Abstract
Escherichia coli K1 meningitis occurs following penetration of the blood-brain barrier, but the underlying mechanisms involved in E. coli penetration of the blood-brain barrier remain incompletely understood. We have previously shown that host cytosolic phospholipase A2α (cPLA2α) contributes to E. coli invasion of human brain microvascular endothelial cells (HBMEC), which constitute the blood-brain barrier, but the underlying mechanisms remain unclear. cPLA2α selectively liberates arachidonic acid from membrane phospholipids. Here, we provide the first direct evidence that host 5-lipoxygenase and lipoxygenase products of arachidonic acid, cysteinyl leukotrienes (LTs), contribute to E. coli K1 invasion of HBMEC and penetration into the brain, and their contributions involve protein kinase C alpha (PKCα). These findings demonstrate that arachidonic acid metabolism regulates E. coli penetration of the blood-brain barrier, and studies are needed to further elucidate the mechanisms involved with metabolic products of arachidonic acid for their contribution to E. coli invasion of the blood-brain barrier.
The mortality and morbidity associated with neonatal Gram-negative bacillary meningitis have remained significant despite advances in antimicrobial chemotherapy and supportive care. Inadequate knowledge of the pathogenesis has contributed to this mortality and morbidity (18-20). Escherichia coli K1 is the most common Gram-negative organism that causes neonatal meningitis. Most cases of neonatal E. coli K1 meningitis develop as a result of hematogenous spread, but the underlying mechanisms involved in E. coli penetration of the blood-brain barrier remain incompletely understood (18-20).
Several lines of evidence from experimental animal models as well as human cases of E. coli K1 meningitis indicate that E. coli penetrates into the brain initially in the cerebral vasculature (3, 21). We have developed the in vitro model of the blood-brain barrier by isolation and cultivation of human brain microvascular endothelial cells (HBMEC) (22, 34, 37). Upon cultivation on collagen-coated Transwell inserts, these HBMEC exhibit morphological and functional properties of tight junction formation and a polarized monolayer. These are shown by our demonstrations of tight junction proteins (such as ZO-1), adherens junction proteins (such as β-catenin), and their spatial separation, limited permeability to inulin (molecular mass, 4,000 Da), and development of high transendothelial electrical resistance (22, 34, 37). We have also developed the animal models of experimental hematogenous meningitis, which mimic the pathogenesis of E. coli meningitis in humans, e.g., hematogenous infection of the meninges (10, 11, 17, 21, 40, 41).
Using these in vitro and in vivo models, we have shown that E. coli invasion of HBMEC is a prerequisite for penetration into the brain and requires specific microbial determinants (10, 11, 17-20, 40, 41). This was shown in animal models of experimental hematogenous E. coli meningitis; mutants of E. coli K1 deleted of the structures contributing to HBMEC invasion (e.g., Ibe proteins and CNF1) were significantly less able to penetrate into the brain than the parent strain despite having similar levels of bacteremia. We subsequently showed that these E. coli K1 determinants interact with their respective host receptors, involving host signaling molecules for efficient invasion of HBMEC (4, 15-20, 33), but the contributions of microbe-host interactions and host signaling molecules to E. coli K1 penetration of the blood-brain barrier remain incompletely understood.
We have shown that host cytosolic phospholipase A2α (cPLA2α) contributes to E. coli K1 invasion of HBMEC (5), but the underlying mechanisms remain unclear. cPLA2α selectively liberates arachidonic acid from the sn-2 position of membrane phospholipids (7) (Fig. 1), and we hypothesize that the contribution of cPLA2α to E. coli K1 invasion of HBMEC is related to arachidonic acid metabolism. In the present study, we showed that 5-lipoxygenase (5-LO) and 5-LO-derived products of arachidonic acid, cysteinyl leukotrienes (LTs) (7, 29) (Fig. 1), contribute to E. coli K1 invasion of HBMEC, and their contributions occur via protein kinase C alpha (PKCα). More importantly, E. coli K1 penetration into the brain was inhibited by gene deletion of cPLA2α and 5-LO as well as by the administration of cPLA2α inhibitor and type 1 cysteinyl LT receptor (CysLT1) antagonist (Fig. 1).
FIG. 1.
A schematic diagram for cytosolic phospholipase A2α (cPLA2α), 5-lipoxygenase (5-LO), 5-LO-activating protein (FLAP), and leukotrienes (LTs), LTB4 and cysteinyl LTs (LTC4, LTD4, LTE4), that have been examined in this paper for their contributions to E. coli K1 invasion of the HBMEC monolayer and/or penetration into the brain. cPLA2α selectively liberates arachidonic acid (A.A) from membrane phospholipids. Pharmacological inhibitors of cPLA2α, 5-LO, and FLAP and antagonists of CysLT1 and BLT-1 are shown as arrows with ×.
MATERIALS AND METHODS
Bacterial strains and reagents.
E. coli K1 strain RS218 is the cerebrospinal fluid isolate from a neonate with meningitis (16, 17). E. coli K-12 strain HB101 was used as a noninvasive control (16, 17). Arachidonic acid, leukotriene D4 (LTD4), leukotriene B4 (LTB4), MK886, zileuton, MK571, zafirlukast, montelukast, and pranlukast were purchased from Cayman Chemical Company (Ann Arbor, MI) (Fig. 1). Evans blue was purchased from Sigma (St. Louis, MO). Arachidonyl trifluoromethyl ketone (AACOCF3) was purchased from Biomol Laboratories (Plymouth Meeting, PA) (Fig. 1). CP105696 was a gift from Pfizer (Fig. 1). cPLA2α antibody, phospho-cPLA2α antibody specific for serine 505 phosphorylation, and phospho-PKCα antibody were purchased from Cell Signaling Technologies (Danvers, MA), and PKC antibody was from Santa Cruz Biotechnology (Santa Cruz, CA).
E. coli binding and invasion assays in HBMEC.
HBMEC were isolated and characterized as described previously (37). HBMEC (∼70% confluence) were infected with a dialyzed adenovirus (Ad) of AdECFP-cPLA2-wt (where ECFP is enhanced cyan fluorescent protein and wt is wild type), AdECFP-cPLA2-S505A, AdECFP-cPLA2-S515A (29), or Ad5CA dominant negative PKCα or a vector control (8). Strain RS218 grown overnight in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, MI) was resuspended in experimental medium (M199-HamF12 [1:1], containing 5% heat-inactivated fetal bovine serum, 2 mM glutamine, and 1 mM pyruvate) and added in a multiplicity of infection of 100:1 to HBMEC grown in collagen-coated 24-well plates, which were incubated at 37°C in a 5% CO2 incubator for 90 min for binding assays. HBMEC were washed, lysed, and cultured for determination of CFU. The results were calculated as a percentage of the initial inoculum and expressed as percent relative binding compared to percent binding of RS218 in the presence of the vehicle control (dimethyl sulfoxide [DMSO]) or in HBMEC transfected with the vector control. Each set was run in triplicate. The HBMEC invasion assay with gentamicin treatment was performed to determine the number of viable intracellular bacteria recovered from the infected HBMEC, as previously described (10, 11, 17, 40, 41).
Immunoblotting and immunoprecipitation.
The lysates of HBMEC incubated with E. coli were prepared for Western blotting and immunoprecipitation as described previously (4, 15-17, 33).
Mouse model of experimental hematogenous meningitis.
cPLA2α−/− and wild-type mice, either male or female, approximately 10 to 13 weeks old and 18 to 26 g, that had been backcrossed on the BALB/c strain for >10 generations (35) and female 5-LO−/− (129-Alox5tm1Fun/J) and strain-matched wild-type (129SvEv) mice, approximately 9 to 12 weeks old and 18 to 26 g (36), were used. All procedures and handling techniques were approved by The Johns Hopkins Animal Care and Use Committee.
Each mouse received E. coli RS218 (1 × 107 CFU) in 100 μl phosphate-buffered saline (PBS) via the tail vein. One hour later, the mouse chests were opened, and blood from the right ventricle was collected for bacterial cultures (CFU). The animals were then perfused with a mammalian Ringer solution by transcardiac perfusion through a 23-gauge needle inserted into the left ventricle of the heart under a perfusion pressure of about 100 mm Hg. The perfusate exited through a cut in the right atrium. The composition of the mammalian Ringer solution was 132 mM NaCl, 4.6 mM KCl, 2 mM CaCl2, 1.2 mM MgSO4, 5.5 mM glucose, 5.0 mM NaHCO3, and 20 mM HEPES and Na-HEPES, containing 10 mg/ml bovine serum albumin (BSA); the pH of the Ringer solution was maintained at 7.40 to 7.45 by adjustment of the ratio of Na-HEPES to HEPES. Ringer solution used in this study has been shown not to change the microvessel permeability (43). At 30 min after perfusion of Ringer solution, the mice were decapitated. The brains were removed, weighed, homogenized, and cultured for determination of CFU. Since E. coli K1 penetration into the brain is shown to depend on the magnitude of bacteremia (10, 21), bacterial penetration into the brain was also expressed as (number of CFU per gram of brain/number of CFU per milliliter of blood) × 100. Kidneys and spleens were also removed, homogenized, and cultured for determination of bacterial counts, which were expressed as CFU per gram.
Blood-brain barrier permeability.
Evans blue dye extravasation into the brain tissue was used as an indicator of the blood-brain barrier permeability in cPLA2α−/− mice as previously described (14, 32). Briefly, 1.2 μg arachidonic acid in 100 μl PBS was injected through the tail vein 30 min before bacterial injection or 0.5 μg LTD4 or LTB4 in 100 μl PBS was injected through the tail vein 10 min before bacterial injection. At 10 min after bacterial injection, 2% Evans blue in 100 μl PBS was injected through the tail vein, and 50 min later, mice were perfused with Ringer solution for 30 min followed by removal of the brain. After measurement of the protein concentration, 0.5 ml of 60% trichloroacetic acid was added to 0.5-ml brain homogenates, and the samples were placed on ice for 30 min and centrifuged for 30 min at 1,000 × g. Evans blue concentration in the supernatants was measured at 610 nm and calculated according to a standard curve. Evans blue extravasation was expressed as micrograms per milligram of brain protein.
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM). Differences of bacterial counts between different groups of mice were determined by the Wilcoxon rank sum test. Differences of HBMEC binding and invasion were determined by Student's t test. A P value of <0.05 was considered significant.
RESULTS
Host cPLA2α contributes to E. coli K1 invasion of HBMEC and penetration into the brain.
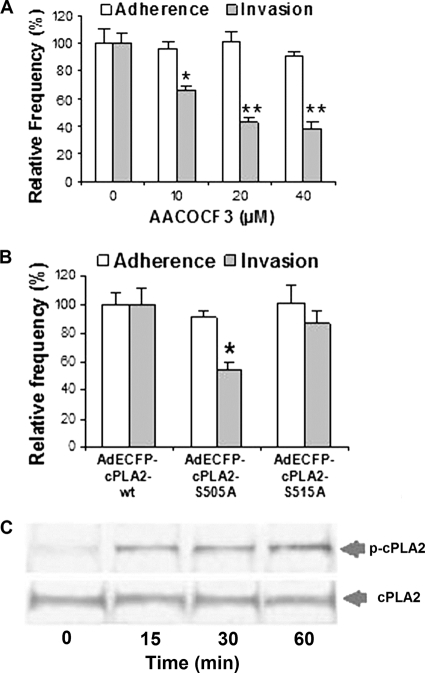
We have previously shown that E. coli K1 invasion of the blood-brain barrier involves host cPLA2α (6). This is shown by our demonstration that AACOCF3 (an inhibitor of cPLA2α [Fig. 1]) inhibited E. coli invasion of HBMEC, and E. coli invasion was defective in BMEC derived from cPLA2α−/− mice compared to BMEC derived from the wild-type mice. However, it remains unclear how cPLA2α contributes to E. coli K1 invasion of the blood-brain barrier. In the present study, we verified that pharmacological inhibition of cPLA2α exhibited a dose-dependent inhibition of E. coli K1 invasion of HBMEC but had no effect on E. coli K1 binding to HBMEC (Fig. 2A). It is important to note that AACOCF3 at 40 μM did not affect the viability of the HBMEC monolayer, as determined by Live/Dead staining (Molecular Probes), and also did not affect bacterial growth, as examined by comparing CFU in experimental medium with AACOCF3 and CFU in medium without AACOCF3 (data not shown). cPLA2α releases arachidonic acid from membrane phospholipids (7), and cPLA2α activation in response to various agonists has been shown to involve serine phosphorylations at S505 and/or S515, as demonstrated by reduced arachidonic acid release in constructs with mutagenesis of serine to alanine (S505A and S515A) (29). We next showed that E. coli K1 invasion was significantly less in HBMEC transfected with the S505A construct than in HBMEC transfected with wild-type cPLA2α, but there was no significant effect of the S515A construct on E. coli invasion of HBMEC (Fig. 2B). These findings suggest that cPLA2α phosphorylation at S505 is likely to be involved in E. coli K1 invasion of HBMEC. We showed that serine 505 phosphorylation of cPLA2α indeed occurred in response to E. coli K1 in a time-dependent manner in HBMEC (Fig. 2C). This finding is consistent with that of our recent report that found that cPLA2α phosphorylation at S505 occurs in response to invasive E. coli K1 but not to noninvasive E. coli K1 in HBMEC (39).
FIG. 2.
cPLA2α contributes to E. coli K1 invasion of HBMEC. (A) HBMEC were pretreated with AACOCF3 at the indicated concentrations (10, 20, and 40 μM) for 60 min followed by bacterial binding and invasion assays. The data shown are means ± SEM from three independent experiments. Each experiment was performed in triplicate. *, P < 0.05; **, P < 0.01 (one-way analysis of variance [ANOVA] followed by Dunnett's test, compared to the vehicle control [DMSO]). (B) cPLA2α phosphorylation at serine 505 is involved in E. coli K1 strain RS218 invasion of HBMEC but not binding. HBMEC transfected with the cPLA2α wild type or cPLA2α S505A or cPLA2α S515A were examined for E. coli binding and invasion. The data shown are means ± SEM from two independent experiments. Each experiment was performed in triplicate. *, P < 0.05 (Student's t test, comparison between the AdECFP-cPLA2α mutant and the wild type). (C) HBMEC were incubated with E. coli K1 strain RS218 at 37°C for various time points and lysed, and lysates were analyzed for phospho-cPLA2α at serine 505 and cPLA2α by Western blotting using specific anti-S505 phospho-cPLA2α or cPLA2α antibodies, respectively.
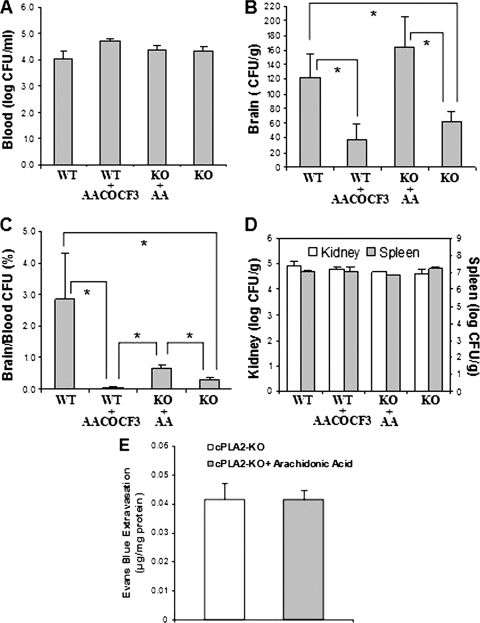
The role of cPLA2α in E. coli K1 penetration into the brain was next examined using gene deletion and pharmacological inhibition in the mouse model of experimental hematogenous meningitis. We examined and compared the ability of E. coli K1 to penetrate into the brains of cPLA2α−/− mice to its ability to penetrate into the brains of the wild-type BALB/c mice. Each animal received 1 × 107 CFU of E. coli K1 strain RS218 via the tail vein. One hour later, the blood specimens were obtained for determination of CFU, and the animals were perfused with sterile Ringer solution until the perfused solution became colorless. The brains and nonbrain organs (kidneys and spleens) were removed, weighed, homogenized, and cultured for determinations of CFU. The magnitude of bacteremia did not differ significantly between the two groups of cPLA2α−/− and wild-type animals, as shown by similar bacterial counts in the blood (Fig. 3A), but bacterial penetration into the brain, as assessed by the bacterial counts recovered from the brain, was significantly less in cPLA2α−/− mice than in the wild-type animals (Fig. 3B). These findings demonstrate that the ability of E. coli K1 to penetrate into the brain was significantly less in cPLA2α−/− mice than in the wild-type mice and that significantly decreased penetration into the brains of cPLA2α−/− mice was not the result of having lower levels of bacteremia. This concept is also shown by the percentage of bacterial counts in the brain compared to those in the blood, which was significantly less in 5-LO−/− mice than in the wild-type animals (Fig. 3C).
FIG. 3.
cPLA2α contributes to E. coli K1 penetration into the brain. Bacterial counts in the blood (CFU per milliliter) (A), brain (CFU per gram) (B), and kidney and spleen (CFU per gram) (D) were determined 1 h after intravenous injection of E. coli K1 strain RS218. (B and C) E. coli K1 penetration into the brain was significantly less in cPLA2α-deficient mice (KO) than in the wild-type mice (WT). AACOCF3 significantly inhibited E. coli penetration into the brains of the wild-type mice, while arachidonic acid (AA) rescued the decreased E. coli penetration into the brain of cPLA2α−/− mice to the level in the wild-type animals. Data shown are means ± SEM. *, P < 0.05, Wilcoxon rank sum test, comparison between WT (n = 10 samples), WT plus AACOCF3 (n = 3), KO plus AA (n = 3), and KO (n = 12). (E) Arachidonic acid did not affect the blood-brain barrier permeability. Evans blue dye extravasation into the brain tissue was used as an indicator of the blood-brain barrier permeability. Evans blue extravasation was expressed as micrograms per milligram of brain protein. Data shown are means ± SEM (n = 3).
Of interest, the levels of E. coli K1 penetration into the nonbrain organs (kidneys and spleens) were similar between cPLA2α−/− and wild-type animals, as shown by similar bacterial counts recovered from the kidneys and spleens (CFU per gram) (Fig. 3D). These findings suggest that host cPLA2α contributes to E. coli K1 penetration, specifically into the brain.
We next examined the effect of pharmacological inhibition of cPLA2α on E. coli K1 penetration into the brain and nonbrain organs. Intravenous administration of AACOCF3 (4 mM in 50 μl PBS, a dose which inhibits cPLA2α activity in mice [13]) 30 min before bacterial injection significantly decreased the brain penetration of E. coli in the wild-type mice to the level observed in cPLA2α−/− mice (Fig. 3B and C). In contrast, AACOCF3 did not affect the bacterial counts in the blood, kidneys, and spleens (Fig. 3A and D). These in vitro and in vivo findings with gene deletion and pharmacological inhibition indicate that cPLA2α contributes to E. coli K1 invasion of HBMEC and penetration specifically into the brain.
We next showed that intravenous administration of arachidonic acid (1.2 μg/mouse in 50 μl PBS, a dose which restores cPLA2α-dependent vascular responses in cPLA2α−/− mice [12]) 30 min before bacterial injection significantly increased E. coli penetration into the brain in cPLA2α−/− mice (Fig. 3B and C) without affecting the bacterial counts in the blood, kidneys, and spleens (Fig. 3A and D). The enhancement of E. coli K1 penetration into the brain by arachidonic acid in cPLA2α−/− mice was not accompanied by any changes in the blood-brain barrier permeability, as shown by no significantly increased extravasation of intravenously administered Evans blue dye into the brains of animals with or without arachidonic acid administration (Fig. 3E).
Role of 5-LO in E. coli K1 invasion of HBMEC and penetration into the brain.
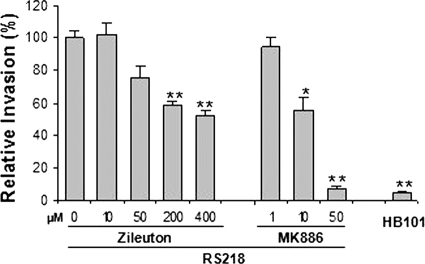
Leukotrienes (LTs) are synthesized from arachidonate by 5-LO and 5-LO-activating protein (FLAP) (7, 30) (Fig. 1), and we next examined whether the contribution of cPLA2α to E. coli invasion of HBMEC and penetration into the brain is related to lipoxygenated metabolites of arachidonic acid. We first examined the roles of 5-LO and FLAP in E. coli K1 binding to and invasion of HBMEC by using their pharmacological inhibitors (zileuton and MK886 for 5-LO and FLAP, respectively) (Fig. 1). Pretreatment of HBMEC with zileuton and MK886 significantly inhibited E. coli K1 invasion of HBMEC in a dose-dependent manner (Fig. 4), while zileuton and MK886 did not affect E. coli binding to HBMEC (data not shown). Zileuton at 400 μM and MK886 at 50 μM did not affect the HBMEC viability, as determined by Live/Dead staining (Molecular Probes), and also did not affect bacterial growth, as examined by comparing CFU in experimental medium with these inhibitors to CFU in medium without these inhibitors.
FIG. 4.
5-LO and FLAP are involved in E. coli K1 invasion of HBMEC. E. coli K1 invasion of HBMEC was dose-dependently inhibited by 5-LO and FLAP inhibitors (zileuton and MK886, respectively). A laboratory E. coli K-12 strain, HB101, was used as a negative control for HBMEC invasion. The data shown are means ± SEM from three independent experiments. Each experiment was performed in triplicate. *, P < 0.05; **, P < 0.01 (Student's t test, compared to the vehicle control [DMSO] of strain RS218).
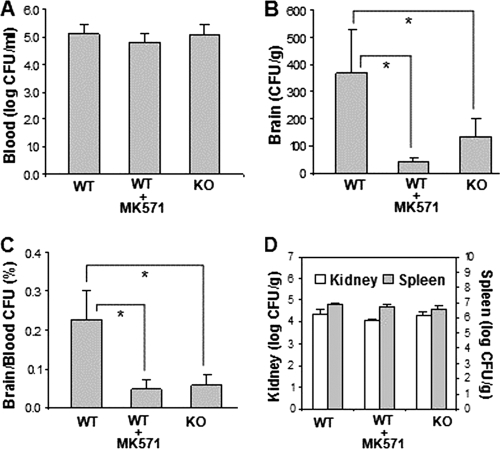
The role of 5-LO in E. coli K1 penetration into the brain was subsequently examined in 5-LO−/− mice compared to the strain-matched wild-type 129/SvEv mice. The magnitudes of bacteremia were similar between the two groups of 5-LO−/− and wild-type animals (Fig. 5A), but the bacterial counts recovered from the brains were significantly less in 5-LO−/− mice than in the wild-type animals (Fig. 5B and C). This in vivo finding of significantly decreased E. coli K1 penetration into the brain of 5-LO−/− mice, together with the in vitro finding of a dose-dependent inhibition of HBMEC invasion by pharmacological inhibition of 5-LO and FLAP, indicates that endogenous LT biosynthesis via 5-LO and FLAP is likely to play an important role in E. coli K1 invasion of HBMEC and penetration into the brain. In contrast, bacterial counts recovered from the kidneys and spleens did not differ significantly between the two groups of 5-LO−/− and wild-type animals (Fig. 5D).
FIG. 5.
5-LO and cysteinyl leukotrienes are involved in E. coli K1 penetration into the brain. Bacterial counts in blood (CFU per milliliter) (A), brain (CFU per gram) (B), and kidney and spleen (CFU per gram) (D) were determined 1 h after intravenous injection of E. coli K1 strain RS218. (B and C) E. coli K1 penetration into the brain was significantly less in 5-LO knockout mice (KO) than that in the wild-type mice (WT). MK571 significantly inhibited E. coli penetration into the brains of the wild-type mice. Data shown are means ± SEM. *, P < 0.05 (comparison between WT [n = 7] and WT plus MK571 [n = 5] or KO [n = 7]).
Cysteinyl LTs contribute to E. coli K1 invasion of HBMEC and penetration into the brain.
LTs, such as LTB4 and cysteinyl LTs (LTC4, LTD4, and LTE4), exhibit their biological actions via ligation with specific G-protein-coupled receptors (GPCRs), which include BLT-1 for LTB4 and CysLT1 for cysteinyl LTs (7, 30) (Fig. 1).
We next examined which class of LTs is involved in E. coli K1 invasion of HBMEC using CysLT1 antagonists (MK571, zafirlukast, montelukast, and pranlukast) and the BLT-1 antagonist (CP105696) (Fig. 1). All four CysLT1 antagonists were effective in inhibiting E. coli invasion of HBMEC in a dose-dependent manner, while the BLT-1 antagonist failed to exhibit any inhibition (Fig. 6). These antagonists did not affect the HBMEC viability and also did not affect bacterial growth. These findings suggest that cysteinyl LTs, not LTB4, are likely to contribute to E. coli K1 penetration of the blood-brain barrier. This concept was supported by demonstrating that one of the CysLT1 antagonists (MK571) significantly decreased E. coli penetration into the brain of wild-type mice to the level observed in 5-LO−/− mice (Fig. 5B and C), without affecting the bacterial counts in the blood, kidneys, and spleens (Fig. 5A and D). These in vitro and in vivo findings demonstrate that cysteinyl LTs are likely to contribute to E. coli K1 penetration of the blood-brain barrier.
FIG. 6.
Cysteinyl LTs, not LTB4, are involved in E. coli K1 invasion of HBMEC. E. coli K1 invasion of HBMEC was dose-dependently inhibited by the CysLT1 antagonists (MK571, zafirlukast, montelukast, and pranlukast) but not by the BLT1 antagonist (CP105696). A laboratory E. coli K-12 strain, HB101, was used as the negative control for HBMEC invasion. The data shown are means ± SEM from three independent experiments. Each experiment was performed in triplicate. **, P < 0.01 (Student's t test, compared to the vehicle control [DMSO]).
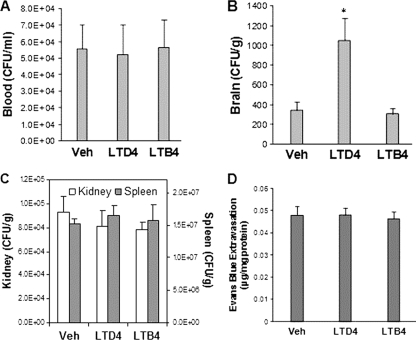
We showed that arachidonic acid enhances E. coli K1 penetration into the brain of cPLA2α−/− mice (Fig. 3B and C) and next examined whether its enhancement is related to arachidonic acid metabolites derived by 5-LO and FLAP, i.e., LTB4 and cysteinyl LTs. Administration of exogenous cysteinyl LTs (LTD4, 500 ng in 100 μl PBS, a dose which increases systemic vascular permeability in mice [27]) 10 min before bacterial injection significantly increased E. coli K1 penetration into the brain in cPLA2α−/− mice (Fig. 7B) without affecting bacterial counts in the blood, kidneys, and spleens (Fig. 7A and C), and the LTD4-mediated increased bacterial penetration into the brain was not accompanied by any changes in the blood-brain barrier permeability (Fig. 7D). In contrast, LTB4 (500 ng in 100 μl PBS) did not affect E. coli counts in the blood and also did not affect E. coli penetration into the brain, kidneys, and spleens (Fig. 7A, B, and C). These findings demonstrate that host-derived cysteinyl LTs are likely to contribute to E. coli invasion of HBMEC and penetration specifically into the brain. These findings support the concept that the decreased E. coli penetration into the brain of cPLA2α−/− mice is likely to be related to its failure to elaborate 5-LO metabolites of arachidonic acid, cysteinyl LTs. Taken together, these findings demonstrate that arachidonic acid released by host cPLA2α contributes to E. coli K1 invasion of HBMEC and penetration specifically into the brain, and its contribution is most likely to stem from its metabolic products, cysteinyl LTs.
FIG. 7.
LTD4, not LTB4, enhanced E. coli K1 penetration into the brain in cPLA2α−/− mice. Bacterial counts in blood (CFU per milliliter) (A), brain (CFU per gram) (B), and kidney and spleen (CFU per gram) (C) were determined at 1 h after intravenous injection of E. coli K1 strain RS218. (A to C) LTD4 or LTB4 (500 ng in 100 μl PBS) was injected through the tail vein 10 min before bacterial injection. Data shown are means ± SEM (n = 4). *, P < 0.05 (Student's t test, compared to the vector control [5% ethanol]). (D) LTD4 or LTB4 did not affect the blood-brain barrier permeability in cPLA2α−/− mice. Evans blue dye extravasation into the brain tissue was used as an indicator of the blood-brain barrier permeability. Evans blue extravasation was expressed as micrograms per milligram of brain protein. Data shown are means ± SEM (n = 4).
Host cPLA2α and cysteinyl LTs contribute to E. coli K1 invasion of HBMEC via PKCα.
Our findings so far demonstrate that host cPLA2α, 5-LO, and cysteinyl LTs (Fig. 1) contribute to E. coli K1 invasion of HBMEC and penetration into the brain, but their underlying mechanisms remain unclear. PKC is a family of at least 12 serine/threonine kinases that transduce multiple signals in the regulation of a variety of cellular functions, which include actin cytoskeleton rearrangements (9, 23). We have shown that E. coli K1 invasion of HBMEC requires host cell actin cytoskeleton rearrangements, as shown by the demonstration that the pretreatment of HBMEC with cytochalasin D and latrunculin A (microfilament-disrupting agents) inhibited E. coli invasion of HBMEC (31). We therefore examined whether the contribution of cPLA2α, 5-LO, and cysteinyl LTs to E. coli K1 invasion of HBMEC involves PKC.
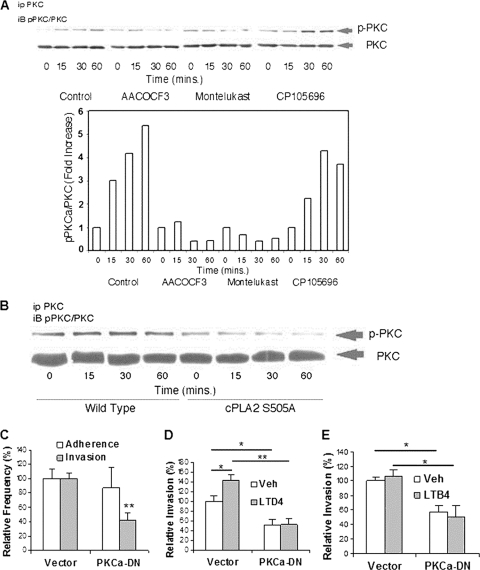
We showed that PKCα contributes to E. coli K1 invasion of HBMEC. This was shown by our demonstrations that PKCα activation (i.e., serine phosphorylation) occurs in response to E. coli K1 in HBMEC (Fig. 8A and B), and E. coli K1 invasion was significantly decreased in HBMEC expressing dominant negative PKCα compared to the control vector-transfected HBMEC (Fig. 8C, D, and E). More importantly, PKCα activation in response to E. coli K1 was inhibited by the cPLA2α inhibitor and CysLT1 antagonist but not by the BLT-1 antagonist (Fig. 1 and 8A). Also, PKCα activation in response to E. coli K1 did not occur in HBMEC transfected with cPLA2 S505A (Fig. 8B). These findings demonstrate that host cPLA2α and cysteinyl LTs contribute to E. coli K1 invasion of HBMEC, most likely involving PKCα. This concept was further supported by our demonstration that exogenous cysteinyl LT (LTD4, 1 μM), not LTB4 (1 μM), significantly enhanced E. coli K1 invasion of control vector-transfected HBMEC compared to that of HBMEC transfected with the vehicle control but failed to exhibit such an enhancement in HBMEC expressing dominant negative PKCα (Fig. 8D and E). Taken together, these findings demonstrate that PKCα is downstream of cPLA2α and cysteinyl LTs in E. coli K1 penetration of the blood-brain barrier.
FIG. 8.
cPLA2α, 5-LO, and cysteinyl LTs contribute to E. coli invasion of HBMEC via PKCα. (A) HBMEC incubated with E. coli K1 strain RS218 at 37°C for various times in the presence of inhibitors/antagonists or the vehicle control were immunoprecipitated with PKCα antibody and then assessed for phospho-PKC by Western blotting with phospho-PKC antibody. The HBMEC lysates were also examined for the total amounts of PKC. The bands of phosphorylated and total PKC shown by a Western blot were calculated by ImageJ software, and ratios of phospho-PKC to PKC are expressed as fold increases compared to that at time zero. (B) Effect of cPLA2α S505A on PKCα activation. HBMEC transfected with the cPLA2α wild type or cPLA2α S505A were incubated with E. coli K1 strain RS218 for various times. The HBMEC lysates were immunoprecipitated with PKCα antibody and subsequently Western blotted with phospho-PKC antibody. The HBMEC lysates were examined for the total amounts of PKC as described above. (C) PKCα is involved in E. coli RS218 invasion of HBMEC. HBMEC transfected with the dominant negative PKCα construct or the vector control were used for bacterial binding and invasion assays. The data shown are means ± SEM from three separate experiments. Each experiment was performed in triplicate. **, P < 0.01 (Student's t test, comparison between the Ad5CA dominant negative PKCα [PKCa-DN] and the vector control). (D and E) Effects of LTD4 or LTB4 on E. coli K1 strain RS218 invasion of HBMEC transfected with the dominant negative PKCα construct or the vector control. HBMEC were pretreated with 1 μM LTD4 (D) or 1 μM LTB4 (E) for 30 min. Ethanol (0.5%) was used as the vehicle control (Veh). The data shown are means ± SEM from two independent experiments. Each experiment was performed in triplicate. *, P < 0.05; **, P < 0.01 (Student's t test, comparison between the Ad5CA dominant negative PKCα [PKCα-DN] and the vector control).
DISCUSSION
E. coli K1 penetration of the blood-brain barrier occurs as the result of microbial invasion of HBMEC, involving specific host signaling molecules (18-20), but the microbe-HBMEC interactions and host signaling molecules involved in E. coli K1 penetration of the blood-brain barrier remain incompletely understood.
In the present study, we demonstrated that host cPLA2α contributes to E. coli K1 invasion of HBMEC and penetration specifically into the brain, and its contribution is related to lipoxygenated metabolites of arachidonic acid, cysteinyl LTs. This was shown by our demonstrations that (i) gene deletion and pharmacological inhibition of cPLA2α prevented E. coli invasion of HBMEC and penetration into the brain but not into nonbrain organs (Fig. 1, 2, and 3), (ii) pharmacological inhibition of 5-LO and FLAP inhibited E. coli invasion of HBMEC (Fig. 1 and 4), (iii) gene deletion of 5-LO prevented E. coli penetration into the brain but not into nonbrain organs (Fig. 5), (iv) pharmacological inhibition of cysteinyl LTs, formed via LT biosynthetic pathways involving cPLA2α and 5-LO and acting via the CysLT1, prevented E. coli invasion of HBMEC and penetration into the brain but not into nonbrain organs (Fig. 1, 5, and 6), and (v) exogenous administration of LTD4, but not LTB4, enhanced E. coli K1 penetration into the brain but not into nonbrain organs in cPLA2α−/− mice (Fig. 1 and 7). This is the first demonstration that 5-LO and cysteinyl LTs contribute to E. coli K1 penetration of the blood-brain barrier in vitro and in vivo.
Our studies also showed that the contribution of cPLA2α, 5-LO, and cysteinyl LTs to E. coli K1 penetration was specific to the brain, and E. coli K1 penetration into nonbrain organs (kidneys and spleens) was not affected by gene deletion and/or pharmacological inhibition of cPLA2α, 5-LO, and cysteinyl LTs. The reasons for this selective contribution of cPLA2α, 5-LO, and cysteinyl LTs to E. coli penetration into the brain are not clear. We have previously shown that E. coli K1 binding and invasion occurred in HBMEC but not in nonbrain endothelial cells, such as human umbilical vein endothelial cells, human iliac vein endothelial cells, and human aortic arterial endothelial cells, and E. coli K1 binding to and invasion of HBMEC requires specific microbe-host interactions (18-20). We showed that cPLA2α activation occurs in response to specific microbial determinants contributing to E. coli K1 binding to and invasion of HBMEC (e.g., NlpI, OmpA, FliC, IbeC) (20, 39), and studies are needed to determine whether the involvement of those microbial determinants in cPLA2α activation and arachidonic acid metabolism is in part responsible for selective contribution of cPLA2α, 5-LO, and cysteinyl LTs to E. coli K1 penetration, specifically into the brain.
Arachidonic acid and LTs have been shown to increase systemic vascular permeability (2, 42), and we showed that LTs increased ear concentration of systemically administered Evans blue dye (indicative of systemic vascular permeability) (data not shown). In contrast, the enhancement of E. coli K1 penetration into the brain by arachidonic acid and LTD4 in cPLA2α−/− mice occurred without affecting the blood-brain barrier permeability. These findings are in principle consistent with those of our previous studies, where E. coli K1 penetration of the blood-brain barrier was not associated with any changes in the blood-brain barrier integrity (38).
Another novel finding of our study is the demonstration that the contribution of host cPLA2α, 5-LO, and cysteinyl LTs to E. coli K1 invasion of HBMEC involves PKCα. These findings differ from those of other investigators who showed that PKCα contributes to cPLA2α phosphorylation in immortalized rat brain endothelial cells (1), PKCα can regulate cPLA2α in monocytes (24), PKCδ is involved in LTD4-induced stress fiber formation in intestinal epithelial cells (26), and PKC regulates LTD4-stimulated cellular functions and the functional consequences of CysLT1 activation in airway smooth muscle cells (6). Our finding that PKCα is downstream of cPLA2α and cysteinyl LTs in E. coli K1 invasion of HBMEC is also supported by the demonstration that PKCα activation in response to E. coli K1 was prevented by inhibition of cPLA2α and cysteinyl LTs and that LTD4 enhanced E. coli K1 invasion in vector-transfected HBMEC but not in HBMEC expressing dominant negative PKCα. These findings suggest that the contribution of cPLA2α-, 5-LO-cysteinyl LT-PKCα pathways to E. coli penetration appears to be unique to the blood-brain barrier, and additional studies are needed to elucidate how the pathways involving cPLA2α, 5-LO, cysteinyl LTs, and PKCα contribute to E. coli invasion in HBMEC and penetration specifically into the brain.
LTs have been shown to be involved in respiratory diseases, allergic diseases, and cardiovascular diseases (30), but the roles of LTs in microbial penetration of the blood-brain barrier have not been reported. Our findings demonstrate for the first time that E. coli K1 exploits 5-LO- and FLAP-derived eicosanoids, cysteinyl LTs, for penetration of the blood-brain barrier, the essential step required for development of E. coli meningitis.
Our findings also demonstrate for the first time differential effects of terminal LTs, LTB4 and cysteinyl LTs, in their contribution to E. coli K1 invasion of HBMEC and penetration into the brain. These are shown by our demonstrations that (i) pharmacological inhibition of CysLT1 but not of BLT-1 inhibited E. coli invasion of HBMEC (Fig. 1 and 6), (ii) cysteinyl LTs (LTD4) but not LTB4 enhanced E. coli invasion of vector-transfected HBMEC (Fig. 8D and E), (iii) LTD4 but not LTB4 enhanced E. coli penetration into the brain in cPLA2α−/− mice (Fig. 7), and (iv) PKCα activation in response to E. coli K1 was inhibited by the CysLT1 antagonist but not by the BLT-1 antagonist (Fig. 8A). The mechanisms involved with these terminal LTs for their differential contributions to the pathogenesis of E. coli meningitis remain unclear, and additional studies are needed to clarify this issue.
Increasing resistance to antimicrobial agents is an important factor contributing to mortality and morbidity associated with E. coli sepsis and meningitis (25, 28). On top of demonstrating a novel role of arachidonic acid metabolism in regulating E. coli K1 invasion of the blood-brain barrier, our findings suggest that counteracting host signaling molecules used by meningitis-causing pathogens for their invasion of the blood-brain barrier (as shown here with the cPLA2α inhibitor, 5-LO inhibitor, and CysLT1 antagonists) may limit the exposure to emerging antimicrobial-resistant bacteria and might provide an innovative strategy for prevention and/or therapy of E. coli meningitis.
Acknowledgments
This work was supported by NIH grants NS26310 and AI84984.
We thank V. Natarajan for providing adenovirus constructs with dominant negative PKCα or the vector control. The animal experiments were approved by the Animal Care and Use Committee of Johns Hopkins University.
Editor: S. M. Payne
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Anfuso, C. D., G. Lupo, L. Romeo, G. Giurdanella, C. Motta, A. Pascale, C. Tirolo, B. Marchetti, and M. Alberghina. 2007. Endothelial cell-pericyte cocultures induce PLA2 protein expression through activation of PKCα and the MAPK/ERK cascade. J. Lipid Res. 48:782-793. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T., K. L. Black, K. Ikezakim, K. N. Chen, and D. P. Becker. 1991. Intracarotid infusion of leukotriene C4 selectively increases blood-brain permeability after focal ischemia in rats. J. Cereb. Blood Flow Metab. 11:638-643. [DOI] [PubMed] [Google Scholar]
- 3.Berman, P. H., and B. Q. Banker. 1966. Neonatal meningitis: a clinical and pathological study of 29 cases. Pediatrics 38:6-24. [PubMed] [Google Scholar]
- 4.Chung, J. W., S. J. Hong, K. J. Kim, D. Goti, M. F. Stins, S. Shin, V. L. Dawson, T. M. Dawson, and K. S. Kim. 2003. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J. Biol. Chem. 278:16857-16862. [DOI] [PubMed] [Google Scholar]
- 5.Das, A., L. Asatryan, M. A. Reddy, C. A. Wass, M. Stins, S. Joshi, J. V. Bonentre, and K. S. Kim. 2001. Differential role of cytosolic phospholipase A2 in the invasion of brain microvascular endothelial cells by Escherichia coli and Listeria monocytogenes. J. Infect. Dis. 184:732-737. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande, D. A., R. M. Pascual, S. Wang, D. M. Eckman, E. C. Riemer, C. D. Funk, and R. B. Penn. 2007. PKC-dependent regulation of the receptor locus dominates functional consequences of cysteinyl leukotriene type 1 receptor activation. FASEB J. 21:2335-2342. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh, M., D. E. Tucker, S. A. Burchett, and C. C. Leslie. 2006. Properties of the group IV phospholipase A2 family. Prog. Lipid Res. 45:487-510. [DOI] [PubMed] [Google Scholar]
- 8.Gorshkova, I., D. He, E. Berdyshev, P. Usatuyk, M. Burns, S. Kalari, Y. Zhao, S. Pendyala, J. G. Garcia, N. J. Pyne, D. N. Brindley, and V. Natarajan. 2008. Protein kinase C-epsilon regulates sphingosine 1-phosphate-mediated migration of human lung endothelial cells through activation of phospholipase D2, protein kinase C-zeta, and Rac1. J. Biol. Chem. 283:11794-11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hryciw, D. H., C. A. Pollock, and P. Pronnik. 2005. PKCα-mediated remodeling of the actin cytoskeleton is involved in constitutive albumin uptake by proximal tubule cells. Am. J. Physiol. Renal Physiol. 288:F1227-F1235. [DOI] [PubMed] [Google Scholar]
- 10.Huang, S.-H., C. Wass, Q. Fu, N. V. Prasadarao, M. Stins, and K. S. Kim. 1995. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect. Immun. 63:4470-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, S. H., Y. H. Chen, Q. Fu, M. Stins, Y. Wang, C. Wass, and K. S. Kim. 1999. Identification and characterization of an Escherichia coli invasion gene locus ibeB required for penetration of brain microvascular endothelial cells. Infect. Immun. 67:2103-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichinose, F., R. Ullrich, A. Sapirstein, R. C. Jones, J. V. Bonventre, C. N. Serhan, K. D. Bloch, and W. M. Zapol. 2002. Cytosolic phospholipase A2 in hypoxic pulmonary vasoconstriction. J. Clin. Invest. 109:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyvas, A., and S. David. 2004. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron 41:323-335. [DOI] [PubMed] [Google Scholar]
- 14.Kaya, M., R. Kalayci, M. Küçük, N. Arican, I. Elmas, H. Kudat, and F. Korkut. 2003. Effect of losartan on the blood-brain barrier permeability in diabetic hypertensive rats. Life Sci. 73:3235-3244. [DOI] [PubMed] [Google Scholar]
- 15.Khan, N. A., S. Shin, J. W. Chung, K. J. Kim, S. Elliott, Y. Wang, and K. S. Kim. 2003. Outer membrane protein A and cytotoxic necrotizing factor-1 use diverse signaling mechanisms for Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microb. Pathog. 35:35-42. [DOI] [PubMed] [Google Scholar]
- 16.Khan, N. A., Y. Kim, S. Shin, and K. S. Kim. 2007. FimH-mediated Escherichia coli K1 invasion of human brain microvascular endothelial cells. Cell Microbiol. 9:169-178. [DOI] [PubMed] [Google Scholar]
- 17.Khan, N. A., Y. Wang, K. J. Kim, J. W. Chung, C. A. Wass, and K. S. Kim. 2002. Cytotoxic necrotizing factor 1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 277:15607-15612. [DOI] [PubMed] [Google Scholar]
- 18.Kim, K. S. 2001. Escherichia coli translocation at the blood-brain barrier. Infect. Immun. 69:5217-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, K. S. 2003. Pathogenesis of bacterial meningitis: from bacteremia to neuronal injury. Nat. Rev. Neuroscience 4:376-385. [DOI] [PubMed] [Google Scholar]
- 20.Kim, K. S. 2008. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 6:625-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, K. S., H. Itabashi, P. Gemski, J. Sadoff, R. L. Warren, and A. S. Cross. 1992. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J. Clin. Invest. 90:897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, Y. V., F. DiCello, C. S. Hillaire, and K. S. Kim. 2004. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. Am. J. Physiol. Cell Physiol. 286:C31-C42. [DOI] [PubMed] [Google Scholar]
- 23.Larsson, C. 2006. Protein kinase C and the regulation of the actin cytoskeleton. Cell. Signal. 18:276-284. [DOI] [PubMed] [Google Scholar]
- 24.Li, Q., V. Subbulakshmi, C. M. Oldfield, R. Aamir, C. M. Weyman, A. Wolfman, and M. K. Cathcart. 2007. PKCα regulates phosphorylation and enzymatic activity of cPLA2 in vitro and in activated human monocytes. Cell. Signal. 19:359-366. [DOI] [PubMed] [Google Scholar]
- 25.López-Cerero, L., M. De Cueto, C. Saenz, D. Navarro, C. Velasco, J. Rodríguez-Baño, and A. Pascual. 2008. Neonatal sepsis caused by a CTX-M-32-producing Escherichia coli isolate. J. Med. Microbiol. 57:1303-1305. [DOI] [PubMed] [Google Scholar]
- 26.Massoumi, R., C. Larsson, and A. Sjolander. 2002. Leukotriene D4 induces stress-fibre formation in intestinal epithelial cells via activation of RhoA and PKCdelta. J. Cell Sci. 115:3509-3515. [DOI] [PubMed] [Google Scholar]
- 27.Moos, M. P. W., J. D. Mewburn, F. W. K. Kan, S. Ishii, M. Abe, K. Sakimura, K. Noguchi, T. Shimizu, and C. D. Funk. 2008. Cysteinyl leukotriene 2 receptor-mediated vascular permeability via transendothelial vesicle transport. FASEB J. 22:4352-4362. [DOI] [PubMed] [Google Scholar]
- 28.Ortega, M., F. Marco, A. Soriano, M. Almela, J. A. Martínez, A. Muñoz, and J. Mensa. 2009. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J. Antimicrob. Chemother. 63:568-574. [DOI] [PubMed] [Google Scholar]
- 29.Pavicevic, Z., C. C. Leslie, and K. U. Malik. 2008. cPLA2 phosphorylation at serine-515 and serine-505 is required for arachidonic acid release in vascular smooth muscle cells. J. Lipid Res. 49:724-737. [DOI] [PubMed] [Google Scholar]
- 30.Peters-Golden, M., and W. R. Henderson. 2007. Leukotrienes. N. Engl. J. Med. 357:1841-1854. [DOI] [PubMed] [Google Scholar]
- 31.Prasadarao, N. V., C. A. Wass, M. F. Stins, H. Shimada, and K. S. Kim. 1999. Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect. Immun. 67:5775-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pu, H., K. Hayashi, I. E. Andras, S. Y. Eum, B. Hennig, and M. Toborek. 2007. Limited role of COX-2 in HIV Tat-induced alterations of tight junction protein expression and disruption of the blood-brain barrier. Brain Res. 1184:333-344. [DOI] [PubMed] [Google Scholar]
- 33.Reddy, M. A., P. V. Nemani, C. A. Wass, and K. S. Kim. 2000. Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in E. coli K1 invasion of human brain microvascular endothelial cells. J. Biol. Chem. 275:36769-36774. [DOI] [PubMed] [Google Scholar]
- 34.Rüffer, C., A. Strey, A. Janning, K. S. Kim, and V. Gerke. 2004. Cell-cell junctions of dermal microvascular endothelial cells contain tight and adherens junction proteins in spatial proximity. Biochemistry 43:5360-5369. [DOI] [PubMed] [Google Scholar]
- 35.Sapirstein, A., H. Saito, S. J. Texel, T. A. Samad, E. O'Leary, and J. V. Bonventre. 2005. Cytosolic phospholipase A2α regulates induction of brain cyclooxygenase-2 in a mouse model of inflammation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288:R1774-R1782. [DOI] [PubMed] [Google Scholar]
- 36.Serezani, C. H., C. D. M. Aronoff, S. Jancar, P. Mancuso, and M. Peters-Golden. 2005. Leukocytes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 106:1067-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stins, M. F., F. Gilles, and K. S. Kim. 1997. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 76:81-90. [DOI] [PubMed] [Google Scholar]
- 38.Stins, M. F., J. L. Badger, and K. S. Kim. 2001. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathogen 30:19-28. [DOI] [PubMed] [Google Scholar]
- 39.Teng, C., Y. Tseng, D. Pearce, Y. Xie, M. Paul-Satyaseela, and K. S. Kim. 2010. NlpI contributes to Escherichia coli K1 strain RS218 interaction with human brain microvascular endothelial cells. Infect. Immun. 78:3090-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, Y., and K. S. Kim. 2002. Role of OmpA and IbeB in Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo. Ped. Res. 51:559-563. [DOI] [PubMed] [Google Scholar]
- 41.Wang, Y., S. H. Huang, C. A. Wass, M. F. Stins, and K. S. Kim. 1999. The gene locus yijP contributes to Escherichia coli K1 invasion of brain microvascular endothelial cells. Infect. Immun. 67:4751-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whittle, I. R., I. R. Piper, and J. D. Miller. 1991. The contribution of arachidonic acid to the aetiology and pathophysiology of focal brain oedema; studies using an infusion oedema model. Acta Neurochir. (Wien) 113:57-68. [DOI] [PubMed] [Google Scholar]
- 43.Zhu, L., D. Schwegler-Berry, V. Castranova, and P. He. 2004. Internalization of caveolin-1 scaffolding domain facilitated by Antennapedia homeodomain attenuates PAF-induced increase in microvessel permeability. Am. J. Physiol. Heart Circ. Physiol. 286:H195-H201. [DOI] [PubMed] [Google Scholar]