished inflammatory response paralleled by the expansion of Treg cells (21). In the present study we found that CD4+ CD25+ T cells from patients with active disease had higher levels of expression of TLR2 than those from patients who had received treatment but that the level of expression in the control group was similar to that in the AD group. Hence, the elevated level of expression of this receptor in patients with active disease may be related to the increased number of CD4+ CD25+ T cells observed.
Although we did not observe differences in the expressions of CD103 and CCR5 between the groups, we found that these molecules are expressed predominantly in CD4+ CD25+ T cells. Mice deficient in CCR5 exhibit increased resistance to infection by Leishmania major, characterized by a low parasite burden and an increased number of IFN-γ-producing cells, which in turn is associated with a reduced number of regulatory T cells (43). In a similar study it was demonstrated that CCR5-deficient mice infected with P. brasiliensis have a reduced fungal burden and smaller number of CD4+ CD25+ T cells in their lesions (25).
A comparison of the functional activities of CD4+ CD25+ Treg cells from patients with active disease, patients who had received treatment, and healthy individuals revealed that these cells are able to reduce the proliferative response of allogeneic PBMCs or autologous CD4+ CD25− T cells but that the level of this activity was higher in the former group. This result agrees with the findings reported previously by Cavassani et al. (13). Our results also indicated that after effective treatment, the number of CD4+ CD25+ T cells as well as their suppressive activity return to levels similar to those observed for healthy individuals. Interestingly, in the absence of CD4+ CD25+ T cells, the proliferative responses of CD4+ CD25− T cells to the mitogen ConA and specific antigen cAg were similar for patients with active disease and patients who had received treatment, which was not the case before the cells had been separated.
The use of a Transwell system allowed us to confirm that the suppressive activity of Treg cells from patients with active disease and, notably, healthy individuals as well as patients who had received treatment is dependent on cell-cell contact. The results also showed that there are certain differences in the mechanisms used by cells from each of the groups analyzed here.
Cell-cell contact-mediated suppression is exerted by different mechanisms, which include CD95-CD95L-induced apoptosis, competition for engagement with costimulatory molecules mediated by CTLA-4, and suppressor signals induced by the ligation of the surface-bound TGF-β with its receptor in effector cells (40, 41). The suppression exerted by TGF-β is believed to be an important mechanism used by regulatory T cells (2, 28). A recent study showed that CTLA-4 signaling of CD25+ cells enhances the suppressive signal by increasing the availability of TGF-β in the contact region between the cells (30). As can be seen here, our results revealed an increase in the level of expression of membrane-bound TGF-β in regulatory T cells from patients with active paracoccidioidomycosis.
In experiments conducted to analyze the role of suppressor cytokines (IL-10 and TGF-β), we first showed that the supplementation of media with recombinant IL-10 alone or combined with recombinant TGF-β has effects on PBMC proliferation similar to those observed for cultures in which CD4+ CD25+ T cells were present. The neutralization of TGF-β and IL-10 in cocultures of PBMCs or CD4+ CD25− T cells with CD4+ CD25+ T cells from patients with active disease completely abrogated the suppressive activity of the latter. It is interesting that, unlike supplementation with recombinant TGF-β, which had only a partial effect on cell proliferation, the addition of anti-TGF-β neutralizing antibody completely eliminated the suppressive effect of Treg cells. This result may be attributable to the neutralization of both soluble and membrane-associated TGF-β.
Our results showed that PBMCs from patients with active disease express greater amounts of IL-10 and TGF-β mRNA than those from healthy individuals. Although the role of the production of suppressor cytokines (IL-10 and TGF-β) by regulatory T cells remains unclear (40) and our results do not allow us to establish the cellular source of these cytokines, it can reasonably be supposed that the presence of regulatory T cells in cocultures increases the level of production of these proteins, at least in the AD group. We found that CD4+ CD25+ cells suppress the production of IL-10 by cells from the TD or C group, while, on the contrary, they induced its production by cells from the AD group. These results may indicate that in the C and TD groups, the major source for this cytokine could be other than Treg cells, probably Th2 cells, which are suppressed by the addition of CD4+ CD25+ cells. Differently, in AD patients, the source of IL-10 (and TGF-β) is probably CD4+ CD25+ cells, explaining the elevation of these cytokine levels in the presence of Treg cells.
Taken together, our results suggest that the activation of regulatory T cells (CD4+ CD25+) contributes to immune suppression during paracoccidioidomycosis infection and that the mechanism by which these cells exert their suppressive effect is mediated, at least partially, by cell-cell contact and the production of IL-10 and TGF-β.
Acknowledgments
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grants 06/59624-0 and 07/50183-3 and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
We have no financial conflicts of interest.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 19 July 2010.
REFERENCES
- 1.Agostini, M., E. Cenci, E. Pericolini, G. Nocentini, G. Bistoni, A. Vecchiarelli, and C. Riccardi. 2005. The glucocorticoid-induced tumor necrosis factor receptor-related gene modulates the response to Candida albicans infection. Infect. Immun. 73:7502-7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, J., D. Q. Tran, M. Pesu, T. S. Davidson, H. Ramsey, J. J. O'Shea, and E. M. Shevach. 2008. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J. Exp. Med. 205:1975-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baecher-Allan, C., and D. A. Hafler. 2004. Suppressor T cells in human diseases. J. Exp. Med. 200:273-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkaid, Y., and B. T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353-360. [DOI] [PubMed] [Google Scholar]
- 5.Benard, G., M. A. Hong, G. M. Del Negro, L. Batista, M. A. Shikanai-Yasuda, and A. J. Duarte. 1996. Antigen-specific immunosuppression in paracoccidioidomycosis. Am. J. Trop. Med. Hyg. 54:7-12. [DOI] [PubMed] [Google Scholar]
- 6.Benard, G., M. J. Mendes-Giannini, M. Juvenale, E. T. Miranda, and A. J. Duarte. 1997. Immunosuppression in paracoccidioidomycosis: T cell hyporesponsiveness to two Paracoccidioides brasiliensis glycoproteins that elicit strong humoral immune response. J. Infect. Dis. 175:1263-1267. [DOI] [PubMed] [Google Scholar]
- 7.Benard, G., C. C. Romano, C. R. Cacere, M. Juvenale, M. J. Mendes-Giannini, and A. J. Duarte. 2001. Imbalance of IL-2, IFN-gamma and IL-10 secretion in the immunosuppression associated with human paracoccidioidomycosis. Cytokine 13:248-252. [DOI] [PubMed] [Google Scholar]
- 8.Brummer, E., E. Castaneda, and A. Restrepo. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 6:89-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calich, V. L., T. A. da Costa, M. Felonato, C. Arruda, S. Bernardino, F. V. Loures, L. R. Ribeiro, R. de Cassia Valente-Ferreira, and A. Pina. 2008. Innate immunity to Paracoccidioides brasiliensis infection. Mycopathologia 165:223-236. [DOI] [PubMed] [Google Scholar]
- 10.Calich, V. L., C. A. Vaz, and E. Burger. 1998. Immunity to Paracoccidioides brasiliensis infection. Res. Immunol. 149:407-417, 499-500. [DOI] [PubMed] [Google Scholar]
- 11.Campanelli, A. P., G. A. Martins, J. T. Souto, M. S. Pereira, M. C. Livonesi, R. Martinez, and J. S. Silva. 2003. Fas-Fas ligand (CD95-CD95L) and cytotoxic T lymphocyte antigen-4 engagement mediate T cell unresponsiveness in patients with paracoccidioidomycosis. J. Infect. Dis. 187:1496-1505. [DOI] [PubMed] [Google Scholar]
- 12.Campanelli, A. P., A. M. Roselino, K. A. Cavassani, M. S. Pereira, R. A. Mortara, C. I. Brodskyn, H. S. Goncalves, Y. Belkaid, M. Barral-Netto, A. Barral, and J. S. Silva. 2006. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J. Infect. Dis. 193:1313-1322. [DOI] [PubMed] [Google Scholar]
- 13.Cavassani, K. A., A. P. Campanelli, A. P. Moreira, J. O. Vancim, L. H. Vitali, R. C. Mamede, R. Martinez, and J. S. Silva. 2006. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J. Immunol. 177:5811-5818. [DOI] [PubMed] [Google Scholar]
- 14.Fallarino, F., U. Grohmann, K. W. Hwang, C. Orabona, C. Vacca, R. Bianchi, M. L. Belladonna, M. C. Fioretti, M. L. Alegre, and P. Puccetti. 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4:1206-1212. [DOI] [PubMed] [Google Scholar]
- 15.Franco, M., M. R. Montenegro, R. P. Mendes, S. A. Marques, N. L. Dillon, and N. G. Mota. 1987. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev. Soc. Bras. Med. Trop. 20:129-132. [DOI] [PubMed] [Google Scholar]
- 16.Guyot-Revol, V., J. A. Innes, S. Hackforth, T. Hinks, and A. Lalvani. 2006. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 173:803-810. [DOI] [PubMed] [Google Scholar]
- 17.Ju, S. T., K. Matsui, and M. Ozdemirli. 1999. Molecular and cellular mechanisms regulating T and B cell apoptosis through Fas/FasL interaction. Int. Rev. Immunol. 18:485-513. [DOI] [PubMed] [Google Scholar]
- 18.Kashino, S. S., R. A. Fazioli, C. Cafalli-Favati, L. H. Meloni-Bruneri, C. A. Vaz, E. Burger, L. M. Singer, and V. L. Calich. 2000. Resistance to Paracoccidioides brasiliensis infection is linked to a preferential Th1 immune response, whereas susceptibility is associated with absence of IFN-gamma production. J. Interferon Cytokine Res. 20:89-97. [DOI] [PubMed] [Google Scholar]
- 19.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 20.Loures, F. V., A. Pina, M. Felonato, E. F. Araujo, K. R. Leite, and V. L. Calich. Toll-like receptor 4 signaling leads to severe fungal infection associated with enhanced proinflammatory immunity and impaired expansion of regulatory T cells. Infect. Immun. 78:1078-1088. [DOI] [PMC free article] [PubMed]
- 21.Loures, F. V., A. Pina, M. Felonato, and V. L. Calich. 2009. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J. Immunol. 183:1279-1290. [DOI] [PubMed] [Google Scholar]
- 22.Mamoni, R. L., and M. H. Blotta. 2006. Flow-cytometric analysis of cytokine production in human paracoccidioidomycosis. Cytokine 35:207-216. [DOI] [PubMed] [Google Scholar]
- 23.Mamoni, R. L., and M. H. Blotta. 2005. Kinetics of cytokines and chemokines gene expression distinguishes Paracoccidioides brasiliensis infection from disease. Cytokine 32:20-29. [DOI] [PubMed] [Google Scholar]
- 24.Mamoni, R. L., S. A. Nouer, S. J. Oliveira, C. C. Musatti, C. L. Rossi, Z. P. Camargo, and M. H. Blotta. 2002. Enhanced production of specific IgG4, IgE, IgA and TGF-beta in sera from patients with the juvenile form of paracoccidioidomycosis. Med. Mycol. 40:153-159. [DOI] [PubMed] [Google Scholar]
- 25.Moreira, A. P., K. A. Cavassani, F. S. M. Tristao, A. P. Campanelli, R. Martinez, M. A. Rossi, and J. S. Silva. 2008. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J. Immunol. 180:3049-3056. [DOI] [PubMed] [Google Scholar]
- 26.Mota, N. G., M. T. Rezkallah-Iwasso, M. T. Peracoli, R. C. Audi, R. P. Mendes, J. Marcondes, S. A. Marques, N. L. Dillon, and M. F. Franco. 1985. Correlation between cell-mediated immunity and clinical forms of paracoccidioidomycosis. Trans. R. Soc. Trop. Med. Hyg. 79:765-772. [DOI] [PubMed] [Google Scholar]
- 27.Musatti, C. C., M. T. Rezkallah, E. Mendes, and N. F. Mendes. 1976. In vivo and in vitro evaluation of cell-mediated immunity in patients with paracoccidiodomycosis. Cell. Immunol. 24:365-378. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 194:629-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Netea, M. G., R. Sutmuller, C. Hermann, C. A. Van der Graaf, J. W. Van der Meer, J. H. van Krieken, T. Hartung, G. Adema, and B. J. Kullberg. 2004. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172:3712-3718. [DOI] [PubMed] [Google Scholar]
- 30.Oida, T., L. Xu, H. L. Weiner, A. Kitani, and W. Strober. 2006. TGF-beta-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J. Immunol. 177:2331-2339. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira, S. J., R. L. Mamoni, C. C. Musatti, P. M. Papaiordanou, and M. H. Blotta. 2002. Cytokines and lymphocyte proliferation in juvenile and adult forms of paracoccidioidomycosis: comparison with infected and non-infected controls. Microbes Infect. 4:139-144. [DOI] [PubMed] [Google Scholar]
- 32.Restrepo, A., M. Restrepo, F. de Restrepo, L. H. Aristizabal, L. H. Moncada, and H. Velez. 1978. Immune responses in paracoccidioidomycosis. A controlled study of 16 patients before and after treatment. Sabouraudia 16:151-163. [PubMed] [Google Scholar]
- 33.Roberts, T., N. Beyers, A. Aguirre, and G. Walzl. 2007. Immunosuppression during active tuberculosis is characterized by decreased interferon-gamma production and CD25 expression with elevated forkhead box P3, transforming growth factor-beta, and interleukin-4 mRNA levels. J. Infect. Dis. 195:870-878. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi, S., T. Takahashi, and Y. Nishizuka. 1982. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J. Exp. Med. 156:1577-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaguchi, S., T. Takahashi, and Y. Nishizuka. 1982. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J. Exp. Med. 156:1565-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi, S., K. Wing, and M. Miyara. 2007. Regulatory T cells—a brief history and perspective. Eur. J. Immunol. 37(Suppl. 1):S116-S123. [DOI] [PubMed] [Google Scholar]
- 37.Satoguina, J. S., T. Adjobimey, K. Arndts, J. Hoch, J. Oldenburg, L. E. Layland, and A. Hoerauf. 2008. Tr1 and naturally occurring regulatory T cells induce IgG4 in B cells through GITR/GITR-L interaction, IL-10 and TGF-beta. Eur. J. Immunol. 38:3101-3113. [DOI] [PubMed] [Google Scholar]
- 38.Shevach, E. M., and G. L. Stephens. 2006. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat. Rev. Immunol. 6:613-618. [DOI] [PubMed] [Google Scholar]
- 39.Sutmuller, R. P., M. H. den Brok, M. Kramer, E. J. Bennink, L. W. Toonen, B. J. Kullberg, L. A. Joosten, S. Akira, M. G. Netea, and G. J. Adema. 2006. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 116:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vignali, D. A., L. W. Collison, and C. J. Workman. 2008. How regulatory T cells work. Nat. Rev. Immunol. 8:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Boehmer, H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6:338-344. [DOI] [PubMed] [Google Scholar]
- 42.Xu, G., and Y. Shi. 2007. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res. 17:759-771. [DOI] [PubMed] [Google Scholar]
- 43.Yurchenko, E., M. Tritt, V. Hay, E. M. Shevach, Y. Belkaid, and C. A. Piccirillo. 2006. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J. Exp. Med. 203:2451-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziegler, S. F. 2006. FOXP3: of mice and men. Annu. Rev. Immunol. 24:209-226. [DOI] [PubMed] [Google Scholar]