Abstract
Yersinia pestis is the causative agent of plague, a rapidly fatal infectious disease that has not been eradicated worldwide. The capsular Caf1 protein of Y. pestis is a protective antigen under development as a recombinant vaccine. However, little is known about the specificity of human T-cell responses for Caf1. We characterized CD4 T-cell epitopes of Caf1 in “humanized” HLA-DR1 transgenic mice lacking endogenous major histocompatibility complex class II molecules. Mice were immunized with Caf1 or each of a complete set of overlapping synthetic peptides, and CD4 T-cell immunity was measured with respect to proliferative and gamma interferon T-cell responses and recognition by a panel of T-cell hybridomas, as well as direct determination of binding affinities of Caf1 peptides to purified HLA-DR molecules. Although a number of DR1-restricted epitopes were identified following Caf1 immunization, the response was biased toward a single immunodominant epitope near the C terminus of Caf1. In addition, potential promiscuous epitopes, including the immunodominant epitope, were identified by their ability to bind multiple common HLA alleles, with implications for the generation of multivalent vaccines against plague for use in humans.
Plague was recently classified as a reemerging disease by the World Health Organization (4), and Yersinia pestis, the causative agent, is regarded as a candidate biological weapon (20, 34, 39). The bubonic form of plague is transmitted by fleas from rodents to humans but the highly infectious pneumonic form passes directly from human to human by aerosols against which vaccination is thought to be less effective (37). Animal models have been especially important in the study of the pathogenesis of plague as a result of the scarcity of patients with Y. pestis infection or the opportunity to evaluate the protective efficacy of vaccines in humans.
The 100-kb plasmid pFra unique to Y. pestis bears the Caf operon encoding the capsular F1 (Caf1) protein and a number of other proteins required for export and polymerization of the protein capsule on the bacterial cell surface, as well as Caf1R which regulates the temperature-dependent expression of Caf1 upon infection of mammals (51). The capsule was shown to confer resistance to phagocytosis by macrophages in vitro (12), but it has now been clearly demonstrated that the lack of Caf1 has minimal impact on virulence in vivo (6, 11, 45). Instead, virulence is primarily associated with the Lcr plasmid-borne temperature-regulated type III secretion system that encodes a series of Yersinia outer proteins or Yops (5, 28, 37). The Lcr-encoded V protein facilitates the injection of Yops into the cytosol of phagocytes, leading to the inhibition of phagocytosis and inflammatory cytokine release, as well as directly suppressing neutrophil chemotaxis (44). The enteropathogenic yersiniae also directly suppress T-cell responses (18). Dendritic cells (DC) are early targets of Yops (7), although it has been shown that Y. pestis disrupts antigen presentation by DC considerably less than has been demonstrated for the enteropathogenic yersiniae (40) and so would be expected to have less impact on the induction of acquired immunity. However, plague is rapidly fatal in the naive host as a result of the virulence factors suppressing innate immunity prior to the induction of acquired immunity (26, 27). Consequently, enhancing acquired immunity through prophylactic vaccination is a rational strategy to increase the resistance of exposed individuals.
Resistance against plague correlates with the magnitude of antibody titers specific for Y. pestis antigens in synergy with CD4 and CD8 T-cell-derived gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (13, 37, 46). Thus, T cells operate at two levels, by providing help for opsonic and neutralizing antibody responses and as effectors releasing inflammatory cytokines to recruit and activate phagocytes to kill the pathogen at the site of infection. Yops are candidate protective antigens (21, 25, 43) and have been shown to induce IFN-γ responses (26), although YopE, YopK, and YopN were shown to be immunogenic but not protective in mice (25). The immunogens most studied are Caf1 and V proteins, and immunization with adjuvant containing Caf1 alone (35) or in combination with V antigen (14, 15, 47, 50) protects susceptible strains of mice and nonhuman primates (3), as well as inducing significant immune responses in humans (48, 49). Protection has also been achieved by adoptive transfer of Caf1-loaded DC (9, 24) or by passive immunization with monoclonal IgG antibodies specific for Caf1 (2, 19), both approaches leading to enhanced phagocytosis and killing of Y. pestis.
Although CD4 epitopes of plague antigens have been defined for some inbred mouse strains (23, 31, 33, 36), no characterization has previously been undertaken of binding to and presentation by human HLA class II molecules. In this report we characterized CD4 T-cell epitopes of Caf1 that bind the common human major histocompatibility complex (MHC) class II allele HLA-DR1 in HLA transgenic mice by multiple approaches.
MATERIALS AND METHODS
Antigens.
The Caf1 gene of Y. pestis was cloned, and recombinant Caf1 protein (rCaf1) expressed in E. coli, and purified as previously described (29). Synthetic peptides of 20 amino acids in length and overlapping by 10 amino acids based on the complete Caf1 sequence, including the signal peptide (accession no. Q65AJ6 [P26948]) were synthesized by J. Gray, Institute of Cell and Molecular Biosciences, University of Newcastle, Newcastle, United Kingdom.
Mice.
HLA-DR1 transgenic (DR1-tg) mice carrying full-length genomic constructs for HLA-DRA1*0101 and HLA-DRB1*0101 crossed for more than six generations to C57BL/6 Ab-null mice, thus lacking expression of endogenous mouse MHC class II molecules, were generated by us as described previously (1), and bred in-house. The experiments described were performed under the terms of Animals (Scientific Procedures) Act 1986 and authorized by the Home Secretary, Home Office, United Kingdom.
Lymph node proliferation assay.
Groups of three or four 8- to 12-week-old DR1-tg mice were immunized in one footpad with 25 μg of rCaf1 in Titermax adjuvant (Sigma-Aldrich) or 25 μg of synthetic peptides, and popliteal lymph nodes were removed 7 days later for ex vivo assay.
All cells were grown in culture medium (RPMI 1640 medium containing 3 mM l-glutamine, 50 μM 2-mercaptoethanol, 10% [vol/vol] fetal bovine serum, and 30 μg of gentamicin/ml; Sigma Chemical Co., Poole, Dorset, United Kingdom). Proliferation assays were performed on lymph node cells from groups of DR1-tg mice immunized with rCaf1 or each of the 16 synthetic peptides representing the complete Caf1 sequence. Popliteal lymph node cells were cultured in the presence or absence of a range of doses of rCaf1 or synthetic peptides for 72 h at 3 × 105 per well in 200 μl of culture medium in 96-well round bottom microtiter plates. Cells were labeled with 14.8 kBq of tritiated thymidine (TRA310; specific activity, 74 GBq/mmol; Amersham International, Buckinghamshire, United Kingdom) for the final 6 h of culture before harvesting on glass fiber membranes and quantitation of radioactivity by using a liquid scintillation counter (Microbeta Trilux; Perkin-Elmer, Waltham, MA). The results are shown for individual mice from groups of at least three as the mean counts per minute (cpm) of triplicate wells, and responses were considered positive when antigen stimulation exceeded twice the cpm of cells cultured in the absence of antigen.
T-cell hybridoma assay.
T-cell lines were generated from the lymph node cells of rCaf1-immunized DR1-tg mice by periodic restimulation with antigen and irradiated spleen cells and expansion in recombinant interleukin-2 (rIL-2) as described previously (8). rCaf1-specific T-cell lines were used to generate T-cell hybridomas by polyethylene glycol fusion of T-cell lines with BW5147 (TCRα−β−) cells (a gift from P. Marrack, Denver, CO). T-cell hybridomas were shown to express CD4, CD3ɛ, and TCRαβ by flow cytometry and secreted IL-2 in response to rCaf1- or peptide-pulsed bone marrow macrophages used as antigen-presenting cells.
Bone marrow macrophages were generated as described previously (16), by culture of femoral bone marrow cells for 6 days in bacteriological petri dishes in the culture medium described above supplemented with 5% horse serum, 1 mM sodium pyruvate, 10 mM HEPES buffer (Sigma), and 5% of a culture supernatant from the L929 cell line as a source of macrophage colony-stimulating factor (M-CSF). Cells were harvested with a cell scraper (∼107 per petri dish) and shown to be at least 90% macrophages by flow cytometry based on the expression of F4/80.
Macrophages were plated at 4 × 104/well into flat-bottom 96-well plates and activated by treatment with 1 ng of IFN-γ (R&D Systems, Abingdon, United Kingdom)/ml overnight at 37°C in a humidified CO2 incubator. The next day, macrophages were treated with culture medium alone or a range of doses of rCaf1 or synthetic peptides in triplicate wells for 3 h before adding T-cell hybridoma cells (4 × 104/well). Plates were incubated for a further 24 h and then frozen. The responses of T-cell hybridomas were determined as the amount of IL-2 released in a bioassay measuring the proliferation of IL-2-dependent CTLL-2 cells (3 × 104/well) in the presence of T-cell hybridoma culture supernatants diluted 1:2. The bioassay was incubated for 24 h in triplicate wells of flat-bottom 96-well microtiter plates, the cells were labeled with 3H-thymidine as described above, and the results are plotted as the mean cpm of triplicate wells ± the standard deviation. The experiments were repeated at least twice, and the data for a representative experiment are shown.
IFN-γ ELISPOT assay.
Popliteal lymph nodes were removed 7 days after footpad immunization of DR1-tg mice with 25 μg of Caf1 or peptides. Lymph nodes were teased in single cell suspension, washed, and resuspended in HL-1 serum-free media (BioWhittaker, Lonza, Slough, United Kingdom), supplemented with l-glutamine and penicillin-streptomycin (Gibco). The frequency of cells producing IFN-γ was quantified by enzyme-linked immunospot (ELISPOT) assay (Diaclone; Tepnel Research Products and Services, Besançon, France), carried out according to the manufacturer's protocol. In brief, 96-well hydrophobic polyvinylidene difluoride membrane-bottom plates (MAIP S45; Millipore) were prewet with ethanol before coating them with 10 μg of anti-IFN-γ monoclonal antibody (MAb) overnight at 4°C. The plates were then washed with phosphate-buffered saline (PBS), blocked with 2% skimmed milk for 2 h, and washed, and then antigen and lymph node cells (2 × 105 cells/well) were added to appropriate wells. The plates were incubated for 72 h at 37°C in 5% CO2. The cells were discarded, and the plate was washed three times with PBS-Tween 20 (0.1%), followed by incubation for 90 min at room temperature with biotinylated anti-IFN-γ. After a further wash, streptavidin-alkaline phosphatase conjugate was added, and the plates were incubated at room temperature for 1 h. After a further wash, 100 μl of BCIP (5-bromo-4-chloro-3-indolylphosphate) and nitroblue tetrazolium was added. When spots were visible, the contents of the wells were discarded, and the plates were thoroughly washed with tap water, incubated overnight at 4°C, and air dried. The spots were then counted by using an automated ELISPOT reader (Autoimmun Diagnostika, Strasbourg, France). Mice immunized with rCaf1 were screened in triplicate for responses to all 16 Caf1 peptides individually, and mice immunized with peptides were tested for responses to the immunizing peptide and the two flanking peptides. Concanavalin A (5 μg/ml) was used as a positive control, and cells incubated in culture medium only as negative control for each mouse and results were expressed as spot-forming cells (SFC) per 106 cells. Positive responses were defined as the mean SFC of more than two standard deviations above the mean of the negative control.
HLA-DR peptide-binding assay.
HLA-DR molecules were purified from the Epstein-Barr virus (EBV) B-lymphoblastoid cell lines by affinity chromatography using the monomorphic MAb L243 (38). Binding of peptides to HLA-DR heterodimers was assessed by competitive enzyme-linked immunosorbent assay (ELISA) as previously reported (38) on an automated workstation. Briefly, HLA-DR molecules were diluted in DM buffer (10 mM phosphate, 150 mM NaCl, 1 mM n-dodecyl β-d-maltoside, 10 mM citrate [pH 6.0]) with an appropriate biotinylated peptide and serial dilutions of competitor peptides. Unlabeled forms of the biotinylated peptides were used as reference peptides to assess the validity of each experiment. The sequences and 50% inhibitory concentrations (IC50s) of reference peptides were as follows: HA 306-318 (PKYVKQNTLKLAT) for DRB1*0101 (4 nM), DRB1*0401 (8 nM), and DRB1*1101 (7 nM); YKL (AAYAAAKAAALAA) for DRB1*0701 (3 nM); A3 152-166 (EAEQLRAYLDGTGVE) for DRB1*1501 (48 nM); MT 2-16 (AKTIAYDEEARRGLE) for DRB1*0301 (100 nM); and B1 21-36 (TERVRLVTRHIYNREE) for DRB1*1301 (37 nM). After 24 to 72 h of incubation at 37°C, the samples were neutralized with 50 μl of 450 mM Tris-HCl (pH 7.5), 0.3% bovine serum albumin, and 1 mM DM buffer and applied to 96-well Maxisorp ELISA plates (Nunc, Denmark) previously coated with 10 μg of L243 MAb/ml. Samples were allowed to bind to the antibody-coated plates for 2 h at room temperature. Bound biotinylated peptide was detected by incubating streptavidin-alkaline phosphatase conjugate (GE Healthcare, Saclay, France) and, after washings, by adding 4-methylumbelliferyl phosphate substrate (Sigma, France). The emitted fluorescence was measured at 450 nm upon excitation at 365 nm. The peptide concentration that prevented binding of 50% of the labeled peptide (i.e., the IC50) was determined. The data are expressed as the relative affinity, the ratio of the IC50 of test the peptide to the IC50 of the reference peptide, which is a high binder to the corresponding HLA-DR molecule. Average and standard error values were calculated from three independent experiments.
Regions of the Caf1 sequence that bind HLA-DRB1*0101 were predicted by using the IEDB analysis resource based on a consensus method that averages the ranking of three independent algorithms—Sturniolo, SMM_align, and ARB (average relative binding)—by comparing the score of a peptide against the scores of five million random 15-amino-acid peptides selected from Swiss-Prot database: the smaller the percentile rank, the higher the affinity (41, 42).
RESULTS
T-cell responses of Caf1-immunized DR1-tg mice.
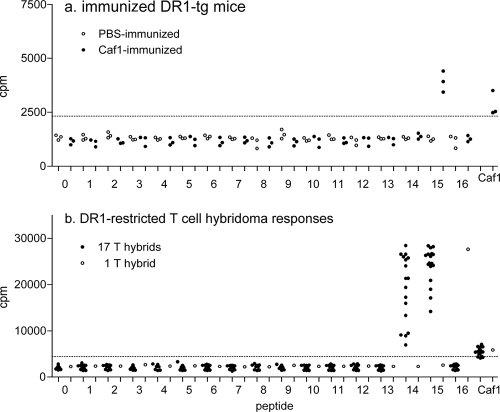
To identify potential immunodominant CD4 T-cell epitopes of Caf1, groups of DR1-tg mice were immunized in the footpad with rCaf1 in adjuvant or with PBS in adjuvant as a control. Proliferation responses of popliteal lymph node cells to Caf1 and each of 16 overlapping peptides representing the complete Caf1 sequence including the signal peptide (Table 1) were assayed in vitro 7 days later (Fig. 1a). A T-cell proliferation response was detected in all rCaf1-immunized mice to rCaf1 and to peptide 15 but not to any of the other Caf1 peptides. Sham (PBS)-immunized mice did not proliferate above background in response to any of the peptides.
TABLE 1.
Caf1 peptides and epitopes
| Caf1 peptide | Sequencea | IEDB predictionb | IEDB epitope IDc |
|
|---|---|---|---|---|
| DR1 | Other | |||
| 1 | MKKISSVIAIALFGTIATAN | 3.4 | 41851 | |
| 2 | ALFGTIATANAADLTASTTA | 8.3 | ||
| 3 | AADLTASTTATATLVEPARI | 21.2 | 66545 (Ad) | |
| 4 | TATLVEPARITLTYKEGAPI | 27.0 | ||
| 5 | TLTYKEGAPITIMDNGNIDT | 28.1 | 65115 | |
| 6 | TIMDNGNIDTELLVGTLTLG | 19.9 | ||
| 7 | ELLVGTLTLGGYKTGTTSTS | 18.2 | 36138 (Ab) | |
| 8 | GYKTGTTSTSVNFTDAAGDP | 36.4 | ||
| 9 | VNFTDAAGDPMYLTFTSQDG | 40.7 | 70067 | 70067 (DQ8) |
| 10 | MYLTFTSQDGNNHQFTTKVI | 41.1 | 43064 (DQ8) | |
| 11 | NNHQFTTKVIGKDSRDFDIS | 44.8 | 45121 | |
| 12 | GKDSRDFDISPKVNGENLVG | 62.8 | ||
| 13 | PKVNGENLVGDDVVLATGSQ | 46.8 | ||
| 14 | DDVVLATGSQDFFVRSIGSK | 46.5 | 7887 | |
| 15 | DFFVRSIGSKGGKLAAGKYT | 10.6 | 8268 | 70822 (Ad) |
| 16 | GGKLAAGKYTDAVTVTVSNQ | 26.8 | 19899 | 162 (Ab) |
| Cd | PKYVKQNTLKLATGM | 11.3 | 48236 | |
The signal sequence is indicated in boldface.
The highest consensus score for any core within the peptide and the highest binders are indicated in boldface.
That is, the identification number in IEDB (http://www.immuneepitope.org/).
Peptide HA 306-320, the reference peptide for HLA-DRB1*0101.
FIG. 1.
Proliferation responses of Caf1-immunized mice. T-cell responses to Caf1 peptides. (a) Groups of DR1-tg mice were immunized with 25 μg of rCaf1 (solid symbols) or the equivalent volume of PBS (open symbols) in adjuvant, and the proliferation responses of draining lymph node cells to Caf1 and 16 overlapping synthetic peptides representing the complete Caf1 sequence were determined. The results for the highest response to a range of peptide doses are shown. Scatter plots show responses of individual mice as mean cpm of triplicate wells for each antigen after tritiated thymidine labeling (see Materials and Methods). (b) T-cell hybridomas were generated from Caf1-immunized DR1-tg mice and assayed for IL-2 production in response to Caf1 and the 16 Caf1 peptides, measured as the proliferation of the IL-2-dependent CTLL-2 cell line, and the responses are shown of individual T-cell hybridomas to Caf1 and all 16 peptides expressed as cpm of triplicate wells of CTLL-2 cells labeled with tritiated thymidine, as described in Materials and Methods. The horizontal dotted lines indicate twice the mean cpm in the absence of antigen, and values greater than this were considered positive responses.
T-cell lines propagated from popliteal lymph node cells from two Caf1-immunized mice were used to generate cloned T-cell hybridomas. A total of 18 T-cell hybridomas responded modestly to Caf1, and 17 of the T-cell hybridomas also responded to both peptides 14 and 15 but none of the other Caf1 peptides (Fig. 1b). The remaining T-cell hybridoma recognized Caf1 and peptide 16 (Fig. 1b).
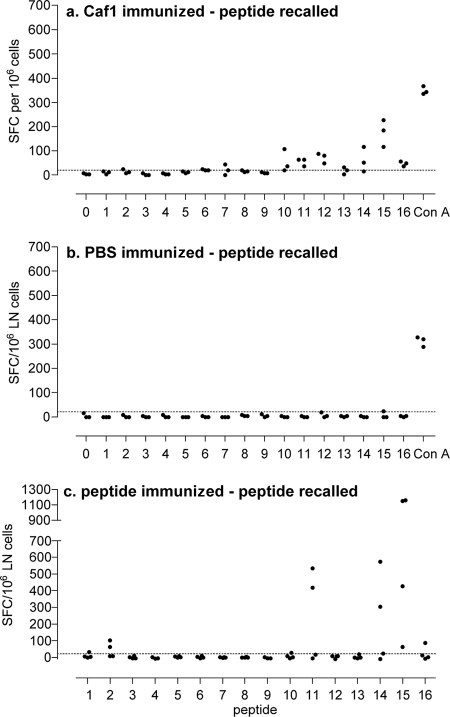
Additional groups of three mice were immunized with Caf1, and the frequency of popliteal lymph node cells responding to in vitro stimulation with Caf1 peptides by the production of IFN-γ was measured by ELISPOT assay. Peptide 7 and peptides 10 to 16 induced IFN-γ-producing cells above background from popliteal lymph nodes of one or more Caf1-immunized mice (Fig. 2a). Responses of the greatest magnitude were seen to peptides 11, 12, 14, and 15 (Fig. 2a). The positive control concanavalin A induced the largest responses in all mice, and none of the peptides were stimulatory for lymph node cells of the sham (PBS)-immunized mice (Fig. 2b).
FIG. 2.
Lymph node IFN-γ responses after Caf1 or peptide immunization. Groups of DR1-tg mice were immunized with 25 μg of rCaf1 (a) or sham-immunized with PBS in adjuvant (b), and lymph node cells were evaluated for IFN-γ responses by ELISPOT assay after stimulation in vitro with each of the synthetic peptides separately or concanavalin A as a positive control as described in Materials and Methods. Alternatively, separate groups of three mice were immunized with 50 μg of each of the synthetic peptides and response recalled to the immunizing peptide (c). The results are expressed as spot-forming cells (SFC) per 106 cells for individual mice of a group, and responses were considered positive if the values were greater than the mean plus 2 standard deviations of cells in the absence of antigen, as shown by the horizontal line.
T-cell responses of peptide-immunized DR1-tg mice.
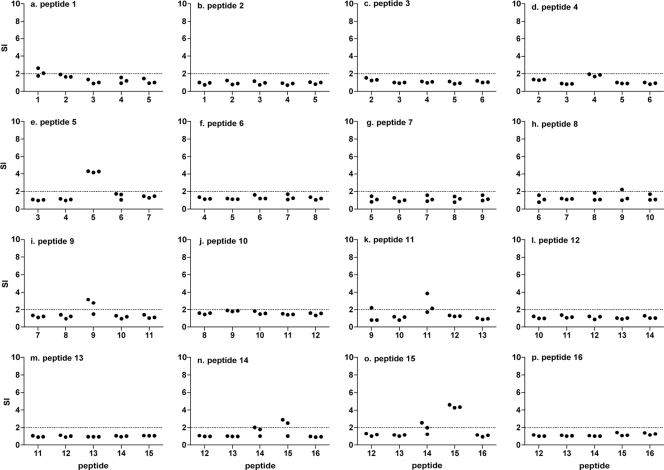
There is increasing evidence for the importance of subdominant and cryptic epitopes during infections since these responses can become more prominent in a disease context (42). For this reason, groups of DR1-tg mice were immunized with individual Caf1 peptides to identify any additional cryptic and subdominant epitopes in peptides that did not recall detectable responses from Caf1-immunized mice (Fig. 3). The responses of peptide-immunized mice to immunizing and flanking peptides can also be used to determine whether epitopes are shared between two adjacent peptides. Lymph node cells were assayed for proliferation responses to the immunizing and flanking peptides.
FIG. 3.
Lymph node proliferation responses after synthetic peptide immunization. Separate groups of DR1-tg mice were immunized with 50 μg of one of the 16 Caf1 peptides in adjuvant. The proliferation responses of draining lymph node cells were measured as in Fig. 1 in response to Caf1 and the immunizing and flanking peptides (a to p). Scatter plots show responses of individual mice as the stimulation index calculated as the mean cpm of triplicate wells in the presence of peptide divided by the mean cpm in the absence of antigen. The horizontal dotted lines indicate twice the mean cpm in the absence of antigen, and values above this were considered positive responses. Proliferation responses of peptide-immunized mice.
All mice immunized with peptide 15 responded to the immunizing peptide, some also responding to peptide 14 (Fig. 3n and o). Mice immunized with peptide 14 also responded to both peptides 14 and 15, suggesting that the immunodominant epitope seen in Caf1-immunized mice is in fact shared between the two peptides 14 and 15. One or more of the mice immunized with peptides 1, 5, 9, and 11 also responded to the immunizing peptides but not either flanking peptide (Fig. 3a, e, i, and k), and the remaining peptides did not induce proliferation responses in peptide-immunized mice (Fig. 3b, c, d, f, g, h, j, l, m, and p).
Separate groups of mice were immunized with individual Caf1 peptides for measurement of IFN-γ-producing cell frequencies by ELISPOT assay. Peptides 2, 11, 14, 15, and 16 induced IFN-γ in one or more mice; responses of the largest magnitude were induced by peptide 15 (Fig. 2c). In each case, recall responses were induced by the immunizing peptide but not flanking peptides either side in each case, except that peptide 14-immunized mice also responded to peptide 15 (data not shown).
Peptide binding to HLA-DR molecules.
All 16 Caf1 peptides were tested for binding to a total of seven common HLA-DR alleles, including DR1 (Table 2). Peptides 1, 2, 5, and 15 bound DR1 with high affinity (relative values of <10) and peptide 16 with intermediate affinity (relative value of <100). The remaining 11 peptides showed a low affinity for DR1. All five DR1-binding peptides also bound between two and four additional DR alleles with high or intermediate affinity in different combinations of DR4, DR7, DR11, and DR15 but not DR3 or DR13 (Table 2). Importantly, the immunodominant peptide identified by the present study in HLA-DR1 transgenic mice was a broad or promiscuous binder and so of likely relevance for potential use for the vaccination of individuals of multiple common HLA haplotypes.
TABLE 2.
Binding of Caf1 peptides to DR molecules
| Peptide | Relative affinitya of: |
||||||
|---|---|---|---|---|---|---|---|
| DR1 | DR3 | DR4 | DR7 | DR11 | DR13 | DR15 | |
| 1 | 1 | >1,000 | 559 | 1 | >1,357 | >2,733 | 0.1 |
| 2 | 12 | 561 | 0.4 | 24 | 8 | >2,733 | 64 |
| 3 | 539 | 158 | 4 | 59 | 700 | >2,733 | >208 |
| 4 | 545 | 17 | 313 | 93 | 2 | 68 | 43 |
| 5 | 10 | >1,000 | 280 | 36 | >1,357 | >2,733 | 80 |
| 6 | 630 | 800 | 612 | 36 | 849 | >2,733 | >208 |
| 7 | 1,319 | 574 | 0.2 | 414 | 19 | 2,000 | 133 |
| 8 | 2,582 | >1,000 | 4 | 239 | >1,357 | >2,733 | >208 |
| 9 | >2,563 | >1,000 | >1,250 | >3,365 | >1,357 | >2,733 | >208 |
| 10 | 504 | >1,000 | 15 | 3 | 949 | >2,733 | 111 |
| 11 | 121 | 7 | 875 | 478 | 30 | 469 | >208 |
| 12 | >2,563 | >1,000 | >1,250 | 1,656 | 4 | 1,286 | 119 |
| 13 | >2,563 | 1 | 2 | 1,600 | >1,357 | >2,733 | >208 |
| 14 | 519 | 4 | 74 | 5 | 35 | >2,733 | 7 |
| 15 | 2 | >238 | 27 | 34 | 34 | >626 | 53 |
| 16 | 79 | >1,000 | 1 | 5 | 980 | >2,733 | 148 |
Values are expressed as the relative affinity, i.e., the ratio of the IC50 of the test peptide to the IC50 of a reference peptide chosen as a high binder for each allele. High- and intermediate-affinity vales were interpreted as follows: <10, high affinity; <100, intermediate affinity (both types of values are indicated in boldface).
Regions of the Caf1 sequence that bind HLA-DRB1*0101 were predicted by using the IEDB analysis resource and expressed as the highest consensus score for any core within each of the 16 Caf1 peptides (Table 1). Peptides 1, 2, and 15 were the highest binders, giving values comparable to those of the reference peptide containing the influenza virus hemagglutinin epitope HA 306-318 (22). The prediction data correlated with the three Caf1 peptides showing the best binding to HLA-DRB1*0101 (Table 2). Peptide 5 was also a high-affinity binder but was not predicted by the methods used here.
DISCUSSION
Relatively little is known about the specificity of human T-cell responses to Y. pestis. This is important in a number of ways: (i) to gain a better understanding of subversion of immunity in the pathogenesis of plague, (ii) to design epitope vaccines, and (iii) from a perspective of fundamental immunology, to contribute to our understanding of the interface between bacterial infection and host T-cell immunity. Because of the logistical problems inherent in identifying Y. pestis immune human subjects, we have here undertaken complete analysis HLA-DR1-restricted CD4 T-cell epitopes of Caf1 of Y. pestis by multiple approaches. We used mice transgenic for HLA DRB1*0101 in the absence of endogenous mouse MHC class II so that all T-cell responses detected were DR1 restricted and epitopes could be identified in the absence of any competing MHC class II products. Vaccination with Caf1 protects mice against plague effectively (35) but induces relatively poor T-cell responses in vivo (31) (Fig. 1). It is therefore of considerable interest to dissect the basis of Caf1 immune recognition in more detail. We show here that the HLA-DR-restricted CD4 T-cell response is dominated by recognition of a single Caf1 epitope and that this response is likely to have broad-ranging applicability to human populations since the epitope binds with varying affinity to all of the HLA-DR alleles tested.
The single immunodominant epitope of Caf1 was identified within peptide 15 (Caf1141-160), overlapping with the sequence in peptide 14 (Caf1131-150) and demonstrated by multiple approaches. Peptide 15 was the only peptide to recall T-cell responses consistently in Caf1-immunized mice measured both as proliferation and as IFN-γ-secreting cell frequency. The presence of an epitope shared by peptides 14 and 15 was confirmed by proliferation responses and IFN-γ-secreting cell frequency in peptide-immunized mice, and both peptides 14 and 15 were also recognized by the majority of T-cell hybridomas generated from Caf1-immunized mice. However, lymph node T cells taken directly ex vivo from Caf1-immunized mice recognized peptide 15 but not 14, possibly showing a dominance of a particular fine specificity for peptide 15 in the in vivo T-cell repertoire. In addition, it was shown that peptide 15 was among the five peptides that bound DR1 with high affinity and was the only peptide from the mature Caf1 protein predicted as an epitope by consensus of the combined algorithms of the IEDB analysis resource.
Peptides 7, 10, 11, 12, 13, and 16 also stimulated IFN-γ-producing cells, although not recalling proliferation responses in Caf1-immunized mice, suggesting additional immunodominant or subdominant epitopes in these peptides. Of these, only peptides 11 and 16 induced IFN-γ-producing cells in peptide-immunized mice. The presence of an epitope on peptide 16 was confirmed by the specificity of one of the T-cell hybridomas generated from Caf1-immunized mice. The epitope on peptide 16 is distinct from that on peptide 14/15 because neither the peptide 16-specific T-cell hybridoma nor peptide 16-immunized mice recognized peptide 15. Peptide 16 also bound DR1 with intermediate affinity and was one of the higher-scoring peptides by epitope prediction. The epitope on peptide 11 was also confirmed in proliferation and IFN-γ assays following peptide immunization, although this peptide bound HLA-DR with low affinity.
All five peptides that bound DR1 elicited a T-cell response in at least one of the functional assays. However, several peptides (peptides 4 and 6 to 14), recognized by T cells in one or more of the assays, did not show detectable binding to HLA-DR1, suggesting that strong binding to a particular MHC class II allele is not a prerequisite for a functional helper T-cell epitope. Additional peptides (peptides 4, 5, 8, and 9) were shown to include epitopes by peptide immunization using both proliferation and IFN-γ assays. The lack of strong responses to these peptides in Caf1-immunized mice identify them as cryptic epitopes (30).
An unexpected outcome of the present study was the identification of one or more DR1-restricted epitopes in the signal sequence of Caf1. All 20 amino acids of peptide 1 and the first 11 amino acids of peptide 2 represent the signal sequence of Caf1 (17). T-cell responses to peptide 1 in Caf1-immunized mice were not detected (Fig. 1a) and would not be expected because the mature rCaf1 protein lacks the signal sequence. However, a marginal response in lymph node proliferation assay was detected in one mouse immunized with peptide 1 (Fig. 3) and in separate experiments cells from one peptide-immunized mouse responded by IFN-γ production (Fig. 2c). Peptide 1 also bound with high affinity to DR1, as well as DR7 and DR15 (Table 2), and scored highest in epitope prediction analysis. Similar results were obtained for peptide 2. Thus, the data suggest the presence of at least one CD4 T-cell epitope within the signal sequence by several approaches. In addition, we have infected DR1-transgenic mice with Y. pestis and measured T-cell responses as secreted IFN-γ upon in vitro recall to the Caf1 peptide set, and some mice responded to peptides 1 and 2 (data not shown), suggesting that after cleavage from Caf1, the signal sequence is available for antigen presentation during infection. It remains to be determined whether the location of CD4 T-cell epitopes within the signal sequence of proteins from bacteria is a general phenomenon.
Although the functional mapping of T-cell epitopes was confined to HLA-DR1, we also examined the binding of all 16 Caf1 peptides to seven different, common HLA-DR alleles. Remarkably 15 of the 16 Caf1 peptides bound with high or intermediate affinity to one or more of the DR alleles tested, including alleles of the same (DR1, DR4, DR7, and DR11) or different HLA-DR supertypes (10). In particular, peptides 2, 14, and 15 demonstrated the greatest promiscuous binding properties, each binding to five of the seven alleles. Of the remaining peptides, three bound to three alleles, five bound to two alleles, and three bound to a single allele, and peptide 9 was the only peptide that bound none of the DR alleles. Epitopes that bind multiple HLA alleles are termed promiscuous or universal (32) and would be particularly valuable for use in epitope-defined vaccines. In addition, we have previously mapped HLA-DQ8-restricted CD4 T-cell epitopes of Caf1, as well as the immunodominant mouse H-2d- and H-2b-restricted epitopes of Caf1, including one within peptide 15 (Table 1) (31).
In conclusion, this is the first report identifying HLA-restricted CD4 T-cell epitopes of a major plague vaccine antigen. In particular, we have shown that the T-cell response after Caf1 vaccination is focused on a single immunodominant epitope with properties of a promiscuous HLA class II binder.
Acknowledgments
This study was conducted with funding from NIH-NIAID contract HHSN266200400084C.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.Altmann, D. M., D. C. Douek, A. J. Frater, C. M. Hetherington, H. Inoko, and J. I. Elliott. 1995. The T-cell response of HLA-DR transgenic mice to human myelin basic protein and other antigens in the presence and absence of human CD4. J. Exp. Med. 181:867-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, G. W., P. L. Worsham, C. R. Bolt, G. P. Andrews, S. L. Welkos, A. M. Friedlander, and J. P. Burans. 1997. Protection of mice from fatal bubonic and pneumonic plague by passive immunization with monoclonal antibodies against the F1 protein of Yersinia pestis. Am. J. Trop. Med. Hyg. 56:471-473. [DOI] [PubMed] [Google Scholar]
- 3.Chichester, J. A., K. Musiychuk, C. E. Farrance, V. Mett, J. Lyons, V. Mett, and V. Yusibov. 2009. A single component two-valent LcrV-F1 vaccine protects non-human primates against pneumonic plague. Vaccine 27:3471-3474. [DOI] [PubMed] [Google Scholar]
- 4.Cornelis, G. R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. U. S. A. 97:8778-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis, G. R. 2002. The Yersinia Ysc-Yop type III weaponry. Nat. Rev. Mol. Cell. Biol. 3:742-752. [DOI] [PubMed] [Google Scholar]
- 6.Davis, K. J., D. L. Fritz, M. L. Pitt, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative yersinia-pestis in African green monkeys (Cercopithecus aethiops). Arch. Pathol. Lab. Med. 120:156-163. [PubMed] [Google Scholar]
- 7.De Leo, F. R., and B. J. Hinnebusch. 2005. A plague upon the phagocytes. Nat. Med. 11:927-928. [DOI] [PubMed] [Google Scholar]
- 8.Delvig, A. A., and J. H. Robinson. 1998. Two T-cell epitopes from the M5 protein of viable Streptococcus pyogenes engage different pathways of bacterial antigen processing in mouse macrophages. J. Immunol. 160:5267-5272. [PubMed] [Google Scholar]
- 9.Do, Y., C. G. Park, Y. S. Kang, S. H. Park, R. M. Lynch, H. Lee, B. S. Powell, and R. M. Steinman. 2008. Broad T-cell immunity to the LcrV virulence protein is induced by targeted delivery to DEC-205/CD205-positive mouse dendritic cells. Eur. J. Immunol. 38:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doytchinova, I. A., and D. R. Flower. 2005. In silico identification of supertypes for class II MHCs. J. Immunol. 174:7085-7095. [DOI] [PubMed] [Google Scholar]
- 11.Drozdov, I. G., et al. 1995. Virulent non-capsulate Yersinia pestis variants constructed by insertion mutagenesis. J. Med. Microbiol. 42:264-268. [DOI] [PubMed] [Google Scholar]
- 12.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 70:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elvin, S. J., and E. D. Williamson. 2004. Stat 4 but not Stat 6 mediated immune mechanisms are essential in protection against plague. Microb. Pathog. 37:177-184. [DOI] [PubMed] [Google Scholar]
- 14.Eyles, J. E., G. Sharp, E. D. Williamson, I. D. Spiers, and H. O. Alpar. 1998. Intra nasal administration of poly-lactic acid microsphere co-encapsulated Yersinia pestis subunits confers protection from pneumonic plague in the mouse. Vaccine 16:698-707. [DOI] [PubMed] [Google Scholar]
- 15.Eyles, J. E., I. D. Spiers, E. D. Williamson, and H. O. Alpar. 1998. Analysis of local and systemic immunological responses after intra-tracheal, intra-nasal, and intra-muscular administration of microsphere co-encapsulated Yersinia pestis sub-unit vaccines. Vaccine 16:2000-2009. [DOI] [PubMed] [Google Scholar]
- 16.Fischer, H. G., B. Opel, K. Reske, and A. B. Reske-Kunz. 1988. Granulocyte-macrophage colony-stimulating factor-cultured bone marrow-derived macrophages reveal accessory cell function and synthesis of MHC class II determinants in the absence of external stimuli. Eur. J. Immunol. 18:1151-1158. [DOI] [PubMed] [Google Scholar]
- 17.Galyov, E. E., O. Y. Smirnov, A. V. Karlishev, K. I. Volkovoy, A. J. Denesyuk, I. V. Nazimov, K. S. Rubtsov, V. M. Abramov, S. M. Dalvadyanz, and V. P. Zavyalov. 1990. Nucleotide-sequence of the yersinia-pestis gene encoding f1 antigen and the primary structure of the protein: putative T-cell and B-cell epitopes. FEBS Lett. 277:230-232. [DOI] [PubMed] [Google Scholar]
- 18.Gerke, C., S. Falkow, and Y. H. Chien. 2005. The adaptor molecules LAT and SLP-76 are specifically targeted by Yersinia to inhibit T-cell activation. J. Exp. Med. 201:361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill, J., C. Copse, S. Leary, A. J. Stagg, E. D. Williamson, and R. W. Titball. 2003. Synergistic protection of mice against plague with monoclonal antibodies specific for the F1 and V antigens of Yersinia pestis. Infect. Immun. 71:2234-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inglesby, T. V., D. T. Dennis, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, J. F. Koerner, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, M. Schoch-Spana, and K. Tonat. 2009. Plague as a biological weapon: medical and public health management. JAMA 283:2281-2290. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov, M. I., B. L. Noel, R. Rampersaud, P. Mena, J. L. Benach, and J. B. Bliska. 2008. Vaccination of mice with a Yop translocon complex elicits antibodies that are protective against infection with F1-negative Yersinia pestis. Infect. Immun. 76:5181-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardetzky, T. S., J. C. Gorga, R. Busch, J. B. Rothbard, J. L. Strominger, and D. C. Wiley. 1990. Peptide binding to HLA-DR1: a peptide with most residues substituted to alanine retains MHC binding. EMBO J. 9:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan, A. A., J. P. Babu, G. Gupta, and D. N. Rao. 2008. Identifying B- and T-cell epitopes and studying humoral, mucosal and cellular immune responses of peptides derived from V antigen of Yersinia pestis. Vaccine 26:316-332. [DOI] [PubMed] [Google Scholar]
- 24.Kingston, R., F. Burke, J. H. Robinson, P. A. Bedford, S. M. Jones, S. C. Knight, and E. D. Williamson. 2007. The fraction 1 and V protein antigens of Yersinia pestis activate dendritic cells to induce primary T-cell responses. Clin. Exp. Immunol. 149:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leary, S., K. F. Griffin, E. E. Galyov, J. Hewer, E. D. Williamson, A. Holmstrom, A. Forsberg, and R. W. Titball. 1999. Yersinia outer proteins (YOPs) E, K, and N are antigenic but non-protective compared to V antigen in a murine model of bubonic plague. Microb. Pathog. 26:159-169. [DOI] [PubMed] [Google Scholar]
- 26.Li, B., and R. Yang. 2008. Interaction between Yersinia pestis and the host immune system. Infect. Immun. 76:1804-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marketon, M. M., R. W. De Paolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 309:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto, H., and G. M. Young. 2009. Translocated effectors of Yersinia. Curr. Opin. Microbiol. 12:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, J., E. D. Williamson, J. H. Lakey, M. J. Pearce, S. M. Jones, and R. W. Titball. 1998. Macromolecular organisation of recombinant Yersinia pestis F1 antigen and the effect of structure on immunogenicity. FEMS Immunol. Med. Microbiol. 21:213-221. [DOI] [PubMed] [Google Scholar]
- 30.Moudgil, K. D., and E. E. Sercarz. 2005. Understanding crypticity is the key to revealing the pathogenesis of autoimmunity. Trends Immunol. 26:355-359. [DOI] [PubMed] [Google Scholar]
- 31.Musson, J. A., M. Morton, N. Walker, H. M. Harper, H. V. McNeill, E. D. Williamson, and J. H. Robinson. 2006. Sequential proteolytic processing of the capsular Caf1 antigen of Yersinia pestis for MHC class II-restricted presentation to T lymphocytes. J. Biol. Chem. 281:26129-26135. [DOI] [PubMed] [Google Scholar]
- 32.Panina-Bordignon, P., A. Tan, A. Termijtelen, S. Demotz, G. Corradin, and A. Lanzavecchia. 1989. Universally immunogenic T-cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur. J. Immunol. 19:2237-2242. [DOI] [PubMed] [Google Scholar]
- 33.Parent, M. A., K. N. Berggren, I. K. Mullarky, F. M. Szaba, L. W. Kummer, J. J. Adamovicz, and S. T. Smiley. 2005. Yersinia pestis V protein epitopes recognized by CD4 T cells. Infect. Immun. 73:2197-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prentice, M. B., and L. Rahalison. 2007. Plague. Lancet 369:1196-1207. [DOI] [PubMed] [Google Scholar]
- 35.Reddin, K. M., T. J. Easterbrook, S. M. Eley, P. Russell, V. A. Mobsby, D. H. Jones, G. H. Farrar, E. D. Williamson, and A. Robinson. 1998. Comparison of the immunological and protective responses elicited by microencapsulated formulations of the F1 antigen from Yersinia pestis. Vaccine 16:761-767. [DOI] [PubMed] [Google Scholar]
- 36.Shim, H.-K., J. A. Musson, H. Harper, H. McNeill, N. Walker, H. Flick-Smith, A. A. von Delwig, E. D. Williamson, and J. H. Robinson. 2006. Mechanisms of MHC class II-restricted processing and presentation of the V antigen of Yersinia pestis. Immunology 119:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smiley, S. T. 2008. Immune defense against pneumonic plague. Immunol. Rev. 225:256-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Texier, C., S. Pouvelle, M. Busson, M. Hervé, D. Charron, A. Ménez, and B. Maillère. 2000. HLA-DR restricted peptide candidates for bee venom immunotherapy. J. Immunol. 164:3177-3184. [DOI] [PubMed] [Google Scholar]
- 39.Titball, R. W., and E. D. Williamson. 2003. Second and third generation plague vaccines. Adv. Exp. Med. Biol. 529:397-406. [DOI] [PubMed] [Google Scholar]
- 40.Velan, B., E. Bar-Haim, A. Zauberman, E. Mamroud, A. Shafferman, and S. Cohen. 2006. Discordance in the effects of Yersinia pestis on the dendritic cell functions manifested by induction of maturation and paralysis of migration. Infect. Immun. 74:6365-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vita, R., L. Zarebski, J. A. Greenbaum, H. Emami, I. Hoof, N. Salimi, R. Damle, A. Sette, and B. Peters. 2010. The Immune Epitope Database 2.0. Nucleic Acids Res. 38:D854-D862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel, T. U., H. Horton, D. H. Fuller, D. K. Carter, K. Vielhuber, D. H. O'Connor, T. Shipley, J. Fuller, G. Sutter, V. Erfle, N. Wilson, L. J. Picker, and D. I. Watkins. 2002. Differences between T-cell epitopes recognized after immunization and after infection. J. Immunol. 169:4511-4521. [DOI] [PubMed] [Google Scholar]
- 43.Wang, S., S. Joshi, I. Mboudjeka, F. Liu, T. Ling, J. D. Goguen, and S. Lu. 2008. Relative immunogenicity and protection potential of candidate Yersinia pestis antigens against lethal mucosal plague challenge in BALB/c mice. Vaccine 26:1664-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welkos, S., A. Friedlander, D. McDowell, J. Weeks, and S. Tobery. 1998. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb. Pathog. 24:185-196. [DOI] [PubMed] [Google Scholar]
- 45.Welkos, S. L., K. M. Davis, L. M. Pitt, P. L. Worsham, and A. M. Freidlander. 1995. Studies on the contribution of the F1 capsule-associated plasmid pFra to the virulence of Yersinia pestis. Contrib. Microbiol. Immunol. 13:299-305. [PubMed] [Google Scholar]
- 46.Williamson, E. D. 2009. Plague. Vaccine 27:D56-D60. [DOI] [PubMed] [Google Scholar]
- 47.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 1997. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine 15:1079-1084. [DOI] [PubMed] [Google Scholar]
- 48.Williamson, E. D., H. C. Flick-Smith, E. L. Waters, J. Miller, I. Hodgson, C. S. Le Butt, and J. Hill. 2007. Immunogenicity of the rF1+rV vaccine for plague with identification of potential immune correlates. Microb. Pathog. 42:11-21. [DOI] [PubMed] [Google Scholar]
- 49.Williamson, E. D., and R. W. Titball. 2002. Vaccines against dangerous pathogens. Br. Med. Bull. 62:163-173. [DOI] [PubMed] [Google Scholar]
- 50.Yamanaka, H., T. Hoyt, R. Bowen, X. Yang, K. Crist, S. Golden, M. Maddaloni, and D. W. Pascual. 2009. An IL-12 DNA vaccine coexpressing Yersinia pestis antigens protects against pneumonic plague. Vaccine 27:80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zav'yalov, V., A. V. Zavialov, G. Zav'yalova, and T. Korpela. 2010. Adhesive organelles of Gram-negative pathogens assembled with the classical chaperone/usher machinery: structure and function from a clinical standpoint. FEMS Microb. Rev. 34:1-62. [DOI] [PubMed] [Google Scholar]